ABSTRACT
Aspergillosis, candidiasis, and cryptococcosis are the most common cause of mycoses-related disease and death among immune-compromised patients. Adhesins are cell-surface exposed proteins or glycoproteins of pathogens that bind to the extracellular matrix (ECM) constituents or mucosal epithelial surfaces of the host cells. The forces of interaction between fungal adhesins and host tissues are accompanied by ligand binding, hydrophobic interactions and protein-protein aggregation. Adherence is the primary and critical step involved in the pathogenesis; however, there is limited information on fungal adhesins compared to that on the bacterial adhesins. Except a few studies based on screening of proteome for adhesin identification, majority are based on characterization of individual adhesins. Recently, based on their characteristic signatures, many putative novel fungal adhesins have been predicted using bioinformatics algorithms. Some of these novel adhesin candidates have been validated by in-vitro studies; though, most of them are yet to be characterised experimentally. Morphotype specific adhesin expression as well as tissue tropism are the crucial determinants for a successful adhesion process. This review presents a comprehensive overview of various studies on fungal adhesins and discusses the targetability of the adhesins and adherence phenomenon, for combating the fungal infection in a preventive or therapeutic mode.
KEYWORDS: Adhesin, adherence phenomenon, adhesion, host–pathogen interaction, morphotypes, GPI-anchored proteins, infectious propagules
1. Fungal adhesins
Adhesion of pathogenic microbes to host tissue is the primary step of infection and is crucial for the persistence of the pathogen in the host (Pwj et al. 2013). This phenomenon is mediated by microbial cell-surface molecules called adhesins that promote attachment of microbes to the host tissue. Specificity of infecting (more efficiently) a particular host or tissue (tissue tropism) is determined by interactions between adhesins and their specific receptors on surface of the host-tissue (Klemm and Schembri 2000). Fungal adhesins are mostly proteins or glycoproteins, and both the carbohydrate or protein parts of these molecules have been entailed in interacting with the host-tissue/-molecules to facilitate adhesion (Tronchin et al. 2008). Role of fungal adhesins in infecting and colonising host has been established by various researchers. The most notable of these studies involves constructing deletion mutants of putative adhesins, or blocking of their function to demonstrate the resultant decline in the adherence and virulence of fungal morphotype(s). In mycology, the most widely studied adhesins are of Candida albicans; however, in recent times new adhesins have been identified for most of recurrently occurring fungal infections, caused by the other Candida spp., Aspergillus fumigatus (Afu), Blastomyces dermatitidis, Coccidioides immitis, Cryptococcus neoformans, Paracoccidioides brasiliensis, and Histoplasma capsulatum (Perlroth et al. 2007).
Cell wall components of fungi provide rigidity and protect them against environmental constrains (Paul 2007). Carbohydrates like chitin, β-1,3/1,6-glucan comprise approximately 60−70% of the total mass of cell wall in Candida spp. Proteins in the cell wall fungi are mostly linked covalently to β-glucan through GPI anchor. Majority of these cell-wall proteins are implicated in the regulation of primary host–pathogen interactions, as adhesins, aspartyl proteases, dismutases, and phospholipases (Pwj et al. 2013). Biotechnological advancements and use of sophisticated techniques have enhanced our understanding regarding the role of fungal adhesins in mediating adhesion, aggregation, biofilm formation, and inhibiting the fungus specific host immune response. Most of characterised adhesins are proteins with a relatively complex N-terminal domain that mediates specific peptide–peptide, peptide–sugar and other peptide–ligand interactions (Klis et al. 2001). The GPI-anchored adhesins comprise of conserved sequences: an N-terminus endoplasmic reticulum (ER) signal peptide and a C-terminus peptide for GPI-ylation in the ER membrane (Verstrepen and Klis 2006). Downstream the N-terminal domain resides a variable domain with low complexity and abundance of serine/threonine tandem repeats. Greater adherence capacity of these glycoproteins is attributed to presence of longer repeat regions, whereas shorter repeats culminate in decreased adherence, possibly because the effector N-terminal domain remains engraved in the cell wall in such cases (Verstrepen and Klis 2006).
2. Opportunistic fungal pathogens and invasive infections
Mycoses infecting immunocompromised individuals but not the healthy ones come under the category of opportunistic human fungal pathogens. They can cause a wide range of complications including mild to severe, localised to systemic and superficial to invasive infections. Invasive fungal infections (IFIs) can cause severe deep tissue, disseminated bloodstream and respiratory tract infections (Warnock 2007). According to revised definition of IFIs proposed by “EORTC/MSG Consensus Groups”, they are categorised into three groups: 'proven', 'probable', and 'possible', based on certain defined diagnostic criteria (B J et al. 2018). Pathogenic fungi are leading cause of many superficial, mucosal and invasive infections among humans. In the iatrogenic environment, there is a high incidence of IFIs, particularly in the people with compromised immune system. IFIs are arduous to diagnose and treat, and therefore, lead to higher rates of morbidity and mortality. Marked risk of infection is associated with the immunocompromised individuals with neutropenia, solid organ or bone marrow transplants, HIV infection, haematological disorders and patients undergoing chemotherapy and immunosuppressant drug therapies (Aikawa et al. 2005; Baddley 2011; Badiee and Hashemizadeh 2014). Approximately, 90% of the documented IFI-related mortality is caused by ten notorious species of fungi including: Aspergillus fumigatus (Aspergillosis), Blastomyces dermatitidis (Blastomycosis), Candida albicans (Candidiasis), Cryptococcus neoformans (Cryptococcosis), Rhizopus oryzae (Mucormycosis), Coccidioides immitis (Coccidioidomycosis), Pneumocystis jirovecii (Pneumocystis), Histoplasma capsulatum (Histoplasmosis), Paracoccidioides brasiliensis (Paracoccidioidomycosis), Penicillium marneffei (Penicilliosis) (Brown et al. 2012).
As a causative agent of invasive infection, the fungus must first adhere to the mucosal tracts and then, actively invade the tissue with the help of enzymatic and cytopathic virulent factors. Morphological transformation strategies are adopted by many fungal pathogens in the course of ensuing invasive infection. Several humans opportunistic mycoses are dimorphic and capable of undergoing morphogenetic transition between their yeast and hyphal forms, thereby inclining them as per the requirement of phases of colonisation in host and invasion. Four genera of fungi, Candida, Aspergillus, Cryptococcus and Pneumocystis, are known to be responsible for approximately 90% of all fungal-infection-related deaths (Brown et al. 2012; Shourian and Qureshi 2019).
Candida albicans is known to display prominent adherence ability to the host-tissue. About 25–30% of cancer/leukaemia patients enduring chemotherapy are predisposed to oropharyngeal candidiasis (Rolston and Bodey 2003). Dissemination of fungi in the blood stream of leukaemic patients with oropharyngeal candidiasis can lead to its systemic form, which makes it more cumbersome to treat(Degregorio et al. 1982). C. albicans strains have been reported to adhere, colonise and disseminate the murine alimentary tract devoid of intestinal bacteria (Westwater et al. 2007). Different morphotypes of C. albicans including spore, hyphae and pseudo-hyphae interact differently with host immune cells. Switching of morphotypes of C. albicans between the spore and hyphal forms is associated with its virulence. Cell wall structures of the hyphal form(s) are distinct from the spore-form. The hyphae of C. albicans cause profound damage to the host tissues, whereas the spores are efficient in evading the host immunity. Among total incidences of acute and disseminated candidiasis most of the infections are manifested by four species of Candida, namely, C. albicans, C. glabrata, C. parapsilosis and C. tropicalis (Hajjeh et al. 2004; Pfaller and Diekema 2004a). C. glabrata has been documented as a rapidly emerging non-albicans species of Candida resistant to azole antifungal agents (Pfaller and Diekema 2004a), whereas, relatively smaller fraction of Candida-related blood stream infections are known to be driven by C. guilliermondii, C. krusei, C. dubliniensis, C. lusitaniae and C. rugosa (Pfaller and Diekema 2004a).
Aspergillus fumigatus, a mould belonging to the eurotiomycetes class, is the most frequent opportunistic human pathogen among other genera of the class and is known to cause allergic bronchopulmonary aspergillosis (ABPA), aspergilloma, and acute invasive aspergillosis (IA) infections in immunocompromised individuals with high rates of morbidity and mortality (Latgé 2003; Beauvais et al. 2007). The pathogen is often characterised by greenish cone-shaped conidia, from 2.5 to 3 µm in diameter, produced in chains, whereas some isolates of A. fumigatus have no pigments and produce white conidia (J P Latgé 1999). A. fumigatus is identified as the most abundant fungal pathogen in the air, causing serious and generally fatal invasive infections in immunocompromised individuals (Andriole 1993), wherein the spores (conidia) of A. fumigatus may establish an invasive disease in the lung tissue by the development of hyphae. The hyphae grow by the extension of the tips that generates significant pressure on the tip, enough for penetrating the substrata (Money 2001). Exact data have not been recorded; however, it has been predicted that fungal hyphae are capable of exerting a total force equivalent to 0.01–0.1 N/m2 on their hyphal tips. For comparison, 0.1 N/m2 is the opposite resistance force exerted by an 8% agar (w/v) gel (Hube and Naglik 2001). Additionally, the tips of hyphae also secrete hydrolysing enzymes capable of degrading host-cellular and molecular components that facilitate the infiltration of growing hyphae into the surrounding host-tissue (Gow et al. 2002). Hope et al. developed a double-layer model of the pulmonary alveolus with lung endothelial cells cultured at the bottom of the cell culture inserts and a type II pneumocyte line cultured on the upper plane. On adding A. fumigatus conidia to the upper chamber of this model, the conidia germinated and formed hyphae that subsequently invaded the abluminal surface of endothelial cells (Hope et al. 2007). The invasion of A. fumigatus from the luminal surface of the endothelial cells is mediated by the formation of pseudopodia that enveloped the hyphae. The formation of these pseudopodia is accompanied by the accumulation of a dense network of microfilaments around the endocytes (Kamai et al. 2009). Other common Aspergillus species responsible for causing allergic and invasive aspergillosis in humans include A. flavus. A. niger and A. terreus (Krishnan et al. 2009).
Cryptococcus neoformans is ubiquitous opportunistic aetiological agent found in soil dwellings, avian excreta and air, causing pulmonary cryptococcosis in immunocompromised individuals upon exposure of its spores. The most commonly manifested cryptococcal infection is cryptococcal meningoencephalitis of brain that is associated with approximately 50% mortality (Williamson 2017). The hallmark feature of C. neoformans is a polysaccharide capsule that surrounds the cell body. In immuno-compromised individuals (HIV patients, recipients of bone marrow and solid organ transplants) exposure of cryptococcal spores from environment causes the fungus to colonise the host’s alveolar tissue causing characteristic inflammation and nodulation in the lungs. After evading the exerted host defence, it can gradually disseminate to other organs including the central nervous system through breaching blood brain barrier and causing inflammation in meninges layer. The initial infection is latent without any notable symptoms resulting in more severe conditions in later stages of disseminated systemic infection. Besides Cryptococcus sp., the earlier mentioned invasive mycoses can also cause brain infections like meningitis. The outer capsular components protect C. neoformans from phagocytosis by alveolar macrophages and support their persistance inside, coping with oxidative stress and breach the blood brain barrier through transcytosis by brain microvascular endothelial cells (BMECs) (Shourian and Qureshi2019; Brown et al. 2012; Liu et al. 2012).
Pneumocystis jirovecii (aka, P. carinii) was discovered in HIV patients in 1980. Initially, it was misidentified as a protozoan but is now recognised as a fungus (Edman et al. 1988). Pneumocystis carinii pneumonia (PCP) is one of the major opportunistic mycotic infections of AIDS manifested patients and is considered most common AIDS defining illness of the modern era (Fei et al. 2009). Interestingly, the mortality of PCP is 10% in HIV-positive patients and 30–60% in HIV-negative immunocompromised patients. An abnormally intense inflammatory response in HIV-negative patients is believed to be responsible for the higher mortality in these cases (Mansharamani et al. 2003; Kotani et al. 2017).
Zygomycetes infections are rare, reaching an annual rate of 1.7 infections per million people, inside the United States (Rees et al. 1998 Pfaller and Diekema 2004a). Infections with these organisms are typically acute and unexpectedly progressive, with mortality rates of 70–100% (Gonzalez et al. 2002). The most ordinary etiologic mediator of zygomycosis encompasses members of the genera Rhizopus, Mucor and Absidia. These organisms lead to infections in immunocompromised people, particularly with ketoacidosis, diabetes neutropenia and corticosteroid therapy (Gonzalez et al. 2002). Zygomycetes have a marked tendency for blood vessels (angioinvasion) and produces numerous emboli with associated myocardial infarction and contiguous tissue necrosis (Pfaller and Diekema 2004b).
Penicillium marneffei, a dimorphic fungus, causes penicilliosis in healthy as well as immunocompromised individuals and is mostly endemic to Southeast Asian countries. This fungus was first identified in bamboo rats for manifesting infection. Later on, it was found to infect HIV infected individuals in endemic regions causing cutaneous skin lesions. The mortality rates are high because of poor early diagnosis and prophylaxis (Sirisanthana and Supparatpinyo 1998).
Histoplasma capsulatum is the causative agent of acute pulmonary histoplasmosis and disseminated Histoplasmosis. Repeated exposure to the spores by inhalation results in acute pulmonary histoplasmosis affecting host’s respiratory tract. When the immune cells of host are unable to clear the pathogen or the fungus adopts certain escape tricks to host immune clearance mechanisms, it disseminates systemically, leading to more severe disseminated Histoplasmosis (Goodwin et al. 1980).
Blastomycosis is another opportunistic infection caused by dimorphic fungus Blastomyces dermatitidis. This infection is mostly prevalent in the riverside regions of Mississippi and Ohio rivers in America and Canada (Khuu et al. 2014). Common habitats for this fungus include soil with dead and decaying materials. Spores are predominantly air-borne and come in contact with host respiratory system by inhalation. When host defence mechanisms are insufficient to combat, it undergoes morphogenetic transition to colonise the infected tissue and further disseminates to other organs, leading more severe systemic spread (Saccente and Woods 2010).
Besides these common species of popular opportunistic fungi, in the recent years, several less virulent strains of Aspergillus, Candida, Cryptococcus and Histoplasma have attained higher virulence. Some of emerging fungi leading to recent outbreaks includes Candida auris, previously known for causing superficial fungal infection, such as otitis, is now adapted to cause more severe invasive diseases like bloodstream infections (BSI). C. auris mediated bloodstream fungemia has been recorded in many countries mainly in South Korea (Lee et al. 2011), India (Anuradha Chowdhary et al. 2013), South Africa (Magobo et al. 2014), Kuwait (Emara et al. 2015), Pakistan (A. Chowdhary et al. 2014) and Venezuela (Calvo et al. 2016). This strain is resistant to the first line antifungal agents viz., fluconazole, amphotericin B and echinocandins (Lockhart et al. 2017). Another fungus with notable emergence of virulence is Cryptococcus gattii with its two serotypes (B and C) (KJ and Varma 2006) and four molecular types (VGI, VGII, VGIII and VGIV). Whole genome sequencing of this strain suggested its close identity with Cryptococcal neoformans. However, unlike C. neoformans, which is known to target immune suppressed individuals, C. gattii has predilection for individuals with intact immunity (Shourian and Qureshi 2019). C. gattii outbreaks have already been reported from North American Pacific Northwest region (Perfect 1989), tropical northern Australia (Fisher et al. 1993), Papua New Guinea (Seaton 1996), sub Saharan Africa (Litvintseva et al. 2005), Vancouver Islandand Western Canada (Kidd et al. 2004); however, its occurrence is believed to be more widespread (Byrnes et al. 2011). Different studies suggested an overall mortality rate of 13–33% among patients infected with C. gatti (https://www.cdc.gov/fungal/diseases/cryptococcosis-gattii/statistics.html).
Looking at the statistics for the prevalence of these fungal infections suggests that each year >200,000 immunocompromised individuals are affected with invasive aspergillosis with more than 50% (in high risk patients) estimated mortality (Garcia‐Vidal et al. 2008; Guinea et al. 2010). Approximately, 3 million individuals worldwide are affected with chronic pulmonary aspergillosis, especially patients with preexisting health conditions, such as, asthma and other pulmonary diseases, where it results in nearly 15% mortality (Nam et al. 2010; Denning et al. 2011). More than 52,000 deaths per annum have been recorded in patients suffering from Pneumocystis jirovecii pneumonia/Pneumocystis, especially in HIV patients (Walzer et al., 2008). A fatal mycotic brain infection is caused by Cryptococcus meningitis and as estimated by U.S. Centre for Disease Control and Prevention (CDC), every year approximately 220,000 new cases of cryptococcal meningitis are recorded with nearly 181,000 deaths of patients. The situation worsens in some developing countries with poor medical facilities and higher prevalence of immunocompromised individuals particularly due to HIV infection. Most of the deaths because of cryptococcal meningitis in the recent past were recorded in sub-Saharan Africa (https://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/statistics.html). Worldwide, disseminated Candida blood stream infections are diagnosed in approximately 400,000 individuals each year. Estimated and recorded mortality rates for these infections are 42 % and 27% respectively (Morgan 2005; Brown et al. 2012).
Coccidioidomycosis, also called “valley fever”, is caused by the exposures to spores of soil dwelling fungus Coccidioides immititis. This fungus is endemic to arid regions of Mexico and United States. Approximately, 111,717 cases of coccidioidomycosis were reported during the years 1998–2011 from different states of US (“Increase in Reported Coccidioidomycosis – United States, 1998–2011,” 2013).
