Abstract
Objectives
Countries throughout the world are experiencing COVID-19 viral load in their populations, leading to potential transmission and infectivity of asymptomatic COVID-19 cases. The current systematic review and meta-analysis aims to investigate the role of asymptomatic infection and transmission reported in family clusters, adults, children and health care workers, globally.
Study design
Systematic review and meta-analysis.
Methods
An online literature search of PubMed, Google Scholar, medRixv and BioRixv was performed using standard Boolean operators and included studies published up to 17 August 2021. For the systematic review, case reports, short communications and retrospective studies were included to ensure sufficient asymptomatic COVID-19 transmission data were reported. For the quantitative synthesis (meta-analysis), participant data from a collection of cohort studies focusing on groups of familial clusters, adults, children and health care workers were included. Inconsistency among studies was assessed using I2 statistics. The data synthesis was computed using the STATA 16.0 software.
Results
This study showed asymptomatic transmission among familial clusters, adults, children and health care workers of 15.72%, 29.48%, 24.09% and 0%, respectively. Overall, asymptomatic transmission was 24.51% (95% confidence interval [CI]: 14.38, 36.02) among all studied population groups, with a heterogeneity of I2 = 95.30% (P < 0.001). No heterogeneity was seen in the population subgroups of children and health care workers. The risk of bias in all included studies was assessed using the Newcastle Ottawa Scale.
Conclusions
For minimising the spread of COVID-19 within the community, this study found that following the screening of asymptomatic cases and their close contacts for chest CT scan (for symptomatic patients), even after negative nucleic acid testing, it is essential to perform a rigorous epidemiological history, early isolation, social distancing and an increased quarantine period (a minimum of 14–28 days). This systematic review and meta-analysis supports the notion of asymptomatic COVID-19 infection and person-to-person transmission and suggests that this is dependent on the varying viral incubation period among individuals. Children, especially those of school age (i.e. <18 years), need to be monitored carefully and follow mitigation strategies (e.g. social distancing, hand hygiene, wearing face masks) to prevent asymptomatic community transmission of COVID-19.
Keywords: COVID-19, Asymptomatic transmission, Hand hygiene, Social distancing, Age, Children
Introduction
Symptomatic COVID-19 viral infection is a significant risk factor for transmission of the disease within the general public. The major signs of COVID-19 infection include fever, dyspnoea, a dry cough and diarrhoea; these symptoms are reported to last up to 14 days, with a median incubation period of 9–12 days. Aerosol transmissions occur through sneezing or coughing and are reported to be the primary route of person-to-person infection.1 However, simulation studies have also observed asymptomatic COVID-19 person-to-person transmission.2 Polymerase chain reaction (PCR)-based assays are recommended in managing asymptomatic transmission of the virus.3 He et al. reported the first case of asymptomatic transmission of COVID-19 on 21 February 2020.4 Asymptomatic infection was reported as ‘hidden coronavirus infections’ (‘infections’ or ‘covert coronavirus infections’).5 The COVID-19 prevention and control protocol (6th edition) states that asymptomatic COVID-19 cases should remain in quarantine for 14 days and that they should have two negative nucleic acid tests before being discharged. Worldwide, interest in asymptomatic COVID-19 infections and their transmission potential had increased.6 In China, around 86% of asymptomatic COVID-19 transmission was undocumented before travel restrictions were introduced.6
To date, asymptomatic COVID-19 cases have been reported among family clusters,7, 8, 9, 10, 11, 12 pregnant women,13 , 14 adults,15, 16, 17, 18, 19, 20, 21, 22, 23, 24 children,1 , 25 , 26 health care workers27, 28, 29 and travellers.30, 31, 32, 33, 34 Considering the potential transmission of asymptomatic COVID-19 within the community, this study aimed to collate data from the general population, as well as vulnerable groups from different backgrounds, and perform a meta-analysis. Previous studies have reported the proportion of COVID-19 infections attributable to asymptomatic transmission to be around 20%, with some variation depending on the population group. In this study, a meta-analysis was performed that considered different population groups.
Methods
Study design
A systematic review and meta-analysis were performed.
Data sources and search strategy
For the systematic review and meta- analysis PRISMA guidelines were followed.35 , 36 The following Boolean operators were used to search the PubMed database, Google scholar, medRxiv and BioRixv: ‘asymptomatic transmission’, ‘((COVID-19) AND (Coronavirus)) AND (Asymptomatic transmission)’, ‘((COVID-19) OR (Coronavirus)) AND (asymptomatic transmission)’, ‘(SARS-CoV-2) AND (asymptomatic transmission)’, ‘(2019-nCoV) AND (asymptomatic transmission)’, ‘(Wuhan pneumonia) AND (asymptomatic transmission)’, ‘(Wuhan flu) AND (asymptomatic transmission)’, ‘(2019-nCoV acute respiratory disease) AND (asymptomatic transmission)’ and ‘(2019-nCoV respiratory syndrome) AND (asymptomatic transmission)’. This study included published literature in English language up to 17 August 2021..
Inclusion and exclusion criteria
For the meta-analysis, the present study included cohort studies that reported asymptomatic person-to-person transmission among clusters. Studies that were published in the English language were included.
Data collection and study selection
Details of authors, sample size and numbers reported for the asymptomatic infection of COVID-19 were extracted and recorded independently. Data extraction was done separately by two independent reviewers, and disagreement was settled by a joint discussion. The data were carefully checked to minimise the risk of duplication.
