Purpose of review
The gut microbiota has emerged as a key conduit in mental health and is a promising target for interventions. This review provides an update on recent advances in using microbiota-targeted approaches for the management of mental health.
Recent findings
Approaches that have emerged as microbiota-targeted interventions in the management of mental health include probiotics, prebiotics, synbiotics, fecal microbiota transplant as well as diet. Among these approaches, probiotic supplementation has been investigated most prominently, providing promising evidence for its use in improving mood and anxiety. There is also growing interest in the use of multistrain probiotics, whole dietary interventions or combined approaches, with encouraging results emerging from recent studies.
Summary
Although the current literature preliminarily supports targeting the microbiota to manage mental health and use as adjuvant therapies for certain brain disorders, large gaps remain and especially data including clinical cohorts remains scarce. Research studies including larger cohorts, well-characterized clinical populations and defined duration and dosage of the intervention are required to develop evidence-based guidelines for microbiota-targeted strategies.
Keywords: gut-targeted interventions, microbiota, microbiota-gut-brain axis
INTRODUCTION
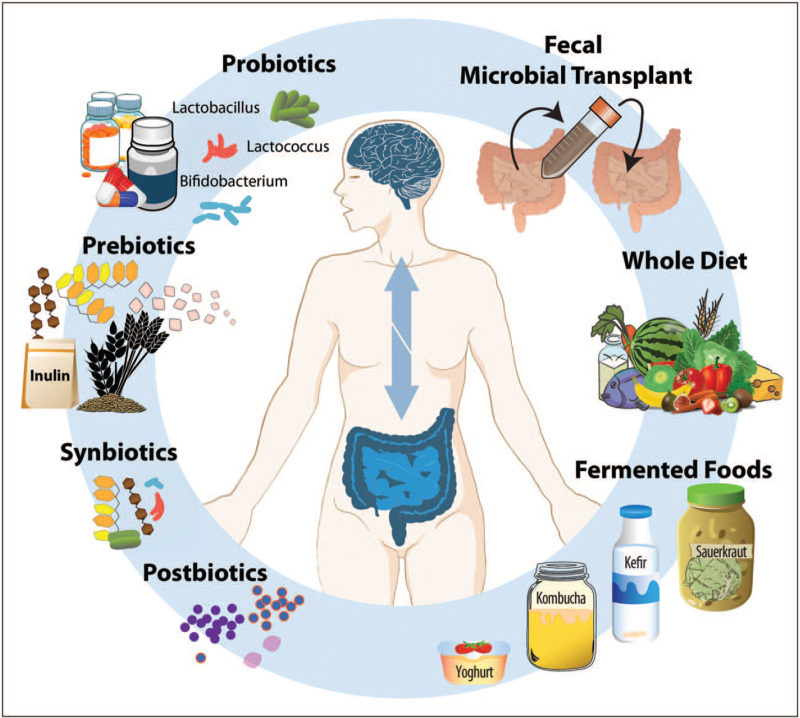
As a collection of trillions of bacteria, viruses, fungi, and archea residing on various parts of the human body, with the most densely populated area being the gastrointestinal tract, the gut microbiota has emerged as a central conduit in the regulation of important body processes, including brain and behavior. More than 1,000 species (a taxonomical classification of bacteria) have been identified in the human gut, associated with different functions and positive or negative effects for the host. Importantly, evidence is accumulating on the impact of the microbiota on mood and behavior and in clinical studies, differences in microbiota composition between diseased and healthy cohorts have been described [1▪]. However, despite the apparent common findings among studies, such as increased abundance of potential pro-inflammatory and lower concentration of health-promoting bacteria, identifying a disease-specific microbial profile remains challenging. Nevertheless, the involvement of the gut microbiota in mental disease processes and the potential to modify the microbiota through external factors, makes microbiota-targeted interventions a promising avenue for the development of new therapeutic approaches. Collectively, the exogenous factors whose benefit to mental health is partially mediated by the gut microbiota are referred to as psychobiotics [2]. Although the term originally referred to probiotics, additional approaches can now be regarded as potential psychobiotics, including prebiotics, synbiotics, postbiotics, fecal microbiota transplants, and dietary agents. A visual representation can be found in Fig. 1. This review outlines the state of the literature and latest advances in the development of microbiota-targeted interventions for the management of mental health and psychiatric disorders and discusses the gaps in knowledge that need to be filled in order to include gut-targeted therapies into the care of patients.
