Abstract
Background:
Data on HIV-1 controllers in Africa are scarce. We report the proportion of HIV-1 controllers in a group of adults prospectively monitored with frequent viral load measurements as part of a clinical trial in West Africa.
Methods:
For the Temprano trial, antiretroviral therapy (ART)-naive HIV-1 infected adults with no criteria for starting ART were randomized to start ART immediately or defer ART until the WHO starting criteria were met. Plasma viral load was measured every 6 months. The trial follow-up was 30 months. We considered all Temprano participants randomized to defer ART. Patients with all semestrial viral <2000 copies/ml and still off ART at month 30 were defined as HIV-1 controllers. Controllers with all viral loads <50 copies/ml were defined as elite controllers, the rest as viremic controllers.
Results:
Of the 1023 HIV-1-infected adults randomized in the Temprano deferred-ART group, 18 (1.8%) met the criteria for classification as HIV controllers, of whom seven (0.7%) were elite controllers and 11 (1.1%) viremic controllers. The HIV-1 controllers had low peripheral blood mononuclear cell HIV-1 DNA and low inflammatory marker levels. They maintained high CD4+ cell count and percentages and had a low morbidity rate.
Discussion:
HIV controllers exist in Africa at a proportion close to that reported elsewhere. They represent a small fraction of all HIV-1-infected patients but raise important questions. Further studies should assess whether starting ART might represent more risk than benefit for some controllers, and where it does, how to identify these patients before they start ART.
Keywords: adults, elite controllers, HIV-1 controllers, sub-Saharan Africa, viremic controllers
Introduction
Untreated HIV-1 infection leads to AIDS and death in the vast majority of patients. A minority of HIV-1-infected persons, known as long-term non-progressors, remain asymptomatic and maintain high CD4+ cell count without the use of antiretroviral therapy (ART) [1–4]. A smaller group, known as HIV-1 controllers, control HIV-1 replication spontaneously and persistently without treatment [4–6]. Factors associated with control of the virus include a low reservoir level, a strong HIV-specific T-CD4 and T-CD8 cells immune response, the presence of alleles HLA-B∗57 and HLA-B∗27, and the presence of antiviral restriction factors, among several other immune or genetic factors [7–12].
Data on HIV-1 controllers in sub-Saharan Africa are scarce [13–15] and opportunities to record new data are getting fewer. In the past, many HIV-1-infected individuals living in sub-Saharan Africa had poor access to routine viral load monitoring before they were put on ART. Because ART is now recommended for all HIV-1-infected individuals irrespective of their CD4+ cell count, data on monitored patients who are not on ART will not be recorded in future.
Here we describe the proportion of HIV-1 controllers identified in Côte d’Ivoire, West Africa, in a group of HIV-infected adults people who were followed without ART in the framework of a clinical trial from 2008 to 2015.
Methods
Patients and follow-up
Temprano ANRS 12136 was a 2 × 2 factorial plan randomized trial of early ART and isoniazid preventive therapy (IPT), the methods and results for which have been reported previously [16]. In summary, 2056 HIV-infected, ART-naive adults with no criteria for starting ART were randomized to start ART immediately (early-ART group) or defer starting until the WHO criteria for starting ART were met (deferred-ART group); to be given or not given 6 months of IPT. The CD4+ cell count and HIV-1 plasma viral load (VL) were measured on enrollment and every 6 months thereafter. At the end of the trial, the HIV-1 DNA in the peripheral blood mononuclear cells (PBMCs) was measured in all patients and several plasma inflammatory markers were measured in the first 477 participants, using blood samples that had been taken on enrollment and frozen at −80°C [17,18].
The primary analysis showed that the participants randomized to early-ART had a lower risk of severe morbidity at 30 months [16]. The long-term follow-up analysis showed that participants randomized to IPT and those with low plasma soluble vascular cell adhesion molecule-1 (sVCAM-1) and soluble cluster of differentiation 14 (sCD14) at baseline had a lower risk of mortality [17,19].
Here, we report data from enrollment to month 30 for all trial participants with a baseline plasma HIV-1 RNA of <2000 copies/ml who were randomized to the deferred-ART group.
Definitions
We defined as HIV-1 controllers (HIC) patients still alive and off ART at month 30, who had at least three VLs measurements between baseline and month 30 and for whom all available VLs were below 2000 copies/ml. Controllers with every VL measurement below 50 copies/ml were defined as elite controllers, and the rest as viremic controllers. One blip interim value of above 50 copies/ml (EC) or above 2000 copies/ml (VL) occurring within a significant morbidity event was accepted [20]. All other patients were classified as progressors. In this report, we distinguish progressors who did not start ART (progressors-no ART) from those who started ART (progressors-ART) during trial follow-up.
Serological, immunological and virological assays
All participants in the Temprano trial were tested at least twice for HIV serology. Those who had a negative baseline plasma HIV-1 RNA were tested a third time, using different techniques. The tests were carried out following the recommendations in force in the national HIV program of Côte d’Ivoire (two different ELISA tests, one sensitive and one specific and discriminating for HIV-1 and HIV-2). People who were positive for HIV-2 alone were not included in the trial. People with a positive serology for HIV-1 alone, or for both HIV-1 and HIV2, were included. The latter represented 2.5% of all those included [16].