Quality assessment
The Newcastle Ottawa scale (cohort studies) was used to evaluate the selected studies in the current systematic review and meta-analysis.35 , 37
Publication bias
Possible publication bias in this study was assessed for the included cohort studies.38
Statistical analyses and data synthesis
After extracting the results, studies were pooled and the effect of asymptomatic COVID-19 transmission was examined through the random effects method. For continuous outcomes, the standard error (SE) with 95% confidence intervals (CIs) were calculated. Heterogeneity between studies was assessed using the I2 statistic (I2 values indicating the existence of heterogeneity were assessed according to Higgins and colleagues).35 , 39 , 40 Data for the meta-analysis were collated.35 , 41 Data synthesis was conducted using the STATA 16.0 software.
Patient and public involvement
There was no direct patient or public involvement in this systematic review and meta-analysis.
Results
Literature search
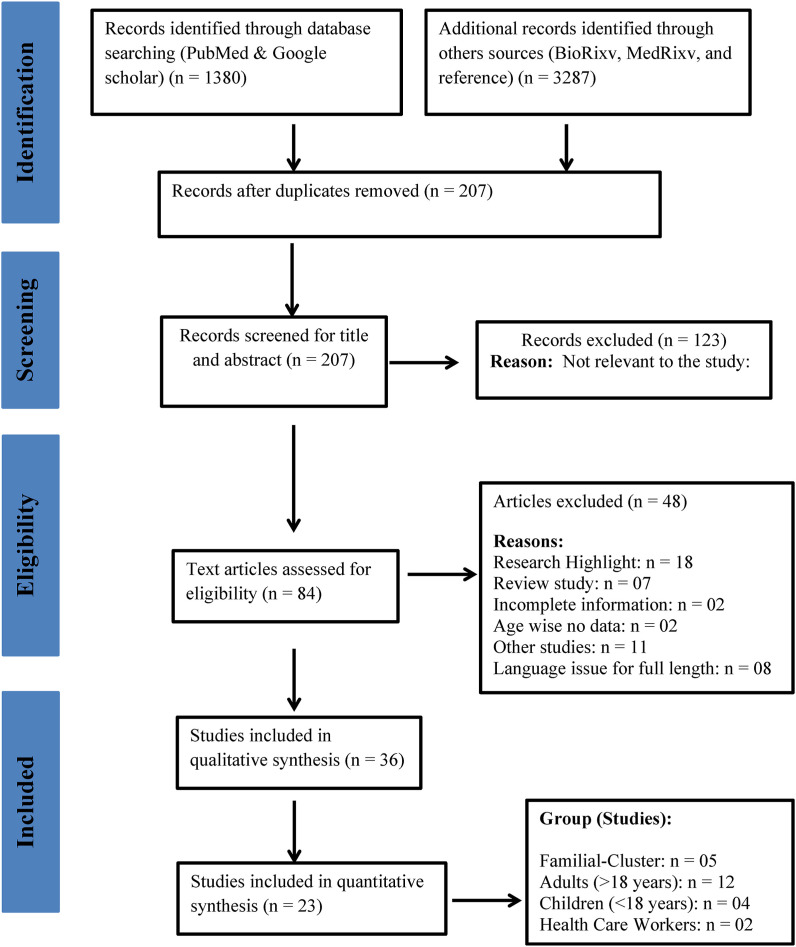
The literature search and screening were performed according to the PRISMA chart (Fig. 1 ). Initially, 4667 published research articles were identified using the online database search. After removing 4460 duplicate publications, 207 research articles were shortlisted. After screening the title and abstracts, 123 articles were excluded and 84 full-text articles were assessed for eligibility. A further 48 studies were excluded because they were research highlight reports, review studies, had incomplete information, reported no age-specific data, were classified as ‘other’ non-relevant studies or had language issues. For the qualitative synthesis, 36 articles were selected and 23 studies were included in the meta-analysis. Studies were grouped into the following population subgroups: family clusters (n = 5), adults (n = 12), children (n = 4) and health care workers (n = 2).
Fig. 1.
PRISMA chart.
