Abstract
Stereotyped movements (“stereotypies”) are semi-voluntary repetitive movements that are a prominent clinical feature of autism spectrum disorder. They are described in first-person accounts by people with autism as relaxing and that they help focus the mind and cope in overwhelming sensory environments. Therefore, we generally recommend against techniques that aim to suppress stereotypies in individuals with autism. Further, we hypothesize that understanding the neurobiology of stereotypies could guide development of treatments to produce the benefits of stereotypies without the need to generate repetitive motor movements. Here, we link first-person reports and clinical findings with basic neuroanatomy and physiology to produce a testable model of stereotypies. We hypothesize that stereotypies improve sensory processing and attention by regulating brain rhythms, either directly from the rhythmic motor command, or via rhythmic sensory feedback generated by the movements.
Keywords: autism, challenging behavior, stereotypies, stereotyped movements, self-stimulatory behaviors, stimming, fidgeting, attention, sensory processing, efference copy, corollary discharge, oscillations, axon branching
Imagine you’re on your second cup of coffee, sitting in Grand Rounds watching a semi-useful presentation. Your right knee bounces rhythmically, as it often does when watching talks. Your neighbor taps your shoulder gently to get you to stop because it’s distracting them. Later, when reading through some paper charts, you twirl your pen around your thumb or tap it on the page. Meanwhile, at school, your child sits on an exercise ball instead of a chair so they can bounce to get the wiggles out while remaining seated at their desk. They quietly doodle on the corner of their homework while listening to the teacher.
Simultaneously, another child in the class rhythmically rocks their body and periodically flaps their hands. “Alex, please stop that and pay attention,” the teacher says. Alex’s teacher has recommended to Alex’s parents and behavioral therapist that they work on reducing the “stimming” because it keeps Alex from paying attention in class. It’s also part of the reason the other kids at school think Alex is odd. Alex’s parents also wish the stimming would stop because it makes it hard to bring Alex out in public. As soon as they arrive at a store or restaurant, Alex starts body rocking and hand flapping. The parents have tried positive and negative rewards to decrease the stimming, but nothing has worked.
Stereotyped movements or motor “stereotypies” are common. In people deemed “neurotypical”, these behaviors are commonly referred to as “fidgeting.” In people with autism, engaging in these behaviors is often considered problematic and is colloquially referred to as “stimming” (i.e. self-stimulatory behaviors)1. Stereotypies are often perceived as interfering with normal activity. There is a common belief that if a person is stimming, they are not attending to their environment. Thus, in many contexts, especially educational settings, stereotypies are perceived as counterproductive for learning2. They can also be distracting to others. Therefore, behavioral therapies often have a goal of reducing the frequency and intensity of stereotypies.
Here, we propose a new perspective on motor stereotypies. Instead of being a sign that a person with autism is in their own world and isn’t paying attention, we propose that people with autism may perform stereotypies in order to pay attention. What if engaging in these movements helps Alex’s brain process information? What if Alex’s brain is generating this rhythmic movement so they can tune in to what the teacher is saying? What if Alex’s brain generates these stereotypies to help regulate the sensory overload they experience in public places?
We propose that by understanding how and why stereotypies are generated, we can harness those mechanisms to improve sensory processing and attention in people with autism. Here, we propose a testable model of the beneficial purpose of stereotypies. We hypothesize that engaging in stereotyped movements entrains brain rhythms to enhance sensory processing and attention.
Stereotypies are common in people with autism.
Autism is a behaviorally-based diagnosis assigned to people who have disabilities in social communication and who exhibit restricted, repetitive patterns of behavior, interests, and activities. Specifically, the presence of “stereotyped or repetitive motor movements” is a clinical criterion for the diagnosis of autism spectrum disorder3. “Motor stereotypies” can be defined as repetitive, non-goal-directed, rhythmic, patterned movements that stop with distraction and have no accompanying premonitory urge4–6. Examples include hand flapping, body rocking, spinning, repetitive jumping, and finger flicking. Motor stereotypies are just one type of stereotyped behavior. Other examples of stereotyped behavior include atypical use of language (e.g. echolalia), unusual visual inspection of objects, and more complex seemingly “non-functional” behaviors such as aligning objects7. Throughout this paper, we will use the terms “motor stereotypies”, “stereotypies”, and “stimming” interchangeably. We are excluding self-injurious behaviors (SIB) here, as these are beyond the scope of the current discussion.
When do people with autism engage in stereotypies?
Stereotypies can be a visible read-out of a person’s state of being, both negative and positive. The intensity of stereotypies correlates with anxiety8 and the severity of core autism symptoms9. Observational studies of children with autism show that stereotypies increase following a stressful trigger10. Based on self-reports from people with autism, stereotypies can help with feelings of anxiety, nervousness, or feeling “wound up”11–13. People also engage in stereotypies when relaxed and happy14. Stereotypies are described as comforting, calming, and even enjoyable11, 13. Stimming can help organize thoughts, focus, or get rid of excess energy11. This can be especially helpful in environments where the person feels sensory overload: engaging in stereotypies reportedly modulates overwhelming sensory inputs11, 13.