The CD4+ percentage and absolute count (True Count technique on FACScan, Becton Dickinson) and plasma HIV-1 RNA (real-time polymerase-chain reaction assay, generic HIV viral load, Biocentric, threshold of detectability 50 copies/ml) were carried out in the CeDReS laboratory at the Treichville University Hospital in Côte d’Ivoire. PBMCs HIV-1 DNA (real-time PCR, Biocentric, Bandol, France) and inflammatory biomarkers (commercially available enzyme-linked immunosorbent assays) were carried out in France using techniques previously reported [17,18]. The technique was based on the amplification of the long terminal repeat (LTR) gene well adapted to HIV-1 non B sub-types and referring to the 8E5 cell line containing one HIV copy per cell (HIV DNA cell, Biocentric, Bandol, France). The threshold of the technique was five copies of HIV-1 DNA per PCR well. The HIV-1 DNA load was expressed as a proportion of infected cells among the target cells. Results were expressed as the HIV-1 DNA log10 copy number per million PBMC.
Statistical analysis
This is a post-hoc analysis of the Temprano trial data. Continuous variables are described using medians and interquartile ranges (IQR). Categorical variables are described using numbers and percentages. We used Wilcoxon rank sum test to compare the distribution of each inflammatory biomarker at baseline between controllers (elite and viremic controllers in one group) and progressors (ART and no-ART in the other group).
The analysis was conducted using SAS software, version 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Funder, registration and ethics
The Temprano protocol was approved by the Côte d’Ivoire National Ethics Committee for Health Research. It was registered at ClinicalTrials.gov (NCT00495651). Informed consent was given prior to participation in the trial. The study sponsor (Agence Nationale de Recherches sur le Sida et les hépatites virales) played no part in the conduct of the study or interpretation of the data.
Results
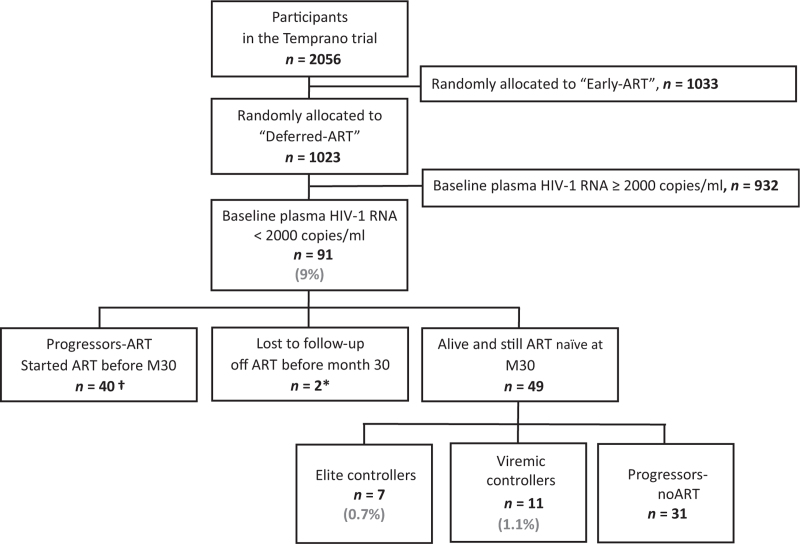
From March 2008 to June 2012, 1023 patients were enrolled on the Temprano and randomly allocated to the deferred-ART group. Ninety one (9%) of them had a baseline plasma HIV-1 RNA below 2000 copies/ml. Of these 91 participants, 40 started ART before month 30, two were lost to follow-up while still off ART, and 49 were still in active follow-up, off ART, at month 30 (Fig. 1).
Fig. 1.
Flow chart.
∗These two patients were lost to follow-up while still off ART and after only one viral load measurement. †These 40 patients started ART before the end of the trial. ART, antiretroviral therapy.
The 49 patients still off ART and alive at month 30 had a total of 211 plasma HIV-1 RNA cell measurements (median 5.0, IQR 4.0–5.0) and 212 CD4+ cell count (median 5.0, IQR 4.0–5.0) measurements between month 6 and month 30, which corresponds to 86.1% and 86.5% of the 245 semestrial plasma HIV-1 RNA and CD4+ cell count measurements scheduled in the protocol between month 6 and month 30. Based on the above definition, seven patients (0.7%) were classified as elite controllers, 11 (1.1%) as viremic controllers, 31 (3.0%) as progressors-no ART and 40 (3.9%) as progressors-ART (Fig. 1). The four groups were similar in age, sex and CD4+ cell count or percentage distribution at baseline. The elite and viremic controllers had lower baseline HIV-1 DNA values than participants in the progressor groups. The percentage of participants with a baseline HIV-1 RNA <50 copies/ml was 64% in viremic controllers, 48% in progressors-no ART and 17% in progressor-ARTs (Table 1). The majority of participants in all groups were women (Table 1).
Table 1.
HIV-1 infected adults randomized in the deferred ART group of Temprano with a plasma HIV-1 RNA below 2000 copies/ml (N = 89a).
