Abstract
Outpatient treatments that limit progression to severe coronavirus disease 2019 (COVID-19) are of vital importance to optimise patient outcomes and public health. Monoclonal antibodies (mAb) demonstrated ability to decrease hospitalizations in randomized, clinical trials. However, there are many barriers to mAb treatment such as patient access and clinician education. There are no data comparing efficacy or safety of available mAbs. We sought to rapidly launch an adaptive platform trial with the goals of enhancing access to treatment, regardless of geography and socioeconomic status, and evaluating comparative efficacy and safety of available mAbs. Within 21 days from idea genesis, we allocated mAb treatment to all patients within the context of this clinical trial. Within 2 months, we closed the gap of the likelihood of receiving mAb, conditional on background positivity rate, between Black and White patients (Black patients 0.238; White patients 0.241). We describe trial infrastructure, lessons learned, and future directions for a culture of learning while doing.
Keywords: COVID-19, SARS-CoV-2, Monoclonal antibodies, Bamlanivimab, Etesevimab, Casirivimab, Imdevimab
1. Monoclonal antibody treatment for COVID-19
Millions worldwide have died from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The pandemic required health systems to rapidly adopt new therapies and promote social and health equity [1]. Monoclonal antibody (mAb) treatment is associated with decreased hospitalization and death in outpatients with mild to moderate coronavirus disease 2019 (COVID-19) [[2], [3], [4], [5]]. Outpatient treatments that limit progression to severe disease are of vital importance to optimise patient outcomes and public health.
Monoclonal antibodies bind to and neutralize SARS-CoV-2, blocking entry of the virus into human cells. A form of passive immunity, mAb are most effective if given early after SARS-CoV-2 infection [2,3]. Randomized, clinical trials in patients with mild to moderate COVID-19 demonstrated reductions in hospitalizations and deaths with mAb treatment compared to placebo [2,3]. Subsequently, the United States Food and Drug Administration (US FDA) issued Emergency Use Authorizations (EUA) for bamlanivimab, bamlanivimab and etesevimab, casirivimab and imdevimab, and sotrovimab for use within 10 days of symptom onset in outpatients with risk factor(s) for progression to severe disease.
2. Do we need an adaptive platform trial to evaluate monoclonal antibody treatment?
There are many unanswered questions about mAb treatment. First, the use of mAb therapy remains low. Is this due to patient access barriers, operational challenges with outpatient infusions, limited awareness of efficacy data among referring clinicians, supply chain issues, or a combination of reasons? [[6], [7], [8]] Second, the published clinical trial data generated hypotheses regarding the optimal patient population for treatment, but many seek added evidence to inform mAb deployment, especially when resources are scarce. Third, while other platform trials are evaluating multiple mAb therapies in various settings (e.g., RECOVERY, ACTIV-2, ACTIV-3), there are no outpatient trials directly comparing all 3 EUA-available mAbs. Fourth, the spread of SARS-CoV-2 variants may impact antibody and vaccine effectiveness, and the emergence of mAb-resistant SARS-CoV-2 variants is a major concern [9]. The EUA for bamlanivimab monotherapy was revoked due to increased frequency of resistant variants and concern of decreased bamlanivimab monotherapy efficacy in this setting, and bamlanivimab and etesevimab distribution was temporarily paused and then resumed based on changing prevalence of variants of concern [10]. An adaptive platform trial could evaluate all available mAbs across subgroups of patients and generate answers to pivotal and evolving clinical questions in a rapid fashion to address these knowledge gaps. Collecting data quickly, and with rigor, would enable clinicians to rapidly adapt to the changing therapeutics landscape and pathogen evolution.
3. A culture of learning while doing
When confronted with complex patients, clinicians often perceive a conflict between the need to “learn” (i.e., randomize patients into clinical trials) versus “do something” (i.e., provide a therapeutic agent that may or may not be helpful or harmful) [11]. Traditionally, research efforts seek to create insights using highly structured settings with careful conditions to limit threats to causal inference. This approach is often costly, slow, and it may not resemble how the intervention will be used in practice. Such studies are often performed in larger hospitals with existing research infrastructure, limiting access of most patients to new treatment options and hindering the external validity of the results. We propose shifting from the traditional research model into one of care with ongoing discovery – providing new therapies to each patient while simultaneously advancing standard practice – that is “learning while doing” [11]. In this model, we optimise the trade-off between learning and doing where little to no sacrifice is made to the conditions of high-quality research yet priority care is ensured to all patients within the system. Indeed, this approach expands the reach of robust learning while doing to many hospitals and healthcare settings often excluded from randomized trials.
