Abstract
Background:
There is growing evidence that 5-fluorouracil (5-FU) combined with therapeutic trauma can effectively induce skin repigmentation in vitiligo patients who are unresponsive to conventional treatments. Previous studies have mainly focused on identifying the antimitotic activity of 5-FU for the treatment of skin cancer, but few studies have investigated its extra-genotoxic actions favoring melanocyte recruitment.
Methods:
We utilized the full thickness excisional skin wound model in Dct-LacZ transgenic mice to dynamically assess the migration of melanocytes in the margins of wounds treated with or without 5-FU. The in-situ expression of CXCL12 was examined in the wound beds using immunofluorescence staining. Quantitative real-time polymerase chain reaction and Western blotting analyses were performed to detect the expression levels of CXCL12 mRNA and protein in primary mouse dermal fibroblasts treated with or without 5-FU. Transwell assays and fluorescein isothiocyanate (FITC)-phalloidin staining were used to observe cell migration and filamentous actin (F-actin) changes of melan-a murine melanocytes.
Results:
Whole mount and cryosection X-gal staining showed that the cell numbers of LacZ-positive melanocytes were much higher in the margins of dorsal and tail skin wounds treated with 5-FU compared with the controls. Meanwhile, CXCL12 immunostaining was significantly increased in the dermal compartment of wounds treated with 5-FU (control vs. 5-FU, 22.47 ± 8.85 vs. 44.69 ± 5.97, P < 0.05). Moreover, 5-FU significantly upregulated the expression levels of CXCL12 mRNA (control vs. 5-FU, 1.00 ± 0.08 vs. 1.54 ± 0.06, P < 0.05) and protein (control vs. 5-FU, 1.00 ± 0.06 vs. 2.93 ± 0.10, P < 0.05) in cultured fibroblasts. Inhibition of the CXCL12/CXCR4 axis suppressed melanocyte migration in vitro using a CXCL12 small interfering RNA (siRNA) or a CXCR4 antagonist (AMD3100).
Conclusion:
5-FU possesses a pro-pigmentary activity through activation of the CXCL12/CXCR4 axis to drive the chemotactic migration of melanocytes.
Keywords: Fluorouracil, Melanocyte, Migration, Fibroblast, Chemokine, Vitiligo
Introduction
Vitiligo is the most common acquired pigmentary disorder, which affects approximately 1% of the world's population, and is characterized by depigmented skin patches resulting from the immune destruction of melanocytes in the epidermis.[1] Ultraviolet B (UVB)-based phototherapy has been clinically proven to be effective in stimulating vitiligo repigmentation, which is usually recommended as the first-line treatment for patients with vitiligo.[2] However, it has been reported that almost 25% of vitiligo patients show a poor response to narrowband (NB)-UVB phototherapy and 48.4% of vitiligo patients fail to respond to photochemotherapy (PUVA) even after 12 months of treatment.[3] It is also a challenge to predict when and where repigmentation will occur in the UVB-treated skin lesions. Phototherapy often requires a long duration of treatment over several months, which significantly reduces patient compliance and increases medical costs.[3] The unsatisfactory benefit of current phototherapy regimens indicates the urgency for novel strategies to trigger and/or hasten skin repigmentation, especially in UVB-resistant patients with vitiligo.
Recently, we and others have observed that the intradermal injection of 5-fluorouracil (5-FU) can achieve significantly better repigmentation of the skin in localized vitiligo patients who exhibited a poor response to UVB phototherapy.[4] The pro-pigmentary effect of 5-FU prompted us to examine whether this nucleobase analogue has an extra-genotoxic activity to change the microenvironment to favor the recruitment of melanocytes from uninjured sites to the vitiliginous skin. In this study, we utilized the full thickness excisional skin wound model in Dct-LacZ transgenic mice that carry the LacZ reporter under control of the Dct promoter,[5] thereby allowing us to dynamically assess the mobilization and migration of melanocytes in the wound-healing process in the presence or absence of 5-FU treatment. Our data provide evidence that 5-FU stimulates dermal fibroblasts to secrete stromal cell derived factor 1 (CXCL12), which recruits and chemoattracts CXCR4-positive melanocytes.[6,7]
Methods
Animal experiments
Homozygous transgenic Dct-LacZ breeder mice were kindly provided by Dr. Ian Jackson (Queen's Medical Research Institute, University of Edinburgh, UK).[5] Mice were housed in cages under specific pathogen-free conditions at the experimental animal facility of the Renmin Hospital of Wuhan University. All experimental protocols were in accordance with the guidelines provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), and were approved by the Animal Care and Use Committee of the Renmin Hospital of Wuhan University. For wound-healing studies, two full-thickness excisional wounds were created on the dorsal skin of male mice using an 8-mm diameter biopsy punch or on their tail skin using a 2-mm diameter biopsy punch. After surgery, the wounds were topically treated with 5% 5-FU (Beyotime Biotechnology, Nanjing, China) for 7 consecutive days; meanwhile, normal saline (0.9% NaCl) was used as a control. To dynamically visualize the numbers of melanocytes in the healing wounds, the neoepithelium tissue at the wound margins was harvested on the third, seventh and twenty-first day after surgery. Those tissue specimens were bisected and processed for X-Gal staining in whole-mount and in cryosections.