| Did not start ART before 30 months | Started ART before 30 months | |||
| Elite controllers n = 7 | Viremic controllers n = 11 | Progressors no ART n = 31 | Progressors ART n = 40 | |
| Baseline | ||||
| Age, years, median (IQR) | 34 (27–49) | 35 (32–44) | 36 (31–42) | 34 (30–42) |
| Sex, female, n (%) | 6 (85.7) | 10 (90.9) | 26 (83.9) | 38 (95.0) |
| WHO clinical stage 1 or 2, n (%) | 7 (100) | 11 (100) | 29 (93.5) | 37 (92.5) |
| Body mass index (kg/m2), median (IQR) | 24.0 (21.4–26.6) | 25.0 (22.8–29.7) | 24.3 (21.8–26.9) | 22.9 (21.0–26.0) |
| Hemoglobin (g/l), median (IQR) | 128 (116–131) | 118 (110- 124) | 114 (107–127) | 109 (99–117) |
| Positive HBs antigen, n (%) | 0 (0) | 1 (9.1) | 2 (6.5) | 3 (7.5) |
| CD4+ cell count/μl, median (IQR) | 595 (508–685) | 514 (471–611) | 556 (476–668) | 451 (336–521) |
| CD4+ percentage, median (IQR) | 31.2 (21.7–35.4) | 28.2 (22.8–32.3) | 25.5 (23.0–28.5) | 21.5 (18.6–25.9) |
| Plasma HIV-1 RNA, n (%) | ||||
| <50 copies/ml | 7 (100) | 7 (63.6) | 15 (48.4) | 7 (17.5) |
| 50–2000 copies/ml | 0 | 4 (36.4) | 16 (51.6) | 33 (82.5) |
| HIV-1 DNA, log10 copies/106 PBMC, median (IQR) | 0.0 (0.0–1.3) | 1.6 (1.4–1.7) | 2.1 (1.9–2.5) | 2.4 (2.0–2.7) |
| Follow-up | ||||
| Received isoniazid Preventive therapy, n (%) | 3 (42.9) | 6 (54.5) | 11 (35.5) | 23 (57.5) |
| Delta CD4+ cell count M0 to M30, median (IQR) | −38 (−166; +62) | +38 (−1; +85) | −71 (−143; +83) | +103 (+34; +245) |
| Serious adverse event (SAE), n (%) | ||||
| Patients with at least one SAE | 0 (0) | 1 (9.1) | 1 (3.2) | 4 (10.0) |
| Number of SAE | 0 | 1b | 1c | 5d |
ART, antiretroviral therapy; IQR, interquartile range; PBMC, peripheral blood mononuclear cells.
The two patients lost to follow-up while still off ART are not included in this table.