FIGURE 1.
Proposed microbiota-targeted interventions for mental health. Several microbiota-targeted interventions have been explored as potential approaches for mental health, including mood and anxiety disorders. These include psychobiotic supplements such as probiotics, prebiotics or synbiotics, as well as dietary approaches or fecal microbial transplants.
Box 1.
no caption available
PROBIOTICS
Probiotics are defined as ’live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’ [3]. The potential psychobiotic effect of probiotics has been studied extensively in the last decade, with the most studied strains being bifidobacteria or lactobacilli. Additionally, there has been a move to using microbial consortia (multiple strains of probiotics) in order to improve efficacy or induce additive and synergistic effects. In healthy populations, reductions in stress, anxiety and depression after probiotic supplementation are repeatedly demonstrated [4▪,5,6,7▪]. In patient populations, recently improved clinical symptoms of depression were observed in an open labeled pilot study in depressed patients using the combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 (CEREBIOME) [8,9] and a reduction in anxiety symptoms in a population with Generalized Anxiety Disorder was elicited through a multistrain probiotic containing B. longum, B. bifidum, B. lactis and L. acidophilus[10▪▪]. However, albeit promising results, randomized controlled trials in clinical populations remain scarce [11]. Additionally, it is important to note that studies using single [12] as well as multiple strains of probiotics do not always lead to the desired outcome [13]. Thus, establishing host factors that impact the personalized efficacy of probiotic supplementation, including habitual diet or baseline microbiota composition, will be important considerations for the development of future probiotic therapies. Of similar importance is strain specificity, meaning that one probiotic strain might be efficacious for a specific subtype in the disease of interest [14▪]. Furthermore, combined approaches could become more important, as demonstrated in a combined dietary–probiotic approach leading to a greater reduction in anxiety symptoms compared to each intervention alone [15]. Together, due to the limited evidence available to-date, the use of probiotics as a stand-alone therapy for mood disorders remains unsupported and some of the positive findings observed in preclinical models are yet to be translated into clinical practice [16▪▪].
PREBIOTICS
Prebiotics are defined as ‘substrates that are selectively utilized by the host microorganisms conferring a healthy benefit’ [17]. In light of potential technical and pragmatic difficulties associated with probiotic approaches, such as ensuring the survival of the probiotic transiting through changing pH levels of the digestive tract, the inability of probiotics to colonize the colon in the long-term, and selection of appropriate strains, supporting the growth of the already resident beneficial bacteria presents an exciting alternative opportunity; however, overall prebiotic approaches in mental health remain less studied when compared to probiotic interventions and the limited and inconclusive data are available from human cohorts demonstrate the need for more well defined clinical cohort studies [18]. For example, in a cohort of healthy females a reduction in preclinical anxiety after a galactooligosaccharide (GOS) intervention was observed [19▪], whereas no effect on depressive symptoms was detected in a population with Major Depressive Disorder (MDD) using the same prebiotic (GOS) [9]. Similar to probiotic interventions, future studies using prebiotics should also consider the impact of host factors, such as baseline microbiota composition [20▪▪], as well as dose and study duration, when evaluating the efficacy or prebiotics in supporting mental health.
It should also be mentioned that besides fermentable carbohydrates, other nutrients have been demonstrated to possess prebiotic properties, such as polyphenols or omega-3 fatty acids, with benefits to mental health and well-being. Polyphenol consumption, for example, has been linked to many health benefits, such as reduction in depressive symptoms, whereas eliciting concordant changes in the gut microbiota [21,22]. Similarly, omega-3 fatty acids have been proposed to restore a eubiotic state in pathological conditions by increasing bifidobacteria and decreasing enterobacteria [23▪], which could be associated with changes in behavior [24]. Thus, while much more investigations into the therapeutic effect are needed, these compounds might become available as microbiota-targeted interventions for mental health disorders.
