Abstract
Coronavirus disease 2019 (COVID-19) vaccines are recommended for all patients with inflammatory bowel disease (IBD).1 Patients with IBD historically have had low vaccine uptake relative to the general population.2 However, a recent survey suggested a rate higher than that of the general population with regard to COVID-19 vaccine intent among the IBD population. Their study was limited being that 96% of the patients surveyed identified as White, and 88% had attained a bachelor’s degree or higher level of education.3 Therefore, these findings may not be representative of the IBD population as a whole. Previous studies have indeed identified disparities in influenza vaccine uptake within the IBD population.4,5
Coronavirus disease 2019 (COVID-19) vaccines are recommended for all patients with inflammatory bowel disease (IBD).1 Patients with IBD historically have had low vaccine uptake relative to the general population.2 However, a recent survey suggested a rate higher than that of the general population with regard to COVID-19 vaccine intent among the IBD population. Their study was limited being that 96% of the patients surveyed identified as White, and 88% had attained a bachelor’s degree or higher level of education.3 Therefore, these findings may not be representative of the IBD population as a whole. Previous studies have indeed identified disparities in influenza vaccine uptake within the IBD population.4 , 5
Disparities of age, sex, race or ethnicity, and geography have been observed in the general population regarding COVID-19 vaccine uptake.6 , 7 These disparities may be related to a multitude of factors: barriers to access, perceived infection risk, health literacy and educational attainment, and mistrust of governmental institutions. Evaluation of social indices at the population level permits identification of health-related disparities. The Health Innovation Program toolkit has been validated as a means to elucidate geographic disparities by stratifying zip codes into urban and rural groupings.8 Similarly, the Neighborhood Atlas uses the Area Deprivation Index (ADI) as a measure of socioeconomic disadvantage—accounting for factors such as income, education, employment, and housing quality—at the level of census block groups and has been used to inform research, health delivery, and policy.9 The ADI is reported as a state decile (range 1–10), in which a high ADI (eg, 10) represents a high degree of neighborhood socioeconomic disadvantage.
Rates of COVID-19 vaccine uptake among patients with IBD in the United States remain unknown. The purpose of this study was to elucidate rates of COVID-19 vaccine uptake among patients with IBD in a single center. We hypothesized that patients with IBD would have high vaccine uptake and that disparities would exist that resemble those observed in the general population.
We performed a single-center, retrospective study evaluating COVID-19 vaccine uptake among adult patients with IBD seen at the University of Wisconsin between November 1, 2020, and April 30, 2021. Completion of a 2-dose messenger RNA vaccine series or 1-dose Janssen vaccine prior to August 1, 2021, was verified in the Wisconsin Immunization Registry (Supplementary Methods).10 We evaluated if COVID-19 vaccination was addressed by the gastroenterology provider at the time of a clinical encounter. Zip codes were allocated to urban or rural groupings using Health Innovation Program toolkit.8 Street-level addresses were used to assign 2018 ADI using the Neighborhood Atlas.9 The primary outcome was completion of a COVID-19 vaccine series. Key secondary outcomes included association between age, sex, race, ethnicity, urban or rural grouping, ADI, and prior influenza vaccination (2020–2021 season) with COVID-19 vaccine uptake.
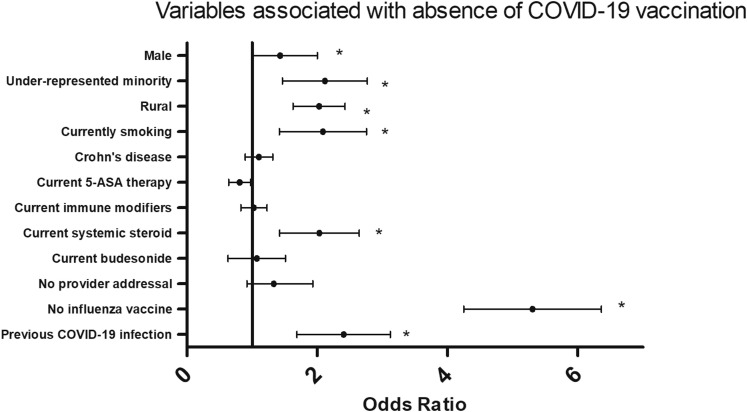
During the study period, 1155 patients with IBD were identified and 131 were excluded. Patients predominantly identified as male (52.7%) and non-Hispanic White (91.1%) (Table 1 ). In total, 84.0% of patients completed a COVID-19 vaccination series, and 75.4% received an messenger RNA vaccine (Supplementary Table 1). Of those who did not complete the vaccine series, the mean age was lower (39.6 ± 14.4 years vs 48.4 ± 17.0 years; P < .001), and patients were more likely to be male (odds ratio [OR], 1.43; 95% confidence interval [CI], 1.01–2.01), identify as an underrepresented minority group (OR, 2.62; 95% CI, 1.48–4.64), or be current smokers (OR, 1.87; 95% CI, 1.05–3.34). The median ADI in the unvaccinated group was higher (2 [interquartile range, 1–4] vs 4 [interquartile range, 2–6]; P < .001), and patients were more likely to reside in a rural zip code (OR, 1.95; 95% CI, 1.38–2.75), have foregone influenza vaccination in 2020–2021 (OR, 5.09; 95% CI, 3.59–7.23), or have been previously infected by COVID-19 (OR, 2.19; 95% CI, 1.28–3.74) (Supplementary Figure 1).