A summary of estimated global burden of invasive opportunistic fungal diseases is displayed in Figure 1.
Figure 1.
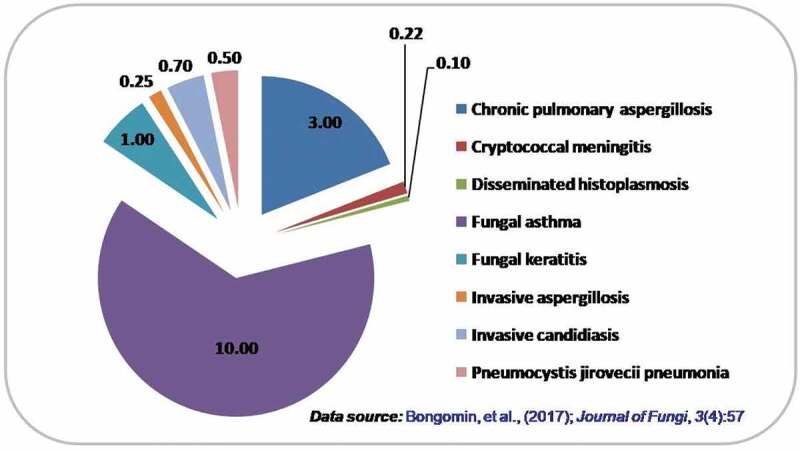
Estimated global burden of fungal diseases annually (in millions)
3. Characterised adhesins of common opportunistic human fungal pathogens
C. albicans, A. fumigatus, Sporothrix schenckii, C. glabrata, C. neoformans and Paracoccidioides brasiliensis are among the few opportunistic fungal pathogens in which the adhesion phenomenon is relatively better studied and certain adhesins have been characterised. For many other common opportunistic fungi, adhesins have sparingly been studied. Table 1 is a compilation of adhesins identified in common opportunistic human fungal pathogens.
Table 1.
List of characterised adhesins from opportunistic fungal pathogens
| Fungus | Adhesin | Morphotype | Ligand | References |
|---|---|---|---|---|
| Aspergillus fumigatus | RodA | Conidia | Collagen | Thau et al. (1994) |
| 37 and 72-kDa proteins | Conidia | Laminin | Tronchin et al. (1997) | |
| 32-kDa protein | Conidia | Fucose | Mendes-Giannini et al. (2000) | |
| Mp1 (Galactomannoprotein) | Swollen conidia | ECM | Upadhyay et al. (2008) | |
| Extracellular Thaumatin domain protein (AfCalAp) | Swollen conidia | Laminin, Fibrinogen, Mice Lung cells | Upadhyay et al. (2009); Liu et al.(2016) |
|
| Hydrophobins | Conidia | ECM | Abad et al. (2010) | |
| CspA | Conidia | ECM | Levdansky et al. (2010) | |
| Alpha-mannosidase | Swollen conidia | Fibrinogen | Upadhyay et al.(2012) | |
| Amidase familyprotein, putative | ||||
| Beta-glucosidase, putative | ||||
| Hypothetical protein | ||||
| Pectate lyase A | ||||
| Oryzin precursor (Alkaline proteinase) (Elastase) | ||||
| A. niger | HF-extractable cell wall putative GPI-anchored mannoprotein – cwpA | Conidia | ECM | Damveld et al. (2005) |
| Blastomyces dermatitidis | 120-kDa protein (WI1)/BAD1g | Yeast form | ECM proteins | Brandhorst et al. (1999) |
| Candida albicans | 55- and 105-kDa proteins | Yeast form | Fibronectin (RGD domain) | Sa et al. (1992) |
| 37-kDa | Yeast form | Laminin | Lo´pez-Ribot et al. (1994) | |
| 60- and 105-kDa glycoproteins | Yeast form | Fibronectin | Klotz et al.(1994) | |
| 68-, 62- and 60-kDa proteins | Yeast form | Laminin | Lo´pez-Ribot et al. (1994) | |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) |
Yeast form | fibronectin and laminin |
Gil-Navarro et al. (1997); Gozalbo et al. (1998) |
|
| Als1p, Ala1p/Als5p | Yeast form | ECM proteins | Sundstrom (1999) | |
| Hwp1 | Yeast form | Mammalian transglutaminases | Staab et al. (1999) | |
| EAP1g | Yeast form | Epithelial cells | Li and Palecek (2003) | |
| Int1 | Yeast form | ECM proteins | de Groot et al. 2004; Zhao et al. 2004 | |
| ALS (Als 1p-Als9p), | Yeast form | ECM proteins | de Groot et al. 2004; Zhao et al. 2004 | |
| EPA and GPI dependent CWPs | Yeast form | ECM proteins | de Groot et al. 2004; Zhao et al. 2004 | |
| CaIff4 | Yeast form | Epithelial cells; Plastic surface |
Kempf et al. 2007 | |
| Alcohol dehydrogenase (Adh1) | hyphae | Vitronectin, fibronectin, laminin | Kozik et al. 2015 | |
| Phosphoglycerate mutase (Gpm1) | hyphae | Vitronectin | Kozik et al. 2015 | |
| Candida glabrata | Epa1p/EPA1g | Yeast form | Host-cell carbohydrates | Cormack et al. 1999 |
| Candida tropicalis | Surface expressed integrin analogue (putative fibronectin receptor) | Yeast form | Fibronectin | Cm and Hostetter 1993 |
| 105-kDa surface expressed fibronectin-binding protein | Yeast form | ECM proteins | Gp and Hostetter 1996 | |
| Fibronectin-binding proteins gi|255722,852 gi|255,728,333 gi|255,727,881 gi|255,732,698 gi|255,722,021 |
Pseudohyphae | fibrinogen | Kozik et al. 2015 | |
| Vitronectin-binding proteins gi|255,722,852 gi|240,134,900 gi|255,728,333 gi|255,727,881 gi|255,732,698 gi|255,722,021 |
Pseudohyphae | Vitronectin | Kozik et al. 2015 | |
| Laminin-binding proteins gi|255,722,852 gi|240,134,900 gi|255,728,333 gi|240,132,975 gi|255,727,881 gi|255,732,698 gi|255,722,021 |
Pseudohyphae | Laminin | Kozik et al. 2015 | |
| Candida parapsilosis | Surface Als like proteins | Pseudohyphae | ECM proteins | Kozik et al. 2015 |
| Fibronectin-binding proteins gi|354,547,941, gi|354,547,939 gi|354,547,813 gi|354,545,198 gi|354,545,113 gi|354,544,475 gi|354,546,116 gi|354,545,980 gi|354,546,805 |
Pseudohyphae | Fibronectin | Kozik et al. 2015 | |
| Vitronectin-binding protein gi|8,927,048 gi|354,545,198 gi|354,545,113 gi|354,544,475 gi|354,546,116 gi|354,545,980 gi|354,546,348 gi|354,545,888 |
Pseudohyphae | Vitronectin | Kozik et al. 2015 | |
| Laminin-binding proteins gi|354,547,939 gi|354,545,113 gi|354,544,475 gi|354,546,805 |
Pseudohyphae | Laminin | Kozik et al. 2015 | |
| Cryptococcus neoformans | MP 15, 98, 88 and 84 kDa proteins | Yeast cells | Lung epithelial cells | Teixeira et al. 2014 |
| Cfl1 | Hyphae | Plastic surface | Wang et al. 2012 | |
| Coccidioides immitis | SOWgpg/rSOWp | Parasitic phase | ECM proteins | Hung et al. 2002 |
| Fusarium oxysporum | β-1,6 glycosyated GPI-CWP -Fem1p (60-kDa) | Microconidia | ECM | Schoffelmeer et al. 2001 |
| Fonsecaea pedrosoi | 50-kDa protein | Conidia | Manose and N-acetilglycosamine | Limongi et al. 2001 |
| Histoplasma capsulatum | 50-kDa protein | Yeast cells | Laminin | McMahon et al. 1995 |
| Heat shock protein (Hsp60) | Yeast cells | CD18 receptors on Macrophage cells, CHO cells | Long et al. 2003 | |
| Metarhizium anisopliae | MAD1 and MAD2 | Conidia | ECM | Wang et al. 2007 |
| Penicillium marneffei | 20-kDa protein | conidia | Laminin and Fibronectin | Hamilton et al. 1998 |
| Cell Wall Mannoprotein Mp1p (58-kDa) | Yeast cells | Concanavalin A (a type of lectin purified from jack beans, binds with mannse residues of glycoproteins) | Cao et al. 1998 | |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) |
Conidia | A549 pneumocytes, fibronectin and laminin | Lau et al. 2013 | |
| Phytophthora cinnamomi | 220 kDa-proteins with thrombospondin type 1 repeats | Zoospores | ECM | Robold and Hardham 2005 |
| Paracoccidioides brasiliensis | 30-kDa; 19- and 32-kDa proteins | Yeast cells | Laminin and ECM proteins | Andreotti et al. 2005 |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Yeast cells | fibronectin, laminin, and type I collagen | Barbosa et al. 2006 | |
| 43-kDa protein | Yeast cells | Laminin | Vicentini et al. 1994 | |
| Pb 14-3-3 protein | Yeast phase | S. cerevisiae cells expressing Pb 14-3-3 adhere to epithelial cell-line A549 | de Oliveira et al. 2015 | |
| Pseudallescheria boydii | Peptidorhamnomannan (PRM) | Conidia | HEp2 cells (human larynx carcinoma cells) | Pinto et al. 2004 |
| Pneumocystis jirovecii | Msg protein | Yeast cells | A549 cell-line | Kutty et al. 2013 |
| Rhizopus oryzae | Conidial surface adhesins | Spores and germ tube | HUVECs, ECM proteins, endothelial cells | Ibrahim et al. 2005 |
| Sporothrix schenckii | Cell wall proteins with MW 90 and 135 kDa | Yeast cells | Human umbilical vein endothelial cells (HUVECs) | Figueiredo et al. 2004 |
| Gp70, Unidentified proteins in 37–92 kDa range | Yeast cells | Fibronectin | Teixeira et al. 2009 | |
| Gp70 glycoprotein | conidia | Dermis of mouse tail | Ruiz-Baca et al. 2009 | |
| Cell surface glycoconjugates with carbohydrate or peptide moieties | Yeast and conidia | Fibronectin | Lima et al. 2001 | |
| Cell wall proteins with MW ≥ 190, 180, 115, 90 and 80 kDa | Yeast form | Epithelial cells | Sandoval-Bernal et al. 2011 | |
| Trichophyton mentagrophytes | Carbohydrate specific adhesins on microconidial surface |
Microconidia | Mannose residues on Chinese hamster ovary (CHO) cells | Esquenazi et al. 2003 |
| Trichophyton rubrum | Lectin-like adhesins on microconidial surface | Microconidia | CHO cells, epithelial and macrophages cells. | D. Esquenazi et al. 2004 |
3.1. Aspergillus fumigatus
Spores of A. fumigatus are capable of adhering to substrates of various nature, including host-cells, receptors and extracellular matrix (ECM) proteins, through polysaccharides, glycoproteins and proteins that cover their cell wall (Figure 2) (Bouchara et al. 1999; Wasylnka and Moore 2000). This filamentous opportunistic fungus also secretes a variety of proteases including alkaline serine protease (Moutaouakil et al. 1993; Monod et al. 1993), aspartic protease (Pep) (Reichard et al. 1994) and metalloproteases (MEPs) (Monod et al. 1993 ;Shende 2018) that help in the initial colonisation of host lung tissue. It is believed that these degradative enzymes are involved in the breakdown of ECM barrier of the host (Latgé 1999). Studies done on human lung carcinoma cell line (A549) suggest that A. fumigatus conidia bind and penetrate the cell surface with an efficiency of 30% (Wasylnka & Moore 2002).
Figure 2.
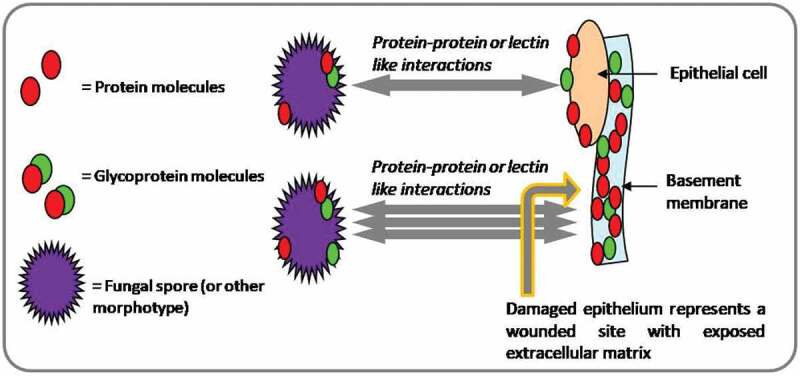
Extracellular matrix (ECM) accessible at the damaged epithelial sites facilitate more pronounced adhesion of fungal morphotypes to host-tissue(s)
Several research groups around the world have attempted to purify the conidial adhesin proteins and glycan components associated with them. However, only a handful of conidial adhesins have been identified and characterised to date in A. fumigatus.
Adhesion capability of A. fumigatus conidia with purified human ECM proteins and its associated glycan components have been demonstrated by certain studies. Annaix et al., have elucidated the interacting sites on human fibrinogen and Afu conidia, involved in the adhesion phenomenon. The D-domain of fibrinogen binds to conidial component of A. fumigatus in a specific and saturable manner. Proteolytic pre-treatment of conidia abolished this binding revealing the protein nature of fibrinogen interacting molecules on their surface (Annaix et al. 1992). Rod A is a GPI- anchored extracellular hydrobhobin protein present on the surface of Afu conidia. Presence of this extremely hydrophobic protein on conidial surface masks their recognition from host immune cells by escaping from crucial dectin-1 and dectin-2 receptors, (Blango et al. 2019), thereby RodA protein is considered conducive to the virulence. A. fumigatus conidial mutants (∆RodA) display decreased capability of adherence to collagen and BSA, but showed affinity for pneumocytes, fibrinogen or laminin (Thau et al. 1994; Sheppard 2011).
Besides RodA, in A. fumigatus at least six morehydrophobins are present namely, RodB, RodC, RodD, RodE, RodF and RodG. Out of these, only RodA and RodB are expressed significantly during sporulation and biofilm formation, respectively. However, RodA has been identified as the sole contributor to the virulence of A. fumigatus by imparting the characteristics of hydrophobicity, structural rigidity, immune-inertia and possibly drug-resistance as well (Valsecchi et al. 2017).
Another study carried out by Peñalver et al. demonstrated that A. fumigatus conidia can effectively adhere to the purified human fibronectin, whereas the binding of fibronectin with its mycelia was not significant. Trypsin treatment of conidia removes the adherence factors off the conidial surface, leading to reduction in binding efficacies. This suggests the proteinaceous nature of these factors. Two polypeptides of 23 kDa and 30 kDa size, purified from conidial surface extracts show interaction with fibronectin (Peñalver et al. 1996).
Affinity purification approach has also been used to identify adhesins with affinity for fibronectin and laminin (Gil et al. 1996; Tronchin et al. 1997). A 37 kDa protein identified through this approach was later characterized as Aspergillus allergen Aspf2 with affinity for laminin (Banerjee et al. 1998).
Interestingly, certain adhesin predictor software programms, such as, 'SPAAN', 'FungalRV' and 'FAApred' etc. have also markedly enhanced the identification, as well as the wetlab characterisation of a putative fungal adhesins and their possible role in the infection. SPAAN is based on artifical neural networks that process the adhesin probability (Pad) of a protein by analysing the amino acid, dipeptide, multiplets frequencies as well as charge and hydrobhobic composition of the proteins (Sachdeva et al. 2005). Fungal RV predicts human pathogenic fungal adhesins and adhesin like proteins based on multifaceted parameters of protein composition similar to SPAAN by employing the Hidden Markov model (HMM) matrix. (Chaudhuri et al. 2011). Another adhesin prediction tool FAApred uses the Support Vector Machine (SVM) based approach for prediction with 86.29% of accuracy (Ramana and Gupta 2010). Nath also developed an adhesin prediction model with 94.9% of accuracy by evaluating various protein sequence based features (Nath 2019).
Upadhyay et al. used bacterial adhesin predictor software SPAAN for identifying putative adhesins of A. fumigatus. From the putative adhesins identified using SPAAN, AfCalAp, an Extracellular thaumatin domain protein, was selected for in vitro characterisation (Upadhyay et al. 2009). The ORF of AfCalA was cloned and recombinantly expressed in competent E. coli cells. Further in vitro binding assays suggested that the recombinant AfCalAp has affinity for laminin, fibrinogen and murine lung cells. Furthermore, anti-AfCalAp antibodies reduced the binding affinity of AfCalAp to laminin in a dose dependent manner. Upon investigation of antigenicity and immunoreactivity of AfCalAp, ABPA patients have been found to have elevated levels of antibodies against AfCalAp (Upadhyay et al. 2009). Adhesin nature of 'AfCalA' was further studied by Liu et al. in 2016. By employing the gene-knock down approach, they observed that the ∆AfCalA mutants and wild type conidia adhered equally efficiently with laminin and A549 cell-line, and concluded that AfCalA is dispensable for adherence of A. fumigatus. However, the mutant conidia were less efficient in invading host tissues than the wild type conidia (Liu et al. 2016). Furthermore, antibodies against AfCalA improved the survival of mice model of IPA. ΔAfcalA mutant showed significantly attenuated virulence in immunosuppressed mice, as the model infected with mutant stain showed improved survival, reduced fungal burden in lung, and decreased lung tissue invasion, suggesting that AfCalA mediates pulmonary invasion, and plays an important role in the progression of invasive pulmonary aspergillosis (Liu et al. 2016).