Stereotypies typically begin unconsciously or involuntarily, but individuals describe intentionally self-perpetuating the movements because of the comfort and control they provide13. The cessation of the movements can be internally or externally motivated. Many report a loss of interest in the behavior, or a feeling that the behavior would be inappropriate to continue11. Others report a feeling of emotional fulfillment, or that something has been completed11. Together, these self-reports of people with autism highlight themes of improved sensory processing, emotional regulation, improved focus, concentration, and attention. Despite the prevalence of stereotypies in people with autism, there is scant research quantifying stereotypies for even basic measurements such as the frequency of the rhythmic movements15, 16.
When do people without autism engage in stereotypies?
Repetitive movements are not unique to individuals with autism. People with intellectual disability, severe vision impairment, or institutional care early in life commonly engage in stereotypies17–19. Neurotypical individuals also engage in repetitive movements, which are typically referred to as fidgeting. Examples include doodling, clicking a pen, rocking in a chair, playing with ones’ hair, humming, chewing gum, or bouncing one’s leg20. These movements are often repetitive or patterned, and are self-initiated. Fidgeting may appear purposeless, but it can have positive effects on attention, concentration, and stress21. In fact, therapies based on repetitive manipulation of objects (like small foam “stress” balls) have been found to increase focus and learning performance in some contexts22.
Linking stereotypies to brain rhythms.
Why is engaging in stereotyped movements so common in the general population, and in particular, in people with autism? We hypothesize that the rhythmic brain signal generated to create the movement and/or the rhythmic sensory feedback produced by the movement entrains brain rhythms to enhance information processing (Figure 1).
Figure 1. Hypothesized role of motor stereotypies in the normalization of brain rhythms in sensory processing areas.
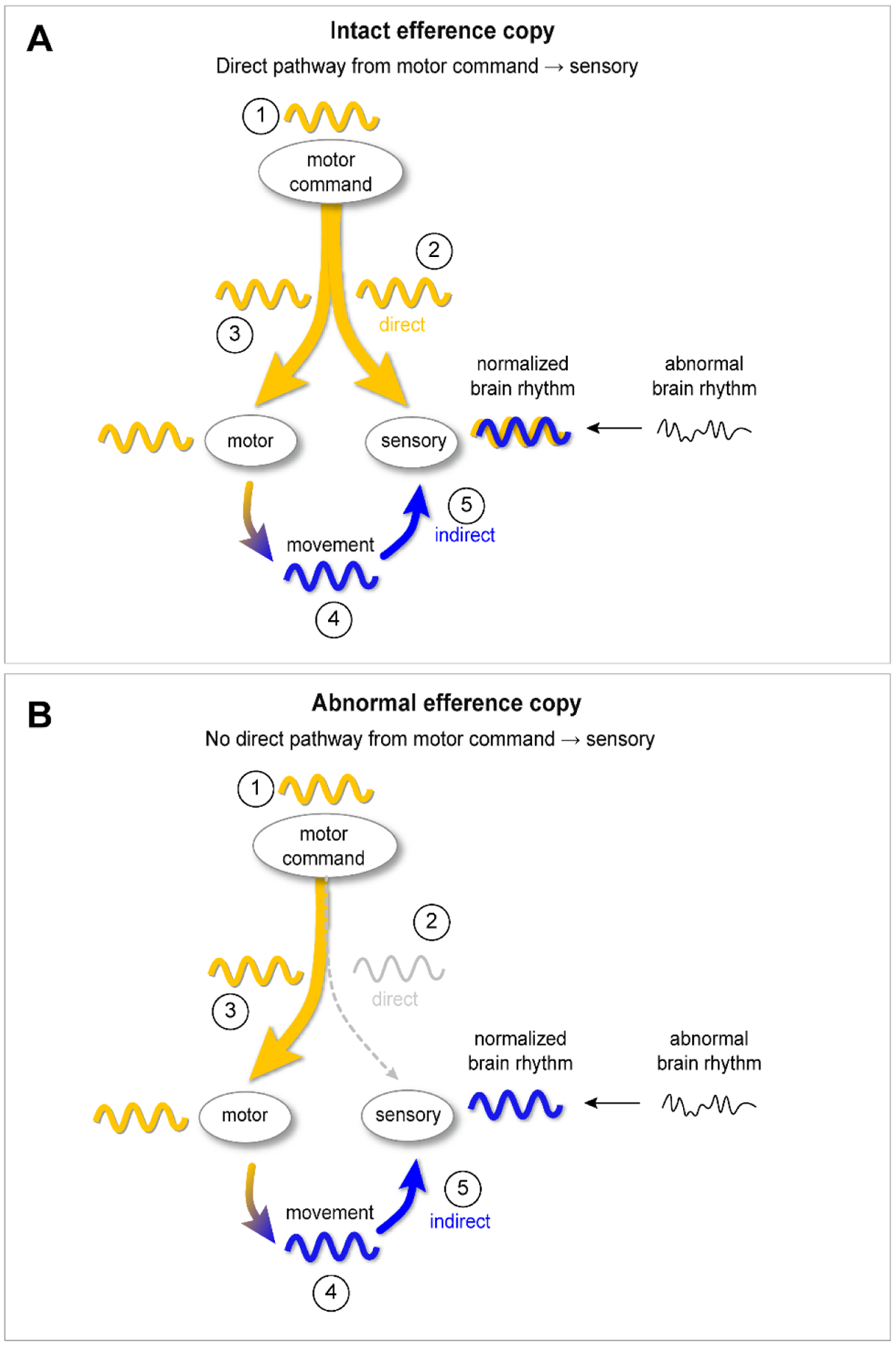
A. Case 1: intact efference copy. 1. The motor command center generates a rhythmic signal. 2. Sensory areas are entrained to the command rhythm via the direct pathway. 3. Because the rhythmic signal is also sent to motor effector areas, a rhythmic movement (the stereotypy) is generated as a “side effect”. 4. The stereotyped / rhythmic movement produces a rhythmic sensory experience. 5. The rhythmic sensory experience modulates brain rhythms in sensory areas (“indirect” pathway).
