Abstract
Background
Attention deficits frequently accompany language impairments in aphasia. Most research on attention in aphasia focuses on selective attention measured by executive control tasks such as the color-word Stroop or Erickson flanker. This is despite ample evidence in neurotypical adults indicating the existence of multiple, distinct attention subtypes. Thus, there is a disconnect between the documented attention impairments in persons with aphasia (PWA) and the literature in neurotypical adults indicating that multiple attention components independently modulate an individual’s interactions with the world.
Aims
This study aimed to use the well-studied Attention Network Test (ANT) to quantify three subtypes of attention (alerting, orienting, and executive control) in PWA and matched controls. It was hypothesized that significant effects of alerting, orienting, and executive control would be observed in both groups, however, the effects would be reduced in PWA compared to the neurotypical controls. It was additionally expected that alerting, orienting, and executive control would not be correlated with one another in either group.
Methods & Procedures
Twenty-two PWA along with 20 age, gender, and education matched controls completed the ANT. Briefly, the ANT consists of a cued-flanker task where the cues provide information about when and where the flanker executive control task will be presented. The combination of cues and flanker targets embedded within the ANT provides measures of alerting, orienting, and executive control. Participants are expected to respond faster and more accurately to the flanker task when cued as to when and where the task will be presented.
Outcomes & Results
In line with previous work, the control group demonstrated significant effects of alerting, orienting, and executive control. However, we only find significant orienting and executive control effects in the aphasia group. Between-group differences were only identified within orienting attention: the control group benefitted more from the orienting cue than the aphasia group. Additionally, alerting, orienting, and executive control were not correlated in the control group, yet, a relationship between orienting and executive control was observed in the aphasia group.
Conclusions
Overall, our findings demonstrate that attention differs between PWA and controls, and that the ANT may provide a more complete picture of attention in aphasia; this may be particularly important when characterizing the relationship between attention and language in aphasia.
Keywords: attention, aphasia, Attention Network Test, stroke, executive function
Introduction
Aphasia is classically thought to be a language-specific disorder. Yet, it is well established that some persons with aphasia (PWA) demonstrate impaired performance on a variety of cognitive skills including attention (Murray, 2012; Murray et al., 1997), memory (Caplan et al., 2013), and executive functions (Fridriksson et al., 2006). Of these cognitive skills, attention may be particularly important for studies of aphasia as it is a necessary foundation for other executive functions that are known to be engaged during language tasks, such as working memory (Baddeley, 1992; Cowan et al., 2005).
Selective attention has been the primary focus of much of the attention research in aphasia as it is proposed to be a critical building block for successful communication. For example, selective attention allows an individual to maintain alertness to critical information while completing a task, or selectively responding to an incoming stimulus (e.g., speech) while ignoring irrelevant and/or distracting information (e.g., background noise). Selective attention in PWA is typically assessed experimentally using classic selective attention paradigms such as the Stroop color-word task (Green et al., 2010; Pompon et al., 2015) and dual-task paradigms (Erickson et al., 1996; Heuer & Hallowell, 2015). These attention tasks particularly tax and measure executive control, i.e., the aspect of selective attention that is related to attending to task-relevant information while suppressing irrelevant or conflicting information. Clinically, the Test of Everyday Attention (TEA) is commonly used to measure selective attention (and also sustained and alternating attention) in PWA (Gordon-Pershey & Wadams, 2017; Murray, 2012; Peach et al., 2017; Robertson et al., 1994). The TEA’s selective attention subtests also mostly engage executive control, e.g., searching for a specific symbol amongst competitors on a map. Findings from this executive control work indicate that PWA exhibit greater declines in performance due to interfering information (Erickson et al., 1996; Heuer & Hallowell, 2015; Pompon et al., 2015), and are less-sensitive to task-related attentional cues meant to facilitate selective attention (Tseng, McNeil & Milenkovic, 1993).
A great deal of work in neurotypical adults has also examined executive control (MacLeod, 1991; Stroop, 1935; Verhaeghen & Cerella, 2002), but a large body of work also indicates that selective attention does not just consist of executive control processes. While several models exist that subdivide attention (e.g., Mirsky, Anthony, Duncan, Ahearn, & Kellam, 1991; Posner & Petersen, 1990; Sohlberg & Mateer, 2010), we focus on Posner and Petersen’s functional-neuroanatomical model of attention networks that divides selective attention into three distinct subsets: alerting, orienting, and executive control (Petersen & Posner, 2012; Posner & Petersen, 1990). Alerting involves achieving and maintaining an alert state while orienting is the selection of specific information from a given stimulus (Fan et al., 2002; Fan & Posner, 2004; Posner & Petersen, 1990). Executive control is a measure of how efficiently a correct response is achieved when relevant stimulus information conflicts with irrelevant stimulus information (Fan et al., 2002; Posner & Petersen, 1990). Performance on these three types of attention have been found to dissociate in neurotypical adults, and performance across the three is only weakly correlated to one another (MacLeod et al., 2010; Roberts, Summerfield, & Hall, 2006; Spagna, Mackie, & Fan, 2015; Stewart & Amitay, 2015). Each of the three attention subtypes are also known to be supported by neuroanatomically-distinct bilateral brain networks in neurotypical adults (Coull et al., 2001; Konrad et al., 2005; Petersen & Posner, 2012; Thiel & Fink, 2007). In PWA and stroke more broadly, left hemisphere damage is also associated with lower performance on a variety of attention measures (Heuer & Hallowell, 2015; Hula et al., 2007; Hula & McNeil, 2008; Kurland, 2011; Murray, 2012; Robin & Rizzo, 1989). Thus, Posner and Petersen’s functional-neuroanatomical model of attention may be particularly important to the study of attention in aphasia as it focuses on three distinct attention subtypes that are supported by distinct anatomical regions which are often damaged in PWA, including the left frontal and parietal cortices. It is therefore likely that many PWA will also present with some degree and combination of alerting, orienting, and executive control impairments that could further contribute to their communication impairments.