Characteristics of the study participants
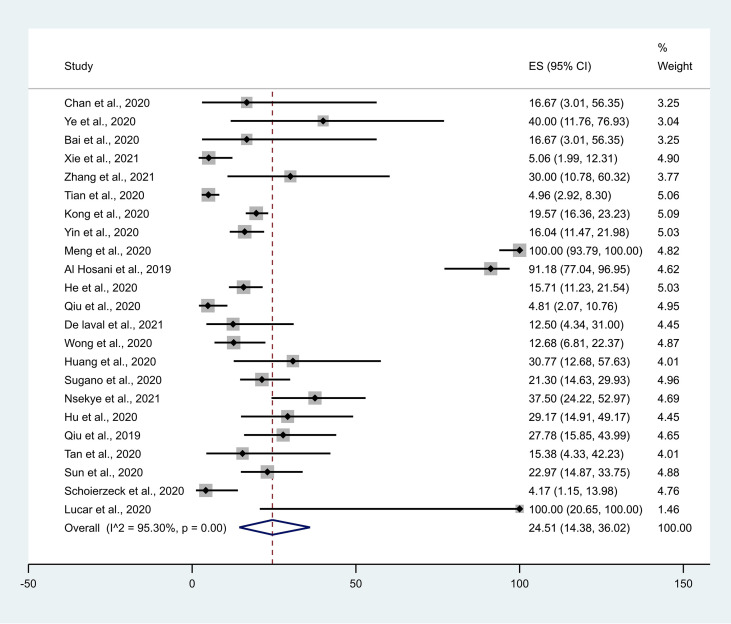
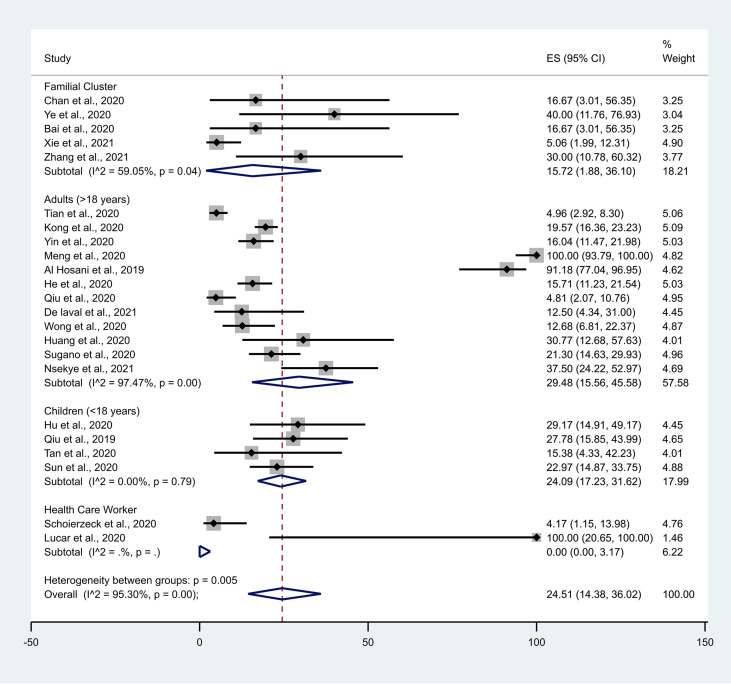
The main components of the included studies are summarised in Table 1 . All published research articles were cohort (observational) study designs. Most of the studies are from China, Korea, the US, Japan and Germany. The current research includes articles published/available online up to 17 August 2021. The current systematic review reports data from 23 studies with a total of 1905 asymptomatic participants. The forest plots of asymptomatic positivity for COVID-19 among the study population (Fig. 2 ) and among different subgroups (Fig. 3 ) are shown.
Table 1.
Characteristics of the included studies.
| Author | Country | Age, years [mean (±SD)/median (IRQ)] | Study Type | Type of test | Major findings |
|---|---|---|---|---|---|
| Family cluster | |||||
| Chan et al., 20208 | China | Family: 36-60 | Cohort | RT-PCR | Supports person-to-person transmission between family |
| Child: 10 | |||||
| Chen et al., 20209 | China | 8.5 ± 0.17 | Case report | RT-PCR | The ability of COVID-19 transmission during the asymptomatic period even after negative viral testing |
| Lu et al., 201910 | China | 8 | Case report | RT-PCR | Supports rigorous investigation in the combination of various testing methods for asymptomatic COVID-19 cases |
| Qian et al., 202011 | China | 6 | Brief report | RT-PCR | Variation in clinical manifestation across individuals was observed |
| Ye et al., 202012 | China | 38 ± 18.38 | Cohort | RT-PCR | Possibility of COVID-19 transmission by the asymptomatic carrier during the incubation period |
| Bai et al., 20207 | China | 20 | Cohort | RT-PCR | Support asymptomatic transmission through a family contact |
| Xie et al., 202156 | China | >18 | Cohort | RT-PCR | Handwashing, social distancing should be done |
| Zhang et al., 202157 | China | >18 | Cohort | RT-PCR | Asymptomatic patients can transmit the disease and improve protective measures. |
| Adults | |||||
| Tian et al., 202021 | China | 47.5 | Cohort | RT-PCR | Early isolation and quarantine for close contacts to prevent asymptomatic transmission |
| Kim et al., 202017 | Korea | 26 (22–47) | Research note | RT-PCR | Supports social distancing to prevent asymptomatic transmission |
| Kong et al., 20208 | China | 37.7 (±19) | Cohort | RT-PCR | Suggest rigorous epidemiological history and chest CT scan as a practical tool to identify the asymptomatic COVID-19 cases in the community |
| Yin et al., 202022 | China | – | Cohort | RT-PCR | No difference in the transmission rate of COVID-19 between asymptomatic and symptomatic cases |
| Meng et al., 202055 | China | 42.60 (±16.56) | Cohort | RT-PCR | Suggest chest CT scan as a vital tool to screen the asymptomatic COVID-19 cases in the community |
| Al Hosani et al., 201915 | UAE | 37 (30–45) | Cohort | RT-PCR | No transmission among household contacts after the implication of strong isolation policies |
| He et al., 202016 | China | – | Cohort | RT-PCR | Significantly smaller transmissibility of asymptomatic cases than symptomatic |
| Qiu et al., 202020 | China | 43 (8–84) | Cohort | RT-PCR | Suggested transmission occurred after 14 days quarantine periods |
| Zhou et al., 202024 | China | – | Short communication | RT-PCR | Recommended rigorous epidemiological history and nucleic acid testing |
| Park et al., 202019 | Korea | 38 (20–0) | Report | RT-PCR | Supports contact tracing, testing and increasing quarantine to prevent asymptomatic COVID-19 transmission in the community |
| De laval et al., 202161 | France | 40 (24–59) | Cohort | RT-PCR | The median incubation day was 4 (1–13) days. |
| Wong et al., 202034 | Brunei | – | Cohort | RT-PCR | Proposes differentiated testing strategies to account for different transmission risk |
| Huang et al., 202060 | China | – | Cohort | RT-PCR | To identify the presence of asymptomatic carriers as early as possible in the community. Infection occurs during the incubation period of asymptomatic cases. |
| Sugano et al., 202058 | Japan | – | Cohort | RT-PCR | Possibility of asymptomatic transmission and the period from exposure to illness ranged from 2 to 17 days. |
| Nsekye et al., 202162 | Rawanda | – | Cohort | RT-PCR | Contact tracing and testing should be done. |
| Children | |||||
| Hu et al., 202025 | China | <15 | Cohort | RT-PCR | Suggest close contact tracing and nucleic acid testing to identify the asymptomatic COVID-19 tracing the community |
| Qiu et al., 201926 | China | 8.3 (±3.5) | Cohort | RT-PCR | Possibility of asymptomatic COVID-19 transmission by close contacts |