B. Case 2: abnormal efference copy. 1. The motor command center generates a rhythmic signal. 2. Because the branched axons have not formed normally, or are not functioning normally, this motor command signal is not directly relayed to sensory areas. 3. The rhythmic signal is sent to motor effector areas and a rhythmic movement (the stereotypy) is generated. 4. The stereotyped / rhythmic movement produces a rhythmic sensory experience. 5. The rhythmic sensory experience modulates brain rhythms in sensory areas (“indirect” pathway).
Brain rhythms help process sensory information.
Electrical rhythms are found at all scales within the nervous system. The summed postsynaptic potentials of populations of neurons produce rhythmic voltage fluctuations that can be detected microscopically (i.e. in single cell recordings) and macroscopically (i.e. with scalp EEG)23, 24. These oscillations reflect neuronal activity but also influence neuronal signaling. For instance, when an excitatory synaptic input coincides with the peak of a voltage oscillation, the neuron has a higher probability of generating an action potential25.
Brain rhythms influence processing of incoming sensory information26, 27. Sampling of sensory inputs is often timed with brain oscillations to optimize information transfer between the external world and the brain28. Through rhythmic movements, sensory receptors are stimulated at rhythmic intervals that coincide with peaks of brain oscillations. For example, in rodents, olfactory sampling via sniffing and tactile sampling through whisking are phase-locked to internally-generated rhythms in the olfactory bulb and brainstem29, 30. In the visual system of primates, the timing of saccades is aligned with rhythmic field potential activity in visual cortex28, 31, 32. Internally-generated oscillations essentially provide regular “windows of opportunity” for information transfer29, 33–35. Even attention itself has been found to be fundamentally rhythmic, with rhythmic changes in perceptual ability during sustained attention tasks36.
Brain rhythms are disrupted in people with autism.
One prominent hypothesis is that autism is a primary disorder of brain oscillations (i.e. an “oscillopathy”)37. Sensory- and motor-related brain oscillations are abnormal in autism37–40, both at rest41, 42 and during active behaviors43–45. Differences are reflected in overall power, but also in how different frequencies of oscillations work together to transmit information (“cross-frequency coupling”)40, 46. Differences in oscillations have been observed in autism throughout the brain in the auditory47, visual40 and motor48 systems. We hypothesize that the repetitive motor command signal that produces stereotypies and/or the rhythmic sensory feedback generated by the movements normalize brain rhythms in people with autism to improve sensory processing (Figure 1). To understand how this might work, we next explore how motor and sensory brain regions are directly linked to each other via “efference copy”.
Initiating a movement generates an electrical signal that travels to both motor effectors and sensory areas via efference copy.
To produce movement, a motor command output signal (“efferent”) is transmitted to the appropriate muscle groups via synaptic transmission. Simultaneously, a duplicate (“copy”) of the motor output signal is sent to sensory areas (Figure 1). Through this “efference copy” (also known as “corollary discharge”), the motor area gives the sensory area a “heads up” that there is a movement coming that is self-generated. The brain then suppresses the response to sensory input generated by the movement49. Efference copy thus allows us to distinguish between sensations produced by self-generated movements from those generated by external stimuli. For instance, during saccadic eye movements, efference copy of motor commands from the superior colliculus informs visual processing centers that the impending disruption in visual information is due to the internally-generated eye movements rather than a change in the external world49–53. Thus, we perceive the visual world as stable despite our eyes moving. Efference copy is also potentially why we can’t tickle ourselves54.
The anatomical substrate for efference copy is axon branching55. By splitting the axon into different branches, single neurons can simultaneously communicate with more than one downstream brain region (Figure 2). Axon branching is an activity-dependent process56 and is developmentally regulated57. There is some evidence for differences in the axonal branching58, 59 and the efference copy system60, 61 in autism and schizophrenia62, and this is an area in need of further research.
Figure 2. Efference copy throughout the sensorimotor system, a simplified schematic illustrating locations of branching axons based on rodent and primate tracing studies.

A. Incoming sensory information is transmitted from peripheral sensory nerves to lower order” (sensory) thalamus and subcortical motor areas (Sherman, 2017). In the case of visual information, one branch routes to the lateral geniculate nucleus and the other copy is sent to the superior colliculus (Chalupa and Thompson, 1980; Crook et al., 2008).