Pyelonephritis in a patient with decompensated diabetes.
Pelvic carcinosis, n = 1.
Pulmonary tuberculosis, n = 3; pyelonephritis, n = 1; profound lymph-node tuberculosis, n = 1.
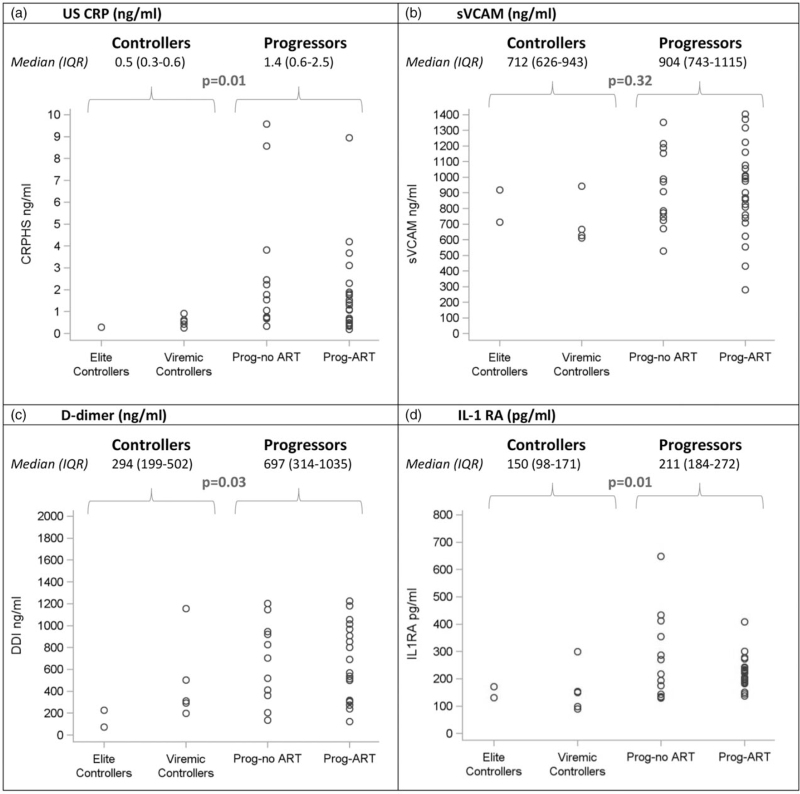
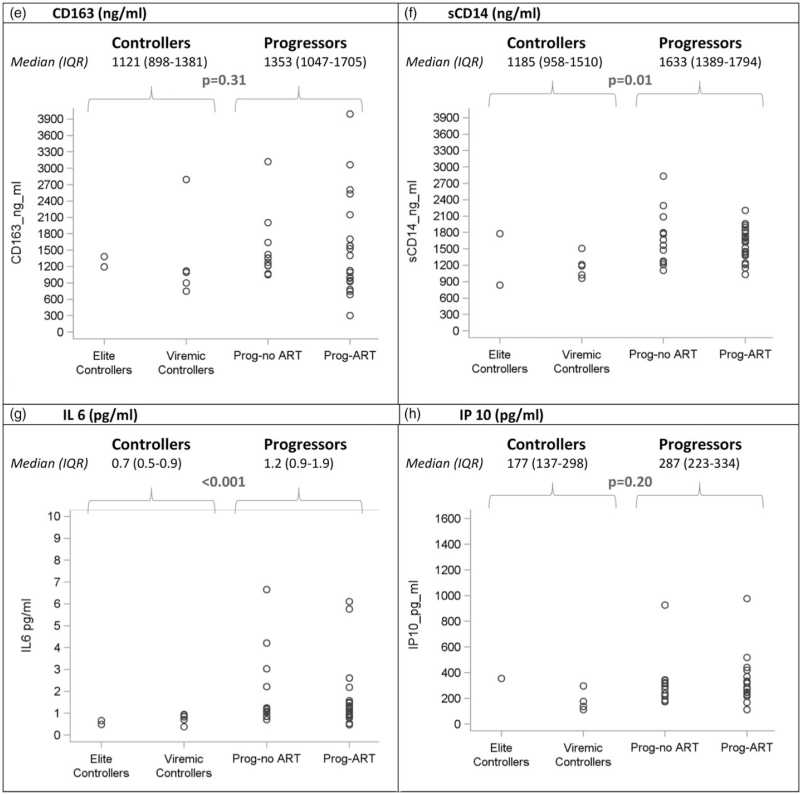
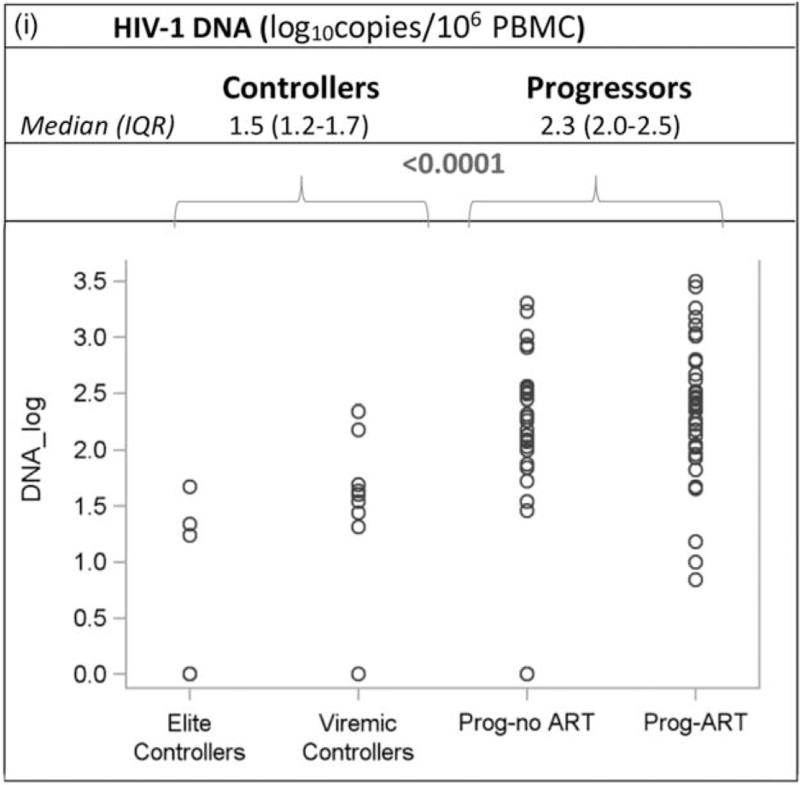
Table 2 shows all recorded CD4+ cell counts and plasma HIV-1 RNA values for participants who matched the definition for elite or viremic controller. Two elite controllers (EC1 and EC7) had a single VL blip above 50 copies/ml, and one viremic controller (VC4) had a single interim VL of above 2000 copies/ml during a documented malaria episode. Of the 30 CD4+ cell count measured for the elite controllers over the entire follow-up, two (6.7%) were below 350 cells/μl 3 (10.0%) were between 350 and 399 cells/μl and 25 (83.3%) were 500 cells/μl or higher. Of the 50 CD4+ cell counts measured for the viremic controllers over the entire follow-up, three (6.0%) were below 350 cells/μl, 19 (38.0%) were between 350 and 499 cells/μl and 28 (56.0%) were 500 cells/μl or higher. Of the 89 patients in the four groups, 43 patients participated in the sub-study of immunoinflammatory markers, including two elite controllers, five viremic controllers, 23 progressors-ART and 13 progressors-no ART. Figure 2 shows the values for all groups. The level of interleukin-1 receptor antagonist, sCD14, ultra-sensitive C-reactive protein, IL-6, D-dimer was significantly lower in controllers compared to progressors. The difference between both groups did not reach significance for IP10, CD163 and sVCAM.
Table 2.
Baseline characteristics and CD4+ and HIV-1 viral load values over time in elite controllers and viremic controllers.