| Tan et al., 20201 | China | – | Cohort | RT-PCR | Possibility of asymptomatic COVID-19 transmission by intrafamilial contact |
| Sun et al., 202059 | China | 5.8 | Cohort | RT-PCR | Both nasopharyngeal and anal swabs should be confirmed negative viral load before declaring full recovery to avoid oral-faecal transmission. |
| Health care Workers | |||||
| Kimball et al., 202027 | USA | – | Report | RT-PCR | Reported rapid transmission among health care worker |
| Schoierzeck et al., 202029 | Germany | 48 | Cohort | RT-PCR | Suggest nucleic acid testing for asymptomatic COVID-19 cases |
| Lucar et al., 202028 | USA | >18 | Cohort | RT-PCR | transmission reported because of prolonged surgery done on asymptomatic COVID-19 case |
| Traveller aged >18 years | |||||
| COVID-19 NERC, 202030 | Korea | >18 | Cohort | RT-PCR | Supports asymptomatic transmission with minor symptoms |
| Mizumoto et al., 202032 | Japan | >18 | Rapid communication | RT-PCR | Support social distancing to prevent the asymptomatic transmission |
| Wan et al., 202033 | China | >18 | Short communication | RT-PCR | Possibility of asymptomatic transmission after 14 days quarantine from asymptomatic COVID-19 case |
| Wong et al., 202034 | Brunei | – | Rapid communication | RT-PCR | Support social distancing & nucleic acid testing of asymptomatic COVID-19 case |
| Le et al., 202054 | China | – | Abstract | – | Support asymptomatic COVID-19 viral transmissibility in the absence of signs and symptoms |
Note: - Missing values (mean/median values were not reported).
IQR, interquartile range; RT-PCT, reverse transcription-polymerase chain reaction.
Fig. 2.
Forest plot of asymptomatic positivity for COVID-19 among the study population. CI, confidence interval.
Fig. 3.
Forest plot of asymptomatic positivity for COVID-19 among different population subgroups. CI, confidence interval.
Quality assessment
The Newcastle Ottawa Scale (for cohort studies) was used for qualitative evaluation of the studies included in the meta-analysis.35 , 37 The risk of bias was assessed based on three domains (selection, comparability and outcome), as highlighted in Table 2 .
Table 2.
Quality Assessment: Cohort study quality according to the Newcastle Ottawa scale.
| Study | Selection∗∗∗∗∗∗ |
Comparability∗∗ |
Outcome∗∗∗∗∗ |
Total Quality Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Family cluster | |||||||||
| Chan et al., 20208 | ∗ | 0 | ∗ | 0 | ∗ | ∗ | 0 | 0 | 4 |
| Ye et al., 202012 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Bai et al., 20207 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Xie et al., 202156 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Zhang et al., 202157 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Adults | |||||||||
| Tian et al., 202021 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Kong et al., 20208 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Yin et al., 202022 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Meng et al., 202055 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Al Hosani et al., 201915 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| He et al., 202016 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Qiu et al., 202020 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| De laval et al., 202161 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Wong et al., 202034 | ∗ | 0 | ∗ | 0 | ∗ | ∗ | 0 | 0 | 4 |
| Huang et al., 202060 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Sugano et al., 202058 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Nsekye et al., 202162 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Children | |||||||||
| Hu et al., 202025 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Qiu et al., 201926 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Tan et al., 20201 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Sun et al., 202059 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Health care Workers | |||||||||
| Schoierzeck et al., 202029 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
| Lucar et al., 202028 | ∗ | 0 | ∗ | 0 | 0 | ∗ | 0 | 0 | 3 |
Note: Selection; 1) Representativeness of the exposed cohort, 2) Selection of the non-exposed cohort, 3) Ascertain exposure, 4) Demonstration that outcome of interest was not present at the start of the study; Comparability; 5) Comparability of cohorts based on the design or analysis controlled for confounders; Outcome: 6) Assessment of outcome, 7) Was follow-up long enough for outcomes to occur, 8) Adequacy of follow-up of cohorts.
∗Newcastle-Ottawa Scale contains 8 items within 3 domain and the total maximum score is 9. A study with score from 7-9, has high quality, 4-6, high risk, and 0-3 very high risk of bias.