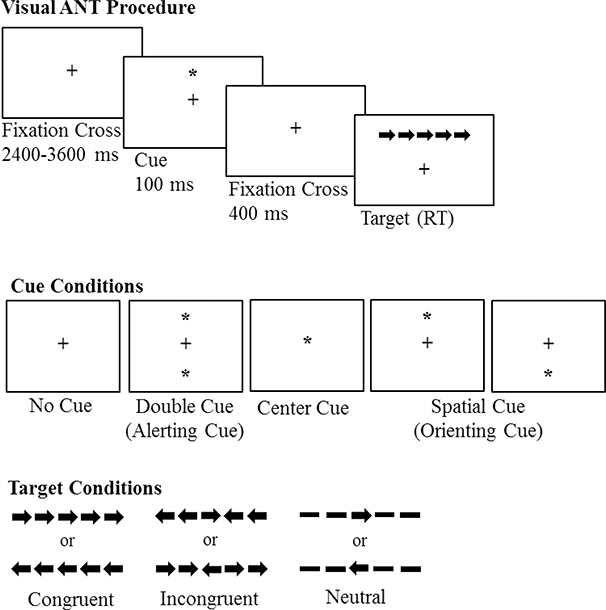
In neurotypical controls, the widely-used Attention Network Test (ANT; Fan et al., 2002) separately measures alerting, orienting, and executive control using a cued-flanker task in the visual modality (Figure 1). The basic premise is that cues provide information prior to a flanker executive control task that facilitate the response time of the executive control task. Orienting cues provide information as to where to direct attention, while alerting cues provide information as to when to direct attention. In clinical populations, the ANT has been used to assess the integrity of each subtype of attention in a variety of populations including stroke (Fan & Posner, 2004; Rinne et al., 2013; Chica et al. 2012), but only rarely in PWA (i.e., n=1 in Laures-Gore & Marshall (2016) and n=5 in Marshall et al., (2018); both related to a mindfulness intervention), and never in comparison to a neurotypical control group.
Figure 1.
Illustration of the procedure, cue conditions, and target conditions for the ANT.
Despite limited use of the ANT in aphasia, alerting and orienting have been examined separately in PWA using other paradigms. One study of orienting attention, which used a spatial-cueing paradigm, found that four individuals with aphasia due to left hemisphere stroke presented with orienting impairments compared to a control group, and that PWA, like those with right hemisphere strokes, did not make use of orienting cues (visual arrows indicating the direction of the upcoming target; Robin & Rizzo, 1989). In fact, the orienting information actually slowed down their response times, suggesting that PWA were negatively affected by attentional cues that are typically helpful to neurotypical controls. Villard and Kiran (2015) and Petry and colleagues (1994) similarly identified PWA to orient more slowly to spatial information relative to neurotypical controls, and specific physical stimulus properties (e.g., orientation, brightness, size, color) have also been shown to draw attention towards specific stimuli in PWA (Heuer et al., 2017). While these few studies suggest that on average PWA likely have impairments in alerting and orienting attention, no study has systematically studied all three attention subtypes of Posner and Petersen’s model in the same group of PWA, or compared their performance to a neurotypical control group. Thus, there is a disconnect between the documented attention impairments in PWA as measured almost exclusively by executive control tasks, and the vast literature in neurotypical adults indicating that the three attentional components of alerting, orienting, and executive control dissociate and all uniquely contribute to an individual’s interactions with the world around them.
The purpose of the present study was to examine alerting, orienting, and executive control abilities in relation to one another within PWA and between PWA and a matched control group. It was hypothesized that significant effects of alerting, orienting, and executive control will be present in both groups, indicating that the ANT is a meaningful measure to collect in PWA to potentially provide further insights into their attention abilities, and allow for better cross-talk between attention research in PWA and in neurotypical controls. It was also hypothesized that the aphasia group would exhibit reduced alerting, orienting, and executive control effects compared to the neurotypical controls, and that alerting, orienting, and executive control measures will not be correlated with one another in either group, coinciding with previous work in stroke and neurotypical controls.
Method
Participants
Participants were 22 chronic PWA (12 females) who experienced a single left hemisphere cerebral stroke11 at least 6 months prior to testing (Table 1). PWA ranged in age from 28 to 80 years (M = 54.64, sd = 12.97), were pre-morbidly right-handed, native speakers of American English, 18+ years of age, with no self-reported history of neurological disease, head trauma, or psychiatric disturbances prior to their stroke. Aphasia classification and severity was determined using the Boston Diagnostic Aphasia Evaluation-III (Goodglass et al., 2000); each stroke participant’s aphasia diagnosis is reported in Table 1. An additional 20 controls (14 females) ranging in age from 31 to 79 years (M = 51.40, sd = 12.82) who were also right-handed, native speakers of American English, 18+ years of age, with no self-reported history of neurological disease, head trauma, or psychiatric disturbances were also recruited. There were no significant differences between the aphasia and control groups in age, gender, or education (Table 2). All participants were monetarily compensated for their participation. Arizona State University’s Institutional Review Board approved all procedures.
Table 1.
