Abstract
Immune checkpoint inhibitor therapy is frequently associated with immune-related adverse events, which occasionally manifest with visual symptoms. Here, we describe a case of unilateral and sudden-onset painless vision loss in an 82-year-old man with metastatic non–small cell lung cancer receiving immunotherapy with the anti–programmed death-ligand 1 agent atezolizumab. Examination demonstrated a right-sided relative afferent pupillary defect, diffusely swollen optic disc, and delayed choroidal and retinal arterial filling on fundus fluorescein angiography, consistent with an arteritic anterior ischemic optic neuropathy. Histology of an ipsilateral temporal artery biopsy revealed a transmural eosinophilic infiltrate without granulomas, while serology revealed the presence of antineutrophil cytoplasmic antibodies. Peripheral eosinophilia was also noted, which preceded treatment by several months. This report highlights the importance of clinician awareness of immune checkpoint inhibitors and their systemic and ophthalmic complications, which rarely appear to extend to eosinophilic temporal arteritis.
Key Words: eosinophilic vasculitis, temporal arteritis, anterior ischemic optic neuropathy, immunotherapy, immune checkpoint inhibitor, programmed cell death 1, PD-L1, atezolizumab
CASE PRESENTATION
An 82-year-old man of European ancestry with a history of metastatic non–small cell lung adenocarcinoma presented to the emergency department of a tertiary medical centre complaining of a 1-week history of sudden-onset painless vision loss in the right eye and a 5-week history of frontal headaches and jaw claudication. On examination, his best-corrected visual acuity was no light perception in the right eye and 6/7.6 in the left eye. He had a right-sided relative afferent pupillary defect. Intraocular pressures were 8 and 11 mm Hg in the right and left eyes, respectively. Extraocular movements were painless without a restricted range of motion. He had large, nontender temporal arteries with sluggish pulsatility to palpation. Dilated funduscopic examination of the right eye revealed a pale macula with a cherry-red spot and a pale and swollen optic nerve head with obscuration of vessels predominantly at the nasal disc margin (Fig. 1A). Aside from mild hypertensive changes, the left fundus was grossly unremarkable (Fig. 1B).
FIGURE 1.
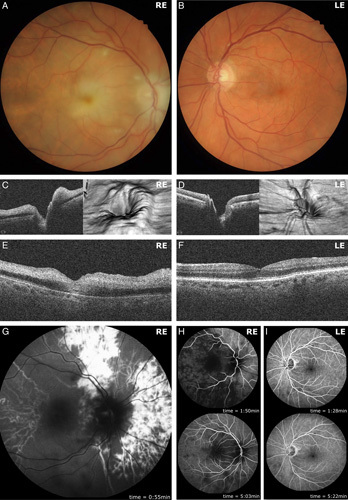
Unilateral optic nerve head swelling and retinal ischemia. Retinal photography of the right fundus demonstrated a swollen, pale optic nerve head, with generalized retinal ischemia evidenced by cotton wool spots and a “cherry-red spot” within the fovea (A). The left fundus by contrast was grossly normal (B). Optic nerve head spectral-domain optical coherence tomography (SD-OCT) imaging of the right eye demonstrated predominantly nasal optic nerve head swelling evident in horizontal cross-section and 3-dimensional projection (C). Left optic nerve head SD-OCT images were unremarkable (D). Horizontal cross-sectional SD-OCT imaging of the right fovea demonstrated hyperreflectivity of the inner retinal layers, characteristic of retinal ischemia (E). SD-OCT imaging of the left fovea, by comparison, was unremarkable (F). Representative fundus fluorescein angiography imaging of the right eye demonstrated profoundly delayed arterial perfusion with patchy choroidal perfusion at 0:55 minutes (G). Retinal arterial perfusion was first observed at 1:50 minutes, with venous and incomplete choroidal perfusion observed at 5:03 minutes (H). Fundus fluorescein angiography imaging of the left eye demonstrated normal arterial, venous, and choroidal perfusion at similar time points of 1:28 and 5:22 minutes following intravenous fluorescein bolus infusion (I). LE indicates left eye; RE, right eye.