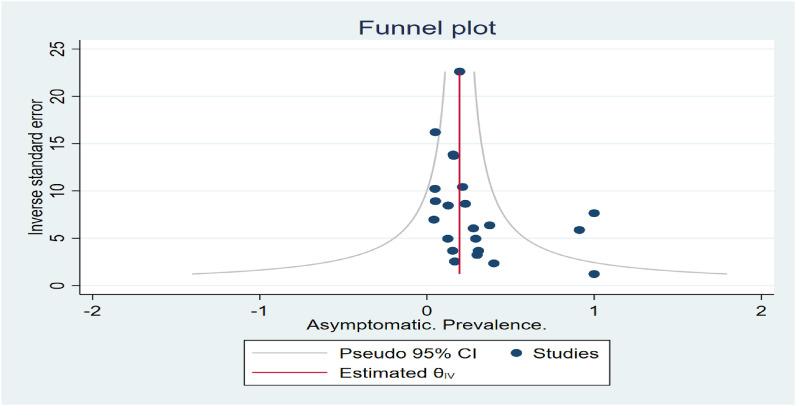
Publication bias
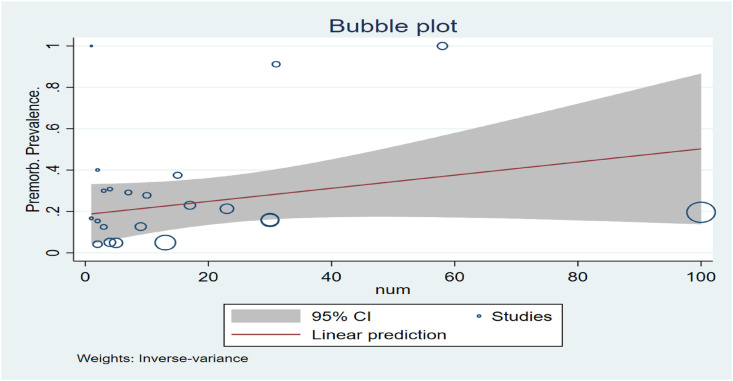
The bubble plot (see Fig. 4 ) shows the study-specific effect size, where the size of each bubble is proportional to the precision of each study. Asymptomatic participants’ funnel plot (standard error) showed no obvious publication bias (Fig. 5 ).
Fig. 4.
Bubble Plot.
Fig. 5.
Funnel Plot.
Meta-analysis
The outcomes of the current meta-analysis (Table 3 ), and forest plots of asymptomatic positivity for COVID-19 among the study population (Fig. 2) and different subgroups (Fig. 3) are shown. A random-effects model was used for the different levels of reported asymptomatic COVID-19 transmission in the community. The current meta-analysis observed heterogeneity among familial clusters (I2 = 59.02%, P = 0.04, with a proportion of 15.72% [95% CI: 1.88, 36.10]) and adults aged ≥18 years (I2 = 97.47%, P < 0.001, with a proportion of 29.48% [95% CI: 15.56, 45.58]). This study did not observe any heterogeneity among children and health care workers, although the random effect model showed the proportion of asymptomatic transmission to be 24.09% (95% CI: 17.23, 31.62) and 0% (95% CI: 0.00, 3.17), respectively. We observed a significant difference (P = 0.005) of heterogeneity between groups (I2 = 95.30%).
Table 3.
The meta-analysis of asymptomatic transmission for COVID-19 among different population subgroups.
| Risk factor (Asymptomatic) Group |
Studies | Asymptomatic Population (n) |
Total Sample Size (N) | Proportion (95% CI) |
I2, P-Value |
|---|---|---|---|---|---|
| Family Cluster | 5 | 11 | 106 | 15.72 (1.88, 36.10) | 59.02%, P = 0.04 |
| Adults (≥18 years age) |
12 | 321 | 1603 | 29.48 (15.56, 45.58) | 97.47%, P < 0.001 |
| Children (<18 years age) |
4 | 36 | 147 | 24.09 (17.23, 31.62) | 0.00%, P = 0.79 |
| Health care Workers | 2 | 3 | 49 | 0.00 (0.00, 3.17) | – |
| Combined (groups) | 23 | 71 | 1905 | 24.51 (14.38, 36.02) | 95.30%, P < 0.001 |
CI, confidence interval.
Discussion
The current study summarised available literature reporting asymptomatic transmission of COVID-19 as retrospective studies and case reports from family clusters, adults, children, health care workers and travellers. The person-to-person asymptomatic transmission was observed among familial clusters in an asymptomatic COVID-19 child (aged 10 years old) who had an abnormal chest CT and in another asymptomatic child with mild chest CT manifestation; family members of these children showed signs of fever and respiratory issues and had a positive COVID-19 test result.8 , 9 The current study suggests that thorough epidemiological investigations, in combination with multiple detection methods (e.g. reverse transcription PCR [RT-PCR], chest CT, rapid IgM-IgG and serum C-reactive protein [CRP] level), asymptomatic carriers in the community who are displaying different (or no) clinical manifestations can be identified.10 , 11 Another study supports the possibility of asymptomatic transmission among familial clusters during the incubation period.12 In addition, in a familial cluster of five COVID-19-positive patients, it was observed that they had contact with other asymptomatic family members who had returned from Wuhan, China, suggesting asymptomatic transmission.7
During any disease outbreak, the unborn babies of pregnant women are at high risk. It has been reported that pregnant women with asymptomatic COVID-19 infection have delivered babies who are negative for the COVID-19 nucleic acid test, suggesting no vertical transmission among neonates born to COVID-19-infected mothers.14 , 42, 43, 44, 45, 46, 47, 48, 49
In Wuhan, a lower COVID-19 fatality rate and higher discharge rate were observed than in Beijing, China. It is essential to identify asymptomatic individuals and implement necessary control measures to prevent transmission.21 In South Korea, 41 COVID-19 asymptomatic adults were identified (confirmed by RT-PCR) out of 213 individuals.17 In another study among 100 asymptomatic cases, 60% developed delayed symptoms and none of the asymptomatic patients died, suggesting that asymptomatic transmission could take place during the incubation period.18 Another study did not observe any difference in the symptomatic and asymptomatic COVID-19 transmission rates among patients.22 In adults, CT imaging of asymptomatic COVID-19 individuals has advantages in highly suspicious cases with negative nucleic acid test results.17 A serological investigation among 31 of 34 adult cases with asymptomatic COVID-19 infection did not require oxygen support during hospitalisation.15 Theoretically, the quantified infection transmission rate shows the estimated risk ratio (RR) of infectivity of symptomatic against asymptomatic to be 3.9% (95% CI: 1.5, 11.8). In asymptomatic adults, the transmission was significantly smaller than in symptomatic cases.16 No gender difference was observed for asymptomatic transmission.20
Further longitudinal surveillance using nucleic acid testing is warranted to identify and assess viral load among asymptomatic COVID-19 adults.24 In one study, four asymptomatic cases were quarantined for 14 days; thus, these individuals were unable to transmit the infection due to proper isolation management.19