Aphasia patient demographics.
| Gender | Age | Months Post Stroke | Years of Education | Aphasia Diagnosis | |
|---|---|---|---|---|---|
| AZ1003 | Female | 48 | 110 | 19 | Mild Broca’s |
| AZ1006 | Male | 60 | 138 | 14 | Severe Broca’s |
| AZ1011 | Female | 73 | 53 | 16 | Mild Anomic |
| AZ1012 | Male | 77 | 85 | 16 | Moderate Wernicke’s |
| AZ1013 | Female | 47 | 258 | 17 | Severe Broca’s |
| AZ1016 | Male | 37 | 142 | 14 | Moderate Broca’s |
| AZ1018 | Female | 43 | 29 | 14 | Mild Broca’s |
| AZ1022 | Female | 46 | 79 | 14 | Moderate Broca’s |
| AZ1028 | Female | 80 | 19 | 24 | Moderate Wernicke’s |
| AZ1030 | Male | 56 | 32 | 16 | Moderate Broca’s |
| AZ1031 | Female | 40 | 63 | 20 | Mild Broca’s |
| AZ1032 | Male | 28 | 20 | 13 | Mild Anomic |
| AZ1033 | Male | 57 | 180; 60 | 14 | Moderate Global |
| AZ1034 | Female | 59 | 110 | 15 | Mild Anomic |
| AZ1035 | Female | 41 | 72 | 17 | Mild Broca’s |
| AZ1036 | Male | 65 | 158 | 15 | Moderate Broca’s |
| AZ1037 | Male | 57 | 13 | 16 | Moderate Broca’s |
| AZ1038 | Male | 54 | 155 | 14 | Moderate Broca’s |
| AZ1039 | Female | 66 | 48 | 14 | Mild Anomic |
| AZ1040 | Female | 54 | 45 | 14 | Mild Broca’s |
| AZ1041 | Female | 59 | 24 | 12 | Mild Anomic |
| AZ1042 | Male | 55 | 37 | 14 | Moderate Broca’s |
Table 2.
Demographic comparisons between aphasia and control groups.
| Aphasia (n=22) | Controls (n=20) | Statistic | |
|---|---|---|---|
| Age | 54.64 (12.97) | 51.40 (12.82) | t(40)=.81, p=.42 |
| Gender (male/female) | 10/12 | 6/14 | χ2(1)=1.06, p=.30 |
| Education (years) | 15.54 (2.67) | 15.20 (2.17) | t(40)=46, p=.65 |
Experimental Design
Participants completed the widely-used ANT (Fan et al., 2002) as part of a larger neuropsychological test battery. Figure 1 includes a schematic of the ANT procedures, cues, and targets. Each trial began with a fixation cross jittered between 2400–3600 milliseconds. Following the offset of the fixation cross, a visual cue was presented in the middle of the screen for 100 milliseconds. Visual cue conditions were as follows: (1) center cues (single asterisk presented in the middle of the screen), (2) double cues (simultaneous presentation of one asterisk above the fixation cross and one asterisk below the fixation cross), (3) spatial cue (single asterisk presented either above or below the fixation cross; spatial cues always predicted the location of the flanker task), and (4) no cue (fixation cross remains in the middle of the screen, but no cueing is provided; i.e., no offset of the fixation cross following the jittered period). Following the offset of the visual cue, the fixation cross was presented on the screen for 400 milliseconds after which time participants completed the flanker task. In the flanker task, participants saw a series of five arrows and indicated via button press whether the center arrow was pointing left or right. The position of the flanker task was randomly assigned to above or below the fixation cross on each trial, such that in half of the trials the flanker arrows appeared above the fixation cross, and in the other half they appeared below. A congruent trial occurred when the center arrow was pointing in the same direction as the flanking arrows, an incongruent trial when the center arrow was pointing in the opposite direction of the flanking arrows, and a neutral trial when the center arrow was not flanked by any arrows, but instead flanked by straight lines. For all flanker trials, participants were instructed to be fast and accurate, and pressed the left arrow on the keyboard if the center arrow pointed left and the right arrow if the center arrow pointed right. Participants completed 180 trials where all cue types and flanker conditions were presented equally. Trial presentation was randomized for each participant. Verbal and written instructions, examples of all stimuli, and 10 practice trials preceded the start of the experiment.
Data Analysis
Alerting, orienting, and executive control (Table 3) were each analyzed in terms of accuracy and reaction time. All responses were included in the accuracy analyses. For the reaction time analyses, reaction times associated with incorrect responses and those greater than 2.5 standard deviations from each participant’s mean were excluded from the analyses; this data trimming procedure was determined a priori based on it being a standard, well-studied approach in psycholinguistic research (Baayen & Milin, 2010; Lachaud & Renaud, 2011; Ratcliff, 1993). This approach aims to capture the middle 85% of the distribution of the reaction time measurements and is based on the assumption that the process of interest is being captured, not other extraneous factors (e.g., brief distractions, button press mistakes, etc.). Consistent with this aim, 1.04% of the data was removed due to incorrect responses and 2.6% due to long reaction times (total: 3.64%) for the aphasia group and 0.81% due to errors and 1.86% due to long reaction times (total: 2.67%) for the control group.
Table 3.