SYNBIOTICS
Synbiotics, ‘a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host’, are emerging as another approach to modulate the gut microbiota and influence mood and behavior. Although the concept of synbiotics was first described 25 years ago, investigations into using synbiotics in managing symptoms of psychiatric disease are still few. Nevertheless, positive outcomes were reported in recent studies using synbiotic supplementation, demonstrating improvements in depression, stress and anxiety levels in specific populations, such as patients undergoing hemodialysis [25] or with coronary artery disease [26] as well as in professional athletes [27].
POSTBIOTICS AND MICROBIAL METABOLITES
Postbiotics and microbial metabolites have attracted interest as another potential gut-targeted intervention. Recently, postbiotics were defined as ‘preparations of inanimate microorganisms and/or their components that confers a health benefit on the host’ [28▪]. Accordingly, postbiotics are deliberately inactivated microbial cells or cell components, which must be present with or without metabolites in order to be effective. These health benefits could be mediated through alterations of the microbiota, enhancing of the intestinal barrier function, modulating of immune or metabolic responses or signaling via the nervous system [28▪]. Due to potential advantages regarding formulation, safety and regulation, the therapeutic use of postbiotics could become more and more available and open up new horizons in the treatment of psychiatric disorders; to-date, however, only a small of number of studies is available. Recent data from animal models support the use of postbiotics as anxiolytic- or antidepressive agents [29] and in human populations, specifically heat-inactivated microbes have shown some benefits in reducing anxiety as well as improving in sleep disturbance in chronically stressed students [30].
Although purified microbial components and products such as short chain fatty acids (SCFA), peptides or exopolysaccharides do not qualify as postbiotics themselves, they can be present in postbiotic preparations [28▪]. Because microbial metabolites, produced from microbial degradation of food components, are one of the mechanisms suggested to underlie the microbiota-brain cross talk, there is also interest in administering these metabolites directly as therapeutic agents. In this regard, data from animal models show promise, for example, in reducing behavioral deficits (depressive-like behavior) and stress responsiveness associated with psychosocial stress [31]. Other data from animal models have shown that administering microbial metabolites directly can elicit the same benefit for brain function as the diet itself [32▪▪,33]. Due to potential challenges in administering microbial products directly, data from human studies are limited. In one recent study, SCFAs directly administered to the colon using specialized pH-dependent colon-delivery capsules attenuated cortisol response to psychosocial stress [34▪]. It should be noted, that microbial metabolites, such as SCFAs, have also been associated with detrimental effects in certain neurological diseases, including Autism Spectrum Disorder [35] or Parkinson's Disease [36], indicating that further research is imperative to understand the use of microbial metabolites as gut-targeted intervention for psychiatric or mood disorders.
FECAL MICROBIAL TRANSPLANT
In light of the brain-modulating potential of the microbiota, the idea of transferring an entire microbial consortium to treat brain-disorders appears to be an exciting opportunity. Especially the potential of a long-lasting reconstructing of the existing ecosystem makes fecal microbial transplant (FMT) an appealing treatment approach for patients with psychiatric diseases and associated microbial imbalances. However, due to possible safety risks and other unknowns, studies investigating the efficacy of FMTs in psychiatric disorders are at very early stages. Data from animal models showing that FMT can be efficacious in reversing adverse behaviors and neuroinflammation and improve anxiety- and depression-like behavior [37▪,38] are establishing a promising basis for clinical trials. However, most human studies to date have been small or case control studies [39]; only one larger human trial including 83 patients with inflammatory bowel disease and co-morbid self-reported depression reported significant improvements in fatigue after FMT [40▪▪]. Thus, insufficient data is currently available to support the use of FMT to treat psychiatric disease in clinical practice and many procedures, such as pretreatment (e.g., antibiotics), route of administration or choice of donors, need to be standardized in order to develop targeted FMT approaches.