Asymptomatic COVID-19 transmission has been observed in children.26 In one study, 24 asymptomatic cases were identified from close contacts of asymptomatic COVID-19 patients.25 Another study supports multiple-site sampling of close contacts1 among children. In a review, it was observed that adults with COVID-19 infection are more likely to show clinical symptoms and radiological manifestations than children, which is in line with previous reports for severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) coronaviruses.50
In a study investigating health care workers in a nursing facility, rapid transmission of COVID-19 was reported in 76 residents; 23 (30.3%) had positive test results, and 13 were asymptomatic on the day of testing, suggesting the possibility of asymptomatic transmission of COVID-19.27 Establishing effective infection control strategies to prevent COVID-19 transmission among frontline health care workers and patients should be addressed urgently and as a priority. In another study, including 48 participants (healthcare worker), two asymptomatic cases become positive, suggesting appropriate testing strategies are essential to prevent outbreaks of COVID-19 within hospital settings.29 In the United States, health care workers who were not wearing respirators were exposed to an asymptomatic COVID-19 patient without developing clinical illness.28
In Korea, COVID-19 was transmitted by 16 infected travellers from other countries; the disease was infectious at this stage, which resulted from close contact with asymptomatic carriers.30 Most of the infections on board the Diamond Princess Cruise ship highlight the asymptomatic transmission of COVID-19 in confined settings. To further mitigate the transmissibility of COVID-19, it may be advised to minimise the number of individuals gathering in confined settings.32 A 36-year-old traveller who returned from Wuhan tested positive for COVID-19 positive and health care workers who were in close contact with this patient also tested positive; however, the patient initially had no symptoms.33 A high proportion of asymptomatic COVID-19 infection (12%) was reported among travellers returning to Brunei. Similarly, an asymptomatic COVID-19 patient showed viral transmissibility without showing any signs or symptoms after travelling in China.31 In another study, it was suggested that testing facilities should be increased to help identify asymptomatic COVID-19 cases.34
Although this study recommends early isolation and social distancing for asymptomatic COVID-19 cases, it is important to recognise that this may lead to psychological and emotional distress (as reported in a qualitative study from the United Kingdom).51 Further studies are warranted based on the ‘one health’ approach to tackle asymptomatic transmission.52 A study by Tao et al. suggested the inclusion of infection fatality rate (IFR) in surveillance data to minimise asymptomatic COVID-19 cases in the community.53
Limitations
There are some limitations in the current systematic review and meta-analysis. A mixed population, a continuous variable, variation in clinical conditions and use of different statistical methods may result in heterogeneity among studies included in the current meta-analysis. Furthermore, the current study only included reported cases of asymptomatic COVID-19 transmission.
Study importance
This is the first study to review the possibility of asymptomatic COVID-19 transmission among different population subgroups in the community. This study also identifies the potential role of isolation, identification of close contacts, social distancing, and testing asymptomatic COVID-19 cases with chest CT scan and nucleic acid testing to minimise the spread of the virus in the community.
Conclusions
Currently, there is no evidence that COVID-19 can be transmitted in the asymptomatic stage; however, results suggest that asymptomatic infections are not limited to one population group (e.g. neonates, children, adults). In young people, it has been suggested that their strong immune status protects against COVID-19 severity. We hypothesise that asymptomatic carriers, either children or adults, should be vigilant as they are capable of transmitting COVID-19 during the incubation period without showing any signs or symptoms. As previous reports support the involvement of lung function in asymptomatic COVID-19 cases, we recommend chest CT scans among symptomatic cases, which is a convenient tool to monitor and track patients in their incubation period.
Author statements
Acknowledgements
The authors thank the Department of Community Medicine and School of Public Health, PGIMER, and the Indian Council of Medical Research, New Delhi.
Ethical approval
Not required.
Funding
None declared.
Competing interests
None declared.
Contributors
Dr Khaiwal Ravindra: Concept design, data extraction, interpretation, final correction and writing the first draft. Mr Vivek Singh Malik: Data extraction, interpretation and writing the first draft. Dr Bijaya K Padhi: Interpretation, internal review of data, review and editing. Dr Sonu Goel: Discussion, review and editing. Dr Madhu Gupta: Internal review of data, review and editing.