Alerting, orienting, and executive control calculations and reaction time interpretations as conventionally defined (Fan, McCandliss, Fossela, Flombaum, & Posner, 2005; Fan et al., 2002; MacLeod et al., 2010). Note, in accuracy, smaller scores equal better alerting, orienting, and executive control.
| Attention Subtype | Calculation | Reaction Time Interpretation |
|---|---|---|
| Alerting | No Cue – Double Cue | Larger scores = better alerting |
| Orienting | Center Cue – Spatial Cue | Larger scores = better orienting |
| Executive Control | Incongruent – Congruent Trials | Smaller scores = better executive control |
Alerting, orienting, and executive control are known to be independent subtypes of attention (Petersen & Posner, 2012; Posner & Petersen, 1990). Therefore, as is consistent with the literature (e.g., Fan et al., 2002; MacLeod et al., 2010), three separate paired samples t-tests were computed to identify significant effects of alerting (no cue versus double cue), orienting (center cue versus spatial cue), and executive control (incongruent versus congruent) within each group. Independent samples t-tests were used to compare alerting (no cue – double cue), orienting (center cue – spatial cue), and executive control effects (incongruent – congruent) between the aphasia and control groups. Exploratory Pearson correlations were used to analyze the independence of the attentional subsystems within each group; these correlations should be interpreted with caution due to the small sample size of each group.
Results
Attention in persons with aphasia and matched controls
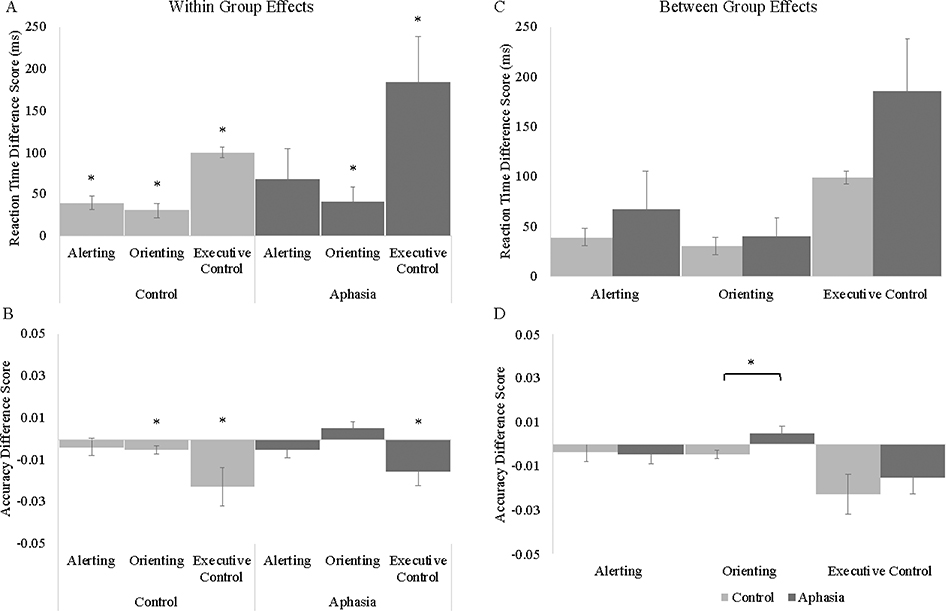
Means and standard errors of the means for all cue and target conditions are reported for reaction time and accuracy in Table 4. Alerting, orienting, and executive control effects are plotted in reaction time and accuracy within each group in Figure 2.
Table 4.
Means and standard errors of the mean for each group in reaction time (milliseconds) and accuracy.
| No Cue | Double | Center | Spatial | Congruent | Incongruent | Neutral | All | ||
|---|---|---|---|---|---|---|---|---|---|
| Controls | RT | 634.24 (39.01) | 594.92 (36.47) | 601.32 (38.60) | 570.81 (38.33) | 570.55 (36.97) | 669.93 (39.74) | 565.09 (36.36) | 600.05 (37.64) |
| ACC | .989 (.006) | .993 (.003) | .989 (.004) | .994 (.002) | .999 (.001) | .976 (.009) | .999 (.001) | .992 (.003) | |
|
| |||||||||
| Aphasia | RT | 1253.36 (147.65) | 1185.45 (131.76) | 1212.23 (134.02) | 1171.84 (138.42) | 1160.04 (130.97) | 1344.96 (161.66) | 1115.53 (123.44) | 1204.57 (136.54) |
| ACC | .986 (.003) | .991 (.004) | .991 (.005) | .985 (.006) | .993 (.003) | .977 (.009) | .994 (.002) | .988 (.004) | |
Figure 2.
Within group effects of alerting (no cue – double cue), orienting (center cue – spatial cue), and executive control (incongruent – congruent) in reaction time (A) and accuracy (B). Between-group comparisons for alerting (no cue – double cue), orienting (center cue – spatial cue), and executive control (incongruent – congruent) in reaction time (C) and accuracy (D). Error bars represent +/− one standard error.
Reaction time
Replicating previous work using the ANT, the control group demonstrated significant effects of alerting (i.e., better performance on double cue trials compared to no cue trials; t(19)=4.89, p<.001), orienting (i.e., better performance on spatial cue trials compared to center cue trials; t(19)=3.46, p=.003), and executive control (i.e., slower responses for incongruent trials compared to congruent trials; t(19)=15.24, p<.001). For the aphasia group, significant orienting (t(21)=2.22, p=.04) and executive control effects (t(21)=3.50, p=.002) were observed, however, unlike in controls, the alerting effect was not significant (t(21)=1.83, p=.08).
Accuracy
For the control group, the alerting effect was not significant (t(19)=.92, p=.37), however, significant effects of orienting (i.e., increased accuracy on the spatial cue trials compared to the center cue trials; t(19)=2.13, p=.05) and executive control (i.e., decreased accuracy for incongruent trials compared to congruent trials; t(19)=2.45, p=.02) were observed. For the aphasia group, the effects of alerting (t(21)=1.13, p=.27) and orienting (t(21)=1.77, p=.09) were non-significant, however, executive control costs were observed (t(21)=2.21, p=.04).