During the pandemic, our large, integrated healthcare system in the US approached treatment of patients with COVID-19 with two goals: i.) enhancing access to treatment, regardless of geography and socioeconomic status, and ii.) coordinating treatment through an integrated, adaptive platform trial. To accomplish these goals, clinician engagement was paramount. In addition, success required leadership investment, a robust data and analytics infrastructure, and therapeutics oversight via system-level treatment guidelines with local collaboration.
4. Preliminary experience with monoclonal antibody treatment and expanding patient access
Prior to the launch of the adaptive platform trial, we developed a robust outpatient infusion infrastructure across a large geographical region in western Pennsylvania and New York. The supply of mAb changed over time. Initially, treatment was only bamlanivimab monotherapy and mAb was only available at outpatient infusion centers [5,12]. After evaluating the evidence, the System COVID-19 Therapeutics Committee determined equivalence existed among the available mAbs. The Committee adopted a therapeutic interchange policy for mAb distribution in December 2020 and updated it with evolving data and federal guidance, first including bamlanivimab monotherapy, bamlanivimab and etesevimab, and casirivimab and imdevimab. Bamlanivimab monotherapy was removed from the policy on March 31, 2021.Bamlanivimab and etesevimab enrollment was paused from June 25 through September 16, 2021 due to federal decisions to temporarily halt distribution based on prevalence of variants of concern (i.e., Beta and Gamma) during that time period. Sotrovimab was added to the platform on July 13, 2021 after initial supply was donated to the system by the manufacturer specifically for use in the trial; this was later transitioned to government-purchased supply allocated via federal Health and Human Services distribution channels. All pharmacies supplying all infusion sites had equal opportunity to order any EUA-available mAb from a central supply facility.
5. Launching a platform trial of monoclonal antibodies for COVID-19 in 21 days
We held a collaborative discussion with the US government on February 17th, 2021 about increasing mAb access (“Do”) while simultaneously addressing the knowledge gaps surrounding mAb treatment (“Learn”). Subsequently, we designed and implemented a pragmatic, open-label, adaptive platform trial integrated with our ongoing, systemwide mAb efforts. This comparative effectiveness evaluation was possible due to preceding placebo-controlled trials confirming the benefits and safety of mAb therapy. The goal was to design an entire program, including outreach, learning, and rapid implementation. The first patient was allocated mAb within the trial on March 10th, 2021, just 21 days later.
Monoclonal antibody access was expanded from outpatient infusion centers to all system EDs on March 23, 2021. We undertook several additional steps to expand awareness and increase the use of mAb therapy across our region. A paper/fax referral process existed for clinicians outside of the health system, including rural clinicians in neighboring states. To reach disadvantaged neighborhoods and patients with limited access to health care, we created a telephone hotline staffed by nurses for patient self-referrals. Using a collaborative relationship with a home infusion company, patients without transportation could receive treatment at home or be transported to an infusion center (at no cost to the patient). Proactively, we identified all patients with a positive SARS-CoV-2 polymerase chain reaction (PCR), or rapid antigen test performed within the system who also met EUA criteria using an EHR-derived screening dashboard. These patients were subsequently called at home by a member of the centralized mAb operations team to assess for symptoms, offer mAb information, and place a mAb referral order (if appropriate). We also educated patients about vaccination during these phone encounters. To further alleviate patient access issues, we created a team of Revenue Cycle analysts to work with all major payors to improve patient cost transparency.
6. The OPTIMISE-C19 platform trial
The OPTIMISE-C19 (Optimizing Treatment and Impact of Monocolonal Antibodies through Evaluation for COVID-19) trial launched on March 10, 2021. This trial was approved by the University of Pittsburgh IRB (STUDY21020179) and UPMC Quality Improvement Review Committee (Project ID 3280) and is listed on ClinicalTrials.gov (NCT04790786). OPTIMISE-C19 evaluates the mAb therapeutic interchange policy and patient outcomes. It is an open-label, pragmatic, randomized platform trial evaluating the comparative effectiveness of COVID-19 specific mAbs with EUA status. The primary outcome was hospital-free days up to day 28 after mAb treatment; secondary outcomes included 28-day mortality and rates of adverse events.
