Stroke is the second leading cause of death and the third leading contributor to the loss of disability-adjusted life-years. The fatality rate of all stroke patients ranges from 15% to 50% at 1 month to 5 years after stroke. Ischemic stroke is the most common type of stroke and accounts for 87% of all stroke cases.[1]
Microglia are resident tissue macrophages in the central nervous system (CNS) and survey the CNS every 2 to 3 h as sentinels. When an ischemic stroke occurs, microglia are rapidly recruited to damaged sites as the first line of defense in the CNS. Microglial activation is dependent on different brain stimuli induced by different factors, leading to tissue damage or repair in stroke patients. Microglia acquire diverse phenotypes in response to different types of activation, exhibit specific biomarkers, and exert distinct biological functions, including proinflammatory and anti-inflammatory activities. In addition, the microglial phenotypic transition is common during the inflammatory response.[1]
Microglial Polarization States
Similar to macrophages, microglia can be activated under different circumstances and exhibit proinflammatory or anti-inflammatory properties [Figure 1].
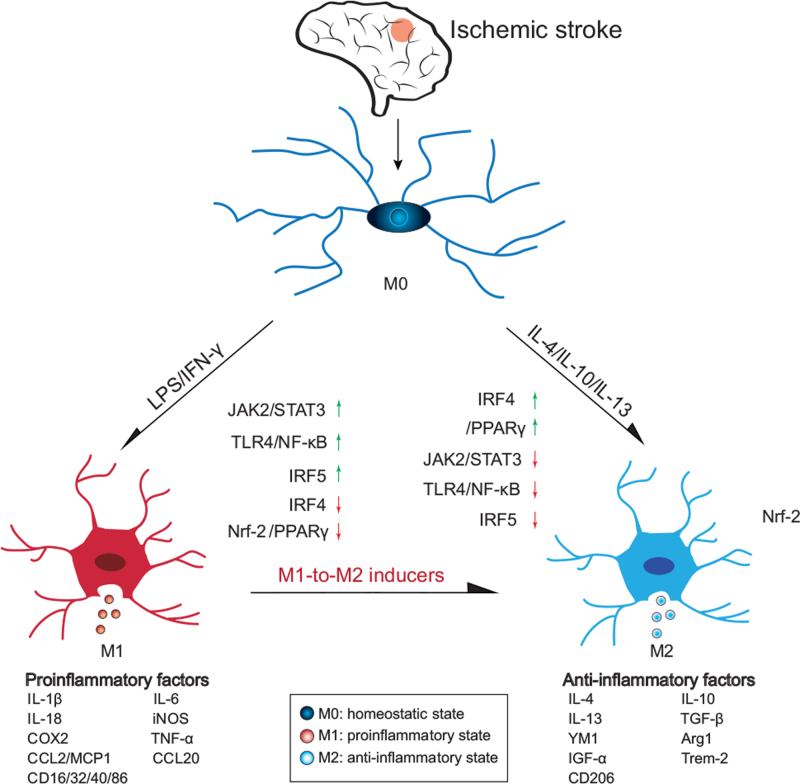
Figure 1.
Polarization states of microglia in ischemic stroke patients. Homeostatic microglia can be activated towards the proinflammatory (M1) and anti-inflammatory (M2) phenotypes. M1 microglia release proinflammatory cytokines, while M2 microglia produce anti-inflammatory cytokines. Moreover, M1 can be converted into M2 microglia by various inducers. Multiple signaling pathways (STAT3, STAT6, TLR4/NF-kB/MAPK, IRF5-IRF4) are involved in this conversion. Arg1: Arginase 1; CCL2: Chemokine ligand 2; CD16: Cluster of differentiation; COX2: Cyclooxygenase 2; IFN-γ: Interferon gamma; IGF-α: insulin-like growth-α; IL: Interleukin; iNOS: Inducible nitric oxide synthase; IRF: Interferon regulatory factor; LPS: Lipopolysaccharide; STAT: Signal transducer and activator of transcription; TNF-α: Tumor necrosis factor-α; Trem: Triggering receptor expressed on myeloid.