Attention in persons with aphasia versus matched controls
Alerting, orienting, and executive control effects are plotted in reaction time and accuracy between each group in Figure 2. Overall, the control group was significantly faster than the aphasia group (t(40)=4.09, p<.001), but the two groups did not differ on accuracy (t(40)=.71, p=.49). For reaction time, no differences in alerting cue benefits (t(40)=.72, p=.48), orienting cue benefits (t(40)=.47, p=.64), or executive control costs were observed (t(40)=1.53, p=.13) between the aphasia and control groups. Since the aphasia group’s reaction times were all significantly longer than the control group’s, we also ran these same statistics using normalized reaction time scores;2 qualitatively we find the same pattern of results (i.e., no group differences for alerting, orienting, or executive control). For accuracy, there were no differences between the two groups for either alerting (t(40)=.18, p=.86) or executive control (t(40)=.65, p=.52), but the control group exhibited a greater orienting cue benefit than the aphasia group (t(40)=2.67, p=.01).
Independence of attention subsystems in persons with aphasia and matched controls
Reaction time
In the control group, alerting did not correlate with orienting (r(18)= −.07, p=.77) or executive control (r(18)=.36, p=.12). Orienting also did not correlate with executive control in the control group (r(18)= −.38, p=.10). For the aphasia group, alerting did not correlate with orienting (r(20)= −.03, p=.88) or executive control (r(20)= −.08, p=.72). Orienting attention negatively correlated with executive control in the aphasia group (r(20)= −.43, p=.05) indicating that better orienting attention is associated with better executive control performance.
Accuracy
For the control group, executive control abilities as measured by accuracy positively correlated with both alerting (r(18)=.61, p=.004) and orienting (r(18)=.79, p<.001), such that better alerting and orienting correlated with poorer executive control. Alerting did not correlate with orienting attention for the control group (r(18)= .24, p=.31). For the aphasia group, alerting did not correlate with either orienting (r(20)=.13, p=.57) or executive control (r(20)= .13, p=.56), but orienting attention did negatively correlate with executive control abilities (r(20)= −.51, p=.02), indicating that better orienting correlated with better executive control abilities in PWA.
Discussion
The relationships between specific types of attention and aphasia are not well-defined, despite attention being a critical cognitive resource for successful communication. Thus, the purpose of this study was to use the ANT to compare alerting, orienting, and executive control abilities in PWA to a matched control group, as well as to assess the independence of these three attentional constructs in PWA. The results indicate that alerting, orienting, and executive control abilities differ in PWA compared to neurotypical adults. The implications of these results are discussed below.
Our findings indicate that the PWA group was able to complete the ANT as instructed and without modifications, as despite their slower performance, accuracy did not differ between the two groups. This ability to investigate multiple subtypes of attention in PWA using the ANT is critical because the vast majority of attention studies in PWA have examined executive control. Thus, it is not clear if PWA exhibit impairments across all attention tasks and if so, whether this impairment is due to a common underlying attention mechanism, general task demands, or executive control in particular. In the present study, we found that orienting accuracy was lower in PWA than the control group, but no group differences were found in accuracy for alerting or executive control. We also did not find group differences in reaction time for any of the attention measures. This finding of impaired orienting attention in PWA relative to controls aligns with previous work using other orienting tasks (Petry et al., 1994; Robin & Rizzo, 1989; Villard & Kiran, 2015), as well as work demonstrating that PWA have greater variability in their ability to utilize spatial-orienting cues (Villard & Kiran, 2015). Since inhibition of task irrelevant information (measured via executive control) and selection of task relevant information (measured via orienting) are affected differently in aphasia, future studies should examine the impact of alerting and orienting on language outcomes, in addition to executive control.
The ANT allowed us to investigate what types of attention are impaired in PWA. As expected, the control group demonstrated significant effects of alerting, orienting, and executive control in reaction time (Fan et al., 2002; Roberts et al., 2006; Spagna et al., 2015; Stewart & Amitay, 2015). Yet, the aphasia group only demonstrated significant orienting benefits and executive control costs in reaction time. The lack of an alerting effect in the aphasia group corresponds to previous work exhibiting PWA to have reduced (auditory) alerting abilities relative to neurotypical controls (Laures, 2005). PWA are also known to have significant inter-individual variability in attention performance (Gordon-Pershey & Wadams, 2017; Murray, 2012; Sturm et al., 1997), and this too may have contributed to the lack of an alerting effect in the aphasia group. Inter-individual variability in aphasia likely arises from numerous factors, including neurological factors such as location of the stroke umbra and disruptions to widespread functional neural networks caused by structural damage. Structurally, the thalamus, brainstem, and bilateral fronto-parietal cortices all support alerting attention (Coull et al., 2001; Konrad et al., 2005; Petersen & Posner, 2012; Rinne et al., 2013; Sturm & Willmes, 2001; Thiel & Fink, 2007), with stronger activation of left versus right fronto-parietal cortices being observed in neurotypical adults during alerting tasks (Fan et al., 2005). Thus, it is possible, that lesions to the left fronto-temporo-parietal language network, which are often associated with aphasia, also include varying amounts of damage to the left frontal and parietal regions that support alerting attention. However, future work is needed to better understand how lesion location impacts alerting, and attention more generally, in PWA.