Cell culture
Primary mouse dermal fibroblasts were prepared from the dorsal skins of 8-week-old adult mice, as described previously.[8] Dermal fibroblasts were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA) with medium changes every 2–3 days. Cells at passage 5 or less were harvested for subsequent experiments. Murine melan-a melanocytes were kindly provided by Dr. Dorothy C. Bennett (St. George's Hospital, London, UK).[9] Melan-a melanocytes were routinely cultured in RPMI1640 medium supplemented with 5% FBS, 100 mmol/L mercaptoethanol, 2 mmol/L L-glutamine, and 200 nmol/L phorbol-12-myristate acetate (PMA) (Sigma Aldrich, St. Louis, MO, USA).
Small interfering RNA (siRNA) transfection
siRNA duplexes targeting the murine CXCL12 gene (siCXCL12) and a non-targeting scrambled control were designed and synthesized by GenePharma (Shanghai, China). The sequence of siCXCL12 was as follows: sense: 5′-UCU GCA UCA GUG ACG GUA ATT-3′; antisense: 5′-UUA CCG UCA CUG AUG CAG ATT-3′. Murine fibroblasts were seeded in 6-well plates. siCXCL12 or the scrambled control reagent was mixed with siRNA transfection medium (Cat#: sc-29528, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and dropped onto the cells according to the manufacturer's instructions. The efficiency of CXCL12 knockdown by these siRNAs was tested using quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting.
β-Galactosidase (X-Gal) staining
The skin samples and 8-μm cryosection sections were fixed with 4% paraformaldehyde for 30 min at room temperature, and then incubated with X-Gal working solution (Beyotime Biotechnology) for 24 h at 37 °C. After washing with PBS, the slides were counterstained with nuclear fast red (Servicebio, Wuhan, China). X-Gal-positive cells were analyzed using an Olympus BX-412 light microscope (Olympus Corp., Tokyo, Japan).
Transwell migration assays
Cell migration was assessed using Transwell cell culture chambers, as previously described.[10] Polyvinylpyrrolidone-free polycarbonate filters with a 8.0-μm pore size were pre-coated with 10 mg/mL collagen IV (C6745, Sigma Aldrich) and placed on the lower surface of Transwell chambers (Costar, 3422, Cambridge, MA, USA). The filters were dried overnight at room temperature. The coated filters were washed extensively in PBS and then dried immediately before use. Melanocytes (1 × 104) were seeded into the upper chambers, the lower chambers were seeded with 3 × 104 fibroblasts in the preceding 24 h with or without treatment with 50 μmol/L 5-FU. After 24 h, the filters were fixed in methanol and stained with crystal violet. Cells that had migrated to the lower surface were counted in five microscopic fields using a 20 × objective in at least three independent experiments. To block CXCR4, melanocytes were pretreated with 10 μmol/L AMD3100 (a specific CXCR4 antagonist, Cat# A5602, Sigma Aldrich) for 1 h and cocultured with fibroblasts.[11]
Immunofluorescent and fluorescein isothiocyanate (FITC)-phalloidin staining
Frozen tissue sections and cells grown on glass-coverslips were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100 for 10 min, and blocked in 10% normal goat serum for 1 h. The anti-CXCL12/SDF1 rabbit polyclonal antibody (Cat#: AF6612, Beyotime Biotechnology, 1:100) was diluted in blocking buffer and placed on the cells at 4°C overnight. After the incubation, slides were washed three times in PBS and then incubated with goat anti-rabbit IgG (AS1109; Aspen Biotechnology, Wuhan, China; 1:100) for 1 h at 37 °C. For filamentous actin (F-actin) staining, cells on coverslips were incubated with FITC-phalloidin (Cat#: C1033, actin-tracker green, Beyotime Biotechnology) in PBS for 2 h at room temperature. Nuclei were stained using 4′6′-diamidino-2-phenylindole (DAPI) solution for 10 min at room temperature. Imaging was performed using an FV1200 (Olympus Corporation) confocal microscope.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNAs were isolated from fibroblasts or epidermal tissues using Trizol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and first strand complementary cDNAs were synthesized using a PrimeScript RT regent kit (TaKaRa Biotech, Beijing, China). qRT-PCR was conducted using a SYBR Premix Ex TaqTM kit II (TaKaRa) with SYBR green real-time PCR mix containing 10 μmol/L forward and reverse primers of the CXCL12 gene (forward: 5′-CCT GAG GAA GGC TGA CCT CCG T-3′; reverse: 5′-AGC TCC ATT GTG CAC GGG CG-3′). Real-time PCR was performed using an ABI 7500 Real-Time PCR System and cycle parameters as follows: denaturation at 95°C for 2 min; 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. The purity of each qRT-PCR product was checked by its dissociation curve. Primers were purchased from Sangon Biotech (Shanghai, China) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, forward: 5′-AAG GTC ATC CCA GAG CTG AA-3′; reverse: 5′-CTG CTT CAC CAC CTT CTT GA-3′) was used as the reference gene. Relative quantification of gene expression levels for target and reference genes were performed using the 2−△△Ct method and was based on Ct values.