His non–small cell lung adenocarcinoma had been diagnosed 3 years before presentation. A lobectomy attempted at diagnosis had been aborted due to the surgical finding of miliary metastatic spread throughout the lung parenchyma. He subsequently underwent 3 cycles of palliative chemotherapy with carboplatin and paclitaxel, followed by 12 cycles of maintenance pemetrexed. Five months before presentation, he commenced palliative immunotherapy with the programmed death-ligand 1 (PD-L1) inhibitor atezolizumab. His past medical history was significant for type 2 diabetes mellitus (treated with gliclazide, metformin, and dapagliflozin), hypertension (treated with amlodipine), and depression (treated with venlafaxine). Aside from bilateral cataract surgery, his past ophthalmic history was unremarkable.
Spectral-domain optical coherence tomography images of the right eye demonstrated predominantly nasal optic disc swelling which was evident in horizontal cross-section and 3-dimensional projections (Fig. 1C). Ischemic changes were observed in the right macula, with the characteristic feature of hyperreflectivity of the inner retinal layers (Fig. 1E). Fundus fluorescein angiography demonstrated profound arterial perfusion delays within the retinal and choroidal circulation, with patchy choroidal perfusion at 55 seconds (normal: 10–15 s; Fig. 1G), followed by delayed retinal arterial perfusion at 1:50 minutes (normal: 1–3 s after dye is first seen in the choroid). Choroidal perfusion remained incomplete by 5:03 minutes (Fig. 1H). Spectral-domain optical coherence tomography imaging of the left optic nerve head and left fovea was unremarkable (Figs. 1D, F). Fluorescein angiography of the left eye demonstrated normal arterial, venous, and choroidal filling at comparable time points (Fig. 1I). These clinical findings were consistent with multivessel occlusion of the central retinal artery and the long and short posterior ciliary arteries.
Laboratory investigations at presentation revealed a prolonged erythrocyte sedimentation rate [75 mm/h (reference range: 1–35 mm/h)] and an elevated C-reactive protein [65.4 mg/L (reference <6 mg/L)]. Computed tomography angiography of the carotid and cerebral arteries demonstrated a focus of moderate stenosis within the cavernous segment of the left internal carotid artery. This finding was not associated with significant hemodynamic changes within the proximal intracranial or ophthalmic arteries.
A preliminary diagnosis of arteritic anterior ischemic optic neuropathy (AAION) was made, and high-dose corticosteroid therapy was initiated (3 d of intravenous methylprednisolone followed by 1 mg/kg oral prednisolone). Biopsy of the right temporal artery was performed 3 days after presentation, with histopathology demonstrating features consistent with eosinophilic temporal arteritis including an intense transmural inflammatory infiltrate with a predominance of eosinophils, without distinct granulomas (Fig. 2).
FIGURE 2.
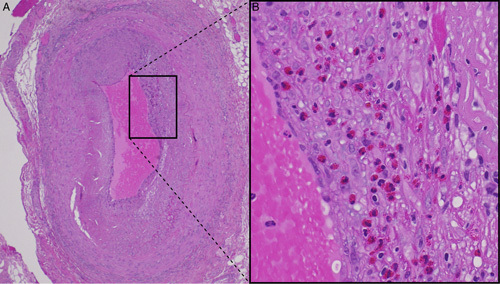
Eosinophilic temporal arteritis. Hematoxylin and eosin staining of right temporal artery biopsy specimens at ×4 (A) and ×20 (B) magnification demonstrating transmural eosinophilic infiltrate without granulomas. The black box in (A) shows the region represented in higher magnification in (B).
A retrospective review of the patient’s pathology revealed persistent peripheral eosinophilia for 13 months before the presentation (Fig. 3). Autoimmune serology revealed an antinuclear antibody titer of 1:2560 (speckled and nucleolar) and the presence of antineutrophil cytoplasmic antibody (intermediate pattern) with an anti–proteinase-3 antibody titer of 7 IU/mL (reference ≤5 IU/mL).
FIGURE 3.