Unlike in reaction time, only executive control costs were observed within accuracy for the aphasia group. This observed speed-accuracy tradeoff in the aphasia group, particularly within alerting and orienting attention, indicates that reaction time may be a more sensitive measure of attention abilities in PWA. This finding does not coincide with past studies reporting PWA to be less accurate but not slower than controls on an alerting task (Laures, 2005), or PWA to be less accurate and slower than controls on an orienting task (Villard & Kiran, 2015). However, it does correspond with other studies that solely used reaction time measures (i.e., PWA complete orienting tasks more slowly than controls; Petry et al., 1994; Robin & Rizzo, 1989), as well as with the broader speed-accuracy tradeoff literature showing older adults consistently favor accuracy over speed, regardless of directions or task type (Brébion, 2001; Forstmann et al., 2011; Heitz, 2014; Hertzog et al., 1993; Smith & Brewer, 1995; Starns & Ratcliff, 2010). Since PWA are typically 65 years and older (Ellis et al., 2018), the ability of the ANT to measure attention using both reaction time and accuracy may make it a valuable clinical tool to characterize attention deficits in aphasia, as the reaction time measures may better capture the slower processing of PWA that is known to negatively impact communication abilities (LaCroix et al., 2020; Love et al., 2008). However, future studies are first needed to establish the reliability and validity of the ANT in PWA.
Previous work demonstrates that alerting, orienting, and executive control are distinct attention subtypes supported by separate neural resources in neurotypical adults (Fan et al., 2002, 2005; Petersen & Posner, 2012). An exploratory correlational analysis in our aphasia group suggests that the independence of these attention subsystems may change following a left hemisphere stroke. Particularly our results indicate that alerting and orienting, and alerting and executive control remain distinct, but not orienting and executive control. While these results should be interpreted with caution due to the small sample size, the direct relationship between orienting and executive control in aphasia (in reaction time and accuracy) may stem from each being supported by distinct, yet adjacent, bilateral fronto-parietal networks (Corbetta & Shulman, 2002; Dosenbach et al., 2008). A lesion affecting any part of the frontal or parietal cortices, will likely have a negative impact on both orienting and executive control. However, future work is needed to assess the independence of each attention component and their corresponding neural substrates in aphasia.
Executive control is traditionally calculated in ANT studies by subtracting congruent trials from incongruent trials (e.g., Fan et al., 2002). However, this subtraction creates potential issues with interpretation as congruent trials have been shown to produce facilitation effects (see MacLeod, 1991 for a review). This may attribute increased interference to incongruent trials as it is difficult to parse facilitation from interference when using this contrast. Using neutral trials as a baseline may provide a more accurate measurement of executive control as in theory, neutral trials neither inhibit nor facilitate processing, and this may be particularly important for quantifying attention abilities in clinical populations. The use of neutral trials as a baseline in our executive control calculation produces similar results as using the congruent trials: significant executive control costs for both groups in reaction time (control: t(19)=15.98, p<.001; aphasia: t(21)=4.78, p<.001), but the neutral trials also identify differences between the two groups on executive control abilities in reaction time (t(40)=2.45, p=.002), suggesting that in addition to orienting attention, executive control may also be impaired in aphasia. This slight change in results between using congruent or neutral trials as the baseline warrants future work exploring the relative contributions of each to the measure of executive control in aphasia.
Conclusion
The well-studied ANT was used to assess alerting, orienting, and executive control attention in PWA and matched controls. Significant orienting and executive control effects were observed in both groups, but the alerting effect was only significant in the control group. We additionally find differences in orienting attention between the two groups, as well as a relationship between orienting and executive control in the aphasia group. Overall, our findings demonstrate that the ANT may be an effective measure of attention in aphasia. Our results additionally suggest the need to separately assess all three subtypes of attention in PWA to gain a more complete picture of attention abilities; this may be particularly important when characterizing the relationship between attention and language in aphasia.
Acknowledgments
This work was supported by NIH DC009659 (PI: G. Hickok) and the American Heart Association pre-doctoral fellowship #18PRE33990328 (A. LaCroix).
Footnotes
Disclosure Statement
The authors report no potential conflict of interest.
Data availability statement: The data that support the findings of this study are available from the corresponding author (A.L.), upon reasonable request.
One participant (AZ1033) had two strokes ten years apart.