B. “Higher order” thalamic neurons receive driving input from L5 of cortex (Sherman, 2016). Individual higher order thalamic neurons in mediodorsal thalamus and pulvinar project to multiple cortical regions (Giguere and Goldman-Rakic, 1988; Rockland et al., 1999), and on the way, thalamocortical neurons send axon branches to the thalamic reticular nucleus TRN (Kuramoto et al., 2017). Neurons in the TRN can have axons that branch to distinct thalamic nuclei (Lee et al., 2014).
C. Individual Layer 6 corticothalamic neurons may send recurrent axon branches to cortical Layer 4 (Lee et al., 2012; White and Keller, 1987).
D1. Individual Layer 5 neurons in the prefrontal cortex of mice send axons to both higher order (mediodorsal) thalamus and pons (Collins et al., 2018). Individual Layer 5 corticofugal projection neurons in motor, somatosensory and visual cortices in rats send axon collaterals to the thalamus (Deschênes et al., 1994). Individual Layer 5 corticofugal axons in rat motor cortex send collaterals to multiple subcortical targets including subthalamic nucleus, basal ganglia, and superior colliculus (Kita and Kita, 2012).
D2. Individual Layer 5 pyramidal cells in rat sensorimotor cortex project to the brainstem or spinal cord and send axon collaterals to striatum (Donoghue and Kitai, 1981). This is also seen in the primate primary motor cortex, where corticofugal projections to the brainstem also branch to the striatum (Parent and Parent, 2006).
D3, D4. Individual Layer 5 neurons in rat somatosensory barrel cortex project to pons, thalamus, and superior colliculus (Veinante el al., 2000). Individual Layer 5 neurons in the prefrontal cortex of mice send axons to both higher order (mediodorsal) thalamus and pons (Collins et al-, 2018).
E1. Individual cerebellar fastigial nucleus (cFN) neurons project to both the superior colliculus and thalamus (Katoh et al., 2000).
E2. Additionally, individual cFN neurons branch to both the left and right deep layers of the superior colliculus (Katoh & Benedek, 2003). Of note, within the cerebellum, there is a high degree of axonal branching in mossy fibers, climbing fibers (Mason & Gregory, 1984), and Purkinje cells (Yang et al., 2014).
We hypothesize that stereotypies entrain abnormal brain rhythms to improve sensory processing.
In our proposed model (Figure 1), there are two potential routes by which stereotypies could regulate brain rhythms in sensory areas: “direct” (via efference copy) or “indirect” (via sensory feedback).
By the direct route, a repetitive motor command signal is generated with the goal of entraining abnormal rhythms in the sensory system. The rhythmic motor command is conveyed to sensory areas via efference copy. The motor command signal thus directly influences rhythms in the sensory brain regions through synaptic transmission. Because of efference copy, the motor command signal is also transmitted to motor effectors. Thus, stereotyped movements are produced as a “side effect” of the brain’s attempt to use rhythmic motor commands to regulate sensory area oscillations.
By the indirect pathway, the rhythmic sensory signal generated by stereotyped movements is what entrains sensory areas rhythms. Rhythmic sensory stimuli drive rhythmic voltage responses63 in primary sensory cortices and other brain regions like the hippocampus64 and prefrontal cortex65. The rhythmic sensory signal generated by stereotyped movements could therefore entrain or modulate66 brain rhythms in sensory areas. Through this indirect route, purely externally-generated rhythms (e.g. by watching fans spinning or proprioceptively by swinging), could entrain sensory areas via rhythmic sensory inputs.
In addition to activating sensory regions via the indirect route, externally-generated rhythmic sensory signals may also engage the efference copy system and thus exert influence on brain rhythms via the direct route. For instance, seemingly “passive” activities like being rocked in a swing actually engages core muscles to stabilize body posture by counterbalancing externally-generated forces on the body. Thus, the rhythmic motor command generated to activate core muscles while swinging could rhythmically engage sensory areas via the direct path. Similarly, watching a fan spin likely engages the efference copy system by triggering saccadic eye movements and optokinetic nystagmus. Thus seemingly “passive” stereotypies could engage either the indirect pathway, the direct pathway, or both.
The direct versus indirect pathway framework provides a model to generate testable hypotheses about the role of top-down (direct) versus bottom-up (indirect) signaling driving a person to engage in stereotyped movements. For example, if efference copy is abnormal or underdeveloped in autism60, then sensory perception areas may not receive direct copies of motor commands during self-generated movements. As shown in the bottom panel of the Figure 1, we would therefore predict that the indirect pathway would be required to obtain the benefit of stereotypies. If, on the other hand, the efference copy system is functioning normally in autism, then stereotypies could provide benefit through the activation of the direct pathway, the indirect pathway, or both (Figure 1, top panel).
Based on evidence that rhythmic sensory inputs entrain neural oscillations66, we hypothesize that the brain rhythms generated by stereotypies (either via the direct or indirect pathway) improve signal processing in sensory areas by providing discrete “windows of opportunity” for incoming sensory signals67. Further, stereotyped movements reinforce the cycle of action and perception20, creating a situation where the mismatch is low between expectations and reality. Because stereotypies are repetitive and thus predictable, these movements might provide a stream of sensory input to the brain with low prediction error. This could provide a regular baseline rhythm onto which signals generated by external inputs can be layered.
Outstanding questions.