Western blotting analysis
Fibroblasts were harvested and washed in PBS and then lysed in extraction buffer. Protein contents were determined using a bicin-choninic acid assay kit (Pierce, Rockford, IL, USA). Equal amounts of each protein extract (20 μg per lane) were separated by 10% sodium dodecylsulfate polyacrylamide gel electrophoresis. Following transfer to polyvinylidene fluoride membranes and blocking with 5% nonfat milk diluted in tris buffered saline (TBS), the membranes were incubated with antibodies to CXCL12 (Cat#: AF6612, Beyotime Biotechnology, 1:200) and GAPDH (Cat#: ab37168; Abcam [Cambridge, MA, USA]; 1:2000) overnight at 4°C. GAPDH was used as a loading control. The polyvinylidene fluoride membranes were washed with TBS containing Tween-20 (TBS-T) and then were further incubated with horseradish peroxidase-conjugated secondary antibodies (AS1107; Aspen Biotechnology) at a dilution of 1:10,000 for 1 h at room temperature. Each membrane was then washed again and protein bands were visualized using an enhanced chemiluminescence (ECL) detection system (Vazyme, Nanjing, China). The intensity of each band was quantified using Image-J software (National Institutes of Health, Bethesda, MD, USA) and was normalized against GAPDH.
Statistical analysis
All data are expressed as means ± standard deviation (SD) of at least three independent experiments. Statistical analyses were performed with SPSS version 19.0 (IBM SPSS, Armonk, NY, USA) and GraphPad Prism 8 (San Diego, CA, USA) software. Comparisons were made using an unpaired two-tailed Student's t test. One-way analysis of variance (ANOVA) was used to evaluate differences between three or more groups followed by Tukey post-hoc test. P < 0.05 is considered to be statistically significant.
Results
5-FU stimulates the mobilization and migration of melanocytes in a murine skin wound-healing model
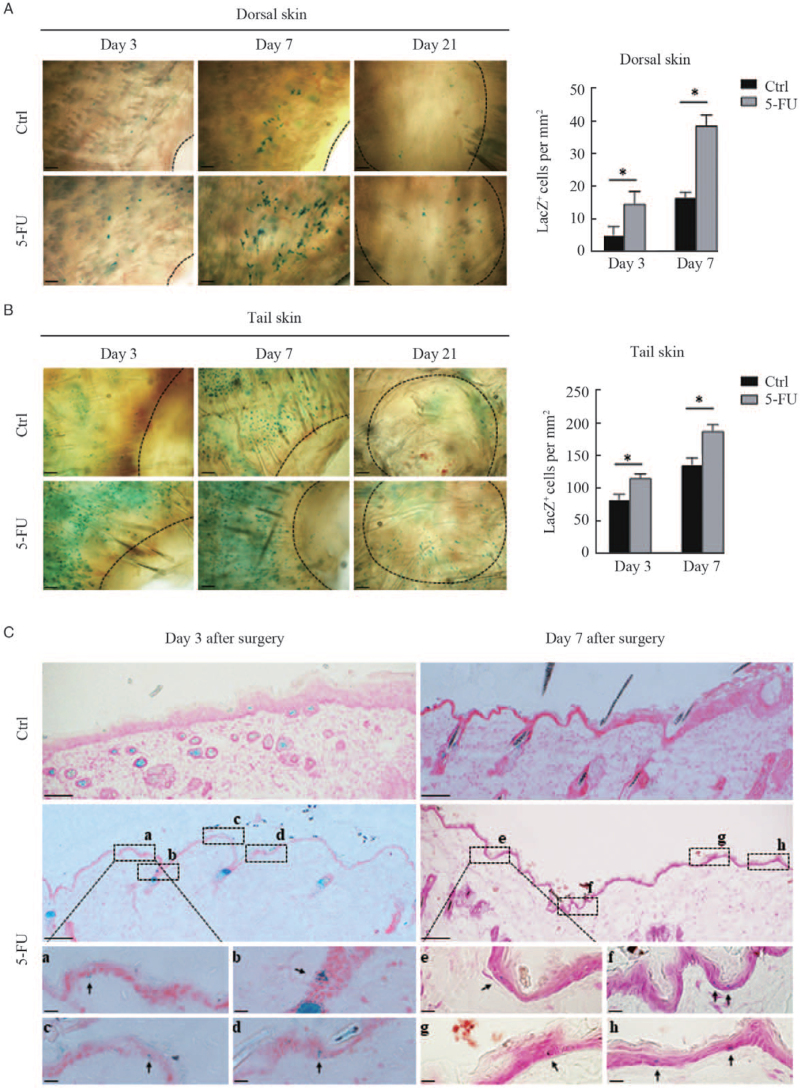
Emerging evidence indicates that 5-FU in combination with therapeutic trauma such as micro-needling[12] and fractional laser[13] can substantially improve skin repigmentation in recalcitrant lesions of vitiligo patients. To investigate whether 5-FU potentially stimulates the mobilization and migration of melanocytes, we first created full-thickness excisional wounds on the dorsal and tail skins of Dct-lacZ transgenic mice, and then dynamically visualized the changes of LacZ-positive melanocytes in the neoepithelium tissue of the healing wounds. The skin specimens were biopsied from the wound margins on the third, seventh, and twenty-first day after surgery and were subjected to whole-mount X-gal staining. Figure 1 shows that lacZ-positive cells were seen on the third day and were much more pronounced on the seventh day after surgery in the wound margins of both dorsal and tail skin. Interestingly, the numbers of lacZ-positive cells were significantly higher in the margins of wounds treated topically with 5% 5-FU than that in the untreated control. X-gal staining of cryosections of the dorsal skin wound margins showed that lacZ-positive cells were found in the outer root sheath (ORS) and in the interfollicular epidermis of the dorsal skin wound margins treated with 5% 5-FU, whereas in the untreated control groups it was barely seen [Figure 1C]. These results implied that 5-FU possesses an unexpected role in the stimulation of the mobilization and migration of melanocytes during the wound-healing process.
