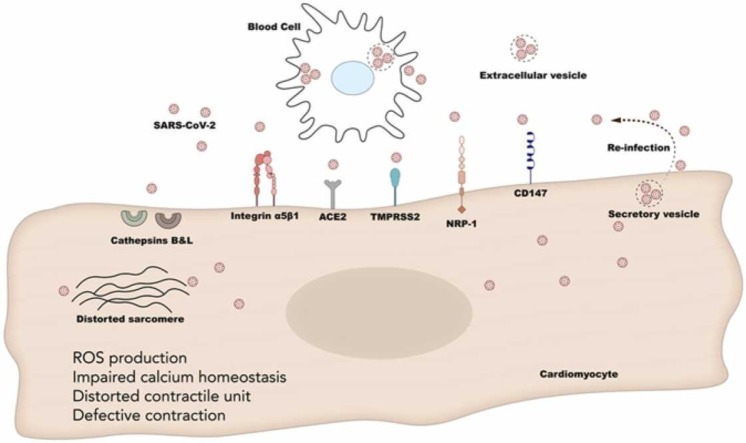
Abstract
SARS-CoV-2 causes respiratory illness with a spectrum of systemic complications. However, the mechanism for cardiac infection and cardiomyocyte injury in COVID-19 patients remains unclear. The current literature supports the notion that SARS-CoV-2 particles access the heart either by the circulating blood cells or by extracellular vesicles, originating from the inflamed lungs, and encapsulating the virus along with its receptor (ACE2). Both cardiomyocytes and pericytes (coronary arteries) express the necessary accessory proteins for access of SARS-CoV-2 particles (i.e. ACE2, NRP-1, TMPRSS2, CD147, integrin α5β1, and CTSB/L). These proteins facilitate the SARS-CoV-2 interaction and entry into the pericytes and cardiomyocytes thus leading to cardiac manifestations. Subsequently, various signaling pathways are altered in the infected cardiomyocytes (i.e. increased ROS production, reduced contraction, impaired calcium homeostasis), causing cardiac dysfunction. The currently adopted pharmacotherapy in severe COVID-19 subjects exhibited side effects on the heart, often manifested by electrical abnormalities. Nonetheless, cardiovascular adverse repercussions have been associated with the advent of some of the SARS-CoV-2 vaccines with no clear mechanisms underlining these complications. We provide herein an overview of the pathways involved with cardiomyocyte in COVID-19 subjects to help promoting pharmacotherapies that can protect against SARS-CoV-2-induced cardiac injuries.
Keywords: SARS-CoV-2, Cardiomyocyte, Cardiac disease, Treatment, Vaccine, Infection
Graphical Abstract
1. The SARS-CoV-2 infectivity
The COVID-19 pandemic has now affected most countries worldwide. At the time of this writing, COVID-19 has claimed more than 5.1 million lives with little over 250 million confirmed cases worldwide.
SARS-CoV-2 is a single-stranded RNA virus that causes a host of symptoms, many of which resemble seasonal influenza. The virus contains a spike (S), envelope (E), and membrane (M) protein subunits, which together make up the viral envelope, in addition to a nucleocapsid (N) protein which holds the RNA genome. The S1 and S2 subunits of the viral envelope catalyze the attachment and fusion of the virus, respectively, with the host cell membrane [1]. SARS-CoV-2 binds primarily to the Angiotensin-Converting Enzyme II receptor (ACE2), which is highly expressed in the lungs and the myocardium [2]. Nonetheless, the molecular mechanisms underlying the virus-host cell interactions remain under extensive investigation. Recent reports indicated a peculiar CGG inserts (coding for arginine) in the spike protein coding sequence providing a gain-of-function for the virus for efficient spreading in humans [3].