Table 1.
COVID-19 Vaccine Series Completion Stratified by Independent Variables
| Completed COVID-19 Vaccine Series (n = 860) | Did not Complete COVID-19 Vaccine Series (n = 164) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Age, y | 48.4 ± 17.0 | 39.6 ± 14.4 | — | <.01a |
| Male | 442 (51.4) | 98 (60.0) | 1.43 (1.01–2.01) | .04b |
| Race | — | <.01c | ||
| American Indian, Alaskan Native | 6 (0.7) | 1 (0.6) | — | — |
| Asian | 15 (1.7) | 0 (0.0) | — | — |
| Black | 22 (2.6) | 14 (8.5) | — | — |
| Native Hawaiian, Pacific Islander | 1 (0.1) | 0 (0.0) | — | — |
| White | 804 (93.5) | 145 (88.4) | — | — |
| Hispanic/Latino | 25 (2.9) | 10 (6.1) | 2.17 (1.02–4.61) | .04b |
| BMI, kg/m2 | 28.0 ± 6.6 | 28.1 ± 6.5 | — | .94a |
| Current smoking | 50 (5.8) | 17 (10.4) | 1.87 (1.05–3.34) | .03b |
| Charlson comorbidity index | 1.0 (0–2) | 0.0 (0–1) | — | <.01d |
| Rural | 235 (27.4) | 69 (42.7) | 1.95 (1.38–2.75) | <.01b |
| ADI state decile | 2 (1–4) | 4 (2–6) | — | <.01d |
| Crohn’s disease | 464 (54.0) | 91 (55.5) | 1.06 (0.76–1.49) | .7b |
| Duration of IBD diagnosis, y | 11 (5–20) | 8 (4–14) | — | <.01d |
| IBD appointments (2020) | 1 (0–2) | 1 (1–2) | — | .09d |
| IBD appointments (2021) | 1 (1–1) | 1 (0.25–1) | — | .16d |
| Current mesalamine | 317 (36.8) | 51 (31.1) | 0.77 (0.54–1.11) | .16b |
| Current immune-modifying therapy | — | .90c | ||
| Azathioprine or 6-MP monotherapy | 79 (9.2) | 12 (7.3) | — | — |
| Methotrexate monotherapy | 4 (0.5) | 0 (0.0) | — | — |
| Anti-TNF monotherapy | 250 (29.1) | 50 (30.5) | — | — |
| Anti-TNF combination therapye | 66 (7.7) | 10 (6.1) | — | — |
| Vedolizumab therapyf | 80 (9.3) | 18 (11.0) | — | — |
| Ustekinumab therapyg | 47 (5.5) | 11 (6.7) | — | — |
| Current systemic corticosteroids | 60 (7.0) | 20 (12.2) | 1.85 (1.08–3.17) | .02b |
| Current budesonide | 47 (5.5) | 8 (4.9) | 0.89 (0.41–1.91) | .76b |
| Vaccine addressed by IBD provider | 294 (34.2) | 46 (28.0) | 1.33 (0.92–1.93) | .13b |
| Deferred influenza vaccine (2020–2021) | 198 (23.0) | 99 (0.60) | 5.09 (3.59–7.23) | <.01b |
| Documented COVID-19 infectionh | 54 (6.3) | 21 (12.8) | 2.19 (1.28–3.74) | <.01b |
Values are mean ± SD, n (%), or median (interquartile range).