Figure 1.
Visualization of LacZ+ cells in the margins of wounds treated with or without 5-FU. Full-thickness excisional wounds were created in the dorsal and tail skins of Dct-lacZ transgenic mice. The skin was biopsied from the wound margins on the third, seventh, and 21st day after surgery. Representative images of LacZ+ cells in dorsal skin (A, upper panel) and tail skin (B, lower panel) by whole-mount X-Gal staining are shown. The black dashed lines indicate the leading edge of wounds. Scale bars: 100 μm. Histograms showing the number of LacZ+ melanocytes in the wound margins of dorsal and tail skin on the third and seventh day after surgery, expressed as means ± SD of three independent experiments, ∗P < 0.01, compared to controls. (C) Representative images of LacZ+ cells in cryosections of dorsal skin with X-Gal staining are shown. Scale bars: 100 μm. Black rectangles (a–h) indicate regions enlarged below. Scale bar: 20 μm (enlarged images). Black arrows indicate typical LacZ+ melanocytes. 5-FU: 5-fluorouracil; SD: Standard deviation.
Activation of the CXCL12/CXCR4 axis contributes to the 5-FU-induced mobilization and migration of melanocytes
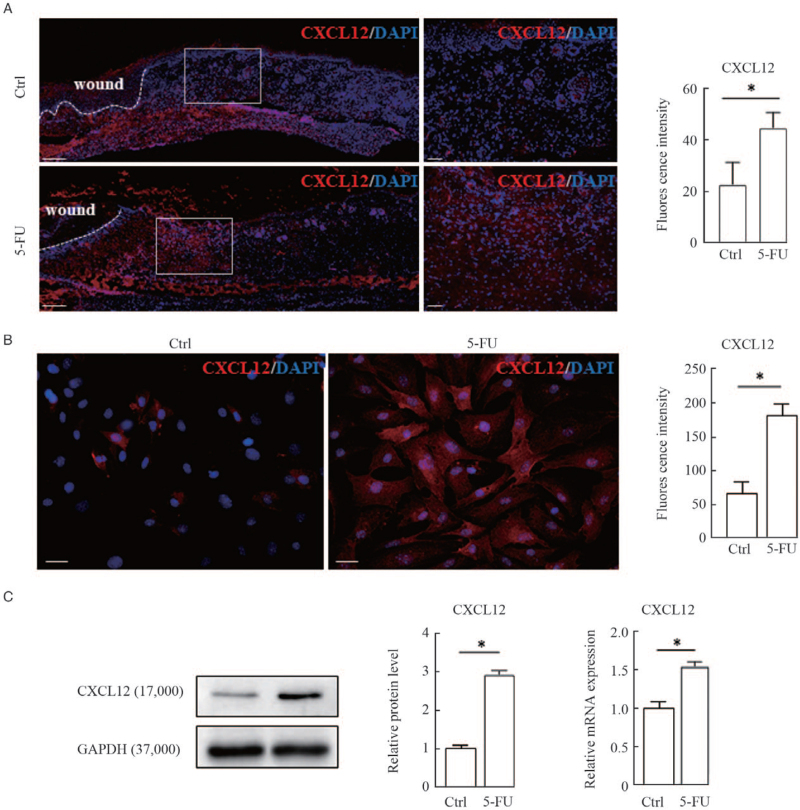
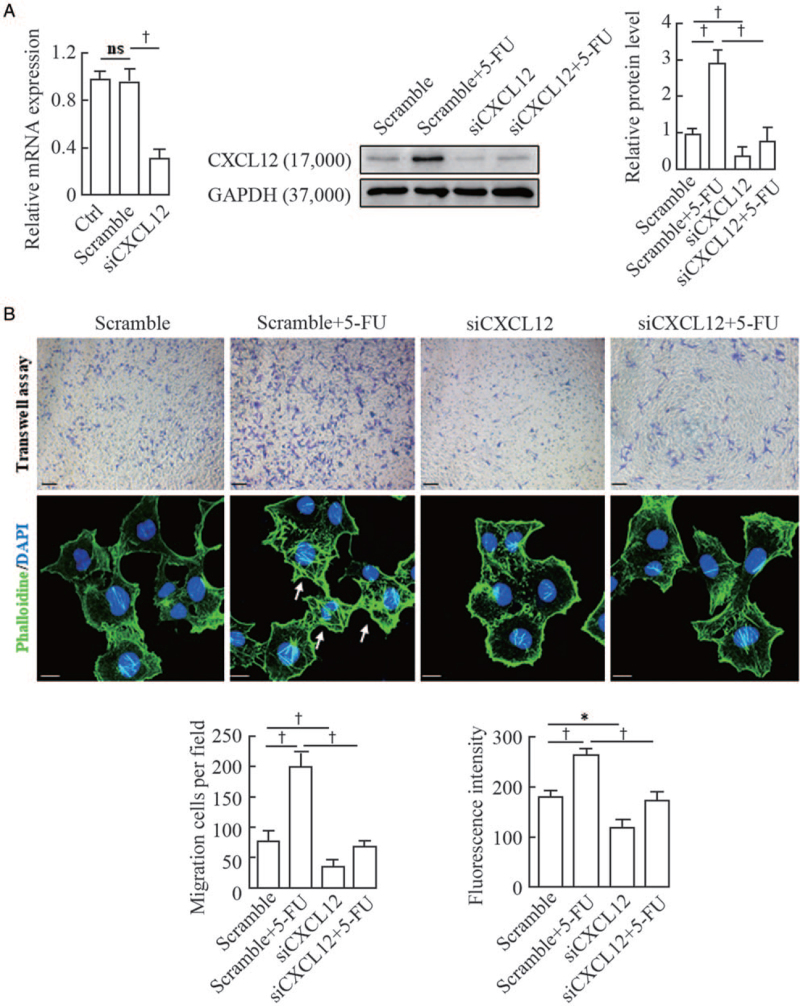
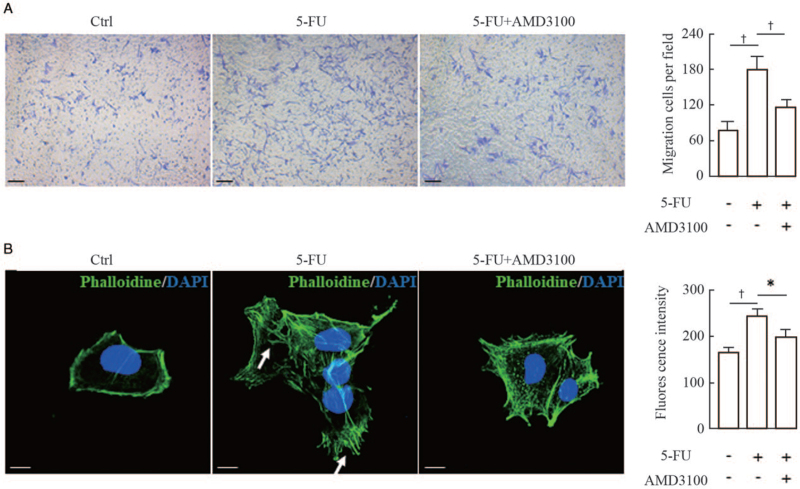
It has been well documented that the chemokine SDF-1/CXCL12 and its receptor CXCR4 play a critical role in regulating the migration and positioning of melanocyte precursors (melanoblasts) during hair follicle formation and cycling.