| Patient | Sex | Age | Baseline HIV-1 DNA a | Baseline HIV-1 RNA b | M6 HIV-1 RNA b | M12 HIV-1 RNA b | M18 HIV-1 RNA b | M24 HIV-1 RNA b | M30 HIV-1 RNA b | Baseline CD4+ cell countc (%) | M6 CD4+ cell countc (%) | M12 CD4+ cell countc (%) | M18 CD4+ cell countc (%) | M24 CD4+ cell countc (%) | M30 CD4+ cell countc (%) |
| EC 1 | F | 34 | 46 | 0 | 0 | 0 | 125 | 0 | 0 | 592 (35%) | 426 (38%) | 598 (37%) | 619 (40%) | 536 (37%) | 528 (31%) |
| EC 2 | F | 35 | 17 | 0 | 0 | 0 | Missing | 0 | 0 | 508 (24%) | 517 (28%) | 735 (32%) | Missing | 1153 (27%) | 342 (16%) |
| EC 3 | M | 26 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 612 (31%) | 624 (40%) | 661 (32%) | 773 (38%) | 565 (45%) | 674 (42%) |
| EC 4 | F | 30 | 0 | 0 | 0 | 0 | Missing | Missing | 0 | 599 (22%) | 498 (23%) | 290 (21%) | Missing | Missing | 359 (20%) |
| EC 5 | F | 27 | 0 | 0 | 0 | 0 | 0 | Missing | Missing | 502 (39%) | 802 (41%) | 595 (45%) | 553 (50%) | Missing | Missing |
| EC6 | F | 49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 782 (34%) | 740 (35%) | 807 (32%) | 1096 (28%) | 929 (31%) | 771 (30%) |
| EC 7 | F | 53 | 0 | 0 | 0 | 188 | 0 | 0 | 0 | 685 (22%) | 787 (26%) | 639 (23%) | 918 (25%) | 761 (24%) | 748 (26%) |
| VC 1 | F | 30 | 27 | 0 | 0 | 0 | 0 | 324 | Missing | 1456 (30%) | 1307 (33%) | 1392 (30%) | 1148 (37%) | 522 (41%) | Missing |
| VC 2 | F | 48 | 43 | 0 | 0 | 0 | 0 | 997 | 0 | 611 (41%) | 578 (37%) | 798 (41%) | 470 (42%) | 464 (42%) | 497 (34%) |
| VC 3 | F | 44 | 0 | 0 | 0 | 0 | 1371 | 0 | 0 | 471 (24%) | 530 (28%) | 557 (26%) | 495 (24%) | 473 (23%) | 589 (33%) |
| VC 4 | F | 24 | 217 | 0 | 499 | 0 | 23324 | 1317 | 215 | 547 (21%) | 445 (21%) | 565 (28%) | 357 (30%) | 575 (25%) | 524 (25%) |
| VC 5 | F | 35 | 35 | 786 | 1022 | 0 | 0 | 708 | Missing | 514 (32%) | 321 (31%) | 383 (35%) | 282 (26%) | 435 (39%) | Missing |
| VC 6 | F | 35 | 40 | 1452 | 715 | 1654 | 869 | 493 | 0 | 504 (23%) | 592 (29%) | 683 (27%) | 666 (26%) | 563 (26%) | 505 (25%) |
| VC 7 | F | 36 | Missing | 1449 | 619 | 0 | 1030 | 0 | 417 | 309 (34%) | 470 (30%) | 420 (28%) | 403 (28%) | 479 (29%) | 347 (30%) |
| VC 8 | F | 32 | 152 | 0 | 0 | 353 | Missing | 901 | 0 | 578 (27%) | 732 (25%) | 557 (27%) | Missing | 514 (25%) | 674 (27%) |
| VC 9 | M | 44 | 40 | 0 | 0 | 0 | 0 | 455 | 413 | 354 (22%) | 378 (24%) | 373 (22%) | 452 (27%) | 438 (27%) | 353 (28%) |
| VC 10 | F | 51 | 20 | 0 | 0 | 0 | 2066 | 715 | 371 | 502 (29%) | 667 (38%) | 540 (36%) | 479 (30%) | 547 (35%) | 578 (35%) |
| VC 11 | F | 34 | 49 | 1377 | Missing | Missing | 1388 | 749 | 453 | 702 (28%) | Missing | Missing | 669 (33%) | 512 (29%) | 787 (29%) |
%, percentage; EC, elite controller; M0, month 0 (baseline); M6, month 6; VC, viremic controller.
Copies per 106 peripheral blood mononuclear cells.
Copies per milliliter.
Per cubic millimeter.
Fig. 2.
Baseline biomarker levels in elite controllers, viremic controllers and progressors.
Each boxplot display the minimum, first quartile (Q1), median, third quartile (Q3), and maximum value of the marker. Dots below and above the minimum and maximum values are outliers. Interferon gamma-induced protein 10 and D-dimer. (a) US CRP (ng/ml). (b) sVCAM (ng/ml). (c) D-dimer (ng/ml). (d) IL-1 RA (pg/ml). (e) CD163 (ng/ml). (f) sCD14 (ng/ml). (g) IL 6 (pg/ml). (h) IP 10 (pg/ml). (i) HIV-1 DNA (log10 copies/106 PBMC). CD163, cluster of differentiation 163; DNA, deoxyribonucleic acid; IL-1-RA, interleukin-1 receptor antagonist; IL-6, interleukin-6; PBMC, peripheral blood mononuclear cells; prog-ART, progressors-ART; prog-no ART, progressors-no ART; sCD14, soluble cluster of differentiation 14; sVCAM-1, soluble vascular cell adhesion molecule-1; US CRP, ultra-sensitive C-reactive protein.
Fig. 2 (Continued).
Baseline biomarker levels in elite controllers, viremic controllers and progressors.
Each boxplot display the minimum, first quartile (Q1), median, third quartile (Q3), and maximum value of the marker. Dots below and above the minimum and maximum values are outliers. Interferon gamma-induced protein 10 and D-dimer. (a) US CRP (ng/ml). (b) sVCAM (ng/ml). (c) D-dimer (ng/ml). (d) IL-1 RA (pg/ml). (e) CD163 (ng/ml). (f) sCD14 (ng/ml). (g) IL 6 (pg/ml). (h) IP 10 (pg/ml). (i) HIV-1 DNA (log10 copies/106 PBMC). CD163, cluster of differentiation 163; DNA, deoxyribonucleic acid; IL-1-RA, interleukin-1 receptor antagonist; IL-6, interleukin-6; PBMC, peripheral blood mononuclear cells; prog-ART, progressors-ART; prog-no ART, progressors-no ART; sCD14, soluble cluster of differentiation 14; sVCAM-1, soluble vascular cell adhesion molecule-1; US CRP, ultra-sensitive C-reactive protein.