Of interest, cardiac involvement with COVID-19 is frequently reported despite the lack of clear mechanisms underlying the development of myocardial cell injury [4], [5], [6]. These mechanisms include direct damage promoted by the interaction of the virus with ACE-2 present in cardiac cells, the injury due to the inflammatory cytokines of the so called “cytokine storm”, and thrombotic events as a cause of myocardial ischemia. We will focus the remaining sections of this review on the various pathways for cardiomyocytes involvement with SARS-CoV-2 and the available data on cardiomyocyte injury and cardiac dysfunction.
2. Cardiomyocyte injury secondary to SARS-CoV-2 infection
As the main contractile element in the heart, injury to cardiomyocytes detrimentally affects cardiac function, added to the poor regenerative capacity of myocardial cells. Shi et al. implicated cardiomyocytes injury, manifesting as increased serum troponin level, as an independent predictor of mortality in patients with SARS-CoV-2 [4]. The signaling pathway through which COVID-19 patients appear to develop myocardial injury is yet to be fully understood however, multiple potential mechanisms have been suggested as detailed in this section.
2.1. Access of SARS-CoV-2 to the heart and strike of cardiomyocytes
The multi-organ failure noticed in COVID-19 patients is still poorly defined, notably cardiac insult and depressed cardiac performance. Original thoughts pointed towards viremia however, evidence in support of this hypothesis was not robust. Besides, the amount of viral particles isolated from the blood of COVID-19 patients was very low, and these viral particles exhibited poor infectivity in cultured cells [7]. On the other hand, large amounts of SARS-CoV-2 mRNA were detected in the blood which is referred to as “RNAemia” [8], [9]. This RNAemia correlated with multi-organ failure and death, and with elevated troponin levels in the blood indicating cardiomyocytes injury [10], in addition to congestive heart failure (CHF) and myocardial infarction [11].
Despite a large number of studies implicating myocarditis in COVID-19 patients, it remains unknown whether cardiomyocytes are permissive to the SARS-CoV-2 infection in case the virus accessed the myocardium. In vivo studies showed that cardiomyocytes express the necessary accessories for the SARS-CoV-2 infection to occur: these include the ACE2 receptor, TMPRSS2, neuropilin-1 receptor (NRP-1), CD147, and integrins (α5β1) [12], [13], [14], [15], [16], [17], [18], [19], [20].
Interestingly, dilated and hypertrophied cardiomyocytes express higher amounts of the ACE2 receptor which could explain the clinical deterioration of COVID-19 patients with cardiac conditions [17], [21], [22]. In the same vein, the existence of the endosomal route of entry of the virus in cardiomyocytes, implicating cathepsins (B and L), makes them more susceptible to the SARS-CoV-2 [23], [24], [25]. While few studies showed viral inclusions in the heart of COVID-19 patients, autopsy studies confirming infection of the myocardial cells are sparse [23], [24]. The SARS-CoV-2 exhibits a high affinity to cardiomyocytes and pericytes in the heart and to a lower extend to macrophages, fibroblasts, or endothelial cells [21], [22], [23]. This implies that the virus in the coronary circulation could infect the pericytes and move forward into the cardiac interstitium. In fact, these viral particles were found in biopsies of human hearts either in the interstitial space, or in inflammatory cells originating from the lungs, which confirms the accessibility of SARS-CoV-2 to the myocardium in COVID-19 patients [26], [27], [28], [29]. Of interest, the first version SARS-CoV showed high infectivity of blood cells, and the current SARS-CoV-2 was also suggested to spread in circulating blood cells from the injured lungs into other tissues, namely the spleen and the heart, with a capacity of replication in these cells (i.e. Monocytes, B and T lymphocytes) [24], [25], [26], [29]. Other studies reported that SARS-CoV-2 employs the endosomal cysteine proteases cathepsins B/L (CTSL and/or CTSB) for cell entry [23], [24], [25]. In fact, both atrial and ventricular cardiomyocytes are possibly susceptible to SARS-CoV-2 infection by involving the CTSB/CTSL for S protein priming. CTSB and CTSL mRNA expression level was found to be high in human embryonic hearts and enriched in cardiomyocytes. This could constitute another cellular mechanism of cardiac injury in COVID-19 subjects [17], [25]. In the same vein, SARS-CoV-2 particles were found in extracellular vesicles along with the ACE2 receptors, which implies patrolling of protected SARS-CoV-2 viral particles in the blood stream of COVID-19 individuals, thus promoting the infection of several tissues including the myocardium [30], [31], [32].