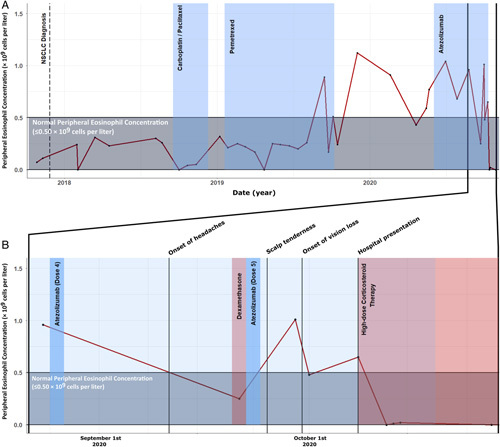
Peripheral eosinophilia predating atezolizumab therapy. Peripheral eosinophilia had been evident for a period of 9 months before commencing of atezolizumab therapy. Before 2019, and in the context of chemotherapy, peripheral eosinophil concentrations were within the normal range. Elevated levels were observed after completion of pemetrexed therapy, and during atezolizumab therapy (A). Normal range eosinophils were subsequently seen in the context of corticosteroid therapy (B). NSCLC indicates non–small cell lung cancer.
The diagnosis was revised to an antineutrophil cytoplasmic antibody–positive eosinophilic vasculitis affecting temporal arteries. Following a multidisciplinary case review, atezolizumab was discontinued, and he was commenced on 10 mg oral methotrexate weekly in addition to 1 mg/kg oral prednisolone. Furthermore, the patient was commenced on trimethoprim and sulfamethoxazole for pulmonary pneumocystis jiroveci prophylaxis and omeprazole for gastric ulcer prophylaxis. Three weeks later, he presented to the emergency department with shortness of breath and fever. He was diagnosed with pneumonia which was managed palliatively. He died 2 days later.
DISCUSSION
We describe a case of sudden-onset vision loss in a man undergoing treatment with the immune checkpoint inhibitor (ICI) atezolizumab (a PD-L1 inhibitor), associated with an eosinophilic temporal arteritis and a unilateral AAION. To our knowledge, this is the first study to provide histopathologic evidence of eosinophilic vasculitis occurring in the context of ICI therapy. In addition, this is the first documented case of an AAION in a patient undergoing anti-PD-L1 therapy.
ICIs are a novel class of monoclonal antibodies used for the treatment of cancer. Collectively, these drugs function by stimulating antitumor immunity through the disinhibition of T cells via cell surface proteins including programmed cell death protein 1 (PD-1), PD-L1, and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4). Though these drugs are typically second or third-line therapeutics for the treatment of various metastatic diseases, their use has revolutionized cancer medicine.1
These therapies have been recognized to result in various immune-related adverse events (irAEs). Accordingly, there is a need for vigilant pharmacosurveillance to determine the nature and frequency of adverse events. As a presumed consequence of T-cell disinhibition, ICIs appear to upregulate various autoimmune processes. The mechanisms by which these processes occur, however, remain to be elucidated.2,3 Evidence suggests that 20%–59% of patients undergoing immunotherapy with anti-PD-1 and anti-PD-L1 inhibitors experience 1 or more irAEs.4 The most commonly affected sites are the endocrine, dermatologic, and gastrointestinal systems. PD-L1 inhibitors have been associated with a slightly lower incidence of irAEs than PD-1 inhibitors, potentially due to the fact that PD-1 inhibition prevents engagement of both PD-L1 and PD-L2 with PD-1, while PD-L1 inhibition prevents only the engagement of PD-L1 with PD-1.4
Though ICI-associated vasculitis is uncommon, it appears to predominantly affect large vessels, resulting in large vessel vasculitis including giant cell arteritis, aortitis, and primary angiitis of the nervous system.3,5 Most cases of ICI-associated vasculitis have been reported with PD-1 or CTLA-4 inhibitor therapy.6 AAION is a consequence of vessel wall inflammation resulting in occlusion of arteries supplying the eye. The most common cause of AAION is giant cell arteritis. Eosinophilic vasculitis by contrast, is a rare cause of AAION.7 We are aware of 6 cases of AAION reported in the context of ICI therapy, each of which involved either a CTLA-4 inhibitor or a PD-1 inhibitor.6,8 A single case of eosinophilic granulomatosis with polyangiitis has been reported previously in a patient undergoing PD-L1 inhibitor therapy. Unfortunately, this association was confounded by concurrent anti-CTLA-4 therapy, and no histopathologic specimens were available to confirm the presence of vasculitis.5
A salient feature of the current case is the retrospective observation of peripheral eosinophilia preceding initiation of therapy by at least 9 months (Fig. 3). Eosinophilia has been observed in cancer patients treated with ICIs9 and is thought by some to be a biomarker of treatment success.10 The PD-1 pathway is intimately involved in the regulation of type 2 innate lymphoid cells (ILC2) in which it acts as a metabolic checkpoint; activated ILC2s secrete high amounts of Th2 cytokines including interleukin 5, a specific and potent activator of eosinophils. PD-1 agonism ameliorates ILC2 activation. While formal cytokine studies were not undertaken in this patient, it is conceivable that activation of the PD-1/PD-L1 pathway by atezolizumab led to ILC2 (and thus interleukin 5) mediated eosinophil activation, the scene being set for this with his background eosinophilia. Eosinophilia is not a current contraindication to PD-L1 therapy. We do, however, question its pathoetiological relevance in a patient who subsequently developed an eosinophilic vasculitis after commencing ICI therapy.