The literature is scant on how fidgeting and other stereotyped movements influence attention, sensory processing, and brain rhythms. Further, although many people with autism report improved sensory processing during stimming, whether stereotypies enhance sensory signal processing has yet to be directly tested.
We propose that testing our model in individuals with and without autism represents low-hanging fruit for understanding core features of autism spectrum disorder. The experimental paradigms to examine the relationship between stereotypies, brain rhythms, and sensory processing / attention would be straightforward for laboratories already engaged in these lines of research using functional magnetic resonance imaging, EEG, and behavioral assessments. Despite being a well-recognized clinical feature of autism, shockingly little is known about the phenomenology of stereotyped movements. For instance, there are almost no studies on the amplitude, frequency, and regularity of stereotypies. Clinically, they appear rhythmic, but stereotypies are also complex and variable68–70. Understanding basic parameters like the frequency at which these movements occur16, 71 and the regularity of the movements would help predict the patterns of brain rhythms that generate them and the characteristics of the sensory input created by the movements themselves72. Through updated methods of wearable sensors in combination with video analysis, measurement and analysis of the physiology of stereotypies as they typically and atypically emerge is now possible73. These technologies could also help quantify similarities and differences in stereotypies across neurodevelopmental disorders74, 75 and to establish developmental “growth charts” for typical and atypical stereotyped movements76, 77.
In terms of neurological localization, it still remains unclear or unknown whether the motor command signal for stereotypies is generated subcortically or cortically78, 79. We have therefore been purposely nonspecific in our terminology for the localization for both motor and sensory “areas” in this writing. Thus, it is an open question where in the nervous system (i.e. cortex vs. thalamus vs. basal ganglia vs. cerebellum) modulation of rhythms by motor commands and sensory feedback might be beneficial.
In predicting which frequencies of brain rhythms would be influenced or entrained by stereotypies, it is important to note that the frequency of muscle activation does not necessarily equal the frequency of the brain signal generating the movement (as it is when rhythmic EEG activity is time-locked to clonic movements during seizures). Similarly, rhythmic sensory inputs may generate the same frequency activity in the brain or they may generate harmonics of that frequency (as we commonly see in the photic driving response).
At the microanatomical level, it is unclear how widespread the phenomenon of branched axons is in the brain and the rest of the nervous system80. Importantly, most brain connectivity models do not currently include axon branching in the calculations. We expect that including branched axons in functional and structural connectivity models will change predictions of brain connectivity in typically developing individuals and in those with autism81. Post-mortem brain studies will help determine how early differences in axon branching can be detected. Using animal models, we can test if differences in axon branching in autism are primary or compensatory82, 83.
It is still unclear if and how behaviors that rely on efference copy differ in people with autism. Quantifications of differences in eye84, 85 and body movements86 offer potential biomarkers for the disorder. Because of efference copy, judging the location of a limb in space is more accurate following active movement than after a passive movement87, 88. If the efference copy system is disrupted in autism, we would predict the difference between perception between active and passive movements to be diminished in individuals with autism. Further, if the efference copy system is disrupted in autism, we would also predict that people with autism would be more likely to be able to tickle themselves than people without autism54.
In conclusion, stereotypies are a poorly understood feature of autism spectrum disorder. We hope that by understanding the anatomy and physiology of motor stereotypies, we can make them less stigmatizing and develop ways to harness their benefits to help people with and without autism.
Acknowledgements:
We appreciate helpful discussions and insightful comments on early versions of this manuscript by Drs. April Levin, Sabine Kastner, Michelle Sleater, and MacKenzie Howard.
Support:
This work was supported by NIH / NINDS K08, the PERF Elterman research grant, the Child Neurology Society Dodge Young Investigator Award, the STARS award from The University of Texas System, startup funds from Dell Medical School, and laboratory space from the College of Natural Sciences at UT Austin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Berkson G, Davenport RK: Stereotyped movements of mental defectives. I. Initial survey. Am J Ment Retard 66:849–52, 1962. [PubMed] [Google Scholar]
- 2.Conroy MA, Asmus JM, Sellers JA, et al. : The Use of an Antecedent-Based Intervention to Decrease Stereotypic Behavior in a General Education Classroom. Focus Autism Dev Dis 20:223–230, 2005. [Google Scholar]
- 3.Association AP: Diagnostic and statistical manual of mental disorders (5th ed.). , 2013. [Google Scholar]
- 4.Péter Z, Oliphant ME, Fernandez TV: Motor Stereotypies: A Pathophysiological Review. Front Neurosci-switz 11:171, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer HS: Motor Stereotypies. Semin Pediatr Neurol 16:77–81, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Edwards MJ, Lang AE, Bhatia KP: Stereotypies: A critical appraisal and suggestion of a clinically useful definition. Movement Disord 27:179–185, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham AB, Schreibman L: Stereotypy in autism: The importance of function. Res Autism Spect Dis 2:469–479, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baribeau DA, Vigod S, Pullenayegum E, et al. : Repetitive Behavior Severity as an Early Indicator of Risk for Elevated Anxiety Symptoms in Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry 59:890–899.e3, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Ghanizadeh A: Clinical approach to motor stereotypies in autistic children. Iran J Pediatr 20:149–59, 2010. [PMC free article] [PubMed] [Google Scholar]
- 10.Gritti A, Bove D, Sarno AMD, et al. : Stereotyped movements in a group of autistic children. Funct Neurol 18:89–94, 2003. [PubMed] [Google Scholar]
- 11.Joyce C, Honey E, Leekam SR, et al. : Anxiety, Intolerance of Uncertainty and Restricted and Repetitive Behaviour: Insights Directly from Young People with ASD. J Autism Dev Disord 47:3789–3802, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Rodgers J, Glod M, Connolly B, et al. : The Relationship Between Anxiety and Repetitive Behaviours in Autism Spectrum Disorder. J Autism Dev Disord 42:2404–2409, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Kapp SK, Steward R, Crane L, et al. : “People should be allowed to do what they like”: Autistic adults’ views and experiences of stimming. Autism Int J Res Pract 1362361319829628, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray-Hammond D: The Importance of Stimming for Autistic People. , 2019. Available at: https://themighty.com/2019/01/autism-stimming/.