In addition to the above-mentioned proteins, the NRP-1 is a multidomain cell surface membrane receptor involved in cell adhesion and its ablation causes embryonic lethality and abnormalities in both the nervous and cardiovascular systems [33]. Recently, it has been shown that NRP-1 facilitates SARS-CoV-2 cell entry and infectivity [18]. Thus, the expression of NRP-1 in cardiomyocytes and endothelial cells facilitates the damage of cardiomyocyte by the SARS-CoV-2 leading to cellular injury [34], [35]. In the same vein, the receptor for cyclophilin A (CyPA), CD147, is also characterized as a novel route for SARS-CoV-2 cell entry [36]. This receptor is expressed in both cardiomyocytes and pericytes, which constitutes another route for the infection of these cells by the SARS-CoV-2 particles [19], [37]. Of note, the expression of CD147 is increased in hypertrophied cardiomyocytes from left ventricles, which increases the risk of cardiac injury in subjects suffering from cardiac hypertrophy [19]. Similarly, the α5β1 integrin also facilitate SARS-CoV-2 binding to the ACE receptor [20], and their expression in the pericytes [38] and cardiomyocytes [39] promotes the accessibility of this virus to cardiomyocytes.
Interestingly, the presence of SARS-CoV-2 particles in autopsies of infected hamsters and humans was confirmed, along with the ability of the virus to infect cardiomyocytes and to reduce their contractility by altering the gene expression of contractile proteins and calcium homeostasis. Not only are cardiomyocytes struck by the SARS-CoV-2, but also, they were able to amplify and to release the virus in the extracellular space [40], [41]. These studies show a permissivity of cardiomyocytes to the virus along with an alarming risk of re-infection once these particles are released in the myocardial interstitial milieu ( Fig. 1). All together, these studies indicate that the heart is prone to either direct infection with the SARS-CoV-2 particles, or it is secondarily damaged by high viral loads in the lung (leading to RNAemia/cytokine storm). Therefore, it is imperative to understand the molecular cascades that might be affected by the SARS-CoV-2 infection of cardiomyocytes, thus leading to the impaired cardiac function in COVID-19 patients.
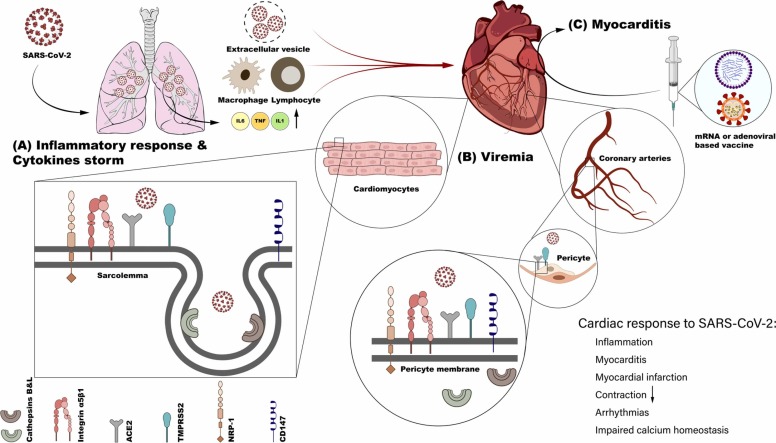
Fig. 1.
Proposed access routes for SARS-CoV-2 particles to the heart. Following access to the lung, SARS-CoV-2 induces an inflammatory reaction leading to the release of inflammatory mediators and recruitment of inflammatory cells (A). SARS-CoV-2 particles exit pulmonary tissues via extracellular vesicles, or in circulating blood cells as “Trojan Horse”. (B) SARS-CoV-2 can either infect pericytes or cardiomyocytes since both express necessary proteins for this process, thus leading to cardiac abnormalities. (C) Myocarditis has been noticed in vaccinated individuals using both the mRNA or the virus-based vaccines.