Microglia can be polarized into a proinflammatory state (M1) by lipopolysaccharide (LPS) or interferon gamma (IFN-γ) stimulation or into an anti-inflammatory state (M2) by interleukin 4 (IL-4)/IL-10/IL-13. M1 microglia release proinflammatory factors and M2 microglia release anti-inflammatory factors different polarization states have different functions in ischemic stroke.[2]
Signaling Pathways Involved in Microglial Activation in Ischemic Stroke
Increasing evidence has suggested that multiple signaling pathways are involved in the transition between the different microglial activation states. Here we describe some important signaling pathways.
Signal transducer and activator of transcription 3 (STAT3) signaling pathway
STAT3 is a potential negative regulator of inflammatory cytokine release, and phosphorylation of STAT3 (p-STAT3) is associated with microglial polarization. STAT3 is activated through tyrosine residue (Tyr705) and serine (Ser727) phosphorylation.[2] STAT3 activation/phosphorylation is associated with both proinflammatory microglial polarization and anti-inflammatory microglial polarization. Under some circumstances, STAT3 activation can promote M2 polarization. The STAT3 signaling pathway was activated during M2 polarization in BV-2 cells treated with panaxatriol saponins, which are traditional Chinese medicines, to treat stroke, while the inhibition of STAT3 activation by Stattic, a selective inhibitor of STAT3, reversed the inhibitory effect of panaxatriol saponins on the inflammatory response and apoptosis in microglia in an oxygen-glucose deprivation/reperfusion (OGD/R) model.[3] The p-STAT3/STAT3 expression level was decreased during proinflammatory microglial activation, and this effect was reversed when microglia were polarized towards the anti-inflammatory state.[4] Under some circumstances, STAT3 activation may promote M1 polarization. p-STAT3 was activated during OGD, while M2 microglial polarization was inhibited.[5] p-STAT3 was also increased by in vivo ischemic/reperfusion injury and in vitro OGD, while this effect was decreased by DZNep, an EZH2 inhibitor, which could promote anti-inflammatory microglial polarization.[6] Milk fat globule-EGF factor 8, also known as lactadherin, reduced OGD-induced neuronal cell apoptosis and p-STAT3 levels, which enhanced M2 microglial polarization.[5] The phosphorylation of JAK1/STAT3 was inhibited by Longxuetongluo capsule at middle and high doses, which suppressed microglial activation and increased neuronal cell viability.[7]
STAT6 signaling pathway
STAT6 activation is a significant factor in M2 microglial polarization. STAT6 was activated in microglia in mouse models of middle cerebral artery occlusion (MCAO) and in stroke patients.[8] STAT6 facilitated efferocytosis, which was essential for microglia-induced neuroprotection.[8] STAT6 knockout increased the number of proinflammatory microglia and reduced the phagocytosis of dead/dying neurons.[8] Arg1, which is traditionally known to protect against neuroinflammation in the ischemic brain, was discovered to be a target of STAT6.[8] STAT6 activation resulted in decreased expression of Arg1 and dysfunctional phagocytosis.[8]
Toll-like receptor (TLR) 4/nuclear factor kappa-B (NF-κB)/mitogen-activated protein kinase (MAPK) signaling pathway