WHOLE DIETARY INTERVENTIONS
The importance of diet in supporting mental health and cognitive function is increasingly recognized. Extensive observational data from large cohorts from a variety of different countries and cultures have linked dietary patterns (e.g., Mediterranean diet, Japanese diet) [41,42] and diet quality [43], including higher fiber [44] and fruit intake [45] to better mental health outcomes. Due to both the established connection between the microbiota and brain and the profound effect of diet on microbiota composition, such as the association between long-term dietary patterns and anti-inflammatory properties of the gut microbiota [46▪▪], a feature that is often underlying mental health disorders, developing microbiota-targeted dietary interventions is of increasing interest in the scientific field and investigations into the potential benefits of healthy diets in mediating the microbiota–brain interaction are starting to emerge. In animal models, microbiota-dependent benefits of intermittent fasting [32▪▪] and a ketogenic diet have been documented [47]. In human cohorts, an 8-week nutrition education program focusing on microbiota-friendly foods decreased depression scores and increased self-rated health and subjected well-being score in an obese population in Japan [48]. Similarly, a 12-month Mediterranean diet program induced increases in microbial taxa that were associated with improved cognition and reduced risk of frailty and inflammation in elderly individuals in a large, multicountry study (Nutrition in Elderly People (NU-AGE) cohort) [49▪▪]. This sparse number of human cohort studies available makes it difficult to provide evidence-based recommendations for the use of specific diets in improving mental health or to treat some symptoms of disease [50,51]. Thus, future high-quality and large cohort studies are imperative to further our understanding of this promising field. Furthermore, the individualized responsiveness to dietary interventions will need to be further investigated in order to determine which diet an individual could benefit from the most. Thereby, various factors could determine an individual's systemic response to the intervention, including microbiota composition [52,53]. Due to the bioavailability of some nutrients (such as dietary fiber and polyphenols) depending on the conversion by resident microbes, determining whether an individual’ s microbiota contains the enzymatic repertoire necessary to benefit from a certain diet will be an important consideration. Thus, with sequencing techniques becoming more readily available, including an individual's microbial profile into the development of nutrition therapy might become a possibility.
FERMENTED FOODS
Although fermented foods have been consumed by humans for centuries, a recent surge in its popularity, due to the many health benefits associated with its consumption, has led to new investigations regarding its use as treatment approaches. Importantly, it has been proposed that the health benefits of fermented foods, ‘foods made through desired microbial growth and enzymatic conversions of food components’, could be associated with the presence of live microorganisms, prebiotics and potential microbial metabolites produced through the fermentation process [54▪]. Some convincing data is presented in the literature regarding the potential of fermented foods to affect microbiota-brain signaling, both from preclinical [55] and clinical studies [56–58]. Although no more recent clinical studies are available and generally interventional studies are sparse, these earlier trials suggest that fermented foods could modify brain activity, stress responses, mood, and anxiety. However, due to heterogeneity in population, small sample sizes, fermented food product used and outcomes measured, drawing any conclusions on the use of fermented foods in supporting mental health is difficult. Nevertheless, results hold promise and the potential of fermented foods to influence pathways underlying psychiatric diseases, such as the microbiota and immune system [59], make the use of fermented foods a promising candidate for gut-targeted intervention.
CONCLUSION
Interest in developing gut-target interventions for brain disorders has exploded with the discovery of the bidirectional signaling between the gut microbiota and the brain. A plethora of review articles is available, outlining the potential of using psychobiotics in the management of psychiatric disease; however, whereas many advances have been made in the field and the current state of the literature provides a promising outlook toward the use of microbiota-targeted interventions as adjuvant therapies for some diseases (such as depression), the likelihood of these therapies replacing pharmacological agents is still in the distant future and especially greater evidence from clinical populations is needed to conclusively provide evidence-based recommendations. Thereby, several aspects are important to note when interpreting the current state of the literature and need to be addressed to further advance the field. The personalized nature of gut-targeted interventions is a critical consideration and factors determining responsiveness to an intervention (including availability of microbial enzymatic repertoire) will be essential for the advancement of these personalized approaches. In individuals missing certain microbial functions, it may be that future nutritional interventions will combine dietary approaches with specifically designed probiotics in order for the intervention to be effective. Similarly, choosing the ‘right’ intervention, the dosage as well as duration of treatment needs to be considered. Probiotics often do not colonize the gut and thus do not become members of the gut community, which usually makes it necessary to continue to ingest probiotics. Prebiotics or diet, on the other hand, have the option to change the microbiota; however, whether the microbiota will go back to baseline after the intervention is stopped is to be determined. Thus, it is likely that most gut-targeted approaches will be life-long interventions.