[14,15] First, we examined the in-situ expression of CXCL12 in wound margins on the third day post-surgery using immunofluorescent staining. The results showed that the immunostaining intensities of CXCL12 were dramatically increased in the dermal compartments of wound margins treated with 5-FU compared with the untreated controls [Figure 2A]. To confirm the relevance of CXCL12 upregulation to 5-FU treatment, we established primary cultures of mouse fibroblasts, and then treated those cells with 50 μmol/L 5-FU for 24 h. Immunofluorescence, Western blotting, and qRT-PCR analyses were used to examine the expression levels of CXCL12 protein and mRNA in the 5-FU-treated fibroblasts. As expected, the levels of CXCL12 protein and mRNA were increased significantly in fibroblasts exposed to 5-FU compared with the untreated controls [Figure 2B and 2C]. Finally, two different approaches were utilized to further corroborate this speculation that 5-FU stimulated melanocyte migration through activation of the CXCL12/CXCR4 axis. Murine fibroblasts were transfected with an siRNA to silence CXCL12 expression [Figure 3A] and Transwell migration assays showed that the siCXCL12-transfected fibroblasts lost the chemotaxis stimulation for melanocytes [Figure 3B]. The F-actin staining pattern in melanocytes treated with the conditioned medium from 5-FU-treated fibroblasts appeared as a coarse actin filament pattern throughout the cytoplasm. In contrast, F-actin was distributed in a diffuse or fine network-like pattern in the cytoplasm of melanocytes treated with siCXCL12-transfected fibroblasts. Similar results were observed in experiments in which melanocytes were pretreated with a specific CXCR4 antagonist (AMD3100) [Figure 4]. These findings indicate that the increased melanocyte migration elicited by treatment with 5-FU is caused by the stimulation of dermal fibroblasts to produce paracrine chemokine CXCL12, thereby inducing melanocytes to drive actin polymerization via the CXCL12/CXCR4 axis.