First results from this trial are available as of September 9, 2021 [13]. The primary analysis model was a Bayesian cumulative logistic model that adjusted for treatment location, age, sex, and time (2-week epochs); comparisons between individual mAb were based on the relative odds ratio between a given two arms for the primary outcome. Sample size is determined by case volume throughout the course of the pandemic; Bayesian adaptive designs allow for statistical inference despite variable sample size. Interim analyses are performed every two weeks and the trial continues to enroll until pre-determined statistical thresholds for superiority or inferiority are met.
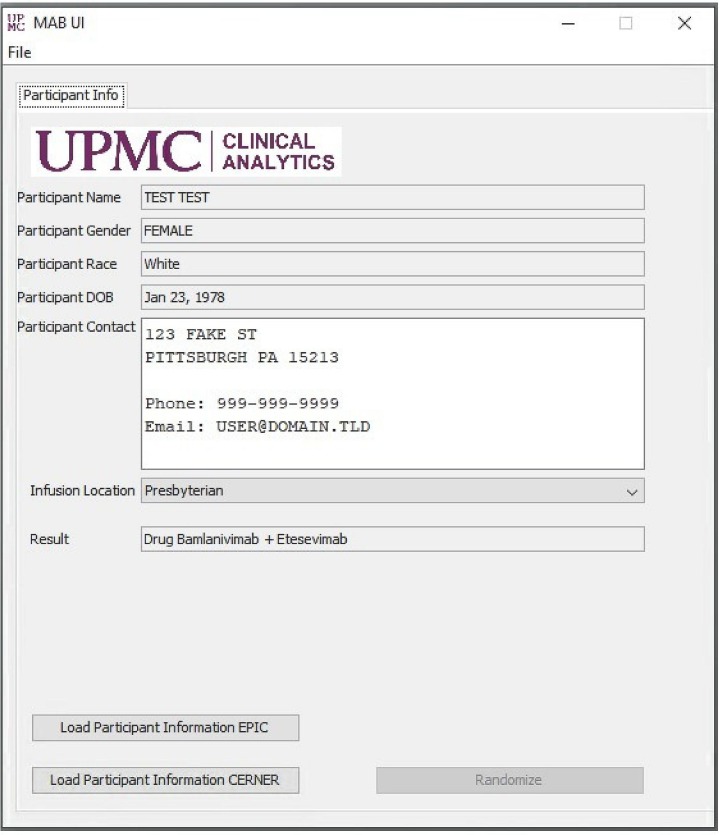
Patients were eligible for OPTIMISE-C19 per FDA EUA criteria [13]. Clinicians ordered a generic mAb referral order, which triggered randomization via a monoclonal antibody assignment application (app) engineered alongside the EHR to link local infusion site mAb inventory to the current patient encounter ( Fig. 1 ). Once the patient's medical record number is entered into the app and the “randomize” button is clicked (by a centralized mAb operations team member for outpatient centers and by a pharmacist for EDs), the app assigns a mAb. Odds of receiving a mAb were equivalent (i.e., 50% when 2 mAb were available, 33.33% when 3 mAb were available). The patient's assignment is automatically recorded by the data and analytics team database.
Fig. 1.
Monoclonal antibody assignment application.
Clinicians in both outpatient settings and EDs review all EUA Fact Sheets with the patient upon referral and inform the patient they may receive any available and authorized mAb treatment. All patients to be infused must agree to treatment within the confines of the EUA prior to treatment. Importantly, clinicians and/or patients can request a specific mAb at any time. The randomization of which mAb is recommended occurs solely within the therapeutic interchange (wherein mAbs are considered equivalent and any mAb can be dispensed based on local inventory). The ultimate decision on what is best for the patient is made jointly by the physician and her patient. To date, no clinician or patient has requested a mAb different from the allocation.