Given that conventional pharmacological treatments might not work for everyone and the promising evidence available regarding the manipulation of the microbiota to elicit health benefits, make gut-targeted intervention an exciting treatment opportunity for patients suffering from psychiatric or psychological disorders. However, multiple unknowns continue to exist and large cohort studies with well defined populations, especially in heterogeneous diseases, and well defined and specific outcome measures are required to fill the huge research gap in the years to come.
Acknowledgements
The authors would like to thank Dr Kenneth J. O’Riordan for his assistance with figure design.
Funding: No specific funding was received for this work.
Financial support and sponsorship
APC Microbiome Ireland is a research center funded by Science Foundation Ireland (SFI), through the Irish Government's National Development Plan [grant number 12/RC/2273 P2]. K.B. has received a postdoctoral fellowship from the Irish Research Council [grant number GOIPD/2019/33]. The Cryan Laboratory is also funded by the Saks-Kavanaugh Foundation.
Conflicts of interest
J.F.C. has been an invited speaker at meetings organized by Mead Johnson, Friesland, Neuropharmex, Yakult, and Alkermes, has been a consultant for Nestle, and has received research funding from Mead Johnson, Cremo, Nutricia, and DuPont. K.B. has been an invited speaker at a meeting organized by Yakult.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Simpson CA, Diaz-Arteche C, Eliby D, et al. The gut microbiota in anxiety and depression – a systematic review. Clin Psychol Rev 2021; 83:101943. [DOI] [PubMed] [Google Scholar]; This systematic review provides a recent update on the microbial differences that can be observed in patients with anxiety and depression. It highlights the potential of the gut microbiota in regulating mood and anxiety and outlines future research needs to further advance the field.
- 2.Sarkar A, Lehto SM, Harty S, et al. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci 2016; 39:763–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill C, Guarner F, Reid G, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014; 11:506–514. [DOI] [PubMed] [Google Scholar]
- 4▪.Wu SI, Wu CC, Tsai PJ, et al. Psychobiotic supplementation of PS128(TM) improves stress, anxiety, and insomnia in highly stressed information technology specialists: a pilot study. Front Nutr 2021; 8:614105. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study offers insight from a human population of the efficacy in probiotics to alleviate stress and improve mood in an high stress environment. While a pilot study, it provides evidence that probiotics can be efficacious in reducing stress-associated mental and physiological outcomes.
- 5.Patterson E, Griffin SM, Ibarra A, et al. Lacticaseibacillus paracasei Lpc-37® improves psychological and physiological markers of stress and anxiety in healthy adults: a randomized, double-blind, placebo-controlled and parallel clinical trial (the Sisu study). Neurobiol Stress 2020; 13:100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lew LC, Hor YY, Yusoff NAA, et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study. Clin Nutr 2019; 38:2053–2064. [DOI] [PubMed] [Google Scholar]
- 7▪.Ma T, Jin H, Kwok L-Y, et al. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol Stress 2021; 14:100294. [DOI] [PMC free article] [PubMed] [Google Scholar]; In addition to compositional changes, this study also investigated functional changes in the microbiota after probiotic supplementation. Thus, it provides insight into potential pathways underlying the stress-relieving and anxiolytic effect of probiotics.