Figure 2.
Upregulation of chemokine CXCL12 expression in wound margins and cultured fibroblasts by 5-FU. (A) Representative images of immunofluorescence staining of CXCL12 (red) and counterstaining with DAPI (blue) in cryosections of dorsal skin wounds are shown. White dashed lines indicate the leading edge of wounds. Scale bars: 200 μm. White rectangles indicate regions enlarged on the right. Scale bar: 50 μm (enlarged images). Histograms showing the comparison of fluorescence intensity for wound margins treated with or without 5-FU are shown on the right. Immunofluorescence staining (B), Western blotting and qRT-PCR (C) were used to examine the expression levels of CXCL12 protein and mRNA in cultured fibroblasts treated with 50 μmol/L 5-FU for 24 h. Representative blots are shown. Data are shown as means ± SD of three independent experiments. ∗P < 0.01 vs. control. 5-FU: 5-fluorouracil; DAPI: diamidino-2-phenylindole; qRT-PCR: Quantitative real-time polymerase chain reaction; SD: Standard deviation.
Figure 3.
Effect of siRNA-mediated knockdown of CXCL12 in murine fibroblasts on the migration and F-actin distribution of melan-a melanocytes. (A) Murine fibroblasts were transfected with CXCL12 siRNA (siCXCL12) and a scrambled control for 48 h. The efficiency of CXCL12 knockdown by siRNAs was tested using qRT-PCR and Western blotting. Representative blots are shown in the middle. The histogram (on the right) shows the densitometric quantification of data with means ± SD of three independent experiments. (B) Melan-a melanocytes were seeded in the upper chambers, and the lower chambers were seeded with siCXCL12-transfected fibroblasts for 24 h of coculture, as described in the Materials and Methods Section. Cells that migrated to the lower surface of the inserts were counted in five microscopic fields using a 20 × objective in at least three independent experiments. A representative image of migrated cells under microscope (upper panel) is shown. Scale bars: 50 μm. Melan-a melanocytes growing on glass coverslips were treated with the conditioned medium from transfected fibroblasts and were subsequently stained for actin with FITC-phalloidin (green). Nuclei were counterstained with DAPI (blue). Representative images of F-actin staining are shown (lower panel). White arrows indicate the distribution of coarse actin bundles. Scale bar: 10 μm. Histograms showing the migrated cells counted in five random fields under microscope (on the left) and the fluorescent intensities of 20 cells (on the right). Data represent means ± SD of three independent experiments. ∗P < 0.05 and †P < 0.01 vs. scramble or scramble + 5-FU. 5-FU: 5-fluorouracil; DAPI: Diamidino-2-phenylindole; F-actin: Filamentous actin; FITC: Fluorescein isothiocyanate; qRT-PCR: Quantitative real-time polymerase chain reaction; siRNA: Small interfering RNA; SD: Standard deviation.
Figure 4.
Effect of AMD3100 (a specific CXCR4 antagonist) on the migration and F-actin distribution of melanocytes. (A) Melan-a melanocytes were seeded into the upper chambers and pretreated with 10 μmol/L AMD3100 for 1 h, and the lower chambers were seeded with murine fibroblasts for 24 h of coculture, as described in the Materials and Methods Section. Cells that migrated to the lower surface of the inserts were counted in five microscopic fields using a 20 × objective in at least three independent experiments. Representative micrographs of migrating cells are shown. Scale bars: 50 μm. Histogram (on the right) shows the number of migrated cells in five random microscopical fields. (B) Melan-a melanocytes growing on glass coverslips were treated with or without 10 μmol/L AMD3100 for 1 h, and were then treated with the conditioned medium from 5-FU-treated fibroblasts and subsequently stained for actin with FITC-phalloidin (green). Nuclei were counterstained with DAPI (blue). Representative images of F-actin staining are shown. White arrows indicate the distribution of coarse actin bundles in the cytoplasm. Scale bars: 10 μm. Histogram (on the right) shows the fluorescent intensities of 20 cells. Data represent means ± SD of three independent experiments. ∗P < 0.05 and †P < 0.01 vs. 5-FU group. 5-FU: 5-fluorouracil; DAPI: Diamidino-2-phenylindole; F-actin: Filamentous actin; SD: Standard deviation.