Alerting: (no cue − double cue)/((no cue + double cue)/2); Orienting: (center cue − spatial cue)/((center cue + spatial cue)/2); Executive Control: (incongruent − congruent)/((incongruent + congruent)/2)
References
- Baayen RH, & Milin P (2010). Analyzing reaction times. International Journal of Psychological Research, 3(2), 12–28. [Google Scholar]
- Baddeley A (1992). Working memory. Science, 255(5044), 556–559. [DOI] [PubMed] [Google Scholar]
- Brébion G (2001). Language Processing, Slowing, and Speed/Accuracy Trade-Off in the Elderly. Experimental Aging Research, 27(2), 137–150. 10.1080/036107301750073999 [DOI] [PubMed] [Google Scholar]
- Caplan D, Michaud J, & Hufford R (2013). Short Term Memory, Working Memory, and Syntactic Comprehension in Aphasia. Cognitive Neuropsychology, 30(2). 10.1080/02643294.2013.803958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC, & Frith CD (2001). The Noradrenergic α2 Agonist Clonidine Modulates Behavioural and Neuroanatomical Correlates of Human Attentional Orienting and Alerting. Cerebral Cortex, 11(1), 73–84. 10.1093/cercor/11.1.73 [DOI] [PubMed] [Google Scholar]
- Cowan N, Elliott EM, Saults JS, Morey CC, Mattox S, Hismjatullina A, & Conway ARA (2005). On the Capacity of Attention: Its Estimation and Its Role in Working Memory and Cognitive Aptitudes. Cognitive Psychology, 51(1), 42–100. 10.1016/j.cogpsych.2004.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, & Petersen SE (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. 10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C, Hardy RY, Lindrooth RC, & Peach RK (2018). Rate of aphasia among stroke patients discharged from hospitals in the United States. Aphasiology, 32(9), 1075–1086. 10.1080/02687038.2017.1385052 [DOI] [Google Scholar]
- Erickson RJ, Goldinger SD, & LaPointe LL (1996). Auditory Vigilance in Aphasic Individuals: Detecting Nonlinguistic Stimuli with Full or Divided Attention. Brain and Cognition, 30(2), 244–253. 10.1006/brcg.1996.0016 [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossela J, Flombaum J, & Posner M (2005). The activation of attentional networks. NeuroImage, 26, 471–479. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, & Posner MI (2002). Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience, 14(3), 340–347. [DOI] [PubMed] [Google Scholar]
- Fan J, & Posner M (2004). Human Attentional Networks. Psychiatrische Praxis, 31, 210–214. 10.1055/s-2004-828484 [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Tittgemeyer M, Wagenmakers E-J, Derrfuss J, Imperati D, & Brown S (2011). The Speed-Accuracy Tradeoff in the Elderly Brain: A Structural Model-Based Approach. Journal of Neuroscience, 31(47), 17242–17249. 10.1523/JNEUROSCI.0309-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Nettles C, Davis M, Morrow L, & Montgomery A (2006). Functional communication and executive function in aphasia. Clinical Linguistics & Phonetics, 20(6), 401–410. 10.1080/02699200500075781 [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, & Barresi B (2000). Boston Diagnostic Aphasia Examination Record Booklet. Lippincott Williams & Wilkins. [Google Scholar]
- Gordon-Pershey M, & Wadams A (2017). The relationship of language and attention in elders with nonfluent aphasia. Cogent Medicine, 4(1), 1356063. 10.1080/2331205X.2017.1356063 [DOI] [Google Scholar]
- Green DW, Grogan A, Crinion J, Ali N, Sutton C, & Price CJ (2010). Language control and parallel recovery of language in individuals with aphasia. Aphasiology, 24(2), 188–209. 10.1080/02687030902958316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz RP (2014). The speed-accuracy tradeoff: History, physiology, methodology, and behavior. Frontiers in Neuroscience, 8. 10.3389/fnins.2014.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Vernon MC, & Rypma B (1993). Age Differences in Mental Rotation Task Performance: The Influence of Speed/Accuracy Tradeoffs. Journal of Gerontology, 48(3), P150–P156. 10.1093/geronj/48.3.P150 [DOI] [PubMed] [Google Scholar]
- Heuer S, & Hallowell B (2015). A novel eye-tracking method to assess attention allocation in individuals with and without aphasia using a dual-task paradigm. Journal of Communication Disorders, 55, 15–30. 10.1016/j.jcomdis.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer S, Ivanova MV, & Hallowell B (2017). More Than the Verbal Stimulus Matters: Visual Attention in Language Assessment for People With Aphasia Using Multiple-Choice Image Displays. Journal of Speech, Language, and Hearing Research : JSLHR, 60(5), 1348–1361. 10.1044/2017_JSLHR-L-16-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hula WD, & McNeil MR (2008). Models of Attention and Dual-Task Performance as Explanatory Constructs in Aphasia. Seminars in Speech and Language, 29(3), 169–187. 10.1055/s-0028-1082882 [DOI] [PubMed] [Google Scholar]
- Hula WD, McNeil MR, & Sung JE (2007). Is there an impairment of language-specific attentional processing in aphasia? Brain and Language, 103(1–2), 240–241. 10.1016/j.bandl.2007.07.023 [DOI] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, Herpertz-Dahlmann B, & Fink GR (2005). Development of attentional networks: An fMRI study with children and adults. NeuroImage, 28(2), 429–439. 10.1016/j.neuroimage.2005.06.065 [DOI] [PubMed] [Google Scholar]
- Kurland J (2011). The Role That Attention Plays in Language Processing. Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 21(2), 47. 10.1044/nnsld21.2.47 [DOI] [Google Scholar]
- Lachaud CM, & Renaud O (2011). A tutorial for analyzing human reaction times: How to filter data, manage missing values, and choose a statistical model. Applied Psycholinguistics, 32(02), 389–416. 10.1017/S0142716410000457 [DOI] [Google Scholar]
- LaCroix AN, Blumenstein N, Tully M, Baxter LC, & Rogalsky C (2020). Effects of prosody on the cognitive and neural resources supporting sentence comprehension: A behavioral and lesion-symptom mapping study. Brain and Language, 203, 104756. 10.1016/j.bandl.2020.104756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laures JS (2005). Reaction time and accuracy in individuals with aphasia during auditory vigilance tasks. Brain and Language, 95(2), 353–357. 10.1016/j.bandl.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Laures-Gore J, & Marshall RS (2016). Mindfulness meditation in aphasia: A case report. NeuroRehabilitation, 38(4), 321–329. 10.3233/NRE-161323 [DOI] [PubMed] [Google Scholar]