References
- 1.Tan X., Huang J., Zhao F., Zhou Y., Li J.-Q., Wang X.-Y. Clinical features of children with SARS-CoV-2 infection: an analysis of 13 cases from Changsha, China. Zhongguo Dang Dai Er Ke Za Zhi Chin J Contemp Pediatr. 2020;22(4):294–298. doi: 10.7499/j.issn.1008-8830.2003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T.-M., Rui J., Wang Q.-P., Zhao Z.-Y., Cui J.-A., Yin L. A mathematical model for simulating the phase-based transmissibility of a novel coronavirus. Infect Dis Poverty. 2020;9(1):24. doi: 10.1186/s40249-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahimi F., Talebi Bezmin Abadi A. Challenges of managing the asymptomatic carriers of SARS-CoV-2. Trav Med Infect Dis. 2020:101677. doi: 10.1016/j.tmaid.2020.101677. Published online April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He G., Sun W., Fang P., et al. The clinical feature of silent infections of novel coronavirus infection (COVID-19) in Wenzhou. J Med Virol. 2020 doi: 10.1002/jmv.25861. Published online 10th April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Kang H., Liu X., Tong Z. Asymptomatic cases with SARS-CoV-2 infection. J Med Virol. 2020:25990. doi: 10.1002/jmv.25990. Published online 22nd May jmv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S.W., Cornforth D.M., Dushoff J., Weitz J.S. The time scale of asymptomatic transmission affects estimates of epidemic potential in the COVID-19 outbreak. Epidemics. 2020;31:100392. doi: 10.1016/j.epidem.2020.100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai Y., Yao L., Wei T., et al. Presumed asymptomatic carrier transmission of COVID-19. J Am Med Assoc. 2020;323(14):1406. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J.F.-W., Yuan S., Kok K.-H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M., Fan P., Liu Z., et al. A SARS-CoV-2 familial cluster infection reveals asymptomatic transmission to children. J Infect Public Health. 2020;13(6):883–886. doi: 10.1016/j.jiph.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu S., Lin J., Zhang Z., et al. Alert for non-respiratory symptoms of Coronavirus Disease 2019 (COVID-19) patients in epidemic period: a case report of familial cluster with three asymptomatic COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25776. Published online 19th March. [DOI] [PubMed] [Google Scholar]
- 11.Qian G., Yang N., Ma A.H.Y., et al. COVID-19 transmission within a family cluster by presymptomatic carriers in China. Clin Infect Dis. 2020:ciaa316. doi: 10.1093/cid/ciaa316. Published online 23rd March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye F., Xu S., Rong Z., et al. Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int J Infect Dis. 2020;94:133–138. doi: 10.1016/j.ijid.2020.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang L., Yan Y., Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020;34(2):75–80. doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu D., Sang L., Du S., Li T., Chang Y., Yang X. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J Med Virol. 2020:25927. doi: 10.1002/jmv.25927. Published online 30th April jmv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Hosani F.I., Kim L., Khudhair A., et al. Serologic follow-up of Middle East respiratory syndrome coronavirus cases and contacts—Abu Dhabi, United Arab Emirates. Clin Infect Dis. 2019;68(3):409–418. doi: 10.1093/cid/ciy503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He D., Zhao S., Lin Q., et al. The relative transmissibility of asymptomatic COVID-19 infections among close contacts. Int J Infect Dis. 2020;94:145–147. doi: 10.1016/j.ijid.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim G. -u., Kim M.-J., Ra S.H., et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.04.040. Published online May S1198743X20302688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong W., Wang Y., Hu J., Chughtai A., Pu H. Comparison of clinical and epidemiological characteristics of asymptomatic and symptomatic SARS-CoV-2 infection: a multi-center study in Sichuan Province, China. Trav Med Infect Dis. 2020:101754. doi: 10.1016/j.tmaid.2020.101754. Published online May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S.Y., Kim Y.-M., Yi S., et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26(8) doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu C., Deng Z., Xiao Q., et al. Transmission and clinical characteristics of coronavirus disease 2019 in 104 outside-Wuhan patients, China. J Med Virol. 2020:25975. doi: 10.1002/jmv.25975. :. Published online May 5 jmv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian S., Hu N., Lou J., et al. Characteristics of COVID-19 infection in beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin G., Jin H. Comparison of transmissibility of coronavirus between symptomatic and asymptomatic patients: reanalysis of the ningbo COVID-19 data. JMIR Public Health Surveill. 2020;6(2) doi: 10.2196/19464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuen K.-S., Ye Z.-W., Fung S.-Y., Chan C.-P., Jin D.-Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10(1):40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou R., Li F., Chen F., et al. Viral dynamics in asymptomatic patients with COVID-19. Int J Infect Dis. 2020;96:288–290. doi: 10.1016/j.ijid.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Z., Song C., Xu C., et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimball A., Hatfield K.M., Arons M., et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility — king county, Washington, march 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucar J., Navalkele B., Becker B.P., Reed C.D., Parham J. Healthcare personnel exposure to a patient with asymptomatic SARS-CoV2 infection during a prolonged surgical intervention. Am J Infect Control. 2020 doi: 10.1016/j.ajic.2020.05.036. Published online June S0196655320303527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwierzeck V., König J.C., Kühn J., et al. First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric dialysis unit. Clin Infect Dis. 2020:ciaa491. doi: 10.1093/cid/ciaa491. Published online 27th April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.COVID-19 National Emergency Response Center Epidemiology and case management team, Korea centers for disease control and prevention. Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res Perspect. 2020;11(1):8–14. doi: 10.24171/j.phrp.2020.11.1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu X., Gao J., Luo X., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID-19) pneumonia. Obstet Gynecol. 