Fig. 2 (Continued).
Baseline biomarker levels in elite controllers, viremic controllers and progressors.
Each boxplot display the minimum, first quartile (Q1), median, third quartile (Q3), and maximum value of the marker. Dots below and above the minimum and maximum values are outliers. Interferon gamma-induced protein 10 and D-dimer. (a) US CRP (ng/ml). (b) sVCAM (ng/ml). (c) D-dimer (ng/ml). (d) IL-1 RA (pg/ml). (e) CD163 (ng/ml). (f) sCD14 (ng/ml). (g) IL 6 (pg/ml). (h) IP 10 (pg/ml). (i) HIV-1 DNA (log10 copies/106 PBMC). CD163, cluster of differentiation 163; DNA, deoxyribonucleic acid; IL-1-RA, interleukin-1 receptor antagonist; IL-6, interleukin-6; PBMC, peripheral blood mononuclear cells; prog-ART, progressors-ART; prog-no ART, progressors-no ART; sCD14, soluble cluster of differentiation 14; sVCAM-1, soluble vascular cell adhesion molecule-1; US CRP, ultra-sensitive C-reactive protein.
For the 40 patients who started ART before month 30, a total of 103 plasma HIV-1 RNA measurements (median 2.0, IQR 2.0–4.0) and 103 CD4+ cell measurements (median 2.0, IQR 2.0–4.0) were carried out between month 6 and starting ART. Of these 40 patients, 31 started ART because they reached at least one clinical or immunological starting criterion from those listed in the WHO guidelines at the time, and nine because they were pregnant. Before they started ART, 22 patients had less than three semestrial VL measurements and 18 patients had three or more. Among the latter, the pre-ART viral loads were all below 2000 copies/ml in three patients. The other 15 all had one or more viral loads of above 2000 copies/ml.
Discussion
Several definitions have been proposed for classifying individuals as HIV controllers [3,4,21,22]. For this study the US definition was used, in which patients not on ART monitored for at least one year are defined as elite controllers if at least three consecutive HIV-1 RNA tests are below 50 copies/ml; and as viremic controllers if at least three consecutive plasma HIV-1 RNA tests are below 2000 copies/ml, with some above 50 copies/ml [6,7].
To our knowledge, this is the first prospective estimate of the proportion of HIV controllers in Western Africa. In this cohort with semestrial viral load monitoring and a mean of six viral load measurements per person, 1.8% of 1023 adults were classified as HIV-1 controllers, including 0.7% of elite controllers and 1.1% of viremic controllers. These rates are higher than those recently estimated in Uganda and Ethiopia [14,15], and within the range of those found in other continents [4,6,14,15,23]. Unlike studies in Europe or North America, the HIV-1 controllers in our study and those in the Ugandan and Ethiopian studies were mostly female, which is consistent with HIV epidemic characteristics in sub-Saharan Africa.
In our study, controllers had low reservoirs and low levels of inflammatory markers. They maintained high CD4+ cell count and percentages in the medium term, and had few episodes of severe morbidity. Whether HIV controllers with such a low risk profile should be considered as a group deserving specific guidelines for starting ART is a debatable issue. [26,27]
On the one hand, it is well known that a few controllers ultimately lose virologic control, increase their viral load, decrease their CD4+ cell count and develop HIV-related diseases [3,4,6,21,22,28–31]. There can be no doubt that controllers who are destined to subsequently lose virologic control should start ART before the loss of control occurs. In addition, even if they maintain low plasma HIV-1 RNA levels and high CD4+ cell count in the long term, controllers may have residual viral replication, persistent immune activation and chronic inflammation. This may increase the risk of various diseases while they still apparently control replication [6,32–35].
On the other hand, an unknown percentage of elite controllers may never lose apparent virologic control or have deleterious residual viral replication if they stay off ART, and therefore may not derive any benefit from ART. The fact that these people represent a small percentage of HIV-1 controllers and a tiny percentage of all HIV-1 patients does not remove the question of their individual care. The recommendation to treat all people infected with HIV is based on a favorable collective benefit/risk ratio. If this rule would disadvantage a small number of individuals with an unfavorable individual benefit/risk ratio, it is the responsibility of the healthcare system to identify these individuals and establish specific guidelines for them.
HIV sub-types were not analyzed in this study. In sub-Saharan Africa, a variety of HIV subtypes are in circulation, with a predominance of subtype C [24]. In Côte d’Ivoire, the predominant subtype is CRF02_AG [25]. The fact that there are apparently as many controllers in an African country with predominant subtype C as in other regions with predominant subtype B may suggests that the HIV subtype may not play a major role in HIV control.