6-MP, 6-mercaptopurine; ADI, Area Deprivation Index; BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; IBD, inflammatory bowel disease; TNF, tumor necrosis factor.
Student’s t test.
Mann-Whitney U test.
Chi-square test.
Odds ratio and 95% CI.
Anti-TNF combination therapy defined as combination of anti-TNF agent and azathioprine, 6-MP, or methotrexate.
Vedolizumab therapy defined as vedolizumab with or without azathioprine, 6-MP, or methotrexate.
Ustekinumab therapy defined as vedolizumab with or without azathioprine, 6-mercaptopureine, or methotrexate
Documented COVID-19 infection includes infection both before and after full vaccination, if applicable, prior to August 1, 2021.
Supplementary Figure 1.
Forest plot of odds ratios with an odds ratio >1 indicating variables that were more likely to be present in those that were not vaccinated against COVID-19. An asterisk indicates statistical significance. Underrepresented minority was defined as those who identified as Black, American Indian/Alaskan Native, Native Hawaiian/Pacific Islander, or Hispanic/Latino. Urban or rural status was defined by the Health Innovation Program toolkit. Immune-modifying therapy was defined as any combination of azathioprine, 6-mercaptupurine, methotrexate, anti-tumor necrosis factor agent, vedolizumab, ustekinumab, or tofacitinib. “No provider addressal” indicates that COVID-19 vaccination was not addressed by the gastroenterology provider at the time of a clinical encounter. “No influenza vaccine” refers to absence of influenza vaccination during the 2020–2021 season. “Previous COVID-19 infection” indicates documented COVID-19 infection prior to COVID-19 vaccination, if applicable, by August 1, 2021.
This is the first U.S. study to describe COVID-19 vaccine uptake and associated disparities in the IBD population. Our observed vaccination rate of 84.0% mirrors the high COVID-19 vaccine intent within the IBD population as previously described.3 High vaccine uptake in our IBD population may be related to perceived risk of severe COVID-19 and frequent interface with the healthcare system, which may have resulted in more opportunities for patient education regarding the safety and efficacy of COVID-19 vaccines in such a population. Moreover, these data indicate that patients with IBD would likely adhere to a 3-dose vaccination series or a booster dose. However, these findings are limited, being that our patients largely identified as non-Hispanic White and had a relatively high degree of neighborhood socioeconomic advantage, which likely also contributed to the high rate of vaccine uptake observed. Our study is further limited by the absence of a matched reference population.
We found that the following variables were associated with increased risked of not being vaccinated against COVID-19: younger age, male sex, identification with an underrepresented minority group, residence in a rural zip code or area of high neighborhood disadvantage, current smoking, low Charlson comorbidity index, shorter duration of IBD diagnosis, current use of systemic corticosteroids, lack of influenza vaccination, and previous COVID-19 infection. Many of these risk factors for deferring COVID-19 vaccination are identical to those previously reported in the general population,6 , 7 implying that these barriers are not unique to IBD. As such, measures to address these disparities at the population level are apt to narrow this gap within the IBD population. Therefore, gastroenterologists should be invested in such measures, which may include partnering with community-based groups, faith leaders, and public health officials in addition to interfacing directly with underserved populations either in person or through telephone outreach to address vaccination.6 , 7 The majority of patients trust their own healthcare providers when it comes to information regarding COVID-19 vaccination,6 providing further incentive for gastroenterologists to take an active role in recommending COVID-19 vaccination for their patients.
In conclusion, we found a high rate of COVID-19 vaccine uptake among patients with IBD. However, we uncovered associated disparities regarding age, sex, and race or ethnicity. Moreover, those who were unvaccinated were more likely to live in a rural zip code or live in a disadvantaged neighborhood. Further studies are needed to investigate interventions developed to address such disparities.
Footnotes
Conflicts of interest This author discloses the following: Freddy Caldera has served as a consultant for Takeda Pharmaceuticals, Celgene, and Arena Pharmaceuticals and received research support from Takeda Pharmaceuticals and Sanofi for work unrelated to the topic of this manuscript. Mary S. Hayney has received research support from Takeda Pharmaceuticals, Dynavax, and Sanofi for work unrelated to the topic of this manuscript; and served as a consultant for GSK vaccines and Seqirus. The remaining authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2021.12.013.