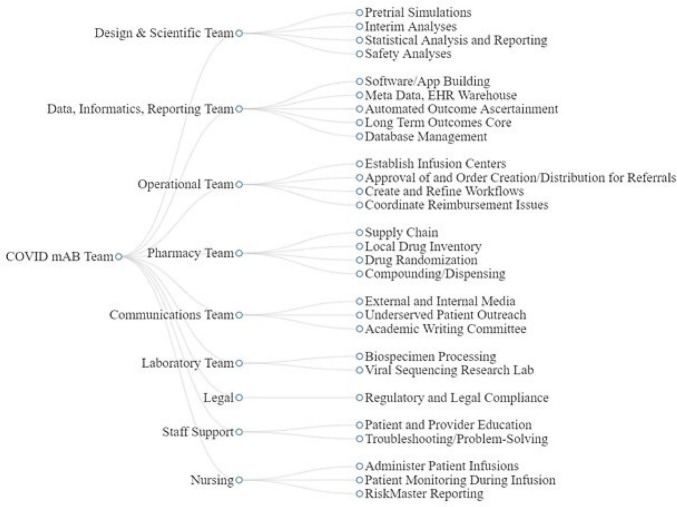
Clinicians, pharmacists, and nurses throughout the system were supported by extensive education and training before the launch of OPTIMISE-C19 and consistently throughout enrollment. External and internal online resources were developed, town halls are held via on-line formats, and direct patient outreach continues. The trial team also works closely with a team of communications experts to share patient stories, engage with local media to raise awareness, and create patient-facing educational materials (Fig. 2 ).
Fig. 2.
COVID-19 monoclonal antibody team.
For outcome ascertainment, the data and analytics team built a system for automated data extraction from the UPMC Clinical Data Warehouse to synthesize data feeds from various EHRs across the inpatient and outpatient care continuum. All extracted data undergo validation by a clinical pharmacist and are reviewed by a system Quality Center nurse to ensure patients are appropriately captured. For patients who have a positive SARS-CoV-2 nasal swab test within the system, we developed infrastructure to retrieve remnant, de-identified samples and perform next-generation sequencing of the Spike gene. This will allow us to assess variant trends of SARS-CoV-2 in our region. Variants in the Pennsylvania catchment are also assessed over time using Global Initiative on Sharing All Influenza Data, to analyze effectiveness based on geographic region while patient-specific variant data is pending [14].
An infusion reaction management guide was created and distributed to all mAb treatment sites for guidance on treatment of any kind of mAb-related adverse event [15]. An attending physician on the Therapeutics Committee oversees all required adverse event reporting to the FDA. Additionally, infusion center representatives document acute reactions on the day of treatment in a secure, electronic file sharing application. Nursing and physician staff also utilize an internal, nonpunitive, patient safety reporting system (“Risk Master”) for adverse reactions and medication errors. Finally, a hospital quality policy was enacted to contact all patients treated with any mAb via telephone at day 28.
7. Priorities for the future of learning health systems and lessons learned
Traditional clinical trial enrollment is cumbersome, and a dramatic shift in the clinical research enterprise is needed [16]. The regulatory and financial requirements of traditional trials hinder the ability of clinicians and researchers to adapt quickly, test multiple therapies simultaneously, collaborate across care areas, and expand trial access to patients outside of academic tertiary centers. While government programs during the COVID-19 pandemic provided institutions with free mAb supply, there are limited resources provided to administer the drug to patients. OPTIMISE-C19 was designed to overcome these barriers and to rapidly evaluate drug effectiveness, alongside system efforts to deliver safe, effective, comprehensive care in a rapid and equitable fashion. While the study has limitations, including lack of patient-level variant data in real time and missing data if patients seek care outside of our EHR, it is the one of the largest comparative effectiveness trial of mAb in the world and spans 49 treatment sites across a wide geographic area, increasing external validity. In creating this platform trial for mAb, we propose three broad priorities for the future of learning health systems and address our lessons learned.
7.1. Create an institutional culture of learning while doing
A collaboration and durable commitment between clinical care teams and researchers, leadership, administrative staff, and data and analytics is essential for a culture of learning while doing. The COVID-19 pandemic has fortunately brought global efforts to decentralize trial enrollment and generate partnerships in research trials across broader populations and healthcare networks, with equity as a core goal. New ways to ask and answer questions may alleviate clinician burden, support clinician autonomy, and expand patient access to new therapies. Routine clinical care and system therapeutic guidance must be intertwined with research endeavors to optimise access and outcomes – in other words, discovery happens during care. This requires extensive efforts in ongoing education, clinician outreach, multimodal communication, and response to feedback. In a “learning while doing” environment, all must embrace the philosophy of decreasing knowledge transfer time from trial results reporting to incorporation into routine care to a very short interval. We used this philosophy in OPTIMISE-C19. For example, bamlanivimab monotherapy exited the trial and clinical care as a treatment option the same day the Therapeutics Committee determined it inferior to combination products, even prior to the FDA decision [10].