Our data are descriptive, their strength being the estimation of the frequency of controllers in an African cohort well followed for 30 months with repeated measurements of viral load. They do not provide new information concerning the mechanism of HIV control. Further studies including human leucocyte antigen (HLA)-typing could provide new insight. Although HLA-B27/HLA-B57 are the main HLA-alleles associated with natural immunity against HIV-infection, there are other HLA-alleles with protective immunity [36], as well as alleles associated with HIV-disease progression [37]. Identifying new alleles associated with control or progression, and exploring the association of HIV-sub-type with certain HLA-type in different regions could be highly informative. In conclusion, this study provides prospective evidence that HIV controllers exist in West Africa at a proportion close to that previously reported in high-income countries. In accordance with the WHO 2015 guidelines, HIV controllers will now start ART immediately. This will have two consequences. First, it will not be known that they HIV controllers since the current definition is based on a number of measurements repeated over time for patients not on ART. Second, if it were shown that a sub-group of controllers incurred more risks than benefits from starting ART, there are currently no markers to identify people in this sub-group before they start ART. Further studies should try to confirm whether this sub-group exists, and if it does, identify markers indicating they need not start ART [38] and explore whether other tests, such as integration of intact proviruses in heterochromatin regions, may allow to identify such persons, even when treated with ART [39,40].
Acknowledgements
The Temprano trial was supported by grants from the French National Agency for AIDS and viral hepatitis research (ANRS, Paris, France; Grants ANRS 12136, ANRS 12224, ANRS 12253): Brigitte Bazin, Géraldine Colin, Jean François Delfraissy, François Dabis, Thierry Menvielle, Claire Rekacewicz.
We are indebted to all patients who participated in this trial.
We also gratefully acknowledge the valuable contributions of the SMIT, CeDReS, CePReF, CMSDS, HGAN, CIRBA, USAC, FSU Com Anonkoua-Kouté, Hôpital El Rapha, Centre La Pierre Angulaire, PACCI, PNLS, PNLT, NPSP, CEPAC international, RIP-plus and INSERM 1219 teams.
Author contributions
S.P.E. and X.A. were co-chairs of the Temprano trial.
J.-B.N., D.G., C.R., S.P.E., X.A. and O.L. formulated the hypotheses and research questions.
A.B., R.M., G.M.K., J.L.C., S.K., C.D., S.P.E. and X.A. recruited and followed the patients and collected clinical data.
A.E., E.G., H.M. and C.R. performed the biological tests.
D.G. and J.B.N. performed the statistical analysis.
J.-B,N., D.G. and X.A. drafted the manuscript. All authors provided critical input into the manuscript.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Jean Baptiste N’takpé and Delphine Gabillard contributed equally to this work.
Serge P. Eholie, Christine Rouzioux, Xavier Anglaret and Olivier Lambotte contributed equally to this work.
References
- 1.Sajadi MM, Constantine NT, Mann DL, Charurat M, Dadzan E, Kadlecik P, et al. Epidemiologic characteristics and natural history of HIV-1 natural viral suppressors. J Acquir Immune Defic Syndr 2009; 50:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okulicz JF, Lambotte O. Epidemiology and clinical characteristics of elite controllers. Curr Opin HIV AIDS 2011; 6:163–168. [DOI] [PubMed] [Google Scholar]
- 3.Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis 2005; 41:1053–1056. [DOI] [PubMed] [Google Scholar]
- 4.Grabar S, Selinger-Leneman H, Abgrall S, Pialoux G, Weiss L, Costagliola D. Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French Hospital Database on HIV. AIDS 2009; 23:1163–1169. [DOI] [PubMed] [Google Scholar]
- 5.Thèze J, Chakrabarti LA, Vingert B, Porichis F, Kaufmann DE. HIV controllers: a multifactorial phenotype of spontaneous viral suppression. Clin Immunol 2011; 141:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okulicz JF, Marconi VC, Landrum ML, Wegner S, Weintrob A, Ganesan A, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis 2009; 200:1714–1723. [DOI] [PubMed] [Google Scholar]
- 7.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis 2008; 197:563–571. [DOI] [PubMed] [Google Scholar]
- 8.Sáez-Cirión A, Sinet M, Shin SY, Urrutia A, Versmisse P, Lacabaratz C, et al. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J Immunol 2009; 182:7828–7837. [DOI] [PubMed] [Google Scholar]
- 9.Lambotte O, Ferrari G, Moog C, Yates NL, Liao H-X, Parks RJ, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 2009; 23:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Côrtes FH, Passaes CP, Bello G, Teixeira SL, Vorsatz C, Babic D, et al. HIV controllers with different viral load cutoff levels have distinct virologic and immunologic profiles. J Acquir Immune Defic Syndr 2015; 68:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sáez-Cirión A, Pancino G, Sinet M, Venet A, Lambotte O. ANRS EP36 HIV CONTROLLERS study group. HIV controllers: how do they tame the virus?. Trends Immunol 2007; 28:532–540. [DOI] [PubMed] [Google Scholar]
- 12.Avettand-Fenoel V, Bayan T, Gardiennet E, Boufassa F, Lopez P, Lecuroux C, et al. Dynamics in HIV-DNA levels over time in HIV controllers. J Int AIDS Soc 2019; 22:e25221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. UNAIDS_Global HIV & AIDS statistics-2019 fact sheet. Available at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_fr.pdf. [Accessed 19 September 2019] [Google Scholar]