- 15.Apartis E: Chapter 8 Clinical neurophysiology in movement disorders. Handb Clin Neurology 111:87–92, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Min C-H: Automatic Detection and Labeling of Self-Stimulatory Behavioral Patterns in Children with Autism Spectrum Disorder. 2017 39th Annu Int Conf Ieee Eng Medicine Biology Soc Embc 2017:279–282, 2017. [DOI] [PubMed] [Google Scholar]
- 17.Chebli SS, Martin V, Lanovaz MJ: Prevalence of Stereotypy in Individuals with Developmental Disabilities: a Systematic Review. Rev J Autism Dev Disord 3:107–118, 2016. [Google Scholar]
- 18.Molloy A, Rowe FJ: Manneristic Behaviors of Visually Impaired Children. Strabismus 19:77–84, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Bos KJ, Zeanah CH, Smyke AT, et al. : Stereotypies in Children With a History of Early Institutional Care. Arch Pediat Adol Med 164:406–411, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrykkad K, Hohwy J: Fidgeting as self-evidencing: A predictive processing account of non-goal-directed action. New Ideas Psychol 56:100750, 2020. [Google Scholar]
- 21.Wilkinson S, Deane G, Nave K, et al. : The Value of Emotions for Knowledge. 101–119, 2019. [Google Scholar]
- 22.Stavley S, Brasell H: Using Stress Balls to Focus the Attention of Sixth-Grade Learners. The Journal of At-Risk Issues, 2006. [Google Scholar]
- 23.Scheeringa R, Fries P: Cortical layers, rhythms and BOLD signals. Neuroimage, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroeder CE, Lakatos P: Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 32:9–18, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lampl I, Reichova I, Ferster D: Synchronous Membrane Potential Fluctuations in Neurons of the Cat Visual Cortex. Neuron 22:361–374, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Haegens S, Golumbic EZ: Rhythmic facilitation of sensory processing: A critical review. Neurosci Biobehav Rev 86, 2018. [DOI] [PubMed] [Google Scholar]
- 27.Buzsáki G, Watson BO: Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci 14:345–67, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroeder CE, Wilson DA, Radman T, et al. : Dynamics of Active Sensing and perceptual selection. Curr Opin Neurobiol 20:172–6, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rojas-Líbano D, Frederick DE, Egaña JI, et al. : The olfactory bulb theta rhythm follows all frequencies of diaphragmatic respiration in the freely behaving rat. Front Behav Neurosci 8:214, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore JD, Kleinfeld D, Wang F: How the brainstem controls orofacial behaviors comprised of rhythmic actions. Trends Neurosci 37:370–380, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito J, Maldonado P, Singer W, et al. : Saccade-Related Modulations of Neuronal Excitability Support Synchrony of Visually Elicited Spikes. Cereb Cortex 21:2482–2497, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowet E, Gips B, Roberts MJ, et al. : Microsaccade-rhythmic modulation of neural synchronization and coding within and across cortical areas V1 and V2. Plos Biol 16:e2004132, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohenkohl G, Bosman CA, Fries P: Gamma Synchronization between V1 and V4 Improves Behavioral Performance. Neuron 100:953–963.e3, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colgin LL: Mechanisms and Functions of Theta Rhythms. Annu Rev Neurosci 36:295–312, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Uhlhaas PJ, Roux F, Singer W: Thalamocortical Synchronization and Cognition: Implications for Schizophrenia? Neuron 77:997–999, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Helfrich RF, Fiebelkorn IC, Szczepanski SM, et al. : Neural Mechanisms of Sustained Attention Are Rhythmic. Neuron 99:854–865.e5, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson CE, Baron-Cohen S: Sensory perception in autism. Nat Rev Neurosci 18:nrn.2017.112, 2017. [DOI] [PubMed] [Google Scholar]
- 38.Orekhova EV, Stroganova TA, Schneiderman JF, et al. : Neural gain control measured through cortical gamma oscillations is associated with sensory sensitivity. Hum Brain Mapp 40:1583–1593, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edgar JC, Lanza MR, Daina AB, et al. : Missing and Delayed Auditory Responses in Young and Older Children with Autism Spectrum Disorders. Front Hum Neurosci 8:417, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seymour RA, Rippon G, Gooding-Williams G, et al. : Dysregulated oscillatory connectivity in the visual system in autism spectrum disorder. Brain 142:3294–3305, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Barstein J, Ethridge LE, et al. : Resting state EEG abnormalities in autism spectrum disorders. J Neurodev Disord 5:24, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berman JI, Liu S, Bloy L, et al. : Alpha-to-Gamma Phase-Amplitude Coupling Methods and Application to Autism Spectrum Disorder. Brain Connectivity 5:80–90, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milne E, Scope A, Pascalis O, et al. : Independent Component Analysis Reveals Atypical Electroencephalographic Activity During Visual Perception in Individuals with Autism. Biol Psychiat 65:22–30, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Snijders TM, Milivojevic B, Kemner C: Atypical excitation–inhibition balance in autism captured by the gamma response to contextual modulation. Neuroimage Clin 3:65–72, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy E, Benítez-Burraco A: Language deficits in schizophrenia and autism as related oscillatory connectomopathies: An evolutionary account. Neurosci Biobehav Rev 83:742–764, 2017. [DOI] [PubMed] [Google Scholar]