The TLR4/NF-κB signaling pathway is responsible for M1 microglial polarization. Multiple studies have indicated that alleviating LPS-induced inflammation is associated with myeloid differentiation factor 88 (MyD88) expression, MAPK and IκBα phosphorylation, and NF-κB P65 nuclear translocation. TLR4 on the microglial cell membrane is the canonical receptor of LPS and other damage-associated molecular patterns. After activation, TLR4 recruits the adaptor protein MyD88, and the downstream NF-κB and MAPK cascades are activated. In an MCAO and reperfusion rat model, the increased levels of the p65 subunit NF-κB and its inhibitor IκB were significantly reduced, and the anti-inflammatory effect was exerted through the TLR4/NF-κB signaling pathway. In OGD-induced BV-2 cells, the TLR4/MyD88 signaling pathway was suppressed by schaftoside, a natural flavonoid compound, and the mRNA and protein levels of proinflammatory cytokines (IL-1β, TNF-α, IL-6) were significantly decreased.[9] Similar effects were also observed by treating the cells with TAK242, a TLR4 inhibitor.[9]
Interferon regulatory factor (IRF) 5-IRF4 signaling pathway
The IRF5–IRF4 axis was discovered to be of importance in neuroinflammatory responses. Overexpression of IRF-5 led to a proinflammatory microglial response, and overexpression of IRF-4 correlated with anti-inflammatory microglial activation. In addition, the expression of IRF-5 and that of IRF-4 had an inhibitory effect on each other. After stroke, the time course of brain microglial cytokine production, such as that of TNF-α, IL-1β, IL-10, and IL-4, showed a correlation with the expression pattern of IRF5/4.[10]
Therapeutic Interventions to Modulate Microglial Polarization States in Ischemic Stroke
Accumulating evidence suggests that the transition from proinflammatory M1 microglia to anti-inflammatory M2 microglia is a promising therapeutic approach to treat ischemic stroke patients.
Bendavia, a mitochondria-targeting tetrapeptide, reduces mitochondrial ROS and inhibits apoptosis.[11] In a tMCAO mouse model, Bendavia exerted antioxidative and anti-inflammatory effects, reduced matrix metalloproteinase (MMP)-9 and TNF-α protein expression levels, and reduced inflammatory microglia/macrophage activation.[11] Schisandrin B mitigated the increased levels of TNF-α, IL-6, and IL-1β in MCAO and reperfusion rat models.[12] Betaine, also known as N-trimethylglycine, exerts an anti-inflammatory and neuroprotective effect. Betaine shifted the microglial polarization state from M1 to M2 in LPS-treated microglial cells. Betaine also reduced the expression of iNOS and CD16/32 and increased the expression of CD206 and Arg1.[13] The compounds involved in ischemic stroke treatment are similar in that they either inhibit the M1 polarization state or promote the M2 polarization state of microglia through some compounds, inhibiting M1 cells and promoting M2 cells simultaneously. The transition could reduce the release of proinflammatory cytokines and facilitate the phagocytosis of damaged cell debris, both of which result in improvements, less damage to neurons, and a better prognosis of ischemic stroke patients.
Targeting microglial polarization provides a new avenue to the ischemic stroke treatment. However, those key genes or chemical compounds that may regulate microglial polarization still need to be identified. In addition, manipulating microglial polarization can also be a potential strategy in the treatment of other neurological disorders, such as Alzheimer's disease, Parkinson's disease, and multiple sclerosis, as summarized in previous reviews.[14]
Future Perspectives
Microglial polarization has been extensively investigated over the past few years and plays a critical role in ischemic stroke. Manipulating the polarization of microglia is a potential therapeutic strategy for ischemic stroke patients. More fundamental work and additional clinical trials are required to fully understand the underlying mechanisms of microglial polarization in ischemic stroke.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81870906), the Natural Science Foundation of Jiangsu Province (No. BK20181156), the Changzhou Science and Technology Support Program (No. CE20205025).
Conflicts of interest
None.