Another predicted adhesin Mp1 (Galactomannoprotein) in A. fumigatus has been characterised in vitro by employing binding assays with purified ECM proteins. The results showed significant binding of recombinant Mp1 adhesin to laminin and fibrinogen. ABPA patients also showed high levels of IgG and IgE antibodies against Mp1 (Upadhyay et al. 2008).
Hydrofluoricacid (HF) extractable CspA (cell surface protein A), a GPI-anchored cell wall protein (CWP) with molecular weight of approximately 75 kDa was studied by Levdanskyet al. (2010). CspA has been found to interact with two other cell-wall associated proteins namely, Ecm33 and Gel2. ΔcspA mutant has been found to exhibit reduced conidial adherence to the substratum. A combined knock out strain with ΔcspA, Δecm33 and Δgel2 significantly reduced the conidial adherence to ECM proteins suggesting its possible role during infection. Overexpression of CspA is associated with the resistance of conidia from phagocytic cell mediated destruction (Levdansky et al. 2010).
AFUA_5G09330 is a 12–29 kDa monomeric cytosolic protein belonging to a large family of fungal proteins and identified as homolog of the CipC-like protein of A. nidulans. Morpho-differentiation of hyphae from conidia is a hallmark of invasive aspergillosis caused by A. fumigatus, AFUA_5G09330 gene is composed of two introns and has been identified as differentially expressed during hyphal stage. CipC like protein homologs have been found to exist most frequently in filamentous forms of fungi than yeasts. Seventeen proteins of the CipC family have been found in the genomes of A. fumigatus, A. clavatus, A. flavus, A. nidulans, A. niger, A. oryzae and A. terreus. AFUA_5G09330 belongs to a family of proteins that is exclusively found in fungi (Bauer et al. 2010).
UDP-galacturonate 4 epimerase3 (Uge3), a key enzyme in the pathway of GAG biosynthesis is thought to be necessary for adhesion of A. fumigatus hyphae to host, as GAGs are essential for adhesion. Mice infected with Δuge3 mutant strain of A. fumigatus lacking N-acetyl galactosamine in its cell wall showed reduced fungal lung burden and increased survival compared to those infected with wild type A. fumigatus strain. The attenuation of virulence and reduced fungal load is considered to be due to the reduced adherence and colony formation by this mutant strain inside the mice lungs (Gravelat et al. 2013).
3.2. Candida albicans
Candida sp. like many other pathogenic fungi isable to densely adhere to various surfaces, including the skin, endothelial tissues and epithelial mucosa of the hosts. Additionally, Candida sp. also adheres to inert surfaces of an object, such as denture prostheses, urinary catheters intravascular and prosthetic heart valves (Busscher et al. 2010; Pwj et al. 2013).
C. albicans and S. cerevisiae possess two classes of covalently linked cCWPs: the dominant class include GPI-dependent CWPs linked to beta- glucan component in the cell wall, second less abundant class is Pir-CWPs (proteins with internal repeats) also linked to beta-glucans. Extracellular cell wall adhesins Als1p and Als3p are GPI-dependent CWPs that adhere to the beta- glucans via GPI-anchor (de Groot et al. 2004).
Hwp1, ALS and EPA family of adhesins are well characterised adhesin proteins of C. albicans. The ALS (agglutinin-like sequence) gene family of adhesin encodes eight cell-surface glycoproteins (Als1p, Als2p, Als3p, Als4p, Als5p, Als6p, Als8p and Als9p). Als proteins consists of three domains: an extracellular N-terminal domain with adhesive function, central domain and a C-terminal domain with abundant N- and O-linked glycosylation (Kapteyn et al. 2000; Zhao et al. 2004).
Structurally related adhesins Hwp1, Als1p and Ala1p/Als5p in C. albicans and Epa1p in C. glabrata have been studied. All of these proteins belong to a class of glycosylphosphatidylinositol-dependent cell wall proteins (GPI-CWP) and have a characteristic N-terminal GPI-anchor facilitating the anchorage of Glycan residues of the cell wall (M JS et al. 2005).
Among these proteins, Als1p (Buurman et al. 1998) and Ala1p (Gaur et al. 1999), also called Als5p (Fu et al. 1998) encoded by members of a large family of ALS genes (agglutinin-like sequence) (Hoyer 2001), have been investigated to have binding affinity for ECM proteins and components of endothelial and epithelial cells (Sundstrom 1999). Δals3 mutant strain showed inefficient binding when tested against BEC and HUVEC cell lines. Adhesion data suggests that the loss of Als3p affects the adhesion of C. albicans more than the loss of Als1p (Zhao et al. 2004).
Hwp1 (hyphal wall protein1) gene is differentially expressed during germ tubes and hyphal stages, and encodes a mannoprotein Hwp1 containing exposed extracellular N-terminal domain (Staab et al. 1996). C. albicans strains lacking Hwpl has been found to form unstable adhesion to buccal epithelial cells of human and has a reduced ability to induce systemic candidiasis in mice (Staab et al. 1999). Human transglutaminase enzyme is thought to catalyse a strong and stable interaction between its substrate and human epithelial cells. The N-terminal domain of Hwp1 is supposed to mimic the transglutaminase substrate. Hwpl plays crucial role in proliferation of C. albicans in keratinised epithelium by interacting with keratinocyte transglutaminase enzyme (Staab et al. 1999). Moreover, surface expressions of Hwp1 have been studied to be more important in mucosal candidiasis (Kartasova et al. 1988; Balish et al. 2001). It is well known that C. albicans binds to different extracellular membrane proteins namely fibronectin, entactin, laminin and collagen, and are considered as potential target molecules when Candida spreads in the host body (Calderone 1993; Pendrak and Klotz 1995; Chaffin et al. 1998). C. albicans has many fibronectin receptors on its surface, including 105-kDa and 60kDa glycoproteins and mammalian homologs of integrins (Sa et al. 1994). At least three fibronectin domain(s) have been implicated in its adherence with C. albicans. 55 kDa and 105 kDa polypeptides have FN domain and show binding activity with fibronectin. 105kD apolypeptide additionally possesses RGD (Argenine, Glycin, and Aspartic acid) sequences. RGD tripeptide residues are involved in integrin-mediated adherence to host ECM. Many ECM proteins have been found to have the RGD sequences. Fungal pathogens contain cell surface proteins that can recognise these regions of their substrate and thereby initiate attachment to the host ECM factors. In vitro study has demonstrated the ability of peptide mimicking the RGD sequences of ECM protein fibronectin in inhibiting the adherence of C. albicans blastospores to endothelial cells (Hostetter 2000).
Although, in C. albicans several molecules with receptor characteristics have been described to be involved in the binding of fibronectin and laminin. An enzyme GAPDH, capable of binding to fibronectin and laminin has been studied and its location was demonstrated in C. albicans cell walls, using immune-electron microscopy. Being capable of binding to the exposed ECM proteins from the cell surface of C. albicans, GAPDH is supposed to play important part in attachment of fungal organism to the host cell thereby progressing infection (Gozalbo et al. 1998). Gozalbo and collaborators (Sa et al. 1994) have shown that the enzyme GAPDH has binding affinity for fibronectin and laminin and could be implicated in the adherence of fungal cells to host tissues, thereby, contributing in virulence. ALA1 gene of C. albicans conferring adherence properties towards ECM proteins, require presence of atleast four contiguous threonine residues for maximum compliance (Gaur et al. 2002).
In C. albicans, a hub protein that connects cellular pathways such as cell wall synthesis, biofilm production, drug resistance to antifungal agents (fluconazole and caspofungin) has been found to be involved and playing important roles in these phenomenon. Mutant strains of SMI1 gene have shown weaker cell wall architecture and susceptibility to antifungal agents. Strain that overexpress this protein has been found to have greater cell wall adhesion by many folds than the wild type strain. It is also involved in hyphal morphogenesis (Martin-Yken et al. 2018). In C. albicans, the EAP1 gene (a putative cell-wall adhesin) has been identified, and in silico analysis indicated that it consists a signal peptide, a GPI-anchor site and has homology with various genes of many yeast encoding CWPs. Saccharomyces cerevisiae, expressing heterologous EAP1 of C. albicans showed improved adherence to HEK293 cell line (Li and Palecek 2003).
3.3 . Candida glabrata
People with old age and compromised immune status are highly susceptible to another potent human opportunistic pathogen namely, Candida glabrata and is the main culprit of invasive candidiasis besides C. albicans, accounting for more than 20% of all blood stream candidiasis in the USA (Desai et al. 2011; Pfaller & Diekema 2007).
C. glabrata often survives the phagocyte(s)-mediated killing and support dissemination of infection. The ability of C. glabrata adherence to mammalian host tissues and various surfaces is governed by numerous surfaces expressed specific adhesins. Presence of various sequences (about 66) encoding GPI-modified adhesin-like wall proteins has been revealed from the C. glabrata genome. These proteins possess modular structure for adhesion as well as an effector domain followed by low complexity region with internal tandem repeats (Pwj et al. 2013). Genome wide analysis of C. glabrata revealed the largest family of adhesins encoding genes, further grouped into seven sub-families (I–VII) based on similarity of N-terminal domain of these adhesins. Lectin like Epithelial adhesins (Epa) are the largest sub-family of adhesins, belonging to sub-family I, consisting of 17 members in CBS138 strain (Kaur et al. 2005) and 23 members in BG2 strain (de Groot et al. 2008) of C. glabrata, are believed to act as important virulent factors in C. glabrata mediated pathogenesis (Desai et al. 2011; Pwj et al. 2013). EPA adhesins are composed of N-terminal binding (A) domain, a central variably glycosylated serine, threonine rich repeats (B) domain and a C-terminal GPI-anchored domain (Diderrich et al. 2015).
N-terminus A domain has been found to have variable specificity and discrimination for binding with host epithelial ligands such as, peptides (Cormack et al. 1999) or glycan components (Zupancic et al. 2008) on epithelial cells. Epa1, Epa6 and Epa7 are well characterised adhesins in C. glabrata acting as lectins and show differential binding affinity for α and β-glycans (Maestre-Reyna et al. 2012). Epa adhesins possess PA14 (Anthrax protective antigen) domain usually found in yeast adhesins (Desai et al. 2011; Pwj et al. 2013).
In silico genome mining analysis and characterisation revealed someof the other proteins having characteristic adhesin domains in C. glabrata (Desai et al. 2011). These adhesins include GPI-anchored Pwp7p (PA14 domain containing Wall Protein), Aed1p (Adherence to Endothelial cells 1) and Aed2p (Adherence to Endothelial cells 2) adhesins having adherence affinity to host endothelial cells. Interestingly, Pwp7p and Aed1p have large intragenic tandem repeats and an exclusive conserved repeat “SHITT” motif thought to be involved in fungal pathogenesis (Desai et al. 2011).
3.4. Cryptococcus neoformans
Cell flocculin 1 (Cfl1), first identified hypha-specific adhesin protein of Cryptococcus neoformans, mostly induced during Cryptococcal sexual development. Cryptococcal Cfl1 adhesin is unique among the most characterised adhesions of opportunistic fungi, as it contains the conserved C-terminal cystine rich, SIGC domain and N-terminal signal peptide that make it secretory.
Cryptococcal strain overexpressing the Cfl1 protein exerts increased flocculation, colonisation and crumpled colony morphology (Wang et al. 2012). Deletion mutation in CPL1 gene affects capsule formation in C. neoformans, a potent virulence factor governing cryptococcal pathogenesis. Cryptococcus expresses, secreted as well as cell surface forms of Cfl1 protein, regulating paracrine intercellular communication. Cfl1 protein homologs have also been identified in Cryptococcus genome namely DHA1, DHA2, CPL1 and CFL105. DHA2 is expressed specifically in yeast form and DHA1 in all cell types predominantly in basidia (Gyawali et al. 2017).
C. neoformans growth can effectively be inhibited by monocytes when grown in fibronectin coated substratum but growth remains unaffected when the interaction occurs between C. neoformans capsule and fibronectin. Rodriguez and his colleagues (Rodrigues et al. 2003) demonstrated that C. neoformans does not bind to this glycoprotein of the ECM and binding to soluble FN is also very poor compared to other fungal models. Both conidia and S. schenckii yeast cells were similarly attached to proteolytic fragments of FN (Lima et al. 2001). Main virulence factor involved in Cryptococcal infection is hydrated polysaccharide capsule composed of glucuronoxylomannan (GXM) and galactosylomannan (GalXM) and mannoproteins (MP) (Kumar et al. 2011). Mannoproteins (MP) accounts for only <1% of total capsular weight (Rodrigues and Nimrichter 2012; Teixeira et al. 2014). Four characterised mannoproteins (MP) – 84, 88, 98 and 115 kDa have been observed to be involved in eliciting immune response inside the host, making them subject of potent vaccine candidature against cryptococcosis (Teixeira et al. 2014).
Mannoproteins have been investigatedto be localised mainly in the inner capsule layer, in proximity to the cell wall, but not connected with GXM or GXMGal (Vartivarian et al. 1989; Jesus et al. 2010). In order to cause infection C. neoformans interacts with various host tissues, namely the endothelial cells, lung epithelia and the blood brain barrier (Goldman et al. 1994). Strains lacking GXM exploit other capsule components as adhesins, to adhere with host cells because mannoproteins in the inner capsule surface become exposed (Teixeira et al. 2014).
3.5. Other fungal pathogens
Dimorphic fungi are the main etiological agents of endemic mycoses that grow at 25°C in the form of filamentous mould, and at 37°C in the form of yeast.It has been found that SOWgp (spherule outer wall glycoprotein) adhesin of recombinant Coccidioides immitis is able to adhere to ECM proteins of mammals. But, after deletion of SOWgp, partial loss in binding of C. immitis spherules to ECM proteins has been recorded, that eventually leads to attenuated virulence (to some extent) in mice models (Pwj et al. 2013).
The members belonging to genus Paracoccidioides are the main causative agents of paracoccidioidomycosis (PCM). Paracoccidioides lutzii and P. brasiliensis are the only two species belonging to Paracoccidioides sp. They have characteristics that allow their growth in unfavourable conditions, allowing them to attachand invade host tissues, culminating in the severe infection. Adhesins, the CWPs facilitate adhesion and are able to mediate interactions between fungi and hosts during infection (de Oliveira et al. 2015). In case of .brasiliensis, their ability to infect Vero cells and the phenomenon of adherence varies depending upon the strain. Recently, a glycoprotein of 30 kDa and pI 4.9 from P. brasiliensis has been found to be a possible adhesin capable of adhering to laminin, and not to other components of ECM, typeI or type IV collagen fibronectin, and may contribute to the virulence of P. brasiliensis (Andreotti et al. 2005).
A 120 kDa glycoprotein (WI-1) is the most studied adhesin in Blastomyces dermatitidis. Now, it is characterised as BAD1 and has been implicated inpromoting the adherence of yeast cells to ECM components of host cells (Brandhorst et al. 2003). Hung and colleagues (Hung et al. 2002) isolated the Coccidioides immitis (SOWg) gene, encoding a glycoprotein component of the outer wall, specifically during the infection phase. Loss of SOWgp gene and in vitro binding of recombinant protein (rSOWp) to ECM proteins (namely: laminin FN collagen type IV) resulted in reduced virulence as there was partial loss in the capacity of infectious spores to adhere to ECM components of mammalians. In the cell surface extracts of Penicillium marneffei, a 20 kDa protein was identified and later characterised as laminin and fibronectin ligand, indicating that these two ligands share the same receptor for binding (Hamilton et al. 1999). A 50 kDa adhesin from the Fonsecaea pedrosoi has been characterised as a receptor, binding to mannose and to N-acetyl-D-glucosamine (Limongi et al. 2001).
4. Adhesins in the virulence
In order to examine the possible role of adhesins in the virulence of the fungal pathogen C. albicans, Rosiana et al. has created a library of 144 single and double mutant of adhesins and have been studied in C. elegans model. Als1ΔIff4Δ, Hyr1ΔAls5Δ, Als7ΔAls5Δ double mutants have been found to be low virulence strains and showed higher percentage worm survival in liquid killing assay upon infection with these mutant stains compared with wild type C. albicans stain. Upon genetic interaction analysis, the double mutant strains of adhesins Als5Δ Eap1Δ, or Als5ΔHwp2Δ conferred significant low virulence as compared to single mutant strains of these adhesin genes showing the synergistic effect of these mutations on virulence (Rosiana et al. 2021). Virulence in Paracoccidioides involve increased expression of multiple adhesin gene at different phases of infection. GP43, malate synthase, glyceraldehyde-3-phosphate dehydrogenase, enolase, 14-3-3 protein and triosephosphate isomerase are few characterised adhesins involved in host–pathogen interaction (de Oliveira et al. 2015).
5. Expression of adhesins in the infectious fungal morphotypes
Morphological changes are required by various eukaryotic pathogens in the process of causing disease. It is now evident, that these transitions are also associated with the progression of infection by human opportunistic fungi. The stored signalling pathways regulate the morphogenetic differentiation in response to host physiological and environmental stimuli and also change the composition of the cell surface as well as the size and shape of the cells during morphogenesis. These subtle changes are required to favour pathogenic events. Most fungi grow through unicellular budding and division or filamentously through apical expansion. Various human pathogenic fungi can switch between two or more morphotypic forms of growth, such as the unicellular and filamentous forms and therefore are called dimorphic fungi. It was established in the 'classical' dimorphic pathogens, that for virulence, a correct morphotype transition is necessary. Genetic mutations or chemical blocking these transitions weaken or negate the potential of the fungal pathogen to confer disease (Wang and Lin 2012).