2.2. SARS-CoV-2 damage induced in cardiomyocytes and the possible effects on signaling cascades
Based on the available data in the literature we shed light on possible signaling pathways that could be altered due to the SARS-CoV-2 strike of cardiomyocytes, which may help us understand the detrimental effects of SARS-CoV-2 on myocardial cells.
The binding of SARS-CoV-2 to the ACE2 receptor mainly leads to two events: (1) internalization of the ACE2 receptor via endogenous transmembrane serine protease 2 (TMPRSS2), and (2) initiation of a cellular process called shedding, managed by A disintegrin and metalloprotease 17 (ADAM17) [22], [42]. This particular enzyme, also known as TNF-α-converting enzyme, is usually expressed at the sarcolemma and facilitates the cleavage of a specific domain in ACE2 which gets internalized along with the virus [43]. Loss of ACE2 favors the binding of angiotensin II (Ang II) with distinct receptors, the angiotensin II type 1 receptor (AT1R) and the angiotensin II type 2 receptor (AT2R) [44]. This damages the cardiomyocytes and consequently leads to demise and replacement with fibrosis, or cardiac hypertrophy, and/or reactive oxygen species (ROS) production, and apoptosis [45], [46]. Further damage to the cardiomyocytes results from loss of ACE2 due to increased concentrations of Ang (1−7), a metabolic product of Ang I that binds to the MAS-receptor and prevents cardiomyocyte growth through inhibition of the MAPK ERK1/2 activity [47]. In parallel, Ang (1−7) activates the phosphatidylinositol 3-kinase (PI3-K)-protein kinase B (Akt)-pathway, resulting in nitric oxide synthase 3 (NOS3) activation and NO generation which causes cardiomyocyte damage [48]. Gomes et al. showed that Ang-(1−7) blocks the nuclear factor of activated T cells (NFAT) from translocation to the nucleus and therefore downregulating hypertrophic genes [49]. The crosstalk between Ang II and Ang (1−7) signaling pathways in the absence of ACE2 in cardiomyocytes would likely lead to more deleterious effects on the heart [50]. Reports from the earlier studies on SARS-CoV-2 indicated that ACE2 activates a downstream signaling cascade from Mas receptors, and this coincided with the activation of both proapoptotic and pro-survival signaling pathways during viral infection and replication [51], [52]. Similar to SARS-CoV-2, cardiac tissue damage was previously reported in MERS-CoV patients; however, the mechanisms of heart injury due to MERS infection remain unclear [53]. In short, ACE2 downregulation by SARS-CoV-2 may weaken its function, diminish its anti-inflammatory role, and its protective role in cardiovascular diseases. The interaction of SARS-CoV-2 with ACE2 unequivocally results in cardiomyocyte damage similarly to that of alveolar cell damage [54].
These pathways further support the virus-induced downregulation of ACE2 and the initiation of a cytokine storm combined with overreactions of the immune system that further promotes injury to the infected cardiomyocytes [6], [55]. As indicated earlier, injured cardiomyocytes by the SARS-CoV-2 have increased expression levels of the chemokine ligand 2 (CCL2), thus leading to monocyte recruitment [56]. The mechanisms responsible for chemokine upregulation in the SARS-CoV-2 infected myocytes have not been fully understood however, based on previous studies, MCP-1 binds only to chemokine receptor 2 (CCR2) [57]. Activation of this receptor is known to promote several downstream signaling pathways including G-proteins, JAK/STAT, and the mitogen-activated protein (MAP) kinase pathways [58], [59]. Recently, a case series of COVID-19 patients with cardiovascular disease were found to be positive for cardiolipin antibody; cardiolipin is a component of the inner mitochondrial membrane and is considered a marker for cellular damage and myocardial injury [60], [61], [62].