- 46.Khan S, Michmizos K, Tommerdahl M, et al. : Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain 138:1394–1409, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gage NM, Siegel B, Roberts TPL: Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Dev Brain Res 144:201–209, 2003. [DOI] [PubMed] [Google Scholar]
- 48.An K, Ikeda T, Yoshimura Y, et al. : Altered Gamma Oscillations during Motor Control in Children with Autism Spectrum Disorder. J Neurosci 38:1229–18, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brooks JX, Cullen KE: Predictive Sensing: The Role of Motor Signals in Sensory Processing. Biological Psychiatry Cognitive Neurosci Neuroimaging 4:842–850, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer B, Weber H: Express saccades and visual attention. Behav Brain Sci 16:553–567, 1993. [Google Scholar]
- 51.Sommer MA, Wurtz RH: Brain Circuits for the Internal Monitoring of Movements*. Annu Rev Neurosci 31:317–338, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Straka H, Simmers J, Chagnaud BP: A New Perspective on Predictive Motor Signaling. Curr Biol 28:R232–R243, 2018. [DOI] [PubMed] [Google Scholar]
- 53.Bansal S, Ford JM, Spering M: The function and failure of sensory predictions. Ann Ny Acad Sci 1426:199–220, 2018. [DOI] [PubMed] [Google Scholar]
- 54.Blakemore S-J, Wolpert D, Frith C: Why can’t you tickle yourself? Neuroreport 11:R11–R16, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Guillery RW, Sherman SM: Branched thalamic afferents: What are the messages that they relay to the cortex? Brain Res Rev 66:205–219, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalil K, Dent EW: Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nat Rev Neurosci 15:nrn3650, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibson DA, Ma L: Developmental regulation of axon branching in the vertebrate nervous system. Development 138:183–195, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zikopoulos B, Barbas H: Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front Hum Neurosci 7:609, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zikopoulos B, Liu X, Tepe J, et al. : Opposite development of short- and long-range anterior cingulate pathways in autism. Acta Neuropathol 136:759–778, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laarhoven T van, Stekelenburg JJ, Eussen MLJM, et al. : Electrophysiological alterations in motor-auditory predictive coding in autism spectrum disorder. Autism Res 12:589–599, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moliadze V, Brodski-Guerniero A, Schuetz M, et al. : Significance of Beta-Band Oscillations in Autism Spectrum Disorders During Motor Response Inhibition Tasks: A MEG Study. Brain Topogr 33:355–374, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parlikar R, Bose A, Venkatasubramanian G: Schizophrenia and Corollary Discharge: A Neuroscientific Overview and Translational Implications. Clin Psychopharm Neu 17:170–182, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nozaradan S, Peretz I, Keller PE: Individual Differences in Rhythmic Cortical Entrainment Correlate with Predictive Behavior in Sensorimotor Synchronization. Sci Rep-uk 6:20612, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martorell AJ, Paulson AL, Suk H-J, et al. : Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition. Cell 177:256–271.e22, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moberly AH, Schreck M, Bhattarai JP, et al. : Olfactory inputs modulate respiration-related rhythmic activity in the prefrontal cortex and freezing behavior. Nat Commun 9:1528, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vialatte F-B, Maurice M, Dauwels J, et al. : Steady-state visually evoked potentials: Focus on essential paradigms and future perspectives. Prog Neurobiol 90:418–438, 2010. [DOI] [PubMed] [Google Scholar]
- 67.Schaefer AT, Angelo K, Spors H, et al. : Neuronal Oscillations Enhance Stimulus Discrimination by Ensuring Action Potential Precision. Plos Biol 4:e163, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thelen E: Kicking, rocking, and waving: Contextual analysis of rhythmical stereotypies in normal human infants. Anim Behav 29:3–11, 1981. [DOI] [PubMed] [Google Scholar]
- 69.Thelen E: Rhythmical stereotypies in normal human infants. Anim Behav 27:699–715, 1979. [DOI] [PubMed] [Google Scholar]
- 70.Goldman S, Greene PE: Stereotypies in autism: a video demonstration of their clinical variability. Frontiers Integr Neurosci 6:121, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goncalves N, Rodrigues JL, Costa S, et al. : Automatic detection of stereotyped hand flapping movements: Two different approaches. 392–397, 2012. [Google Scholar]