The repertoire of cell surface adhesins are tightly regulated during morphotype switches: certain adhesins are solely expressed in the virulent form, while others in the saprotrophic/commensal form. Adhesin, Bad1 of Blastomyces dermatitidis, (a human pathogenic fungus) is expressed particularly in pathogenic yeast form and this protein is responsible for controlling multiple processes during infection (Klein and Tebbets 2007). In C. albicans, adhesin Hwp1 is expressed in hyphae for ensuring fungal adherence to epithelial cells, as Hwp1 adhesin proteins are important for systemic spread of candidiasis (Staab et al. 1999). Candida hyphae express Als3, a type of adhesin, having binding affinity for various host receptors. The binding of the receptors to the adhesin, induces its own endocytosis for facilitating penetration of fungi into epithelial cells (Zhu et al. 2012). On the contrary, C. neoformans adhesin Cfl1 is associated with non-pathogenic filaments, and virulence is attenuated when there is induced Cfl1expression in the form of pathogenic yeast (Wang and Lin 2012).
Taken together, changes in the composition of cell surface of fungal cells are likely to underlie the correlation between virulence and morphogenic transitions. In pathogenic fungi, normally present in open environment, the filamentous form is usually not pathogenic. Nevertheless, these adhesion molecules on the fungal morphological forms help to initiate interactions between the host and pathogen, subsequently establishing infections (Wang and Lin 2012).
Fungal colonisation in host and establishment of infection and systemic circulation is a consequence of down-regulation of filament/spore specific molecules and up-regulation of yeast specific molecules. Thus, the study of these molecules unique to each morphological form could provide new insights into the nature of host–fungal interaction (Wang and Lin 2012).
6. Tissue tropism exhibited by adhesins
Relatively higher binding affinity of a pathogen or molecule towards any specific tissue is known as its tissue-tropism. Adhesins show varying degree of tissue tropism possibly depending on the molecular and cellular composition of the site of infection. Higher tropism of any adhesin towards a tissue (or its inhabitant cells) that is also a cardinal infection site for the pathogen is an important indicator of its possible role in the adhesion phenomenon. Overall tissue tropism of any fungal morphotype is determined by a combination of host and pathogen factors involved (Figure 3). Among the pathogen related factors, differential expression of adhesins in various fungal morphotypes and relative dispersion potential of these morphotypes could be important determinants.
Figure 3.
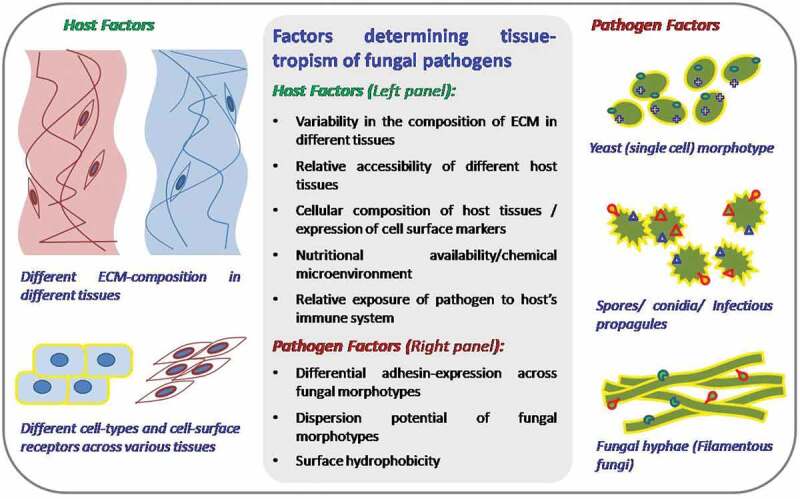
Combination of host and pathogen factors determine the tissue tropism of fungal pathogens
Many fungal adhesins display morphotype-specific expression. Examples include: Hwp1 and Hwp2 adhesins of Candida albicans that are expressed in hyphae (Nobile et al. 2008; Ene and Bennett 2009; Hayek et al. 2010), Bad1, a yeast specific adhesin and important virulent factor of Blastomyces dermatitidis (Brandhorst et al. 2003) and Cell flocculin 1 (Cfl1), a hyphae-specific adhesin of Cryptococcus (Wang et al. 2012, 2013; Tian and Lin 2013). Similarly, AfCalAp, an adhesin of A. fumigatus, appears to display better expression in swollen conidia, than in resting conidia (Upadhyay et al. 2009).
On the other hand, host factors as determinants of tissue tropism may include: Different composition of ECM in various tissues (or across their developmental stages), cellular composition of host tissues and expression of cell surface markers, accessibility of different host tissues to pathogen and relative exposure of pathogen to host’s immune system.
In a tissue, cells are surrounded by ECM that constitutes the larger volume in tissue than cells it surrounds. Along with glycosaminoglycans (GAGs) linked to proteins to form the proteoglycans, the ECM is made up of many proteins, including collagen, elastin, fibronectin, lamininand fibrinogen (in damaged tissues) that are known for their adhesive and structural properties (Alberts et al. 2002). However, the compositions of ECMs vary across various tissues and their developmental stages as per the structural and functional requirement of the tissue (Alberts et al. 2002; Kular et al. 2014). As specific adhesins may have different affinities with various ECM proteins, the relative abundance of these proteins may directly influence of tissue tropism of an adhesin. Similarly, tissue tropism of an adhesin could also be influenced by cellular composition of the tissue and expression of adhesin-specific receptors on the surface of inhabitant cells. We studied tissue tropism of A. fumigatus protein AfCalAp by studying its binding with mice lung and spleen cell suspensions using flow-cytometry. AfCalA binds with significantly affinity with lung cell suspension, which was expected in view of A. fumigatus being a respiratory pathogen (Upadhyay et al. 2009). Among ECM proteins tested, AfCalA shows marked binding with laminin in comparison with fibrinogen and collagen, whereas no interaction could be seen with fibronectin and decorin in the dot-blot assay (Upadhyay et al. 2009). As revealed by another study, AfCalA also interacts with Integrin α5β1 on endothelial and epithelial cells and facilitates endocytosis mediated invasion of the fungus in host tissue (Liu et al. 2016).
In C. albicans hydrophilic vs hydrophobic nature of yeast cells display differential tissue tropism in mice and hydrophobic cells bound more pronouncedly to various tissues (Hazen et al. 1991). Interestingly, in this fungus, thigmotropism (sensing surface contour by hyphae) and chemotropism together are also the proposed determinants of tissue-tropism; however, more studies are required to establish these (Davies et al. 1999)
In C. neoformans, both acapsular (CAP67) strains and wild type (NE-241) show considerably higher tropism towards lung cancer cell-line A549, in comparison with PC3, a control prostate cancer cell line. Binding of CAP67 strain to lung cells is inhibited by a (soluble) mannoprotein-adhesin MP84, revealing its lung-tropism and role in facilitating adhesion of C. neoformans to lung epithelial cells (Teixeira et al. 2014).
Though adhesins of most of fungal pathogens have not been examined across the panel of various cell types of host in terms of their relative tissue tropism, numerous of them have been tested for binding with different ECM proteins (Table 1). This provides an indication of involvement of specific interactions of adhesins even within the group of ECM proteins.
7. Adhesins in biofilm formation
The formation of biofilms by micro-organisms, especially fungi and bacteria, has been extensively studied. Biofilms formed from pathogenic fungi have gained attention in recent years and different species like, yeast, filamentous and dimorphic fungi have been described as capable of developing in community. Biofilms are sessile fungal and microbial communities that adhere strongly to surfaces and among each other; and also, are protected by their own secreted ECM composed mainly of polysaccharides. Adherence is crucial for cells that form biofilms and the interaction between ligand and adherent is maintained by hydrophobic and electrostatic forces. Multiple adhesion molecules work in the successful creation of the biofilm.
C. albicans exhibits specific mechanisms of disease development that overcome the defence and allow colonisation of the mucous tissue.It has been already established that biofilm formation often relates to morphotypic switching and the degree of virulence shown by fungal cells (Baillie and Douglas 1999; Chandra et al. 2001). The expression of C. albicans virulence factors may also vary during different stages of infection (localised and systemic), and host immune response (Naglik et al. 2003). C. albicans can simply grow as biofilm on medical equipments, like urinary catheters and dentures prosthetics further increasing the chance of dissemination to other tissues of the underlying patients.
Biofilm formation involves several steps: attachment and colonisation of yeast cells to the substrate, the subsequent cell proliferation leading to formation of scaffold layer of mycelial network, accumulation and secretion of ECM, and finally dissemination of the cells to other sites (Figure 4) (Nobile and Mitchell 2006). Adherence of Candida cells to mucosal surfaces and/or synthetic material is an early step before they proliferate and consequently form biofilm in the process of infection (Silva et al. 2012). The presence of specific cell-wall proteins, designated normally as adhesins, is a trigger in the modulation of the adhesion process (Silva et al. 2011).
Figure 4.
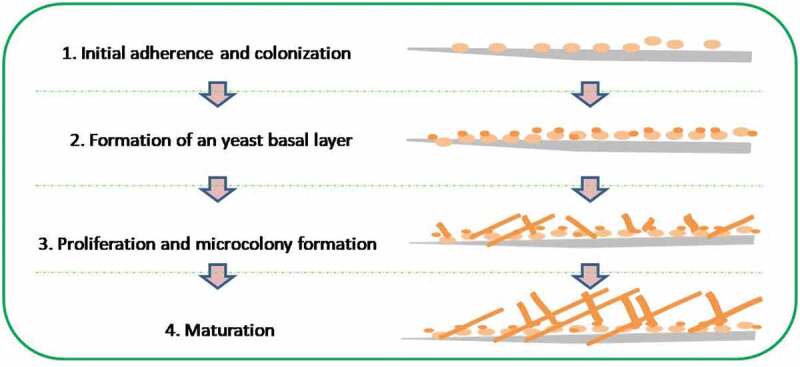
Various phases of biofilm formation by fungi
Fungal cells in maturation phase of biofilm can better withstand the antifungal therapies and host immunological factors compared within dividual fungal cells (Fanning and Mitchell 2012). The propertyof C. albicans biofilm formation is associated with regulatory transcription genes (JS and Mitchell 2011). These genes include BCR1, TEC1 and EFG1. The family of ALS adhesions (agglutinin-like sequence), including eight members (ALS1-ALS9), are expressed by ALS genes that encode cell surface GPI-anchored glycoproteins. In this group, ALS3 is the most prominent contributor in the biofilms formation and is usually upregulated during the infection of the oral mucosal epithelium (Murciano et al. 2012; Zordan & Cormack 2012). Some other proteins that contribute to this phenomenon are HWP1, EAP1, SSA1 lipases (LIP), phospholipases (PLBs) and proteases (SAP) (Wächtler et al. 2011;Barros et al. 2016). These proteins also promote colonisation and thereby infection through degrading the host cell barriers (Barros et al. 2016).
The Hyphal wall protein (Hwp1) is a mannoprotein of the fungal cell wall that promotes Candida cell attachment to the host surface (Henriques et al. 2006). It was also the first cell surface protein described as involved in the biofilm formation of C. albicans in-vivo. The PGA10 cell surface protein and the secreted Pbr1 protein are also described as important for the full adherence of C. albicans biofilms (Sahni et al. 2009). Pga10, also known as RBT51, is a member of CFEM proteins (common in many extracellular fungal membranes) and plays a role in adherence in C. albicans (Pérez et al. 2006). Pérez et al. have shown that Δpga10 mutants form relatively weaker biofilms leading to early detachment from the substrate, compared to the wild type strains. Furthermore, Sahni et al. showed that the adherence in C. albicans to blood cells was lower when the PBR1 gene was eliminated (Sahni et al. 2009).
Upon screening of 531 genes involved in biofilm formation from a library of conditional overexpression strains of C. albicans, 16 genes has been found to increase strain dominance in the multi-stain biofilm. Nine genes (Ihd1/Pga36, Phr2, Pga15, Pga19, Pga22, Pga32, Pga37, Pga42 and Pga59) out of 16 have been found to encode GPI- modified proteins and 8 have been found to be pathogen specific. These genes when overexpressed in the strains modulated the strain abundance and adhereance in multi-strain biofilm (Cabral et al. 2014).
Adhesion in C. glabrata is similar to C. albicans, and is achieved by epithelial adhesins (Epa) that is structurally quite similar to the Als proteins (Silva et al. 2011). Depending on the strain, the family of EPA genes consists of 17–23 genes; though, adhesins, EPA6, EPA1 and EPA7, are the very significant adhesins (Castaño et al. 2005). Deletion in the EPA1 gene reduces in vitro adherence of fungal cells to host epithelial cells (Cormack et al. 1999), and lactose can inhibit the adherence of this adhesin (Sundstrom 2002). Awp (Awp1-7) family of adhesins have been identified and deduced to be involved in the initial stages of biofilm development (de Groot et al. 2008). The gene expression profiling revealed that the expression of Awp adhesins are significantly higher in biofilms. C. glabrata, a hazardous human pathogenic fungus is capable of adhering to mammalian as well asother substrata (Cormack et al. 1999). During screening for the factors required for biofilm formation, it was identified that CgSIR4, CgRIF1 genes and protein kinase genes CgEPA6 and CgYAK1 encode for adhesins (Iraqui et al. 2005). EPA family of gene encoding for CWPs consist CgEpa6 anchored to glycosylphosphatidylinositol (Castaño et al. 2005; Domergue et al. 2005; Iraqui et al. 2005; Kaur et al. 2005). Similar to the cerebellum genes of S. cerevisiae FLO, sub-telomeric groups encoding the EPA genes and are underlying subject of transcriptional silencing (Castaño et al. 2005).
Glycan components of A. fumigatus cell wall is made up of branched and unbranched/linear β- and α-glycans, GAGs, galactomannons, chitosans, chitins and forms the structural scaffold for the wall. β- and α-glycans have shown potent role in hyphal aggregation during the formation of biofilm under in-vitro and in-vivo studies (Müller et al. 2011). α- and β-glycans synthase genes have also been found to be upregulated during biofilm formation by A. fumigatus (Gibbons et al. 2012). A. fumigatus affected patients with predisposed clinical conditions like cystic fibrosis and chronic obstructive pulmonary disease are susceptible and provides natural niches for A. fumigatus colonisation and biofilm formation (Ramage et al. 2011). Like other mycoses, formation of biofilm by A. fumigatus on epithelial cells also occurs through early, intermediate and maturation phases (Kaur and Singh 2014).
8. Factors regulating expression of adhesins
Studying the expression and regulation of adhesins in its various morphotypes may be important for targeting the adherence phenomenon as an anti-fungal strategy. It has been observed that adhesin-expression is a tightly regulated process controlled by different signalling pathways and in many cases the expression of adhesins is modulated in response to different environmental stimuli (such as, carbon/nitrogen starvation, changes in pH, presence/absence of ethanol in the medium, presence of suitable host-environment) and developmental stages of fungus (Guo et al. 2000; Verstrepen et al. 2003; Sampermans et al. 2005; Verstrepen and Klis 2006; Brückner and Mösch 2012; Mayer et al. 2013; Gyawali et al. 2017). These cases can be categorised as under:
8.1. Regulation of adhesin expression under environmental stimuli
FLO proteins are important adhesins of Saccharomyces required for cell-cell and cell-substrate adherence that are induced during nitrogen or carbon source starvation (Verstrepen & Klis 2006; Guo et al. 2000; Reynolds and Fink 2001). Indole acetic acid is also a stimulant for the expression of FLO11 that is considered important for facilitation of yeast infection in wounded sites of plants (Prusty et al. 2004). In Cryptococcus the expression of Dha1 adhesin is dependent on copper availability in the growth medium. It is induced when copper is limited and suppressed when copper is excessive (Gyawali et al. 2017).
In animal pathogens, adhesin expression is also induced in response to the cells perceiving an opportunistic environment for infection, enabling cells to adhere and colonise the host tissues (Verstrepen et al. 2004; Domergue et al. 2005; Verstrepen and Klis 2006). Interestingly, limitation of Nicotinic acid is used as a signal for induction of otherwise silent Candida glabrata adhesin genes – EPA1, EPA6 and EPA7, which mediate the adherence of this fungal pathogen to uroepithelial cells in the murine model of urinary tract infection (Domergue et al. 2005).
In C. neoformans, extracellular secretion of Cfl1 adhesin is used in paracrine communication as a signal for colonisation. It has been observed that, Cfl1 is highly induced during mating colony differentiation, and some amount of this protein is released extracellularly to function as a paracrine signalling molecule. The soluble Cfl1 protein gets enriched in the ECM and acts as an auto-induction signal to stimulate inhabitation of neighbouring cells in that region by using the filamentation signalling pathway (Wang et al. 2013).