Footnotes
How to cite this article: Mao JH, Xu Y, Li BW, Yang YL, Peng Y, Zhi F. Microglia polarization in ischemic stroke: complex mechanisms and therapeutic interventions. Chin Med J 2021;134:2415–2417. doi: 10.1097/CM9.0000000000001711
References
- 1.Zhou G, Wang Y, Gao S, Fu X, Cao Y, Peng Y, et al. Potential mechanisms and perspectives in ischemic stroke treatment using stem cell therapies. Front Cell Dev Biol 2021; 9:646927.doi: 10.3389/fcell.2021.646927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav M, Kumari P, Yadav V, Kumar S. Pharmacological preconditioning with phosphodiestrase inhibitor: an answer to stem cell survival against ischemic injury through JAK/STAT signaling. Heart Fail Rev 2020; 25:355–366. doi: 10.1007/s10741-019-09822-0. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Fan C, Zhao J, Di M, Sui C, Han L, et al. Panaxatriol saponins promote M2 polarization of BV2 cells to reduce inflammation and apoptosis after glucose/oxygen deprivation by activating STAT3. Inflammation 2020; 43:2109–2118. doi: 10.1007/s10753-020-01278-x. [DOI] [PubMed] [Google Scholar]
- 4.Liu ZJ, Ran YY, Qie SY, Gong WJ, Gao FH, Ding ZT, et al. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neurosci Ther 2019; 25:1353–1362. doi: 10.1111/cns.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang YY, Zhang JH. MFG-E8 alleviates oxygen-glucose deprivation-induced neuronal cell apoptosis by STAT3 regulating the selective polarization of microglia. Int J Neurosci 2021; 131:15–24. doi: 10.1080/00207454.2020.1732971. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Zhang M, Zhang X, Fan L, Liu P, Yu L, et al. EZH2 inhibitor DZNep modulates microglial activation and protects against ischaemic brain injury after experimental stroke. Eur J Pharmacol 2019; 857:172452.doi: 10.1016/j.ejphar.2019.172452. [DOI] [PubMed] [Google Scholar]
- 7.Hong Q, Yang Y, Wang Z, Xu L, Yan Z. Longxuetongluo capsule alleviates lipopolysaccharide-induced neuroinflammation by regulating multiple signaling pathways in BV2 microglia cells. J Chin Med Assoc 2020; 83:255–265. doi: 10.1097/JCMA.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 8.Cai W, Dai X, Chen J, Zhao J, Xu M, Zhang L, et al. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight 2019; 4:e131355.doi: 10.1172/jci.insight.131355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou K, Wu J, Chen J, Zhou Y, Chen X, Wu Q, et al. Schaftoside ameliorates oxygen glucose deprivation-induced inflammation associated with the TLR4/Myd88/Drp1-related mitochondrial fission in BV2 microglia cells. J Pharmacol Sci 2019; 139:15–22. doi: 10.1016/j.jphs.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Al Mamun A, Chauhan A, Yu H, Xu Y, Sharmeen R, Liu F. Interferon regulatory factor 4/5 signaling impacts on microglial activation after ischemic stroke in mice. Eur J Neurosci 2018; 47:140–149. doi: 10.1111/ejn.13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai T, Matsubara H, Nakamura S, Hara H, Shimazawa M. The mitochondria-targeted peptide, bendavia, attenuated ischemia/reperfusion-induced stroke damage. Neuroscience 2020; 443:110–119. doi: 10.1016/j.neuroscience.2020.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Fan X, Elkin K, Shi Y, Zhang Z, Cheng Y, Gu J, et al. Schisandrin B improves cerebral ischemia and reduces reperfusion injury in rats through TLR4/NF-(B signaling pathway inhibition. Neurol Res 2020; 42:693–702. doi: 10.1080/01616412.2020.1782079. [DOI] [PubMed] [Google Scholar]
- 13.Shi H, Wang XL, Quan HF, Yan L, Pei XY, Wang R, et al. Effects of betaine on LPS-stimulated activation of microglial M1/M2 phenotypes by suppressing TLR4/NF-kappaB pathways in N9 cells. Molecules (Basel, Switzerland) 2019; 24:367.doi: 10.3390/molecules24020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Huang N, Xu S, Luo Y, Li Y, Jin H, et al. Signaling mechanisms underlying inhibition of neuroinflammation by resveratrol in neurodegenerative diseases. J Nutr Biochem 2021; 88:108552.doi: 10.1016/j.jnutbio.2020.108552. [DOI] [PubMed] [Google Scholar]