- Love T, Swinney D, Walenski M, & Zurif E (2008). How left inferior frontal cortex participates in syntactic processing: Evidence from aphasia. Brain and Language, 107(3), 203–219. 10.1016/j.bandl.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod CM (1991). Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin, 109(2), 163. [DOI] [PubMed] [Google Scholar]
- MacLeod JW, Lawrence MA, McConnell MM, Eskes GA, Klein RM, & Shore DI (2010). Appraising the ANT: Psychometric and theoretical considerations of the Attention Network Test. Neuropsychology, 24(5), 637–651. 10.1037/a0019803 [DOI] [PubMed] [Google Scholar]
- Marshall RS, Laures-Gore J, & Love K (2018). Brief mindfulness meditation group training in aphasia: Exploring attention, language and psychophysiological outcomes. International Journal of Language & Communication Disorders, 53(1), 40–54. 10.1111/1460-6984.12325 [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, & Kellam SG (1991). Analysis of the Elements of Attention: A Neuropsychological Approach. Neuropsychology Review, 2(2), 109–145. [DOI] [PubMed] [Google Scholar]
- Murray LL (2012). Attention and Other Cognitive Deficits in Aphasia: Presence and Relation to Language and Communication Measures. American Journal of Speech-Language Pathology, 21(2), S51–S64. 10.1044/1058-0360(2012/11-0067) [DOI] [PubMed] [Google Scholar]
- Murray LL, Holland AL, & Beeson PM (1997). Auditory processing in individuals with mild aphasia: A study of resource allocation. Journal of Speech, Language, and Hearing Research, 40(4), 792–808. [DOI] [PubMed] [Google Scholar]
- Peach RK, Nathan MR, & Beck KM (2017). Language-Specific Attention Treatment for Aphasia: Description and Preliminary Findings. Seminars in Speech and Language, 38(01), 005–016. 10.1055/s-0036-1597260 [DOI] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The Attention System of the Human Brain: 20 Years After. Annual Review of Neuroscience, 35, 73–89. 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry MC, Crosson B, Gonzalez Rothi LJ, Bauer RM, & Schauer CA (1994). Selective attention and aphasia in adults: Preliminary findings. Neuropsychologia, 32(11), 1397–1408. 10.1016/0028-3932(94)00072-7 [DOI] [PubMed] [Google Scholar]
- Pompon RH, McNeil MR, Spencer KA, & Kendall DL (2015). Intentional and Reactive Inhibition During Spoken-Word Stroop Task Performance in People With Aphasia. Journal of Speech, Language, and Hearing Research : JSLHR, 58(3), 767–780. 10.1044/2015_JSLHR-L-14-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, & Petersen SE (1990). The attention system of the human brain. Annual Review of Neuroscience, 13(1), 25–42. [DOI] [PubMed] [Google Scholar]
- Ratcliff R (1993). Methods for dealing with reaction time outliers. Psychological Bulletin, 114(3), 510. [DOI] [PubMed] [Google Scholar]
- Rinne P, Hassan M, Goniotakis D, Chohan K, Sharma P, Langdon D, Soto D, & Bentley P (2013). Triple dissociation of attention networks in stroke according to lesion location. Neurology, 81(9), 812–820. 10.1212/WNL.0b013e3182a2ca34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KL, Summerfield AQ, & Hall DA (2006). Presentation modality influences behavioral measures of alerting, orienting, and executive control. Journal of the International Neuropsychological Society, 12(04). 10.1017/S1355617706060620 [DOI] [PubMed] [Google Scholar]
- Robertson IH, Ward T, Ridgeway V, & Nimmo-Smith I (1994). The test of everyday attention (TEA). Psychological Corporation. [DOI] [PubMed] [Google Scholar]
- Robin DA, & Rizzo M (1989). The effect of focal cerebral lesions on intramodal and cross-modal orienting of attention. Clinical Aphasiology, 18, 61–74. [Google Scholar]
- Smith GA, & Brewer N (1995). Slowness and age: Speed-accuracy mechanisms. Psychology and Aging, 10(2), 238–247. 10.1037/0882-7974.10.2.238 [DOI] [PubMed] [Google Scholar]
- Sohlberg MM, & Mateer CA (2010). APT-III: Attention process training: A direct attention training program for persons with acquired brain injury. Lash & Associates. [Google Scholar]
- Spagna A, Mackie M-A, & Fan J (2015). Supramodal executive control of attention. Frontiers in Psychology, 6. 10.3389/fpsyg.2015.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starns JJ, & Ratcliff R (2010). The effects of aging on the speed-accuracy compromise: Boundary optimality in the diffusion model. Psychology and Aging, 25(2), 377–390. 10.1037/a0018022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart HJ, & Amitay S (2015). Modality-specificity of Selective Attention Networks. Frontiers in Psychology, 6. 10.3389/fpsyg.2015.01826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643–662. [Google Scholar]
- Sturm W, & Willmes K (2001). On the Functional Neuroanatomy of Intrinsic and Phasic Alertness. NeuroImage, 14(1), S76–S84. 10.1006/nimg.2001.0839 [DOI] [PubMed] [Google Scholar]
- Sturm W, Willmes K, Orgass B, & Hartje W (1997). Do Specific Attention Deficits Need Specific Training? Neuropsychological Rehabilitation, 7(2), 81–103. 10.1080/713755526 [DOI] [Google Scholar]
- Thiel CM, & Fink GR (2007). Visual and Auditory Alertness: Modality-Specific and Supramodal Neural Mechanisms and Their Modulation by Nicotine. Journal of Neurophysiology, 97(4), 2758–2768. 10.1152/jn.00017.2007 [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, & Cerella J (2002). Aging, executive control, and attention: A review of meta-analyses. Neuroscience & Biobehavioral Reviews, 26(7), 849–857. 10.1016/S0149-7634(02)00071-4 [DOI] [PubMed] [Google Scholar]
- Villard S, & Kiran S (2015). Between-session intra-individual variability in sustained, selective, and integrational non-linguistic attention in aphasia. Neuropsychologia, 66, 204–212. 10.1016/j.neuropsychologia.2014.11.026 [DOI] [PubMed] [Google Scholar]