8.2. Morphotype dependent expression of adhesins
Various adhesins from different fungal-pathogens show morphotype-dependent expression pattern. Hwp1 and Hwp2 proteins in Candida albicans, known to be crucial for adherence to host-cells and formation of biofilm, are mainly translated in hyphae; however, they also showed higher expression in opaque cells during mating (Nobile et al. 2008; Ene and Bennett 2009; Hayek et al. 2010; Gyawali et al. 2017). Als3 cell wall adhesin is specifically expressed in hyphal morphotype and plays crucial role in adherence to host epithelial cells (Gong et al. 2020). In Cryptococcus, Cfl1 adhesin is known to regulate the morphology, biofilm-formation and intercellular communication of the fungus. DHA1, DHA2, CPL1 and CFL105 are four additional homologs of this protein, present in Cryptococcus. Among these homologs, expression of Cfl1 and Dha2 is specific to hyphae and yeast respectively, whereas, Dha1 adhesin, though expressed in most cell-types, is mainly enriched at basidia. In Blastomyces dermatitidis, the expression of Bad1-adhesin is specific to yeast form (Brandhorst et al. 2003; Gyawali et al. 2017). Understanding the developmental stage-specificity of these adhesins in relation to predominant fungal morphotypes during different phases of infection may be important for targeting the fungal adherence by targeting stage-specific (dominant) adhesins.
8.3. Signalling involved in the regulation of adhesin expression
Environmental factors are known modulators of adhesin expression. These stimuli are sensed by receptors present on the surface of fungi that in response initiate signalling cascade(s) down to transcription factor(s) directly controlling the expression of individual adhesin or group of adhesins. The cAMP/PKA pathway in many fungi is important as they link development, morphotype-switching and pathogenesis. In A. fumigatus, deleting gpaB or acyA (cAMP/PKA pathway), results in reduced growth and conidiation (Liebmann et al. 2003). Similarly, few more studies also implicate other components of this pathway, such as PkaR (regulatory protein) and PkaC1 (catalytic protein) as crucial for proper growth, germination and precise conidiation (Lin et al. 2015). Signalling pathways involved in adhesin expression are not well defined in literatures. However, a handful of transcriptional regulators are known in the human pathogenic fungi that modulates the adhesin expressions in experimental conditions. In general, signalling cascade involving cAMP, MapK, protein kinases are associated with host–pathogen interaction and adhesin transcription factors regulation, which in turn are implicated in regulation of adhesin expression (M JS et al. 2005).
In C. albicans, a rapamycin sensitive protein kinases TORC1 is known to regulate the expression of adhesins, biofilm formation. Also TORC1 signalling is involved in the transcriptional regulation of many biochemical pathways in response to nutritional niche changes (Flanagan et al. 2017). TORC1 is regulated by the upstream GTPase enzymes Rhb1 and Gtr1. Double mutants ∆Rhb1 and ∆Gtr1 strains have shown hyperflocculation of biofilm and upregulation of genes involved in biofilm formation such as ALS and HWP1 genes (Flanagan et al. 2017). Upon activation of TOR1 with rapamycin in C. albicans increased hyphal transition and cell-cell adherence has been observed (Kim et al. 2019). A zinc-finger protein transcription factor Bcr1p is also required for biofilm formation in C. albicans. The expression analysis showed that Bcr1p activates the genes of the cell surface protein and adhesins. The expression of bcr1 depends on the regulatory protein Tec1p for hyphal differentiation and is a component of down-stream regulatory network for hyphal differentiation affecting the expression of genes encoding cell surface proteins and adhesins. Out of the 22 genes whose expression is most severely affected in the ∆bcr1/∆bcr1 mutant, 11 specify cell surface proteins or enzymes that alter the cell wall. In particular, the expression of several genes of GPI-linked CWPs in mutant ∆bcr1/∆bcr1, for example HYR1, CEPE1, RBT5, ECM331 and adhesins HWP1 and ALS3 are found to be altered. Bcr1p is required for the expression of several genes that govern the properties of the cell surface. The analysis of gene expression showed a high expression of HWP1 and ALS, especially the family of ALS1 genes that showed increased expression and is characteristic of biofilm cells, compared to free cells (Nobile and Mitchell 2005).
HWP1 is supposed to be the first identified morphogenic target located downstream of the signalling machinery and hyphal development regulators in C. albicans. The expression of HWP1 is also influenced by the negative regulators: TUP1 and RBF1. Single and double locus deletions of HWP1 gene results in a moderate conditional defect in the development of hyphae. The expression of HWP1 has been found to be blocked in a ∆EGF1 mutant, as EFG1 positively regulates development of hyphae by encoding a basic helix-loop-helix-type transcription factor. Deletion in EFG1 manifested elongated rod like cells under the controlled laboratory conditions and unable to produce true hyphae. Down-regulation of RBF1 gene in ∆RBF11 mutants results in stimulated filamentous growth revealing its negatively regulatory role in spore to hyphae transition. TUP1 is considered to be involved in the repression of functional genes responsible for differentiation of filament and promoting yeast form. This was evident while studying the deletion mutants of TUP1 homolog in C. albicans resulted in pseudo-hyphal morphology under different conditions for growth. HWP1 works in downstream of the developmental modulators, TUP1, EFG1 and RBF1 during morphotypic advancement of C. albicans (Sharkey et al. 1999).
MedA protein identified in A. nidulans is regulated during development of hyphae and is characterised to be a temporal modulator of central conidiation gene expression by an unknown mechanism. A. nidulan, medA mutants have shown delayed phailide formation as well as lesser number of conidia (Clutterbuck 1969; Aguirre 1993; Busby et al. 1996; Gravelat et al. 2010). The mutant A. fumigatus MedA has been found in conditions of deterioration, in the formation of conidia and biofilms, as well as in the adhesion of pulmonary epithelial cells, in lesions and in vitro stimulation. ΔMedA mutant strains showed attenuated virulence in an AI model of invertebrates and mammals (Gravelat et al. 2010).
Zinc finger transcription factor, Znf2 is the master regulator of morphogenesis and virulence mediated by Cfl1 adhesin protein. Znf2 dictates cell adhesion, filamentation, and sporulation. Cryptococcus strain overexpressing Znf2 elicits defensive host immune response and drops the skill to cause deadly diseases in murine models. Cfl1 is upregulated during the transition from yeast to hypha and covers the surface of the hyphae and contagious spores generated by the hyphae. The overexpression of Cfl1 in yeast cells significantly alters the degree of virulence caused by Cryptococcus. Therefore, Cfl1, which naturally covers the surface of the infectious propagules of Cryptococcus, is implicated in the host–pathogen interaction (Wang et al. 2012).
The Cryptococci yeast culture age and the growth temperature influence the cryptococcal adherence to the pulmonary epithelial cells without alteration in yeast capsule size (Merkel and Scofield 1997). Exposed Cell surface adhesins of C. neoformans capable of binding lung and glial cells are fully expressed during the late stationary phase when the yeasts are grown at 37°C (Merkel and Scofield 1993).
9. Adhesins as potential targets for prevention and therapy
Immunisation with the recombinant N-terminal domain of Als1p adhesin (rAls1p-N) protected BALB/cas well as outbred mice not only from multiple virulent strains of Candida albicans but from those of non- albicans sp. as well (Ibrahim et al. 2005, 2006; Spellberg et al. 2005). Presence of Als family genes in other Candida sp. have been considered responsible for this 'across the species' immunity against Candida (Ibrahim et al. 2006).
A study demonstrating the effects of A. fumigatus on the actin cytoskeleton of pulmonary A549 pneumocytes showed that out of the three main classes of cytoskeleton fibres: microtubules, microfilaments and intermediate filaments, only actin filament undergo significant structural modification in response to infections, including actin aggregate formation, thrashing actin stress fibre, cell blebbing and impaired focal adhesion sites. These alterations could be specifically blocked in A. fumigatus wild type strains by adding 'antipain', a cysteine/serine protease inhibitor, while, the A. fumigatus strain deficient in serine alkaline protease showed insensitivity towards antipain. Approximately fifty percent reduction in fungal-induced A549 cell detachment to plate was observed in the presence of 'Antipain'. Now it is known that A. fumigatus breaks the barrier of alveolar epithelial cells through secreting proteases to act together to disorganise actin filament and devastate cell adhesion to the substrate by changing focal adhesions (Tv et al. 2004). Antibodies against another A. fumigatus adhesin AfCalA reduced its binding with laminin in a dose dependent manner (Upadhyay et al. 2009). AfCalA is absent in resting conidia but in the swollen conidia the protein is surface localised (Upadhyay et al. 2009). Interestingly, in the study of Liu et al., AfCalA knockout mutant showed similar level of binding with A549 cells and laminin as that of wild type strain. However, pretreatment with anti-AfCalA significantly reduced the endocytosis of A. fumigatus by epithelial and vascular endothelial cells (Liu et al. 2016).
In order to promote their adhesion to epithelial surface, Candida sp. express adhesins linked covalently to the β-glucan residues on their cell wall. Adhesins such as, -like sequence (ALS) protein and the hyphal-1 wall (HWP1) protein, contribute in formation of the biofilm and facilitating cell surface adhesion (Nobile et al. 2006a, 2006b). Another key regulatory zinc finger protein named BCR1 is found to play important role in formation of biofilm by stimulating the expression of genes related to other CWP that are involved in formation ofbiofilm in vivo and in vitro experiments. Mutation studies on Bcr1 target genes has shown the Als1 and Hwp1 has significant role in biofilm formation. These Bcr1 target genes can be studied in more detail to draw the better comprehension of the role of these genes in biofilm formation and their therapeutic potential for prevention of the candida infection (Nobile et al. 2006a, 2006b). A hyphae specific adhesin Als3 has been found to be a potential target of recombinant human Serum Amylase A1 acute phase protein. Rh-SAA1 protein induces cell aggregation and death in C. albicans. Als3 double mutant strains showed defective cell aggregation upon treatment with Rh-SAA1 protein and significant higher survival rate. However, reconstituted Als3 strain showed same degree of cell aggregation and death as wild type C. albicans strain. This study suggested the targetebility of Als3 by Rh-SAA1 protein can greatly reduce the infection (Gong et al. 2020).
In Blastomyces dermatitidis, disruption of WI-1 locus resulted in loss of adherence to lung tissue and macrophages, reduced entry of the pathogen into macrophages, as well as decreased virulence in mice model. Reconstitution of WI-1 restored the virulence of B. dermatitidis back to normal (Brandhorst et al. 1999).
In coping with macrophages, some pathogenic fungi have also developed the ability to resist phagocytosis, as evidenced by C. neoformans, which can switch to a variant of the mucosal colony producing a larger GXM capsular polysaccharide, thus reducing the phagocyte efficacy of alveolar macrophages (Fries et al. 2001). Targeting the polysaccharide capsular proteins, transcription factor Znf2 and Cfl1 adhesin protein could potentially reduce the severity of cryptococcal infections (Gyawali et al. 2017). In vitro adhesion of cryptococci and other yeasts with purified glycosphingolipids has already been studied. These studies showed that the Glycosphingolipids, particularly lactosylceramide, may be potential receptor targets on the surface of mammalian cells for yeast morphotypes (Jimenez-Lucho et al. 1990). Cryptococci yeast cells treated with trypsin for 30 minutes lose the capability of adherence to the lung epithelial and glial cells. Formalin treatment of yeasts does not alter cryptococcal adherence to glial and pulmonary epithelial cells indicating resistance to the treatment. By contrast, treating the yeasts with trypsinabrogated their in vitro adherence capacity to glial and lung cells (Merkel and Scofield 1993).
10. Inferences
Fungal pathogenesis is a multifactorial phenomenon, therefore, it is necessary to study the nature of fungal pathogens, their virulence factors and their interaction with host defence components to develop effective antifungal therapies. Although phenomenal progress has been made with respect to the molecular characterisation of various factors of virulence and their interaction with the host; there is a need for further investigation to find out the exact contribution of each virulence factor in the different disease states. Adhesins are important cell surface components, playing important roles in adhering to other cells, or to substrates, live or dead. They have gained interest due to their virulent nature and ability to cause IFIs. Availability of modern genomic and proteomic tools has made it possible to expedite the identification and characterization of the fungal adhesins in recent years. Few studies has imparted the significant role of adhesin mutant fungal strains having reduced adherent ability and subsequent virulence in the mice models. Various studies as mentioned above have established the capability of adhesins targetability for prevention of infection and therapy. As the adhesion phenomenon is multifactorial, the adhesin families in these opportunistic fungi express differentially and coordinate the adhesion phenomenon to host tissues or other surfaces. However, the clear understanding of fungal colonisation and persistence inside the host is still lacking. In future studies on characterisation of new putative adhesins and exploring their mechanism of action would provide their involvement and role the fungal pathogenesis. Importantly, there is a grave need to screen small molecule libraries to identify drug molecules that inhibit or block the interaction of adhesins with ECMs to realise their application potential as antifungal vaccines or therapies.
Acknowledgements
We thank Dr. Penny Joshi, Dept. of Chemistry, DSB Campus, Kumaun University Nainital for her suggestions in drafting this manuscript.
Disclosure of interest
No potential conflict of interest was reported by the author(s).
References
- Abad A, Jv F-M, Bikandi J, Ramírez A, Margareto J, Sendino J, Fl H, Pontón J, Garaizar J, Rementeria A.. 2010. What makes aspergillus fumigatus a successful pathogen? genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol. 27(4):155–182. [DOI] [PubMed] [Google Scholar]
- Aguirre J. 1993. Spatial and temporal controls of the aspergillus brlA developmental regulatory gene. Mol Microbiol. 8(2):211–218. doi: 10.1111/j.1365-2958.1993.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Aikawa N, Kohno S, Shibuya K, Takesue Y, Maesaki S, Yoshida M, Tokimatsu I. 2005. Current status of diagnosis and treatment of invasive fungal infections in Japan: the influence of the new Japanese guidelines. Journal of Infection and Chemotherapy. 11(6):278–287. doi: 10.1007/s10156-005-0413-Z. [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. 2002. Molecular Biology of the Cell. In: Chapter 16, The Cytoskeleton. 4th edition ed. New York: Garland Science; p. 889–962. [Google Scholar]
- Andreotti PF, Monteiro da Silva JL, Am B, Cm S, Benard G, Cp S, MJ M-G. 2005. Isolation and partial characterization of a 30 kDa adhesin from paracoccidioides brasiliensis. Microbes and Infection. 7(5–6):875–881. doi: 10.1016/j.micinf.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Andriole VT. 1993. Infections with aspergillus species. Clin Infect Dis. 17(Suppl Supplement_2):S481–6. doi: 10.1093/clinids/17.Supplement_2.S481. [DOI] [PubMed] [Google Scholar]
- Annaix V, Bouchara JP, Larcher G, Chabasse D, Tronchin G. 1992. Specific binding of human fibrinogen fragment D to aspergillus fumigatus conidia. Infect Immun. 60(5):1747–1755. doi: 10.1128/IAI.60.5.1747-1755.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- B J W, J P F, Rea S, Kaufusi S, B E G, Spalding J. 2018. Epidemiology and clinical features of invasive fungal infection in a US health care network. Open Forum Infectious Diseases. 5(6):187. US: Oxford University Press. doi: 10.1093/ofid/ofy187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddley JW. 2011. Clinical risk factors for invasive aspergillosis. Medical Mycology. 49(S1):S7–S12. doi: 10.3109/13693786.2010.505204. [DOI] [PubMed] [Google Scholar]
- Badiee P, Hashemizadeh Z. 2014. Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J Med Res. 139(2):195–204. [PMC free article] [PubMed] [Google Scholar]
- Baillie GS, Douglas LJ. 1999. Role of dimorphism in the development of Candida albicans biofilms. J Med Microbiol. 48(7):671–679. doi: 10.1099/00222615-48-7-671. [DOI] [PubMed] [Google Scholar]
- Balish E, Warner T, Pierson CJ, Bock DM, Wagner RD. 2001. Oroesophageal candidiasis is lethal for transgenic mice with combined natural killer and T-cell defects. Medical Mycology. 39(3):261–268. doi: 10.1080/mmy.39.3.261.268. [DOI] [PubMed] [Google Scholar]
- Banerjee B, Greenberger PA, Fink JN, Kurup VP. 1998. Immunological characterization of Asp f 2, a major allergen from aspergillus fumigatus associated with allergic bronchopulmonary aspergillosis. Infect Immun. 66(11):5175–5182. doi: 10.1128/IAI.66.11.5175-5182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa MS, Báo SN, Andreotti PF, de Faria FP, Felipe MS, dos Santos Feitosa L, MJ M-G, Cm S. 2006. Glyceraldehyde-3-phosphate dehydrogenase of paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect Immun. 74(1):382–389. doi: 10.1128/IAI.74.1.382-389.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros PP, Ribeiro FC, Rossoni RD, Junqueira JC, Jorge AO. 2016. Influence of candida krusei and candida glabrata on candida albicans gene expression in in vitro biofilms. Arch Oral Biol. 64:92–101. doi: 10.1016/j.archoralbio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Bauer B, Schwienbacher M, Broniszewska M, Israel L, Heesemann J, Ebel F. 2010. Characterisation of the CipC-like protein AFUA_5G09330 of the opportunistic human pathogenic mould. Aspergillus Fumigatus Mycoses. 53(4):296–304. [DOI] [PubMed] [Google Scholar]
- Beauvais A, Schmidt C, Guadagnini S, Roux P, Perret E, Henry C, Jp L. 2007. An extracellular matrix glues together the aerial-grown hyphae of aspergillus fumigatus. Cell Microbiol. 9(6):1588–1600. doi: 10.1111/j.1462-5822.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- Blango MG, Kniemeyer O, Aa. B. 2019. Conidial surface proteins at the interface of fungal infections. PLoS Pathog. 15(9):e1007939. doi: 10.1371/journal.ppat.1007939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchara JP, Sanchez M, Esnault K, Tronchin G. 1999. Interactions between aspergillus fumigatus and host matrix protein. Contributions to microbiologyContribMicrobiol. 2:167–181. [DOI] [PubMed] [Google Scholar]
- Brandhorst T, Wüthrich M, Finkel-Jimenez B, Klein B. 2003. A C-terminal EGF-like domain governs BAD1 localization to the yeast surface and fungal adherence to phagocytes, but is dispensable in immune modulation and pathogenicity of blastomyces dermatitidis. Mol Microbiol. 48(1):53–65. doi: 10.1046/j.1365-2958.2003.03415.x. [DOI] [PubMed] [Google Scholar]
- Brandhorst TT, Wüthrich M, Warner T, Klein B. 1999. Targeted gene disruption reveals an adhesin indispensable for pathogenicity of blastomyces dermatitidis. J Exp Med. 189(8):1207–1216. doi: 10.1084/jem.189.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med. 4(165):165rv13–165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- Brückner S, Mösch HU. 2012. Choosing the right lifestyle: adhesion and development in saccharomyces cerevisiae. FEMS Microbiol Rev. 36(1):25–58. doi: 10.1111/j.1574-6976.2011.00275.x. [DOI] [PubMed] [Google Scholar]
- Busby TM, Miller KY, Miller BL. 1996. Suppression and enhancement of the aspergillus nidulans medusa mutation by altered dosage of the bristle and stunted genes. Genetics. 143(1):155–163. doi: 10.1093/genetics/143.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. 2010. Biofilm formation on dental restorative and implant materials. J Dent Res. 89(7):657–665. doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- Buurman ET, Westwater C, Hube B, Brown AJ, Odds FC, Gow NA. 1998. Molecular analysis of CaMnt1p, a mannosyl transferase important for adhesion and virulence of Candida albicans. Proceedings of the National Academy of Sciences. 95(13):7670–7675. doi: 10.1073/pnas.95.13.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EJ, Bartlett KH, Perfect JR, Heitman J. 2011. Cryptococcus gattii: an emerging fungal pathogen infecting humans and animals. Microbes and Infection. 13(11):895–907. doi: 10.1016/j.micinf.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. gattii Infection Statistics . Centers for disease control and prevention, national center for emerging and zoonotic infectious diseases (NCEZID), division of foodborne, waterborne, and environmental diseases (DFWED). (Accessed on 5th September 2018) https://www.cdc.gov/fungal/diseases/cryptococcosis-gattii/statistics.html.