- 8.Wallace CJK, Milev RV. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry 2021; 12:618279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazemi A, Noorbala AA, Azam K, et al. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr 2019; 38:522–528. [DOI] [PubMed] [Google Scholar]
- 10▪▪.Eskandarzadeh S, Effatpanah M, Khosravi-Darani K, et al. Efficacy of a multispecies probiotic as adjunctive therapy in generalized anxiety disorder: a double blind, randomized, placebo-controlled trial. Nutr Neurosci 2021; 24:102–108. [DOI] [PubMed] [Google Scholar]; This study is the first randomized controlled trial of a probiotic in anxiety disorders. It provides evidence that probiotics could potentially be used as add-on therapies in the management of anxiety disorders. Thus, the study might have important impact for the management of anxiety symptoms and on future research in the field.
- 11.Mörkl S, Butler MI, Holl A, et al. Probiotics and the microbiota-gut-brain axis: focus on psychiatry. Curr Nutr Rep 2020; 9:171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly JR, Allen AP, Temko A, et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun 2017; 61:50–59. [DOI] [PubMed] [Google Scholar]
- 13.Chahwan B, Kwan S, Isik A, et al. Gut feelings: a randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J Affect Disord 2019; 253:317–326. [DOI] [PubMed] [Google Scholar]
- 14▪.Stenman LK, Patterson E, Meunier J, et al. Strain specific stress-modulating effects of candidate probiotics: a systematic screening in a mouse model of chronic restraint stress. Behav Brain Res 2020; 379:112376. [DOI] [PubMed] [Google Scholar]; This study provides an important outlook on the specificity of probiotics strain. Such research will guide the development of specific psychobiotic therapies for mental health.
- 15.Colica C, Avolio E, Bollero P, et al. Evidences of a new psychobiotic formulation on body composition and anxiety. Mediat Inflamm 2017; 2017:5650627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪▪.Nikolova VL, Cleare AJ, Young AH, Stone JM. Updated review and meta-analysis of probiotics for the treatment of clinical depression: adjunctive vs. stand-alone treatment. J Clin Med 2021; 10:647. [DOI] [PMC free article] [PubMed] [Google Scholar]; This analysis provides a most up-to-date review of the literature regarding the efficacy of probiotics in managing depressive symptoms. It highlights that currently probiotics are efficacious in reducing depression symptoms only in conjunction with other therapies, such as antidepressants.
- 17.Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017; 14:491–502. [DOI] [PubMed] [Google Scholar]
- 18.Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev 2019; 102:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪.Johnstone N, Milesi C, Burn O, et al. Anxiolytic effects of a galacto-oligosaccharides prebiotic in healthy females (18–25 years) with corresponding changes in gut bacterial composition. Sci Rep 2021; 11:8302. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this most recent study with a relatively large sample, narrow age range and well defined outcomes in a healthy population, an anxiolytic and bifidogenic effect of the prebiotic was demonstrated. The results contribute to the small but growing level of evidence regarding the use of prebiotics as psychobiotic agents.
- 20▪▪.Leyrolle Q, Cserjesi R, D G H Mulders M., et al. Prebiotic effect on mood in obese patients is determined by the initial gut microbiota composition: a randomized, controlled trial. Brain Behav Immun 2021; 94:289–298. [DOI] [PubMed] [Google Scholar]; This study highlights the importance of host features, specifically microbial, metabolic and immune profile, in determining the response to a prebiotic intervention. such data will be important in the development of personalized psychobiotic approaches.
- 21.Donoso F, Egerton S, Bastiaanssen TFS, et al. Polyphenols selectively reverse early-life stress-induced behavioural, neurochemical and microbiota changes in the rat. Psychoneuroendocrinology 2020; 116:104673. [DOI] [PubMed] [Google Scholar]
- 22.Park M, Choi J, Lee HJ. Flavonoid-rich orange juice intake and altered gut microbiome in young adults with depressive symptom: a randomized controlled study. Nutrients 2020; 12:1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.Vijay A, Astbury S, Le Roy C, et al. The prebiotic effects of omega-3 fatty acid supplementation: a six-week randomised intervention trial. Gut Microbes 2021; 13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the prebiotic effect of omega-3 fatty acids at physiological relevant doses. It therefore supports the notion that some health effects of omega-3 fatty acids could be moderated by the gut microbiota.