Discussion
The repigmentation response of patients with vitiligo to UVB-based phototherapies is initiated by the activation of dormant melanocytes that reside in the epidermis and hair follicles.[14] The residual melanocytes in vitiligo lesions are thought to be immature precursors (also referred to as melanoblasts), which might avoid immune attacks due to their lack of expression of immunogens that are exclusively expressed by mature melanocytes.[15,16] Several clinical observations support the intriguing speculation that 5-FU potentially possesses a pro-pigmentary activity, conferring beneficial effects on the repigmentation of vitiligo, especially in UVB-resistant depigmented areas.[17,18] 5-FU is a pyrimidine analogue that exerts a potent antimitotic activity and has been widely used in the treatment of actinic keratoses, as well as some basal and squamous cell skin cancers.[19] However, relatively few studies have focused on the extra-genotoxic actions of this chemotherapeutic agent. A previous study by Tsuji et al[20] demonstrated that 5-FU exerts a selective cytotoxicity according to the type of epidermal cells. Melanocytes seem to be much less vulnerable than keratinocytes to 5-FU, and therefore, the selective cytotoxicity of 5-FU seems to be useful for pure cultures of melanocytes in vitro. In this study, we investigated the effects of topical application of 5-FU on the mobilization and migration of melanocytes in the Dct-lacZ transgenic mouse model of skin wound-healing. The results obtained from whole-mount and cryosection X-gal staining showed that melanocytes appeared on the third day after surgery and were much more pronounced on the seventh day in the wound margins [Figure 1]. The number of melanocytes was significantly increased in the margins of wounds treated topically with 5-FU compared with the untreated control. Meanwhile, the immunoreactivity of CXCL12 in wound margins on the third day post-surgery was also examined. The results showed that CXCL12-positive immunostaining was clearly observed in the dermis and was augmented in the margins of wounds treated with 5-FU compared with the untreated control, which suggests that CXCL12 upregulation in the dermal compartments of wounds treated with 5-FU likely recruits melanocytes that contribute to the wound healing [Figure 2A].
Although CXCL12 has been accepted as a critical chemokine for recruiting melanocytes by binding to the chemokine receptor CXCR4 on their cell surface,[21] its role in vitiligo repigmentation elicited by 5-FU combined with therapeutic trauma was uncertain. In this study, we also found that 5-FU directly upregulates the expression levels of CXCL12 mRNA and protein in cultured fibroblasts [Figure 2]. When fibroblasts were transfected with CXCL12 siRNA or melanocytes were treated with the CXCR4 antagonist (AMD3100) to inhibit activation of the CXCL12/CXCR4 axis, the chemotactic migration of melanocytes toward the fibroblasts was reduced, as shown by Transwell migration assays [Figures 3 and 4]. Furthermore, the F-actin staining pattern in melanocytes treated with the conditioned medium from 5-FU-treated fibroblasts appeared as a coarse actin filament pattern throughout the cytoplasm, whereas F-actin was distributed in a diffuse or fine network-like pattern in the cytoplasm of melanocytes treated with the conditioned medium from CXCL12-silenced fibroblasts [Figure 3]. These results suggest that CXCL12 acts on melanocytes to produce a motile phenotype by F-actin polymerization.
Collectively, the present study was undertaken to shed light on the molecular mechanisms involved in the pro-pigmentary action of 5-FU to target repigmentation of vitiligo skin. To our knowledge, the enhanced secretion of CXCL12 by dermal fibroblasts exposed to 5-FU treatment has not been previously reported. Thus, the present study is to reveal that the pro-pigmentary activity of 5-FU contributes to the activation of the CXCL12/CXCR4 axis and hence to melanocyte migration. Future work is underway to investigate whether the activation of the CXCL12/CXCR4 axis is abnormal in vitiligo lesions showing good or poor responses to 5-FU treatment. It is worth noting that intradermal injections of 5-FU are only prescribed for patients who have stable vitiligo to avoid incurring the Koebner phenomenon.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (No. 81972919).
Conflicts of interest
None.