- C. neoformans Infection Statistics . Centers for disease control and prevention, national center for emerging and zoonotic infectious diseases (NCEZID), division of foodborne, waterborne, and environmental diseases (DFWED). (Accessed on 5th September 2018) https://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/statistics.html.
- Cabral V, Znaidi S, Walker LA, Martin-Yken H, Dague E, Legrand M, Lee K, Chauvel M, Firon A, Rossignol T, et al. 2014. Targeted changes of the cell wall proteome influence candida albicans ability to form single- and multi-strain biofilms. PLoS Pathog. 10(12):e1004542. doi: 10.1371/journal.ppat.1004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone RA. 1993. Molecular interactions at the interface of candida albicans and host cells. Arch Med Res. 24(3):275–279. [PubMed] [Google Scholar]
- Calvo B, Melo ASA, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, Colombo AL. 2016. First report of candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. Journal of Infection. 73(4):369–374. doi: 10.1016/j.jinf.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Cao L, Chan CM, Lee C, Wong SS, Yuen KY. 1998. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus penicillium marneffei. Infect Immun. 66(3):966–973. doi: 10.1128/IAI.66.3.966-973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño I, Pan SJ, Zupancic M, Hennequin C, Dujon B, Cormack BP. 2005. Telomere length control and transcriptional regulation of subtelomeric adhesins in candida glabrata. Mol Microbiol. 55(4):1246–1258. doi: 10.1111/j.1365-2958.2004.04465.x. [DOI] [PubMed] [Google Scholar]
- Chaffin WL, López-Ribot JL, Casanova M, Gozalbo D, Martínez JP. 1998. Cell wall and secreted proteins of candida albicans: identification, function, and expression. Microbiology and Molecular Biology Reviews: MMBR. 62(1):130–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen candida albicans: development, architecture, and drug resistance. J Bacteriol. 183(18):5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R, Ansari FA, Raghunandanan MV, Ramachandran S. 2011. FungalRV: adhesin prediction and immunoinformatics portal for human fungal pathogens. BMC Genomics. 12(1):1–14. doi: 10.1186/1471-2164-12-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A, Anil Kumar V, Sharma C, Prakash A, Agarwal K, Babu R, Meis JF. 2014. Multidrug-resistant endemic clonal strain of candida auris in India. European Journal of Clinical Microbiology & Infectious Diseases. 33(6):919–926. doi: 10.1007/s10096-013-2027-1. [DOI] [PubMed] [Google Scholar]
- Chowdhary A, Sharma C, Duggal S, Agarwal K, Prakash A, Singh PK, Meis JF. 2013. New clonal strain of candida auris, Delhi, India. Emerg Infect Dis. 19(10):1670–1673. doi: 10.3201/eid1910.130393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck AJ. 1969. A mutational analysis of conidial development in aspergillus nidulans. Genetics. 63(2):317–327. doi: 10.1093/genetics/63.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cm B, Hostetter MK. 1993. Distinct mechanisms of epithelial adhesion for candida albicans and candida tropicalis.Identification of the participating ligands and development of inhibitory peptides. J Clin Invest. 92(4):1840–1849. doi: 10.1172/JCI116775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack BP, Ghori N, Falkow S. 1999. An adhesin of the yeast pathogen candida glabrata mediating adherence to human epithelial cells. Science (New York, N Y). 285(5427):578–582. doi: 10.1126/science.285.5427.578. [DOI] [PubMed] [Google Scholar]
- Damveld RA, Arentshorst M, VanKuyk PA, Klis FM, van den Hondel CA, Ram AF. 2005. Characterisation of CwpA, a putative glycosylphosphatidylinositol-anchored cell wall mannoprotein in the filamentous fungus aspergillus niger. Fungal Genetics and Biology : FG & B. 42(10):873–885. doi: 10.1016/j.fgb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Davies JM, Stacey AJ, Gilligan CA. 1999. Candida albicans hyphal invasion: thigmotropism or chemotropism?. FEMS Microbiol Lett. 171(2):245–249. doi: 10.1111/j.1574-6968.1999.tb13439.x. [DOI] [PubMed] [Google Scholar]
- de Groot PW, De Boer AD, Cunningham J, Dekker HL, de Jong L, Hellingwerf KJ, de Koster C, Klis FM. 2004. Proteomic analysis of candida albicans cell walls reveals covalently bound carbohydrate-active enzymes and adhesins. Eukaryotic Cell. 3(4):955–965. doi: 10.1128/EC.3.4.955-965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot PW, Kraneveld EA, Yin QY, Dekker HL, Gross U, Crielaard W, de Koster CG, Bader O, Klis FM, Weig M. 2008. The cell wall of the human pathogen candida glabrata: differential incorporation of novel adhesin-like wall proteins. Eukaryotic Cell. 7(11):1951–1964. doi: 10.1128/EC.00284-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira HC, JDF DS, Scorzoni L, Marcos CM, Rossi SA, Ac DPES, Pa A, Ra DS, Fusco-Almeida AM, Mendes-Giannini MJ. 2015. Importance of adhesins in virulence of paracoccidioides spp. Front Microbiol. 6:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degregorio MW, Lee WMF, Ries CA. 1982. Candida infections in patients with acute leukemia: ineffectiveness of nystatin prophylaxis and relationship between oropharyngeal and systemic candidiasis. Cancer. 50(12):2780–2784. doi:. [DOI] [PubMed] [Google Scholar]
- Denning D, Pleuvry A, Cole D. 2011. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 89(12):864–872. doi: 10.2471/BLT.11.089441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai C, Mavrianos J, Chauhan N. 2011. Candida glabrata Pwp7p and Aed1p are required for adherence to human endothelial cells. FEMS Yeast Res. 11(7):595–601. doi: 10.1111/j.1567-1364.2011.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diderrich R, Kock M, Maestre-Reyna M, Keller P, Steuber H, Rupp S, Essen LO, Mösch HU. 2015. Structural hot spots determine functional diversity of the candida glabrata epithelial adhesin family. J Biol Chem. 290(4):19597–19613. doi: 10.1074/jbc.M115.655654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domergue R, Castaño I, De Las Peñas A, Zupancic M, Lockatell V, Hebel JR, Johnson D, Cormack BP. 2005. Nicotinic acid limitation regulates silencing of candida adhesins during UTI. Science (New York, N Y). 308(5723):866–870. doi: 10.1126/science.1108640. [DOI] [PubMed] [Google Scholar]
- Edman JC, Kovacs JA, Masur H, Santi DV, Elwood HJ, Sogin ML. 1988. Ribosomal RNA sequence shows pneumocystis carinii to be a member of the fungi. Nature. 334(6182):519–522. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- Emara M, Ahmad S, Khan Z, Joseph L, Al-Obaid I, Purohit P, Bafna R. 2015. Candida auris candidemia in Kuwait, 2014. Emerg Infect Dis. 21(6):1091–1092. doi: 10.3201/eid2106.150270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene IV, Bennett RJ. 2009. Hwp1 and related adhesins contribute to both mating and biofilm formation in candida albicans. Eukaryotic Cell. 8(12):1909–1913. doi: 10.1128/EC.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquenazi D, Alviano CS, de Souza W, Rozental S. 2004. The influence of surface carbohydrates during in vitro infection of mammalian cells by the dermatophyte trichophyton rubrum. Res Microbiol. 155(3):144–153. doi: 10.1016/j.resmic.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Esquenazi D, De Souza W, Sales Alviano C, Rozental S. 2003. The role of surface carbohydrates on the interaction of microconidia of trichophyton mentagrophytes with epithelial cells. FEMS Immunol Med Microbiol. 35(2):113–123. doi: 10.1016/S0928-8244(03)00007-5. [DOI] [PubMed] [Google Scholar]
- Fanning S, Mitchell AP. 2012. Fungal biofilms. PLoS Pathog. 8(4):e1002585. doi: 10.1371/journal.ppat.1002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei MW, Kim EJ, Sant CA, Jarlsberg LG, Davis JL, Swartzman A, Huang L. 2009. Predicting mortality from HIV-associated pneumocystis pneumonia at illness presentation: an observational cohort study. Thorax. 64(12):1070–1076. doi: 10.1136/thx.2009.117846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D, Burrow J, Lo D, Currie B. 1993. Cryptococcus neoformans in tropical northern Australia: predominantly variant gattii with good outcomes. Aust N Z J Med. 23(6):678–682. doi: 10.1111/j.1445-5994.1993.tb04726.x. [DOI] [PubMed] [Google Scholar]
- Flanagan PR, Liu NN, Fitzpatrick DJ, Hokamp K, Köhler JR, Moran GP. 2017. The candida albicans TOR-activating GTPases Gtr1 and Rhb1 coregulate starvation responses and biofilm formation. Msphere. 2(6). doi: 10.1128/mSphere.00477-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries BC, Taborda CP, Serfass E, Casadevall A. 2001. Phenotypic switching of cryptococcus neoformans occurs in vivo and influences the outcome of infection. J Clin Invest. 108(11):1639–1648. doi: 10.1172/JCI13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Rieg G, Fonzi WA, Belanger PH, Edwards JE, Filler SG. 1998. Expression of the candida albicans gene ALS1 in saccharomyces cerevisiae induces adherence to endothelial and epithelial cells. Infect Immun. 66(4):1783–1786. doi: 10.1128/IAI.66.4.1783-1786.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Vidal C, Upton A, Kirby KA, Marr KA. 2008. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clinical Infectious Diseases. 47(8):1041–1050. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur NK, Klotz SA, Henderson RL. 1999. Overexpression of the candida albicans ALA1 gene in saccharomyces cerevisiae results in aggregation following attachment of yeast cells to extracellular matrix proteins, adherence properties similar to those of candida albicans. Infect Immun. 67(11):6040–6047. doi: 10.1128/IAI.67.11.6040-6047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur NK, Smith RL, Klotz SA. 2002. Candida albicans and saccharomyces cerevisiae expressing ALA1/ALS5 adhere to accessible threonine, serine, or alanine patches. Cell Commun Adhes. 9(1):45–57. doi: 10.1080/15419060212187. [DOI] [PubMed] [Google Scholar]
- Gibbons JG, Beauvais A, Beau R, McGary KL, Latgé JP, Rokas A. 2012. Global transcriptome changes underlying colony growth in the opportunistic human pathogen aspergillus fumigatus. Eukaryotic Cell. 11(1):68–78. doi: 10.1128/EC.05102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil ML, Peñalver MC, Lopez-Ribot JL, O’Connor JE, Martinez JP. 1996. Binding of extracellular matrix proteins to aspergillus fumigatus conidia. Infect Immun. 64(12):5239–5247. doi: 10.1128/IAI.64.12.5239-5247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Navarro I, Gil ML, Casanova M, O'Connor JE, Martinez JP & Gozalbo D. 1997. The glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase of Candida albicans is a surface antigen. Journal of Bacteriology, 179(16), 4992–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Lee SC, Casadevall A. 1994. Pathogenesis of pulmonary cryptococcus neoformans infection in the rat. Infect Immun. 62(11):4755–4761. doi: 10.1128/IAI.62.11.4755-4761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Bing J, Guan G, Nobile CJ, Huang G. 2020. The Als3 cell wall adhesin plays a critical role in human serum amyloid A1-induced cell death and aggregation in candida albicans. Antimicrob Agents Chemother. 64(6). doi: 10.1128/AAC.00024-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez CE, Rinaldi MG, Sugar AM. 2002. Zygomycosis. Infect Dis Clin North Am. 16(4):895–914. doi: 10.1016/S0891-5520(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Goodwin RA, Shapiro JL, Thurman GH, Thurman SS, Des Prez RM. 1980. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine. 59(1):1–33. doi: 10.1097/00005792-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Gow NAR, Brown AJP, Odds FC. 2002. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 5(4):366–371. doi: 10.1016/S1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- Gozalbo D, Gil-Navarro I, Azorín I, Renau-Piqueras J, Martínez JP, Gil ML. 1998. The cell wall-associated glyceraldehyde-3-phosphate dehydrogenase of candida albicans is also a fibronectin and laminin binding protein. Infect Immun. 66(5):2052–2059. doi: 10.1128/IAI.66.5.2052-2059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gp D, Hostetter MK. 1996. Evidence for a beta 1 integrin fibronectin receptor in candida tropicalis. J Infect Dis. 174(1):127–132. doi: 10.1093/infdis/174.1.127. [DOI] [PubMed] [Google Scholar]
- Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, Chen D, Xu W, Kravtsov I, Hoareau CM, Vanier G, et al. 2013. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system. PLoS Pathog. 9(8):e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravelat FN, Ejzykowicz DE, Chiang LY, Chabot JC, Urb M, Macdonald KD, al-Bader N, Filler SG, Sheppard DC. 2010. Aspergillus fumigatus MedA governs adherence, host cell interactions and virulence. Cell Microbiol. 12(4):473–488. doi: 10.1111/j.1462-5822.2009.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinea J, Torres-Narbona M, Gijón P, Muñoz P, Pozo F, Peláez T, Bouza E. 2010. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: incidence, risk factors, and outcome. Clinical Microbiology and Infection. 16(7):870–877. doi: 10.1111/j.1469-0691.2009.03015.x. [DOI] [PubMed] [Google Scholar]
- Guo B, Styles CA, Feng Q, Fink GR. 2000. A saccharomyces gene family involved in invasive growth, cell-cell adhesion, and mating. Proc Natl Acad Sci U S A. 97(22):12158–12163. doi: 10.1073/pnas.220420397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyawali R, Upadhyay S, Way J, Lin X. 2017. A family of secretory Proteins is associated with different morphotypes in cryptococcus neoformans. Appl Environ Microbiol. 83(5):e02967–16. doi: 10.1128/AEM.02967-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjeh RA, Sofair AN, Harrison LH, Lyon GM, Arthington-Skaggs BA, Mirza SA, Warnock DW. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol. 42(4):1519–1527. doi: 10.1128/JCM.42.4.1519-1527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AJ, Jeavons L, Youngchim S, Vanittanakom N. 1999. Recognition of fibronectin by penicillium marneffei conidia via a sialic acid-dependent process and its relationship to the interaction between conidia and laminin. Infect Immun. 67(10):5200–5205. doi: 10.1128/IAI.67.10.5200-5205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AJ, Jeavons L, Youngchim S, Vanittanakom N, Hay RJ. 1998. Sialic Acid-dependent recognition of Laminin byPenicillium marneffei conidia. Infect Immun. 66(10):6024–6026. doi: 10.1128/IAI.66.12.6024-6026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek P, Dib L, Yazbeck P, Beyrouthy B, Khalaf RA. 2010. Characterization of Hwp2, a candida albicans putative GPI-anchored cell wall protein necessary for invasive growth. Microbiol Res. 165(12):250–258. doi: 10.1016/j.micres.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Hazen KC, Brawner DL, Riesselman MH, Jutila MA, Cutler JE. 1991. Differential adherence of hydrophobic and hydrophilic candida albicans yeast cells to mouse tissues. Infect Immun. 59(3):907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques M, Azeredo J, Oliveira R. 2006. Candida albicans and candida dubliniensis: comparison of biofilm formation in terms of biomass and activity. Br J Biomed Sci. 63(1):5–11. doi: 10.1080/09674845.2006.11732712. [DOI] [PubMed] [Google Scholar]
- Hope WW, Kruhlak MJ, Lyman CA, Petraitiene R, Petraitis V, Francesconi A, Walsh TJ. 2007. Pathogenesis of aspergillus fumigatus and the kinetics of galactomannan in an in vitro model of early invasive pulmonary aspergillosis: implications for antifungal therapy. J Infect Dis. 195(3):455–466. doi: 10.1086/510535. [DOI] [PubMed] [Google Scholar]
- Hostetter MK. 2000. RGD-mediated adhesion in fungal pathogens of humans, plants and insects. Curr Opin Microbiol. 3(4):344–348. doi: 10.1016/S1369-5274(00)00101-6. [DOI] [PubMed] [Google Scholar]
- Hoyer LL. 2001. The ALS gene family of candida albicans. Trends Microbiol. 9(4):176–180. doi: 10.1016/S0966-842X(01)01984-9. [DOI] [PubMed] [Google Scholar]
- Hube B, Naglik J. 2001. Candida albicans proteinases: resolving the mystery of a gene family. Microbiology. 147(8):1997–2005. doi: 10.1099/00221287-147-8-1997. [DOI] [PubMed] [Google Scholar]