Supplementary Methods
The Wisconsin Immunization Registry (WIR) is a statewide database that tracks immunization rates of Wisconsin residents. It was implemented by the Department of Health and Family Services of the State of Wisconsin and has been available since May 2000, with immunization history backloaded from January 1995. The WIR captures 97% of vaccines administered in the state, including data from both public and private providers, and 98.5% of Wisconsin residents have an active WIR record. The WIR does not capture vaccines administered outside the state, which is why individuals residing outside Wisconsin were excluded from this study, as specified subsequently. All vaccine providers are required to enter coronavirus disease 2019 (COVID-19) vaccines into their immunization registry. The WIR is directly incorporated into our institution’s electronic medical record. At the time of this study, in the state of Wisconsin, COVID-19 vaccines were available at University of Wisconsin clinics, including primary care, in addition to mass immunization clinics and private pharmacies. COVID-19 vaccines were not available at the University of Wisconsin Gastroenterology subspecialty clinics.
Exclusion criteria included death during study period, inactive Wisconsin Immunization Registry, residence outside of Wisconsin, and listing of address as a PO box or correctional center. The study met the requirements for quality improvement as determined by the University of Wisconsin–Madison and was therefore deemed exempt from Institutional Review Board review.
Variables extracted from each chart included age, gender, race, ethnicity, address, body mass index, variables of Charlson comorbidity index, smoking status, type of inflammatory bowel disease diagnosis (ie, Crohn’s disease, ulcerative colitis), duration of IBD diagnosis (years), history of IBD-related surgery (eg, incision and drainage, resection), number of gastroenterology clinic encounters in 2020 and 2021, current inflammatory bowel disease medications, receipt of influenza vaccine (2020–2021 season), addressing of COVID-19 vaccination status by the gastroenterology provider during a clinical encounter, documented COVID-19 infection prior to August 1, 2021, and receipt of COVID-19 vaccine (including vaccine type). Zip codes were identified as “urban” if they fell under the “urban,” “urban advantaged,” or “urban underserved” categorization in the Health Innovation Program toolkit, likewise for “rural.” In-person, video, and telephone visits were considered eligible encounter types. COVID-19 vaccination was considered addressed by the gastroenterology provider if there was documentation of the patient’s COVID-19 vaccination status in a clinic encounter note (eg, “patient fully vaccinated against COVID-19” or “patient declines COVID-19 vaccine”).
Student’s t test, Mann-Whitney U test, chi-square test, and odds ratios with 95% confidence intervals were used for statistical analysis. A P value <.05 was considered significant. Statistical analysis was performed using SPSS version 27 (IBM Corporation, Armonk, NY).
Supplementary Table 1.
COVID-19 Vaccine Uptake
| Did not start vaccination series | 155 (15.1) |
| Started vaccination series | 869 (84.9) |
| Did not complete vaccination series | 9 (0.9) |
| Completed vaccination series | 860 (84.0) |
| Received Janssen | 88 (8.6) |
| Received mRNA vaccine | 772 (75.4) |
| Received Pfizer | 391 (38.2) |
| Received Moderna | 381 (37.2) |
Values are n (%).
COVID-19, coronavirus disease 2019; mRNA, messenger RNA.
References
- 1.Siegel C.A., et al. Gut. 2021;70:635–640. doi: 10.1136/gutjnl-2020-324000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caldera F., et al. Inflamm Bowel Dis. 2021;27:123–133. doi: 10.1093/ibd/izaa055. [DOI] [PubMed] [Google Scholar]
- 3.Dalal R.S., et al. Clin Gastroenterol Hepatol. 2021;19:1730–1732.e2. doi: 10.1016/j.cgh.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beniwal-Patel P., et al. Gastroenterology. 2021;160 S-58. [Google Scholar]
- 5.Beniwal-Patel P., et al. Gastroenterology. 2021;160 S-359. [Google Scholar]
- 6.Murthy B.P., et al. MMWR Morb Mortal Wkly Rep. 2021;70:759–764. doi: 10.15585/mmwr.mm7020e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diesel J., et al. MMWR Morb Mortal Wkly Rep. 2021;70(25):922–927. doi: 10.15585/mmwr.mm7025e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Health Innovation Program. https://www.hipxchange.org/RuralUrbanGroups Available at:
- 9.Kind A.J.H., et al. N Engl J Med. 2018;378:2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith R., et al. Dig Dis Sci. 2021;66:2935–2941. doi: 10.1007/s10620-020-06631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]