- 14.Kayongo A, Gonzalo-Gil E, Gümüşgöz E, Niwaha AJ, Semitala F, Kalyesubula R, et al. Identification of elite and viremic controllers from a large urban HIV ambulatory center in Kampala, Uganda. J Acquir Immune Defic Syndr 2018; 79:394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiros YK, Elinav H, Gebreyesus A, Gebremeskel H, Azar J, Chemtob D, et al. Identification and characterization of HIV positive Ethiopian elite controllers in both Africa and Israel. HIV Med 2019; 20:33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–822. [DOI] [PubMed] [Google Scholar]
- 17.Affi R, Gabillard D, Dunyach-Remy C, Ntakpe J-B, Moh R, Badje A, et al. Association of plasma sVCAM-1 and sCD14 with mortality in HIV-1 infected West African adults with high CD4 counts. J Acquir Immune Defic Syndr 2021; 86:138–145. [DOI] [PubMed] [Google Scholar]
- 18.N’takpe JB, Gabillard D, Moh R, Gardiennet E, Emieme A, Badje A, et al. Association between cellular HIV-1 DNA level and mortality in HIV-1 infected African adults starting ART with high CD4 counts. EBioMedicine 2020; 56:102815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badje A, Moh R, Gabillard D, Guéhi C, Kabran M, Ntakpé J-B, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health 2017; 5:e1080–e1089. [DOI] [PubMed] [Google Scholar]
- 20.Sörstedt E, Nilsson S, Blaxhult A, Gisslén M, Flamholc L, Sönnerborg A, et al. Viral blips during suppressive antiretroviral treatment are associated with high baseline HIV-1 RNA levels. BMC Infect Dis 2016; 16:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurdasani D, Iles L, Dillon DG, Young EH, Olson AD, Naranbhai V, et al. A systematic review of definitions of extreme phenotypes of HIV control and progression. AIDS 2014; 28:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canouï E, Lécuroux C, Avettand-Fenoël V, Gousset M, Rouzioux C, Saez-Cirion A, et al. A subset of extreme human immunodeficiency virus (HIV) controllers is characterized by a small HIV blood reservoir and a weak T-cell activation level. Open Forum Infect Dis 2017; 4:ofx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson AD, Meyer L, Prins M, Thiebaut R, Gurdasani D, Guiguet M, et al. An evaluation of HIV elite controller definitions within a large seroconverter cohort collaboration. PLoS ONE 2014; 9:e86719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naicker P, Sayed Y. Non-B HIV-1 subtypes in sub-Saharan Africa: impact of subtype on protease inhibitor efficacy. Biol Chem 2014; 395:1151–1161. [DOI] [PubMed] [Google Scholar]
- 25.Toni T, d’Aquin, Masquelier B, Minga A, Anglaret X, Danel C, Coulibaly A, et al. HIV-1 antiretroviral drug resistance in recently infected patients in Abidjan, Côte d’Ivoire: a 4-year survey, 2002–2006. AIDS Res Hum Retroviruses 2007; 23:1155–1160. [DOI] [PubMed] [Google Scholar]
- 26. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available at: https://files.aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Accessed 11 February 2020] [Google Scholar]
- 27.Noël N, Saez-Cirion A, Avettand-Fenoël V, Boufassa F, Lambotte O. HIV controllers: to treat or not to treat? Is that the right question?. Lancet HIV 2019; 6:e878–e884. [DOI] [PubMed] [Google Scholar]
- 28.Chereau F, Madec Y, Sabin C, Obel N, Ruiz-Mateos E, Chrysos G, et al. Impact of CD4 and CD8 dynamics and viral rebounds on loss of virological control in HIV controllers. PLoS ONE 2017; 12:e0173893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leon A, Perez I, Ruiz-Mateos E, Benito JM, Leal M, Lopez-Galindez C, et al. Rate and predictors of progression in elite and viremic HIV-1 controllers. AIDS 2016; 30:1209–1220. [DOI] [PubMed] [Google Scholar]
- 30.Madec Y, Boufassa F, Porter K, Prins M, Sabin C, d’Arminio Monforte A, et al. Natural history of HIV-control since seroconversion. AIDS 2013; 27:2451–2460. [DOI] [PubMed] [Google Scholar]
- 31.Noel N, Lerolle N, Lécuroux C, Goujard C, Venet A, Saez-Cirion A, et al. Immunologic and virologic progression in HIV controllers: the role of viral ‘Blips’ and immune activation in the ANRS CO21 CODEX study. PLoS ONE 2015; 10:e0131922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009; 23:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012; 26:2409–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucero C, Torres B, León A, Calvo M, Leal L, Pérez I, et al. Rate and predictors of non-AIDS events in a cohort of HIV-infected patients with a CD4 T cell count above 500 cells/μl. AIDS Res Hum Retroviruses 2013; 29:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crowell TA, Ganesan A, Berry SA, Deiss RG, Agan BK, Okulicz JF, et al. Hospitalizations among HIV controllers and persons with medically controlled HIV in the U.S. Military HIV Natural History Study. J Int AIDS Soc 2016; 19:20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crux NB, Elahi S. Human leukocyte antigen (HLA) and immune regulation: how do classical and non-classical HLA alleles modulate immune response to human immunodeficiency virus and hepatitis C virus infections?. Front Immunol 2017; 8:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elahi S, Shahbaz S, Houston S. Selective upregulation of CTLA-4 on CD8+ T cells restricted by HLA-B∗35Px renders them to an exhausted phenotype in HIV-1 infection. PLoS Pathog 2020; 16:e1008696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eholie SP, Vella S, Anglaret X. Antiretroviral therapy initiation criteria in low resource settings—from ‘when to start’ to ‘when not to start’. AIDS 2014; 28: (Suppl 2): S101–104. [DOI] [PubMed] [Google Scholar]
- 39.Einkauf KB, Lee GQ, Gao C, Sharaf R, Sun X, Hua S, et al. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J Clin Invest 2019; 129:988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang C, Lian X, Gao C, Sun X, Einkauf KB, Chevalier JM, et al. A unique viral reservoir landscape in HIV-1 elite controllers. Nature 2020; 585:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]