- Hung CY, Yu JJ, Seshan KR, Reichard U, Cole GT. 2002. A parasitic phase-specific adhesin of coccidioides immitis contributes to the virulence of this respiratory fungal pathogen. Infect Immun. 70(7):3443–3456. doi: 10.1128/IAI.70.7.3443-3456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Spellberg BJ, Avanesian V, Fu Y, Edwards JE. 2006. The anti-candida vaccine based on the recombinant N-terminal domain of Als1p is broadly active against disseminated candidiasis. Infect Immun. 74(5):3039–3041. doi: 10.1128/IAI.74.5.3039-3041.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Spellberg BJ, Avenissian V, Fu Y, Filler SG, Edwards JE. 2005. Vaccination with recombinant N-terminal domain of Als1p improves survival during murine disseminated candidiasis by enhancing cell-mediated, not humoral, immunity. Infect Immun. 73(2):999–1005. doi: 10.1128/IAI.73.2.999-1005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraqui I, Garcia-Sanchez S, Aubert S, Dromer F, Ghigo JM, d’Enfert C, Janbon G. 2005. The Yak1p kinase controls expression of adhesins and biofilm formation in candida glabrata in a Sir4p-dependent pathway. Mol Microbiol. 55(4):1259–1271. doi: 10.1111/j.1365-2958.2004.04475.x. [DOI] [PubMed] [Google Scholar]
- Jesus MD, Am N, Sk C, Ir L, Nong S, Ca S, Sm L, Casadevall A. 2010. Glucuronoxylomannan, galactoxylomannan, and mannoprotein occupy spatially separate and discrete regions in the capsule of cryptococcus neoformans. Virulence. 1(6):500–508. doi: 10.4161/viru.1.6.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Lucho V, Ginsburg V, HC K. 1990. Cryptococcus neoformans, candida albicans, and other fungi bind specifically to the glycosphingolipid lactosylceramide (gal beta 1-4Glc beta 1-1Cer), a possible adhesion receptor for yeasts. Infect Immun. 58(7):2085–2090. doi: 10.1128/IAI.58.7.2085-2090.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JS F, Mitchell AP. 2011. Genetic control of candida albicans biofilm development. Nat Rev Microbiol. 9(2):109–118. doi: 10.1038/nrmicro2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamai Y, Lossinsky AS, Liu H, Sheppard DC, Filler SG. 2009. Polarized response of endothelial cells to invasion by aspergillus fumigatus. Cell Microbiol. 11(1):170–182. doi: 10.1111/j.1462-5822.2008.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapteyn JC, Hoyer LL, Hecht JE, Müller WH, Andel A, Verkleij AJ, Makarow M, Van Den Ende H, Klis FM. 2000. The cell wall architecture of candida albicans wild-type cells and cell wall-defective mutants. Mol Microbiol. 35(3):601–611. doi: 10.1046/j.1365-2958.2000.01729.x. [DOI] [PubMed] [Google Scholar]
- Kartasova T, van Muijen GN, van Pelt-Heerschap H, van de Putte P. 1988. Novel protein in human epidermal keratinocytes: regulation of expression during differentiation. Mol Cell Biol. 8(5):2204–2210. doi: 10.1128/mcb.8.5.2204-2210.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R, Domergue R, Zupancic ML, Cormack BP. 2005. A yeast by any other name: candida glabrata and its interaction with the host. Curr Opin Microbiol. 8(4):378–384. doi: 10.1016/j.mib.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Kaur S, Singh S. 2014. Biofilm formation by aspergillus fumigatus. Medical Mycology. 52(1):2–9. doi: 10.3109/13693786.2013.819592. [DOI] [PubMed] [Google Scholar]
- Kempf M, Apaire-Marchais V, Saulnier P, Licznar P, Lefrançois C, Robert R, Cottin J. 2007. Disruption of candida albicans IFF4 gene involves modifications of the cell electrical surface properties. Colloids Surf B Biointerfaces. 58(2):250–255. doi: 10.1016/j.colsurfb.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Khuu D, Shafir S, Bristow B, Sorvillo F. 2014. Blastomycosis mortality rates, United States, 1990–2010. Emerg Infect Dis. 20(11):1789–1794. doi: 10.3201/eid2011.131175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, Meyer W. 2004. A rare genotype of cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proceedings of the National Academy of Sciences. 101(49):17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KJ K-C, Varma A. 2006. Do major species concepts support one, two or more species within cryptococcus neoformans ?. FEMS Yeast Res. 6(4):574–587. doi: 10.1111/j.1567-1364.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- Klein BS, Tebbets B. 2007. Dimorphism and virulence in fungi. Curr Opin Microbiol. 10(4):314–319. doi: 10.1016/j.mib.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P, Schembri MA. 2000. Bacterial adhesins: function and structure. International Journal of Medical Microbiology. 290(1):27–35. doi: 10.1016/S1438-4221(00)80102-2. [DOI] [PubMed] [Google Scholar]
- Klis FM, de Groot P, Hellingwerf K. 2001. Molecular organization of the cell wall of candida albicans. Medical Mycology. 39(Suppl 1):1–8. doi: 10.1080/mmy.39.1.1.8-0. [DOI] [PubMed] [Google Scholar]
- Klotz SA, Hein RC, Smith RL, & Rouse JB. 1994. The fibronectin adhesin of Candida albicans. Infection and immunity. 62(10):4679–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani T, Katayama S, Miyazaki Y, Fukuda S, Sato Y, Ohsugi K. 2017. Risk factors for the mortality of pneumocystis jirovecii pneumonia in non-HIV patients who required mechanical ventilation: a retrospective case series study. Biomed Res Int. 1–7. doi: 10.1155/2017/7452604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozik A, Karkowska-Kuleta J, Zajac D, Bochenska O, Kedracka-Krok S, Jankowska U, Rapala-Kozik M. 2015. Fibronectin-, vitronectin- and laminin-binding proteins at the cell walls of candida parapsilosis and candida tropicalis pathogenic yeasts. BMC Microbiol. 15(1):197. doi: 10.1186/s12866-015-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Manavathu EK, Chandrasekar P. 2009. Aspergillus flavus : an emerging non- fumigatus aspergillus species of significance. Mycoses. 52(3):206–222. doi: 10.1111/j.1439-0507.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- Kular JK, Basu S, Sharma RI. 2014. The extracellular matrix: structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng. 5:2041731414557112. doi: 10.1177/2041731414557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Yang M, Bc H, Ml S, Tl D. 2011. Emerging themes in cryptococcal capsule synthesis. Curr Opin Struct Biol. 21(5):597–602. doi: 10.1016/j.sbi.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutty G, England KJ, & Kovacs JA. 2013. Expression of Pneumocystis jirovecii major surface glycoprotein in Saccharomyces cerevisiae. The Journal of infectious diseases, 208(1), 170–179. doi: 10.1093/infdis/jit131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé JP. 1999. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 12(2):310–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé JP. 2003. Aspergillus fumigatus, a saprotrophic pathogenic fungus. Mycologist. 17(2):56–61. doi: 10.1017/S0269915X0300209X. [DOI] [Google Scholar]
- Lau SK, Tse H, Chan JS, Zhou AC, Curreem SO, Lau CC, Yuen KY, Woo PC. 2013. Proteome profiling of the dimorphic fungus penicillium marneffei extracellular proteins and identification of glyceraldehyde-3-phosphate dehydrogenase as an important adhesion factor for conidial attachment. FEBS J. 280(24):6613–6626. doi: 10.1111/febs.12566. [DOI] [PubMed] [Google Scholar]
- Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, Jang HC. 2011. First three reported cases of nosocomial fungemia caused by candida auris. J Clin Microbiol. 49(9):3139–3142. doi: 10.1128/JCM.00319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levdansky E, Kashi O, Sharon H, Shadkchan Y, Osherov N. 2010. The aspergillus fumigatus cspA gene encoding a repeat-rich cell wall protein is important for normal conidial cell wall architecture and interaction with host cells. Eukaryotic Cell. 9(9):1403–1415. doi: 10.1128/EC.00126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Palecek SP. 2003. EAP1, a candida albicans gene involved in binding human epithelial cells. Eukaryotic Cell. 2(6):1266–1273. doi: 10.1128/EC.2.6.1266-1273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann B, Gattung S, Jahn B, Brakhage AA. 2003. cAMP signaling in aspergillus fumigatus is involved in the regulation of the virulence gene pksP and in defense against killing by macrophages. Molecular Genetics and Genomics: MGG. 269(3):420–435. doi: 10.1007/s00438-003-0852-0. [DOI] [PubMed] [Google Scholar]
- Lima OC, Figueiredo CC, Previato JO, Mendonça-Previato L, Morandi V, Lopes Bezerra LM. 2001. Involvement of fungal cell wall components in adhesion of sporothrix schenckii to human fibronectin. Infect Immun. 69(11):6874–6880. doi: 10.1128/IAI.69.11.6874-6880.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limongi CL, Alviano CS, De Souza W, Rozental S. 2001. Isolation and partial characterization of an adhesin from fonsecaea pedrosoi. Medical Mycology. 39(5):429–437. doi: 10.1080/mmy.39.5.429.437. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Sasse C, Gerke J, Valerius O, Irmer H, Frauendorf H, Heinekamp T, Strasburger M, Tran VT, Herzog B, et al. 2015. Transcription factor soma is required for adhesion, development and virulence of the human pathogen aspergillus fumigatus. PLoS Pathog. 11(11):e1005205. doi: 10.1371/journal.ppat.1005205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvintseva AP, Thakur R, Reller LB, Mitchell TG. 2005. Prevalence of clinical isolates of cryptococcus gattii serotype C among patients with AIDS in Sub‐Saharan Africa. J Infect Dis. 192(5):888–892. doi: 10.1086/432486. [DOI] [PubMed] [Google Scholar]
- Liu H, Lee MJ, Solis NV, Phan QT, Swidergall M, Ralph B, Filler SG. 2016. Aspergillus fumigatus CalA binds to integrin α 5 β 1 and mediates host cell invasion. Nature Microbiology. 2(2):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TB, Perlin DS, Xue C. 2012. Molecular mechanisms of cryptococcal meningitis. Virulence. 3(2):173–181. doi: 10.4161/viru.18685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, N P G, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant candida auris on 3 Continents confirmed by whole-genome sequencing and epidemiological analyses. Clinical Infectious Diseases. 64(2):134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KH, Gomez FJ, Morris RE, Newman SL. 2003. Identification of heat shock protein 60 as the ligand on histoplasma capsulatum that mediates binding to CD18 receptors on human macrophages. Journal of Immunology (Baltimore, Md : 1950). 170(1):487–494. doi: 10.4049/jimmunol.170.1.487. [DOI] [PubMed] [Google Scholar]
- López-Ribot JL, Casanova M, Monteagudo C, Sepúlveda P, Martínez JP. 1994. Evidence for the presence of a high-affinity laminin receptor-like molecule on the surface of Candida albicans yeast cells. Infection and Immunity. 62(2):742–6. doi: 10.1128/iai.62.2.742-746.1994. PMID: 8300236; PMCID: PMC186171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M JS M-G, Soares CP, Jlm DS, Andreotti PF. 2005. Interaction of pathogenic fungi with host cells: molecular and cellular approaches. FEMS Immunol Med Microbiol. 45(3):383–394. doi: 10.1016/j.femsim.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Maestre-Reyna M, Diderrich R, Veelders MS, Eulenburg G, Kalugin V, Brückner S, Keller P, Rupp S, Mösch HU, Essen LO. 2012. Structural basis for promiscuity and specificity during candida glabrata invasion of host epithelia. Proc Natl Acad Sci U S A. 109(42):16864–16869. doi: 10.1073/pnas.1207653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magobo RE, Corcoran C, Seetharam S, Govender NP. 2014. Candida auris –associated candidemia, South Africa. Emerg Infect Dis. 20(7). doi: 10.3201/eid2007.131765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansharamani NG, Garland R, Delaney D, Koziel H. 2003. Management and outcome patterns for adult pneumocystis carinii pneumonia, 1985 to 1995. Chest. 118(3):704–711. doi: 10.1378/chest.118.3.704. [DOI] [PubMed] [Google Scholar]
- Martin-Yken H, Bedekovic T, Brand AC, Richard ML, Znaidi S, d’Enfert C, Dague E. 2018. A conserved fungal hub protein involved in adhesion and drug resistance in the human pathogen </R>candida albicans. Cell Surface (Amsterdam, Netherlands). 4:10–19. doi: 10.1016/j.tcsw.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer FL, Wilson D, Hube B. 2013. A possible mechanism of dissemination. J Clin Invest. 96(2):1010–1017. Candida albicans pathogenicity mechanisms. Virulence. 4(2):119–128. McMahon JP, Wheat J, Sobel ME, Pasula R, Downing JF, & Martin WJ.1995. Murine laminin binds to Histoplasma capsulatum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon JP, Wheat J, Sobel ME, Pasula R, Downing JF, Martin WJ 2nd.1995. Murine laminin binds to Histoplasma capsulatum. A possible mechanism of dissemination. J Clin Invest. 96(2):1010–7. doi: 10.1172/JCI118086. PMID: 7635937; PMCID: PMC286381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Giannini MJ, Taylor ML, Bouchara JB, Burger E, Calich VL, Escalante ED, Hanna SA, Lenzi HL, Machado MP, Miyaji M, et al. 2000. Pathogenesis II: fungal responses to host responses: interaction of host cells with fungi. Medical Mycology. 38(Suppl s1):113–123. doi: 10.1080/mmy.38.s1.113.123. [DOI] [PubMed] [Google Scholar]
- Merkel GJ, Scofield BA. 1993. Conditions affecting the adherence of cryptococcus neoformans to rat glial and lung cells in vitro. Journal of Medical and Veterinary Mycology: Bi-monthly Publication of the International Society for Human and Animal Mycology. 31(1):55–64. doi: 10.1080/02681219380000061. [DOI] [PubMed] [Google Scholar]
- Merkel GJ, Scofield BA. 1997. The in vitro interaction of cryptococcus neoformans with human lung epithelial cells. FEMS Immunol Med Microbiol. 19(3):203–213. doi: 10.1111/j.1574-695X.1997.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Money NP. 2001. Biomechanics of invasive hyphal growth. In: Howard RJ, editor. The Mycota VIII. Gow NA (Berlin): Springer-Verlag; p. 3–17. [Google Scholar]
- Monod M, Paris S, Sanglard D, Jaton-Ogay K, Bille J, Latge JP. 1993. Isolation and characterization of a secreted metalloprotease of Aspergillus fumigatus. Infection and immunity. 61(10):4099–4104. doi: 10.1128/IAI.61.10.4099-4104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. 2005. Global trends in candidemia: review of reports from 1995-2005. Curr Infect Dis Rep. 7(6):429–439. doi: 10.1007/s11908-005-0044-7. [DOI] [PubMed] [Google Scholar]
- Moutaouakil M, Monod M, Prevost MC, Bouchara JP, Paris S, Latge JP. 1993. Identification of the 33-kDa alkaline protease of Aspergillus fumigatus in vitro and in vivo. Journal of Medical microbiologyJ Med Microbiol. 39(5):393–399. doi: 10.1099/00222615-39-5-393. [DOI] [PubMed] [Google Scholar]
- Müller FM, Seidler M, Beauvais A. 2011. Aspergillus fumigatus biofilms in the clinical setting. Medical Mycology. 49(Suppl S1):S96–S100. doi: 10.3109/13693786.2010.502190. [DOI] [PubMed] [Google Scholar]
- Murciano C, Moyes DL, Runglall M, Tobouti P, Islam A, Hoyer LL, Naglik JR. 2012. Evaluation of the role of candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PloS One. 7(3):e33362. doi: 10.1371/journal.pone.0033362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik JR, Rodgers CA, Shirlaw PJ, Dobbie JL, Fernandes-Naglik LL, Greenspan D, Agabian N, Challacombe SJ. 2003. Differential expression of candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J Infect Dis. 188(3):469–479. doi: 10.1086/376536. [DOI] [PubMed] [Google Scholar]
- Nam HS, Jeon K, Um SW, Suh GY, Chung MP, Kim H, Koh WJ. 2010. Clinical characteristics and treatment outcomes of chronic necrotizing pulmonary aspergillosis: a review of 43 cases. International Journal of Infectious Diseases. 14(6):e479–e482. doi: 10.1016/j.ijid.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Nath A. 2019. Prediction and molecular insights into fungal adhesins and adhesin like proteins. Comput Biol Chem. 80:333–340. doi: 10.1016/j.compbiolchem.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan QT, Edwards JE, Filler SG, Mitchell AP. 2006a. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2(7):e63. doi: 10.1371/journal.ppat.0020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Mitchell AP. 2005. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Current Biology: CB. 15(12):1150–1155. doi: 10.1016/j.cub.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Mitchell AP. 2006. Genetics and genomics of candida albicans biofilm formation. Cell Microbiol. 8(9):1382–1391. doi: 10.1111/j.1462-5822.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Nett JE, Andes DR, Mitchell AP. 2006b. Function of candida albicans adhesin Hwp1 in biofilm formation. Eukaryotic Cell. 5(10):1604–1610. doi: 10.1128/EC.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Schneider HA, Nett JE, Sheppard DC, Filler SG, Andes DR, Mitchell AP. 2008. Complementary adhesin function in C. albicans biofilm formation. Current Biology: CB. 18(14):1017–1024. doi: 10.1016/j.cub.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LJ. 2007. The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol. 66(2):279–290. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Peñalver MC, O’Connor JE, Martinez JP, Gil ML. 1996. Binding of human fibronectin to aspergillus fumigatus conidia. Infect Immun. 64(4):1146–1153. doi: 10.1128/IAI.64.4.1146-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendrak ML, Klotz SA. 1995. Adherence of candida albicans to host cells. FEMS Microbiol Lett. 129(2–3):103–113. doi: 10.1111/j.1574-6968.1995.tb07566.x. [DOI] [PubMed] [Google Scholar]