Footnotes
How to cite this article: Liao ZK, Hu SH, Han BY, Qiu X, Jiang S, Lei TC. Pro-pigmentary action of 5-fluorouracil through the stimulated secretion of CXCL12 by dermal fibroblasts. Chin Med J 2021;134:2475–2482. doi: 10.1097/CM9.0000000000001689
References
- 1.Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet 2015; 386:74–84. doi: 10.1016/s0140-6736(14)60763-7. [DOI] [PubMed] [Google Scholar]
- 2.Speeckaert R, van Geel N. Vitiligo: an update on pathophysiology and treatment options. Am J Clin Dermatol 2017; 18:733–744. doi: 10.1007/s40257-017-0298-5. [DOI] [PubMed] [Google Scholar]
- 3.Bae JM, Jung HM, Hong BY, Lee JH, Choi WJ, Lee JH, et al. Phototherapy for Vitiligo: a systematic review and meta-analysis. JAMA Dermatol 2017; 153:666–674. doi: 10.1001/jamadermatol.2017.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zohdy HA, Hussein MS. Intradermal injection of fluorouracil versus triamcinolone in localized vitiligo treatment. J Cosmet Dermatol 2018; Epub ahead of print. doi: 10.1111/jocd.12820. [DOI] [PubMed] [Google Scholar]
- 5.Mackenzie MA, Jordan SA, Budd PS, Jackson IJ. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev Biol 1997; 192:99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- 6.Yamada T, Hasegawa S, Hasebe Y, Kawagishi-Hotta M, Arima M, Iwata Y, et al. CXCL12 regulates differentiation of human immature melanocyte precursors as well as their migration. Arch Dermatol Res 2019; 311:55–62. doi: 10.1007/s00403-018-1880-2. [DOI] [PubMed] [Google Scholar]
- 7.Belmadani A, Jung H, Ren D, Miller RJ. The chemokine SDF-1/CXCL12 regulates the migration of melanocyte progenitors in mouse hair follicles. Differentiation 2009; 77:395–411. doi: 10.1016/j.diff.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seluanov A, Vaidya A, Gorbunova V. Establishing primary adult fibroblast cultures from rodents. J Vis Exp 2010; 44:2033.doi: 10.3791/2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei TC, Virador VM, Vieira WD, Hearing VJ. A melanocyte-keratinocyte coculture model to assess regulators of pigmentation in vitro. Anal Biochem 2002; 305:260–268. doi: 10.1006/abio.2002.5665. [DOI] [PubMed] [Google Scholar]
- 10.Su M, Miao F, Jiang S, Shi Y, Luo L, He X, et al. Role of the p53-TRPM1/miR-211-MMP9 axis in UVB-induced human melanocyte migration and its potential in repigmentation. Int J Mol Med 2020; 45:1017–1026. doi: 10.3892/ijmm.2020.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon JE, Kim Y, Kwon S, Kim M, Kim YH, Kim JH, et al. Senescent fibroblasts drive ageing pigmentation: a potential therapeutic target for senile lentigo. Theranostics 2018; 8:4620–4632. doi: 10.7150/thno.26975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mina M, Elgarhy L, Al-Saeid H, Ibrahim Z. Comparison between the efficacy of microneedling combined with 5-fluorouracil vs microneedling with tacrolimus in the treatment of vitiligo. J Cosmet Dermatol 2018; 17:744–751. doi: 10.1111/jocd.12440. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed HA, Mohammed GF, Gomaa AHA, Eyada MMK. Carbon dioxide laser plus topical 5-fluorouracil: a new combination therapeutic modality for acral vitiligo. J Cosmet Laser Ther 2015; 17:216–223. doi: 10.3109/14764172.2014.1003241. [DOI] [PubMed] [Google Scholar]
- 14.Lei TC, Hearing VJ. Deciphering skin re-pigmentation patterns in vitiligo: an update on the cellular and molecular events involved. Chin Med J 2020; 133:1231–1238. doi: 10.1097/cm9.0000000000000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birlea SA, Goldstein NB, Norris DA. Repigmentation through melanocyte regeneration in vitiligo. Dermatol Clin 2017; 35:205–218. doi: 10.1016/j.det.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Riding RL, Harris JE. The role of memory CD8(+) T cells in vitiligo. J Immunol 2019; 203:11–19. doi: 10.4049/jimmunol.1900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khater M, Nasr M, Salah S, Khattab FM. Clinical evaluation of the efficacy of trichloroacetic acid 70% after microneedling vs intradermal injection of 5-fluorouracil in the treatment of nonsegmental vitiligo; A prospective comparative study. Dermatol Ther 2020; 33:e13532.doi: 10.1111/dth.13532. [DOI] [PubMed] [Google Scholar]
- 18.Asilian A, Kazemipour S, Mokhtari F, Iraji F, Shahmoradi Z, Mohaghegh F, et al. Effectiveness of dermabrasion plus 5-fluorouracil vs suction blister in treating vitiligo: a comparative study. Dermatol Ther 2021; 34:e14750.doi: 10.1111/dth.14750. [DOI] [PubMed] [Google Scholar]
- 19.Maghfour J, Kuraitis D, Murina A. Intralesional 5-fluorouracil for treatment of non-melanoma skin cancer: a systematic review. J Drugs Dermatol 2021; 20:192–198. doi: 10.36849/jdd.2021.5518. [DOI] [PubMed] [Google Scholar]
- 20.Tsuji T, Karasek M. A procedure for the isolation of primary cultures of melanocytes from newborn and adult human skin. J Invest Dermatol 1983; 81:179–180. doi: 10.1111/1523-1747.ep12543633. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi A, Hadjur C, Takahashi T, Suzuki I, Hirose K, Mahe YF. Human skin melanocyte migration towards stromal cell-derived factor-1α demonstrated by optical real-time cell mobility assay: modulation of their chemotactic ability by α-melanocyte-stimulating hormone. Exp Dermatol 2013; 22:664–667. doi: 10.1111/exd.12232. [DOI] [PubMed] [Google Scholar]