- 24.Robertson RC, Seira Oriach C, Murphy K, et al. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun 2017; 59:21–37. [DOI] [PubMed] [Google Scholar]
- 25.Haghighat N, Rajabi S, Mohammadshahi M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutr Neurosci 2021; 24:490–499. [DOI] [PubMed] [Google Scholar]
- 26.Moludi J, Khedmatgozar H, Nachvak SM, et al. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: a randomized clinical trial. Nutr Neurosci 2021; 1–10. [DOI] [PubMed] [Google Scholar]
- 27.Quero CD, Manonelles P, Fernández M, et al. Differential health effects on inflammatory, immunological and stress parameters in professional soccer players and sedentary individuals after consuming a synbiotic. a triple-blinded, randomized, placebo-controlled pilot study. Nutrients 2021; 13:1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪.Salminen S, Collado MC, Endo A, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol 2021; 18:649–667. [DOI] [PMC free article] [PubMed] [Google Scholar]; This consensus statement provides a clear definition on postbiotic and summarizes evidence available on health benefits. These clear guidelines will be a foundation for future research on postbiotics and aid in the development of new postbiotic products.
- 29.Wei CL, Wang S, Yen JT, et al. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res 2019; 1711:202–213. [DOI] [PubMed] [Google Scholar]
- 30.Nishida K, Sawada D, Kuwano Y, et al. Health benefits of Lactobacillus gasseri CP2305 tablets in young adults exposed to chronic stress: a randomized, double-blind, placebo-controlled study. Nutrients 2019; 11:1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Wouw M, Boehme M, Lyte JM, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain–gut axis alterations. J Physiol 2018; 596:4923–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪▪.Liu Z, Dai X, Zhang H, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun 2020; 11:855. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that the microbiota is necessary for the health-promoting effect of certain diets. Such data can have important implications for the development of microbiota-targeted dietary approaches.
- 33.Johnson SL, Kirk RD, DaSilva NA, et al. Polyphenol microbial metabolites exhibit gut and blood(-)brain barrier permeability and protect murine microglia against LPS-induced inflammation. Metabolites 2019; 9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪.Dalile B, Vervliet B, Bergonzelli G, et al. Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: a randomized, placebo-controlled trial. Neuropsychopharmacology 2020; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes that directly delivering microbial metabolites (short chain fatty acids) to a human cohort can have stress-alleviating effects. Thus, the study provides novel results form a human cohort supporting the role of microbial metabolites in the microbiota-gut-brain communication.
- 35.Berding K, Donovan SM. Diet can impact microbiota composition in children with autism spectrum disorder. Front Neurosci 2018; 12:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulak A. A controversy on the role of short-chain fatty acids in the pathogenesis of Parkinson's disease. Mov Disord 2018; 33:398–401. [DOI] [PubMed] [Google Scholar]
- 37▪.Rao J, Qiao Y, Xie R, et al. Fecal microbiota transplantation ameliorates stress-induced depression-like behaviors associated with the inhibition of glial and NLRP3 inflammasome in rat brain. J Psychiatr Res 2021; 137:147–157. [DOI] [PubMed] [Google Scholar]; This study shows that fecal microbial transplant can have antidepressive effects through reduction of neuroinflammation. It thus contributes to furthering our understanding on the use of fecal microbial transplant for mental health, especially depression.
- 38.Schmidt EKA, Torres-Espin A, Raposo PJF, et al. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLos One 2020; 15:e0226128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chinna Meyyappan A, Forth E, Wallace CJK, Milev R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatry 2020; 20:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪▪.Johnsen PH, Hilpüsch F, Valle PC, Goll R. The effect of fecal microbiota transplantation on IBS related quality of life and fatigue in moderate to severe nonconstipated irritable bowel: secondary endpoints of a double blind, randomized, placebo-controlled trial. EBioMedicine 2020; 51:102562. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is one of the larger human cohort studies showing efficacy of fecal microbial transplant on depressive symptoms. It thus translates some preclinical findings into clinical cohorts and provides evidence for the potential use of fecal microbial transplant in patients with psychiatric disease.