7.2. Invest in patient outreach, access, and education
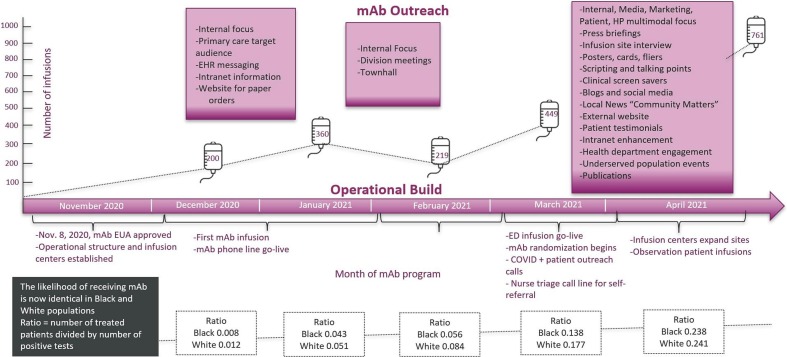
Extensive resources are needed to expand patient outreach, and human resources are the greatest barrier. Leadership commitment is required to dedicate these resources to patient care access. The goal is to address health disparities and improve population outcomes across the entire health system. Similar priorities include broader patient access to mAb infusion and to ensure ongoing clinician and patient education. This investment pays off. Prior to trial launch, 5% of mAb-treated patients identified as Black. Seven weeks later, 18% of mAb-treated patients identified as Black. The likelihood of receiving mAb, conditional on background positivity rate, was one-third lower among Black patients compared to White patients when the mAb program launched. After treating over 1500 patients, the likelihood was the same (mAb treatment to positive test ratio for Black patients 0.238; White patients 0.241, Fig. 3 ). Now, more than 6000 patients have received mAb treatment since trial launch, and the ratio of EUA-eligible patients receiving mAb increased 7.5-fold, even during periods of reduced prevalence of COVID-19 [13]. From March 10 through June 25, 2021, the proportions of eligible White patients receiving mAb increased from 3.1 to 21.6% and eligible Black patients receiving mAb increased from 2.6 to 29.9% [13]. Continued communication of therapeutic guidance and treatment logistics across all locations, key stakeholders, clinicians, and general community members helps support this platform trial success.
Fig. 3.
Monoclonal antibody patient outreach efforts.
7.3. Establish a data infrastructure
Data and analytics teams are key to sustainable and scalable implementation of multicenter platform trials. The embedding of the trial next to the EHR and routine care enhanced enrollment and allowed for rapid translation of new knowledge to the bedside. The continued evaluation of the platform read-outs according to pre-trial simulations requires alignment of bioinformatics teams and statistical analysts. These steps help to coordinate interim analyses, stopping rules, outcome ascertainment, and safety reporting.
8. Conclusions
Efficient, scalable, and inclusive approaches to clinical trial design facilitate a culture of learning while doing. We developed an embedded platform trial of monoclonal antibody treatment in 3 weeks, and expanded trial access to patients from disadvantaged neighborhoods. This approach engages the health care community in a first-of-its-kind trial to learn about novel treatments during a pandemic.
Acknowledgments
Acknowledgements
The authors thank the clinical staff of the UPMC monoclonal antibody infusion centers as well as the support and administrative staff behind this effort, including but not limited to: Allison Hydzik, Larry Hruska, Jennifer Dueweke, Robert Shulik, Amy Lukanski, Rozalyn Russell, Debra Rogers, Jesse Duff, Kevin Pruznak, Jennifer Zabala, Trudy Bloomquist, Daniel Gessel, LuAnn King, Jonya Brooks, Libby Shumaker, Betsy Tedesco, Sarah Sakaluk, Kathleen Flinn, Susan Spencer, Le Ann Kaltenbaugh, Michelle Adam, Meredith Axe, Melanie Pierce, Debra Masser, Theresa Murillo, Sherry Casali, Jim Krosse, Jeana Colella, Rebecca Medva, Jessica Fesz, Ashley Beyerl, Jodi Ayers, Hilary Maskiewicz, Mikaela Bortot, Amy Helmuth, Heather Schaeffer, Janice Dunsavage, Erik Hernandez, Ken Trimmer, Sheila Kruman, Teressa Polcha, and their entire teams. We also thank the U.S. federal government and Pennsylvania Department of Health for the provision of monoclonal antibody treatment.