This study highlights a unique presentation of vision loss resulting from an AAION associated with eosinophilic temporal arteritis in a patient undergoing treatment with the PD-L1 inhibitor atezolizumab. Although this appears to be the first reported case of eosinophilic temporal arteritis associated with ICI therapy, it is unclear if this therapy was a precipitant since eosinophilia predated the commencement of treatment. Nonetheless, with the increasing use of these therapies, and concomitant increase in the number of irAEs, physicians and ophthalmologists alike should be aware of the association and its potential severe visual consequences.
ACKNOWLEDGMENTS
The authors thank Angela Chappell and Carly Emerson (Flinders Medical Centre) for ophthalmic images.
Conflicts of Interest/Financial Disclosures
None reported. All authors have declared that there are no financial conflicts of interest with regard to this work.
Footnotes
E.C.B., S.M., and A.Q. are joint first authors.
O.M.S. and M.D.W. are joint senior authors.
Contributor Information
Ella C. Berry, Email: ella.c.berry@gmail.com.
Sean Mullany, Email: seanmullany@gmail.com.
Alannah Quinlivan, Email: a.quinlivan90@gmail.com.
Amelia Craig, Email: ameliagracecraig@gmail.com.
Julia New-Tolley, Email: jnewtolley@gmail.com.
James Slattery, Email: james_slattery@rocketmail.com.
Shawgi Sukumaran, Email: shawgi.sukumaran@flinders.edu.au.
Sonja Klebe, Email: sonja.klebe@sa.gov.au.
Jamie E. Craig, Email: jamie.craig@flinders.edu.au.
Owen M. Siggs, Email: owen.siggs@gmail.com.
Mihir D. Wechalekar, Email: mihir.w@gmail.com.
REFERENCES
- 1. Xin Yu J, Hubbard-Lucey VM, Tang J. Immuno-oncology drug development goes global. Nat Rev Drug Discov. 2019;18:899–900. [DOI] [PubMed] [Google Scholar]
- 2. Xu C, Chen Y-P, Du X-J, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors—a systematic review. Clin Rheumatol. 2018;37:2579–2584. [DOI] [PubMed] [Google Scholar]
- 4. Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-l1 inhibitors in non-small cell lung cancer: a systematic analysis of the literature. Cancer. 2018;124:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roger A, Groh M, Lorillon G, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) induced by immune checkpoint inhibitors. Ann Rheum Dis. 2019;78:e82. [DOI] [PubMed] [Google Scholar]
- 6. Salem J-E, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Padovano I, Pazzola G, Pipitone N, et al. Anterior ischaemic optic neuropathy in eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome): a case report and review of the literature. Clin Exp Rheumatol. 2014;32:S62–S65. [PubMed] [Google Scholar]
- 8. Mukharesh L, Chwalisz BK. Neuro-ophthalmic complications of immune-checkpoint inhibitors. Semin Ophthalmol. 2021;36:241–249. [DOI] [PubMed] [Google Scholar]
- 9. Scanvion Q, Béné J, Gautier S, et al. Moderate-to-severe eosinophilia induced by treatment with immune checkpoint inhibitors: 37 cases from a national reference center for hypereosinophilic syndromes and the French pharmacovigilance database. Oncoimmunology. 2020;9:1722022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simon SCS, Hu X, Panten J, et al. Eosinophil accumulation predicts response to melanoma treatment with immune checkpoint inhibitors. Oncoimmunology. 2020;9:1727116. [DOI] [PMC free article] [PubMed] [Google Scholar]