- 41.Konishi K. Associations between healthy Japanese dietary patterns and depression in Japanese women. Public Health Nutr 2021; 24:1753–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esgunoglu L, Jennings A, Connole ES, et al. Short-term effects of a Mediterranean-style dietary pattern on cognition and mental wellbeing: A systematic review of clinical trials. Br J Nutr 2021; 1–28. [DOI] [PubMed] [Google Scholar]
- 43.Meller FO, Manosso LM, Schäfer AA. The influence of diet quality on depression among adults and elderly: a population-based study. J Affect Disord 2021; 282:1076–1081. [DOI] [PubMed] [Google Scholar]
- 44.Fatahi S, Matin SS, Sohouli MH, et al. Association of dietary fiber and depression symptom: a systematic review and meta-analysis of observational studies. Complement Ther Med 2020; 56:102621. [DOI] [PubMed] [Google Scholar]
- 45.Taylor AM, Thompson SV, Edwards CG, et al. Associations among diet, the gastrointestinal microbiota, and negative emotional states in adults. Nutr Neurosci 2020; 23:983–992. [DOI] [PubMed] [Google Scholar]
- 46▪▪.Bolte LA, Vich Vila A, Imhann F, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021; 70:1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes that long-term dietary patterns are an important influence on functional properties of the gut microbiota. Given that inflammation is often underlying mental health diseases, these results contribute to the development of microbiota-targeted dietary approaches to improve mental well being.
- 47.Olson CA, Vuong HE, Yano JM, et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 2018; 173:1728–1741. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uemura M, Hayashi F, Ishioka K, et al. Obesity and mental health improvement following nutritional education focusing on gut microbiota composition in Japanese women: a randomised controlled trial. Eur J Nutr 2019; 58:3291–3302. [DOI] [PubMed] [Google Scholar]
- 49▪▪.Ghosh TS, Rampelli S, Jeffery IB, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 2020; 69:1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a large, multicountry human cohort study demonstrating that the Mediterranean diet can change features of the microbiota which are associated with peripheral health markers. It thus provides one of the first human data showing that the microbiota mediates some of the positive effects of diet on human health.
- 50.Adan RAH, van der Beek EM, Buitelaar JK, et al. Nutritional psychiatry: Towards improving mental health by what you eat. Eur Neuropsychopharmacol 2019; 29:1321–1332. [DOI] [PubMed] [Google Scholar]
- 51.Grosso G. Nutritional psychiatry: how diet affects brain through gut microbiota. Nutrients 2021; 13:1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu T, Pan L, Shang Q, Yu G. Fermentation of alginate and its derivatives by different enterotypes of human gut microbiota: Towards personalized nutrition using enterotype-specific dietary fibers. Int J Biol Macromol 2021; 183:1649–1659. [DOI] [PubMed] [Google Scholar]
- 53.Johnson AJ, Vangay P, Al-Ghalith GA, et al. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 2019; 25:789–802. e5. [DOI] [PubMed] [Google Scholar]
- 54▪.Marco ML, Sanders ME, Gänzle M, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat Rev Gastroenterol Hepatol 2021; 18:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this consensus statement, fermented foods are defined, and key classifications are outlined. It thus provides an important guide and considerations to future research studies investigating the effects of fermented foods on the host.
- 55.van de Wouw M, Walsh CJ, Vigano GMD, et al. Kefir ameliorates specific microbiota-gut-brain axis impairments in a mouse model relevant to autism spectrum disorder. Brain Behav Immun 2021; S0889-1591(21)00268-3. [DOI] [PubMed] [Google Scholar]
- 56.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013; 144:1394–1401. 401 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kato-Kataoka A, Nishida K, Takada M, et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef Microbes 2016; 7:153–156. [DOI] [PubMed] [Google Scholar]
- 58.Takada M, Nishida K, Kataoka-Kato A, et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol Motil 2016; 28:1027–1036. [DOI] [PubMed] [Google Scholar]
- 59.Wastyk HC, Fragiadakis GK, Perelman D, et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021; S0092-8674(21)00754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]