Funding statement
This work received no external funding. Sotrovimab was donated by GlaxoSmithKline/VirBio.
Declaration of Competing Interest
None of the authors received any payments or influence from a third-party source for the work presented, and none report any potential conflicts of interest.
No authors have competing financial interests relevant to the context of this manuscript.
References
- 1.Alberti P.M., Lantz P.M., Wilkins C.H. Equitable pandemic preparedness and rapid response: lessons from COVID-19 for pandemic health equity. J. Health Polit. Policy Law. 2020;45(6):921–935. doi: 10.1215/03616878-8641469. (In eng) [DOI] [PubMed] [Google Scholar]
- 2.Chen P., Nirula A., Heller B., et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N. Engl. J. Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinreich D.M., Sivapalasingam S., Norton T., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N. Engl. J. Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb R.L., Nirula A., Chen P., et al. Effect of bamlanivimab as monotherapy or in combination with Etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. Jama. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bariola J.R., McCreary E.K., Wadas R.J., et al. Impact of monoclonal antibody treatment on hospitalization and mortality among non-hospitalized adults with SARS-CoV-2 infection. medRxiv. 2021 doi: 10.1101/2021.03.25.21254322. 2021.03.25.21254322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Increasing Utilization of COVID-19 Monoclonal Antibodies: Considerations and Promising Practices for States. 2021. https://www.nga.org/center/publications/increasing-utilization-monoclonal-antibodies/ Available from. Accessed 23 April 2021.
- 7.Planning Considerations for Monoclonal Antibody Administration. 2021. https://files.asprtracie.hhs.gov/documents/aspr-tracie-covid-19-monoclonal-antibody-therapy-tip-sheet.pdf Available from. Accessed 23 April 2021.
- 8.Gibson A.M., Harris A.K., Spencer B., et al. Rapid implementation of a new ambulatory infusion location for patients with COVID-19 to receive monoclonal antibody therapy. Am. J. Health Syst. Pharm. 2021 doi: 10.1093/ajhp/zxab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum A., Kyratsous C.A. SARS-CoV-2 spike therapeutic antibodies in the age of variants. J. Exp. Med. 2021;218(5) doi: 10.1084/jem.20210198. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab Available from. Accessed 23 April 2021.
- 11.Angus D.C. Optimizing the trade-off between learning and doing in a pandemic. JAMA. 2020;323(19):1895–1896. doi: 10.1001/jama.2020.4984. [DOI] [PubMed] [Google Scholar]
- 12.Bariola J.R., McCreary E.K., Khadem T., et al. Establishing a distribution network for COVID-19 monoclonal antibody therapy across a large health system during a global pandemic. Open Forum Infect. Diseas. 2021 doi: 10.1093/ofid/ofab151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCreary E.K., Bariola J.R., Minnier T., et al. A learning health system randomized trial of monoclonal antibodies for COVID-19. medRxiv. 2021 doi: 10.1101/2021.09.03.21262551. 2021.09.03.21262551. [DOI] [Google Scholar]
- 14.Global Initiative on Sharing Avian Influenza Data Tracking of Variants. 2021. https://www.gisaid.org/ Available at. Accessed on 19 Sep 2021.
- 15.COVID-19 Monoclonal Antibody Toolkit Society of Infectious Diseases Pharmacists (SIDP) 2021. https://sidp.org/COVID19-Monoclonal-Antibody-Toolkit-Guide Available from. Updated 03 Sep 2021. Accessed 19 Sep 2021.
- 16.Angus D.C., Gordon A.C., Bauchner H. Emerging lessons from COVID-19 for the US clinical research enterprise. JAMA. 2021;325(12):1159–1161. doi: 10.1001/jama.2021.3284. [DOI] [PubMed] [Google Scholar]