2020 doi: 10.1097/AOG.0000000000003926. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;(10):25. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan R., Mao Z.-Q., He L.-Y., Hu Y.-C., Wei-Chen Evidence from two cases of asymptomatic infection with SARS-CoV-2: are 14 days of isolation sufficient? Int J Infect Dis. 2020;95:174–175. doi: 10.1016/j.ijid.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong J., Abdul Aziz A.B.Z., Chaw L., et al. High proportion of asymptomatic and presymptomatic COVID-19 infections in air passengers to Brunei. J Trav Med. 2020:taaa066. doi: 10.1093/jtm/taaa066. Published online 5th May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik V.S., Ravindra K., Attri S.V., Bhadada S.K., Singh M. Higher body mass index is an important risk factor in COVID-19 patients: a systematic review. SSRN Electron J. 2020 doi: 10.2139/ssrn.3605087. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nuorti J.P., Butler J.C., Farley M.M., et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342(10):681–689. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 38.Lau J., Ioannidis J.P.A., Terrin N., Schmid C.H., Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krüger K., Mooren F.-C., Pilat C. The immunomodulatory effects of physical activity. Curr Pharmaceut Des. 2016;22(24):3730–3748. doi: 10.2174/1381612822666160322145107. [DOI] [PubMed] [Google Scholar]
- 40.Lasselin J., Alvarez-Salas E., Grigoleit J.-S. Well-being and immune response: a multi-system perspective. Curr Opin Pharmacol. 2016;29:34–41. doi: 10.1016/j.coph.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Pearson-Stuttard J., Blundell S., Harris T., Cook D.G., Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4(2):148–158. doi: 10.1016/S2213-8587(15)00379-4. [DOI] [PubMed] [Google Scholar]
- 42.Chen H., Guo J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen S., Huang B., Luo D.J., et al. [Pregnancy with new coronavirus infection: clinical characteristics and placental pathological analysis of three cases] Zhonghua Bing Li Xue Za Zhi. 2020;49(5):418–423. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y., Peng H., Wang L., et al. Infants born to mothers with a new coronavirus (COVID-19) Front Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dashraath P., Wong J.L.J., Lim M.X.K., et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222(6):521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. Published online March S0163445320301092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y., Li X., Zhu B., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L., Jiang Y., Wei M., et al. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province] Zhonghua Fu Chan Ke Za Zhi. 2020;55(3):166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 49.Zhu H., Wang L., Fang C., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmermann P., Curtis N. COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J. 2020;39(6):469–477. doi: 10.1097/INF.0000000000002700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams S.N., Armitage C.J., Tampe T., Dienes K. Public perceptions and experiences of social distancing and social isolation during the COVID-19 pandemic: a UK-based focus group study. BMJ Open. 2020;10(7) doi: 10.1136/bmjopen-2020-039334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tao J., Zhang X., Zhang X., et al. The time serial distribution and influencing factors of asymptomatic COVID-19 cases in Hong Kong. One Health. 2020;10:100166. doi: 10.1016/j.onehlt.2020.100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao J., Zhang X., Musa S.S., Yang L., He D. High infection fatality rate among elderly and risk factors associated with infection fatality rate and asymptomatic infections of COVID-19 cases in Hong Kong. Front Med. 2021;8:678347. doi: 10.3389/fmed.2021.678347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le T., Takemura T., Moi M.L., Nabeshima T., Nguyen L., Hoang V., et al. Severe Acute Respiratory Syndrome Coronavirus 2 Shedding by Travelers, Vietnam, 2020. Emerg Infect Dis. 2020;26(7):1624–1626. doi: 10.3201/eid2607.200591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meng H., Xiong R., He R., Lin W., Hao B., Zhang L., et al. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J Infect. 2020;81(1):e33–e39. doi: 10.1016/j.jinf.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie W., Chen Z., Wang Q., Song M., Cao Y., Wang L., et al. Infection and disease spectrum in individuals with household exposure to SARS-CoV-2: A family cluster cohort study. J Med Virol. 2021;93(5):3033–3046. doi: 10.1002/jmv.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H., Song S., Chen Z., Bai M., He Z., Fu T., et al. A Cluster Transmission of Coronavirus Disease 2019 and the Prevention and Control Measures in the Early Stage of the Epidemic in Xi'an, China, 2020. Int Med J Experi Clin Res. 2021;27:e929701. doi: 10.12659/MSM.929701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugano N., Ando W., Fukushima W. Cluster of Severe Acute Respiratory Syndrome Coronavirus 2 Infections Linked to Music Clubs in Osaka, Japan. J Infect Dis. 2020;222(10):1635–1640. doi: 10.1093/infdis/jiaa542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun D., Li H., Luv X.X., Xiao H., Ren J., Zhang F.R., et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pedia: WJP. 2020;16(3):251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang K., Zhang J., Wu W., Huang D., He C., Yang Y., et al. A retrospective analysis of the epidemiology, clinical manifestations, and imaging characteristics of familial cluster-onset COVID-19. Ann Trans Med. 2020;8(12):747. doi: 10.21037/atm-20-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Laval F., Grosset-Janin A., Delon F., Allonneau A., Tong C., Letois F., et al. Lessons learned from the investigation of a COVID-19 cluster in Creil, France: effectiveness of targeting symptomatic cases and conducting contact tracing around them. BMC Infect Dis. 2021;21(1):457. doi: 10.1186/s12879-021-06166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nsekuye O., Rwagasore E., Muhimpundu M.A., El-Khatib Z., Ntabanganyimana D., Kamayirese E.N., et al. Investigation of Four Clusters of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Rwanda, 2020. Int J Environ Res Public Health. 2021;18(13):7018. doi: 10.3390/ijerph18137018. [DOI] [PMC free article] [PubMed] [Google Scholar]