- 72.Zalta A, Hou J-C, Thonnat M, et al. : Neural correlates of rhythmic rocking in prefrontal seizures. Neurophysiologie Clinique 50:331–338, 2020. [DOI] [PubMed] [Google Scholar]
- 73.Torres EB, Brincker M, Isenhower RW, et al. : Autism: the micro-movement perspective. Frontiers Integr Neurosci 7:32, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldman S, Temudo T: Hand stereotypies distinguish Rett syndrome from autism disorder. Movement Disord 27:1060–1062, 2012. [DOI] [PubMed] [Google Scholar]
- 75.Gal E, Dyck MJ, Passmore A: The relationship between stereotyped movements and self-injurious behavior in children with developmental or sensory disabilities. Res Dev Disabil 30:342–352, 2009. [DOI] [PubMed] [Google Scholar]
- 76.Symons FJ, Sperry LA, Dropik PL, et al. : The early development of stereotypy and self-injury: a review of research methods. J Intell Disabil Res 49:144–158, 2005. [DOI] [PubMed] [Google Scholar]
- 77.Shultz S, Klin A, Jones W: Neonatal Transitions in Social Behavior and Their Implications for Autism. Trends Cogn Sci 22:452–469, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harris AD, Singer HS, Horska A, et al. : GABA and Glutamate in Children with Primary Complex Motor Stereotypies: An 1H-MRS Study at 7T. Am J Neuroradiol 37:552–557, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Houdayer E, Walthall J, Belluscio BA, et al. : Absent movement-related cortical potentials in children with primary motor stereotypies. Movement Disord 29:1134–1140, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gergues MM, Han KJ, Choi HS, et al. : Circuit and molecular architecture of a ventral hippocampal network. Nat Neurosci 23:1444–1452, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kana RK, Libero LE, Moore MS: Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys Life Rev 8:410–437, 2011. [DOI] [PubMed] [Google Scholar]
- 82.Yang R, Walder-Christensen KK, Kim N, et al. : ANK2 autism mutation targeting giant ankyrin-B promotes axon branching and ectopic connectivity. Proc National Acad Sci 116:15262–15271, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drinjakovic J, Jung H, Campbell DS, et al. : E3 Ligase Nedd4 Promotes Axon Branching by Downregulating PTEN. Neuron 65:341–357, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takarae Y, Minshew NJ, Luna B, et al. : Pursuit eye movement deficits in autism. Brain 127:2584–2594, 2004. [DOI] [PubMed] [Google Scholar]
- 85.Minshew NJ, Luna B, Sweeney JA: Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology 52:917–922, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu D, José JV, Nurnberger JI, et al. : A Biomarker Characterizing Neurodevelopment with applications in Autism. Sci Rep-uk 8:614, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laufer Y, Hocherman S, Dickstein R: Accuracy of reproducing hand position when using active compared with passive movement. Physiotherapy Res Int 6:65–75, 2001. [DOI] [PubMed] [Google Scholar]
- 88.Stock A-K, Wascher E, Beste C: Differential Effects of Motor Efference Copies and Proprioceptive Information on Response Evaluation Processes. Plos One 8:e62335, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chalupa LM, Thompson I: Retinal ganglion cell projections to the superior colliculus of the hamster demonstrated by the horseradish peroxidase technique. Neurosci Lett 19:13–19, 1980. [DOI] [PubMed] [Google Scholar]
- 90.Collins DP, Anastasiades PG, Marlin JJ, et al. : Reciprocal Circuits Linking the Prefrontal Cortex with Dorsal and Ventral Thalamic Nuclei. Neuron 98:366–379.e4, 2018. Available at: http://www.cell.com/neuron/fulltext/S0896-6273(18)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crook JD, Peterson BB, Packer OS, et al. : The Smooth Monostratified Ganglion Cell: Evidence for Spatial Diversity in the Y-Cell Pathway to the Lateral Geniculate Nucleus and Superior Colliculus in the Macaque Monkey. J Neurosci 28:12654–12671, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deschênes M, Bourassa J, Pinault D: Corticothalamic projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Research 664:215 219, 1994. [DOI] [PubMed] [Google Scholar]
- 93.Donoghue JP, Kitai ST: A collateral pathway to the neostriatum from corticofugal neurons of the rat sensory-motor cortex: An intracellular HRP study. J Comp Neurol 201:1–13, 1981. [DOI] [PubMed] [Google Scholar]
- 94.Giguere M, Goldman-Rakic PS: Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. The Journal of Comparative Neurology 277:195 213, 1988. [DOI] [PubMed] [Google Scholar]
- 95.Katoh YY, Benedek G: Cerebellar fastigial neurons send bifurcating axons to both the left and right superior colliculus in cats. Brain Res 970:246–249, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Katoh YY, Arai R, Benedek G: Bifurcating projections from the cerebellar fastigial neurons to the thalamic suprageniculate nucleus and to the superior colliculus. Brain Res 864:308–311, 2000 [DOI] [PubMed] [Google Scholar]