- Pérez A, Pedrós B, Murgui A, Casanova M, López-Ribot JL, Martínez JP. 2006. Biofilm formation by candida albicans mutants for genes coding fungal proteins exhibiting the eight-cysteine-containing CFEM domain. FEMS Yeast Res. 6(7):1074–1084. doi: 10.1111/j.1567-1364.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- Perfect JR. 1989. Cryptococcosis. Infect Dis Clin North Am. 3(1):77–102. doi: 10.1016/S0891-5520(20)30248-8. [DOI] [PubMed] [Google Scholar]
- Perlroth J, Choi B, Spellberg B. 2007. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Medical Mycology. 45(4):321–346. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ. 2004a. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond candida albicans and aspergillus fumigatus. J Clin Microbiol. 42(10):4419–4431. doi: 10.1128/JCM.42.10.4419-4431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ, & International Fungal Surveillance Participant Group . 2004b. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of candida. Clinical Microbiology and Infection?: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 10(Suppl 1):11–23. doi: 10.1111/j.1470-9465.2004.t01-1-00844.x. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 20(2–3):133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto MR, de Sá AC, Limongi CL, Rozental S, Santos AL, Barreto-Bergter E. 2004. Involvement of peptidorhamnomannan in the interaction of pseudallescheria boydii and HEp2 cells. Microbes and Infection. 6(14):1259–1267. doi: 10.1016/j.micinf.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Prusty R, Grisafi P, Fink GR. 2004. The plant hormone indoleacetic acid induces invasive growth in saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 101(12):4153–4157. doi: 10.1073/pnas.0400659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pwj DG, Bader O, De Boer AD, Weig M, Chauhan N. 2013. Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryotic Cell. 12(4):470–481. doi: 10.1128/EC.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, Rajendran R, Gutierrez-Correa M, Jones B, Williams C. 2011. Aspergillus biofilms: clinical and industrial significance. FEMS Microbiol Lett. 324(2):89–97. doi: 10.1111/j.1574-6968.2011.02381.x. [DOI] [PubMed] [Google Scholar]
- Ramana J, Gupta D. 2010. FaaPred: a SVM-based prediction method for fungal adhesins and adhesin-like proteins. PLoS One. 5(3):e9695. doi: 10.1371/journal.pone.0009695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees JR, Pinner RW, Hajjeh RA, Brandt ME, Reingold AL. 1998. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: results of population-based laboratory active surveillance. Clinical Infectious Diseases. 27(5):1138–1147. doi: 10.1093/clinids/27.5.1138. [DOI] [PubMed] [Google Scholar]
- Reichard U, Eiffert H, Ruchel R. 1994. Purification and characterization of an extracellular aspartic proteinase from aspergillus fumigatus. Journal of Medical and Veterinary mycologyJ Med Vet Mycol. 32:(6):427–436. doi: 10.1080/02681219480000581. [DOI] [PubMed] [Google Scholar]
- Reynolds TB, Fink GR. 2001. Bakers’ yeast, a model for fungal biofilm formation. Science (New York, N Y). 291(5505):878–881. doi: 10.1126/science.291.5505.878. [DOI] [PubMed] [Google Scholar]
- Robold AV, Hardham AR. 2005. During attachment phytophthora spores secrete proteins containing thrombospondin type 1 repeats. Curr Genet. 47(5):307–315. doi: 10.1007/s00294-004-0559-8. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, dos Reis FC, Puccia R, Travassos LR, Alviano CS. 2003. Cleavage of human fibronectin and other basement membrane-associated proteins by a cryptococcus neoformans serine proteinase. Microb Pathog. 34(2):65–71. doi: 10.1016/S0882-4010(02)00195-X. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Nimrichter L. 2012. In good company: association between fungal glycans generates molecular complexes with unique functions. Front Microbiol. 3:249. doi: 10.3389/fmicb.2012.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston KVI, Bodey GP. 2003. Fungal Infections. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker. Available from: https://www.ncbi.nlm.nih.gov/books/NBK13518/ [Google Scholar]
- Rosiana S, Zhang L, G H K, A V R, Uthayakumar D, Sukumaran A, Geddes-McAlister J, N V K, Shapiro RS. 2021. Comprehensive genetic analysis of adhesin proteins and their role in virulence of candida albicans. Genetics. 217(2):iyab003. doi: 10.1093/genetics/iyab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa K, Rc H, RL S, JB R. 1994. The fibronectin adhesin of candida albicans. Infect Immun. 62(1):4679–4681. doi: 10.1128/IAI.62.10.4679-4681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa K, RL S, BW S. 1992. Effect of an arginine-glycine-aspartic acid-containing peptide on hematogenous candidal infections in rabbits. Antimicrob Agents Chemother. 36(1):132–136. doi: 10.1128/AAC.36.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccente M, Woods GL. 2010. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 23(2):367–381. doi: 10.1128/CMR.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva G, Kumar K, Jain P, Ramachandran S. 2005. SPAAN: a software program for prediction of adhesins and adhesin-like proteins using neural networks. Bioinformatics. 21(4):483–491. doi: 10.1093/bioinformatics/bti028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni N, Yi S, Daniels KJ, Srikantha T, Pujol C, Soll DR. 2009. Genes selectively up-regulated by pheromone in white cells are involved in biofilm formation in candida albicans. PLoS Pathog. 5(10):e1000601. doi: 10.1371/journal.ppat.1000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampermans S, Mortier J, Soares EV. 2005. Flocculation onset in saccharomyces cerevisiae: the role of nutrients. J Appl Microbiol. 98(2):525–531. doi: 10.1111/j.1365-2672.2004.02486.x. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer EA, Vossen JH, Van Doorn AA, Cornelissen BJ, Haring MA. 2001. FEM1, a Fusarium oxysporum glycoprotein that is covalently linked to the cell wall matrix and is conserved in filamentous fungi. Molecular Genetics and Genomics: MGG. 265(1):143–152. [DOI] [PubMed] [Google Scholar]
- Seaton RA. 1996. The management of cryptococcal meningitis in papua new guinea. P N G Med J. 39(6):67–73. [PubMed] [Google Scholar]
- Sharkey LL, McNemar MD, Saporito-Irwin SM, Sypherd PS, Fonzi WA. 1999. HWP1 functions in the morphological development of candida albicans downstream of EFG1, TUP1, and RBF1. J Bacteriol. 181(17):5273–5279. doi: 10.1128/JB.181.17.5273-5279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DC. 2011. Molecular mechanism of aspergillus fumigatus adherence to host constituents. Curr Opin Microbiol. 14(4):375–379. doi: 10.1016/j.mib.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shourian M, Qureshi ST. 2019. Resistance and tolerance to cryptococcal infection: an intricate balance that controls the development of disease. Front Immunol. 10:66. doi: 10.3389/fimmu.2019.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. 2011. Adherence and biofilm formation of non-Candida albicans candida species. Trends Microbiol. 19(5):241–247. doi: 10.1016/j.tim.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. 2012. Candida glabrata, candida parapsilosis and candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 36(2):288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- Sirisanthana T, Supparatpinyo K. 1998. Epidemiology and management of penicilliosis in human immunodeficiency virus-infected patients. International Journal of Infectious Diseases. 3(1):48–53. doi: 10.1016/S1201-9712(98)90095-9. [DOI] [PubMed] [Google Scholar]
- Spellberg BJ, Ibrahim AS, Avenissian V, Filler SG, Myers CL, Fu Y, Edwards JE Jr.. 2005. The anti-Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infect Immun. 73(9):6191–6193. doi: 10.1128/IAI.73.9.6191-6193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab JF, Bradway SD, Fidel PL, Sundstrom P. 1999. Adhesive and mammalian transglutaminase substrate properties of candida albicans Hwp1. Science (New York, N Y). 283(5407):1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- Staab JF, Ferrer CA, Sundstrom P. 1996. Developmental expression of a tandemly repeated, proline-and glutamine-rich amino acid motif on hyphal surfaces on candida albicans. J Biol Chem. 271(11):6298–6305. doi: 10.1074/jbc.271.11.6298. [DOI] [PubMed] [Google Scholar]
- Sundstrom P. 1999. Adhesins in candida albicans. Curr Opin Microbiol. 2(4):353–357. doi: 10.1016/S1369-5274(99)80062-9. [DOI] [PubMed] [Google Scholar]
- Sundstrom P. 2002. Adhesion in candida spp. Cell Microbiol. 4(8):461–469. doi: 10.1046/j.1462-5822.2002.00206.x. [DOI] [PubMed] [Google Scholar]
- Teixeira PA, Penha LL, Mendonça-Previato L, Previato JO. 2014. Mannoprotein MP84 mediates the adhesion of cryptococcus neoformans to epithelial lung cells. Front Cell Infect Microbiol. 4:106. doi: 10.3389/fcimb.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thau N, Monod M, Crestani B, Rolland C, Tronchin G, Latgé JP, Paris S. 1994. rodletless mutants of aspergillus fumigatus. Infect Immun. 62(10):4380–4388. doi: 10.1128/IAI.62.10.4380-4388.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Lin X. 2013. Matricellular protein Cfl1 regulates cell differentiation. Commun Integr Biol. 6(6):e26444. doi: 10.4161/cib.26444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G, Esnault K, Renier G, Filmon R, Chabasse D, Bouchara JP. 1997. Expression and identification of a laminin-binding protein in aspergillus fumigatus conidia. Infect Immun. 65(1):9–15. doi: 10.1128/IAI.65.1.9-15.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G, Pihet M, Lopes-Bezerra LM, Bouchara JP. 2008. Adherence mechanisms in human pathogenic fungi. Medical Mycology. 46(8):749–772. doi: 10.1080/13693780802206435. [DOI] [PubMed] [Google Scholar]
- Tv K, Jadoun J, Mittelman L, Hirschberg K, OsherovN . 2004. Involvement of secreted aspergillus fumigatus proteases in disruption of the actin fiber cytoskeleton and loss of focal adhesion sites in infected A549 lung pneumocytes. J Infect Dis. 189(11):1965–1973. doi: 10.1086/420850. [DOI] [PubMed] [Google Scholar]
- Upadhyay SK, Basir SF & Madan T. 2008. Characterization of cell wall galactomannoprotein (Mp1) as a potential adhesin of Aspergillus fumigatus. Journal of Allergy and Clinical Immunology, 121(2), S14. [Google Scholar]
- Upadhyay SK, Mahajan L, Ramjee S, Singh Y, Basir SF, Madan T. 2009. Identification and characterization of a laminin-binding protein of aspergillus fumigatus: extracellular thaumatin domain protein (AfCalAp). J Med Microbiol. 58(6):714–722. doi: 10.1099/jmm.0.005991-0. [DOI] [PubMed] [Google Scholar]
- Valsecchi I, Dupres V, Stephen-Victor E, Guijarro J, Gibbons J, Beau R, Beauvais A. 2017. Role of hydrophobins in aspergillus fumigatus. Journal of Fungi. 4(1):2. doi: 10.3390/jof4010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartivarian SE, Reyes GH, Jacobson ES, James PG, Cherniak R, Mumaw VR, Tingler MJ. 1989. Localization of mannoprotein in cryptococcus neoformans. J Bacteriol. 171(12):6850–6852. doi: 10.1128/jb.171.12.6850-6852.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstrepen KJ, Derdelinckx G, Verachtert H, Delvaux FR. 2003. Yeast flocculation: what brewers should know. Appl Microbiol Biotechnol. 61(3):197–205. doi: 10.1007/s00253-002-1200-8. [DOI] [PubMed] [Google Scholar]
- Verstrepen KJ, Klis FM. 2006. Flocculation, adhesion and biofilm formation in yeasts. Mol Microbiol. 60(1):5–15. doi: 10.1111/j.1365-2958.2006.05072.x. [DOI] [PubMed] [Google Scholar]
- Verstrepen KJ, Reynolds TB, Fink GR. 2004. Origins of variation in the fungal cell surface. Nat Rev Microbiol. 2(7):533–540. doi: 10.1038/nrmicro927. [DOI] [PubMed] [Google Scholar]
- Vicentini AP, Gesztesi JL, Franco MF, de Souza W, de Moraes JZ, Travassos LR, Lopes JD. 1994. Binding of paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect Immun. 62(4):1465–1469. doi: 10.1128/IAI.62.4.1465-1469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächtler B, Wilson D, Haedicke K, Dalle F, Hube B. 2011. From attachment to damage: defined genes of candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PloS One. 6(2):e17046. doi: 10.1371/journal.pone.0017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer PD, Evans HE, Copas AJ, Edwards SG, Grant AD, Miller RF. 2008. Early predictors of mortality from Pneumocystis jirovecii pneumonia in HIV-infected patients: 1985–2006. Clin Infect Dis.15;46(4):625–33. doi: 10.1086/526778. PMID: 18190281; PMCID: PMC2735405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Tian X, Gyawali R, Lin X. 2013. Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proc Natl Acad Sci U S A. 110(28):11571–11576. doi: 10.1073/pnas.1308173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhai B, Lin X. 2012. The link between morphotype transition and virulence in cryptococcus neoformans. PLoS Pathog. 8(6):e1002765. doi: 10.1371/journal.ppat.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu T, Gong H, Zhou Q, Wang Y, Sun S, Xie L. 2007. Gene profiling in murine corneas challenged with aspergillus fumigatus. Mol Vis. 13:1226–1233. [PubMed] [Google Scholar]
- Warnock DW. 2007. Trends in the epidemiology of invasive fungal infections. Nippon Ishinkin Gakkai Zasshi. 48(1):1–12. doi: 10.3314/jjmm.48.1. [DOI] [PubMed] [Google Scholar]
- Wasylnka JA, Moore MM. 2000. Adhesion of aspergillus species to extracellular matrix proteins: evidence for involvement of negatively charged carbohydrates on the conidial surface. Infection and immunityInfect Immun. 68(6):3377–3384. doi: 10.1128/IAI.68.6.3377-3384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylnka JA, Moore MM. 2002. Uptake of aspergillus fumigatus conidia by phagocytic and nonphagocytic cells in vitro: quantitation using strains expressing green fluorescent protein. Infection and immunity. 70(6):3156–3163. doi: 10.1128/IAI.70.6.3156-3163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwater C, Schofield DA, Nicholas PJ, Paulling EE, Balish E. 2007. Candida glabrata and candida albicans; dissimilar tissue tropism and infectivity in a gnotobiotic model of mucosal candidiasis. FEMS Immunol Med Microbiol. 51(1):134–139. doi: 10.1111/j.1574-695X.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- Williamson PR. 2017. The relentless march of cryptococcal meningitis. The Lancet Infectious DiseasesLancet Infect Dis. 17(8):790–791. doi: 10.1016/S1473-3099(17)30245-1. [DOI] [PubMed] [Google Scholar]
- Zhao X, Oh SH, Cheng G, Green CB, Nuessen JA, Yeater K, Leng RP, Brown A, Hoyer LL. 2004. ALS3 and ALS8 represent a single locus that encodes a candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology (Reading, England). 150(8):2415–2428. doi: 10.1099/mic.0.26943-0. [DOI] [PubMed] [Google Scholar]
- Zhu W, Phan QT, Boontheung P, Solis NV, Loo JA, Filler SG. 2012. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by candida albicans during oropharyngeal infection. Proc Natl Acad Sci U S A. 109(35):14194–14199. doi: 10.1073/pnas.1117676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan R, Cormack B. 2012. Adhesins on Opportunistic Fungal Pathogens. In: Calderone RA, Clancy, CJ., ed. Candida and Candidiasis: ASM Press, Washington DC, 2nd Edition. ISBN-9781555817176, 1555817173. pp 243-259. [Google Scholar]
- Zupancic ML, Frieman M, Smith D, Alvarez RA, Cummings RD, Cormack BP. 2008. Glycan microarray analysis of candida glabrata adhesin ligand specificity. Mol Microbiol. 68(3):547–559. doi: 10.1111/j.1365-2958.2008.06184.x. [DOI] [PubMed] [Google Scholar]