An additional possible involvement for SARS CoV-2 to directly damage the cardiomyocytes is the activation of phospholipase C (PLC) and protein kinase C (PKC) pathways as a consequence of Ang II binding to AT1-R [63]. PKC is usually activated by the phosphorylation of the active segment of the phosphoinositide-dependent kinase (PDK1) and the phosphorylation of the hydrophobic motif by mTOR Complex 2 (mTORC2) [64]. The activation of at least one of the 12 isoforms of PKC has been reported to mediate hypertrophy and survival signaling in cardiomyocytes [65]. PKC also phosphorylates the sodium-calcium exchanger (NCX), which interferes with the cardiac electrical activity and calcium homeostasis in cardiomyocytes [66]. These changes in calcium homeostasis combined with reduced contractility (down to complete cessation in beating) were recently documented in SARS-CoV-2 infected cardiomyocytes [40], [67]. Furthermore, many of these above-mentioned pathways signal through the mammalian target of rapamycin (mTOR) that could initiate autophagy in cardiomyocytes [68].
The largely released cytokines such as TNFα, IFNγ, and IL-4 promote cellular pro-inflammatory pathways by different cytokine receptors. For example, binding of TNFα to the TNFα receptor 1 (TNFR1) recruits several adaptor proteins, out of which TRADD-containing complex activates the IKK complex, thus resulting in IκBα phosphorylation and subsequent ubiquitin-dependent proteasomal degradation and activation of NF-κB [69]. Subsequently, this activates several well-studied apoptotic signaling pathways, including JNK and p38 MAP kinases, caspase 8, and the NF-κB pathway that all together damage the cardiomyocytes [70], [71]. Interestingly, NF-κB signaling is also activated by IL-6 leading to cardiotoxic activation of cell death pathways [72]. In addition, the release of interleukin-4 (IL-4) is often associated with cardiac fibrosis during hypertension and heart failure [73].
Thus, it is imperative to extend our understanding with regard to the molecular dynamics of the SARS-CoV-2 in cardiomyocytes to prevent their damage/loss. Nonetheless, some of the cardiac injuries stated in this section may not occur during infection but later, after clearance from SARS-CoV-2, which falls within the category of the virus long-term sequelae including myocarditis, fibrosis, myocardial infarction, and sustained hypertension. In fact, the ability of the SARS-CoV-2 to increase the production of reactive oxygen species in several cells (lung and blood) including cardiomyocytes puts the heart at high risk of pathological remodeling [40], [56].
3. Causes of cardiovascular manifestations during and post-COVID-19 remission
Due to the cellular injury in the heart, many questions related to myocardial sequelae in COVID-19 patients remain unanswered calling for a profound characterization and follow-up of cardiac hemodynamics during and post-COVID-19 recovery. While the longest reported period of viral shedding in surviving patients was 37 days, the symptoms may continue for up to 60 days after remission from illness [74], [75]. Irrespective of CVD history, myocardial injuries are prevalent in COVID-19 patients, including acute myocardial infarction and increased troponin release associated with higher mortality rates [65], [66], [76]. Almost 12% of patients without a known history of CVD had elevated troponin or experienced cardiac arrest during hospitalization in China [5].
Of interest, coagulation markers increase during the COVID-19 episodes but sustain (or peak) post-COVID-19 which favors the development of strokes, myocardial infarction, and ischemic diseases leading to multiple organs dysfunction/failure [77], [78], [79]. More profound complications extend to thrombosis in larger parts of the circulation including deep veins, arteries, and cardiac chambers [80], [81], [82]. There are several markers for the severity of coagulation with D-dimer being the most significant one [83], [84]. Of the various factors contributing to blood coagulation in COVID-19 individuals, the developed “cytokine storm” perpetuates a hypercoagulable state [83], [85]. To this effect, patients with coronary artery disease (CAD) are more prone to the detrimental effects of the cytokine storm through an exhibition of inflamed plaque instability and rupture, after recovery from COVID-19, thus leading to post-COVID-19 MI-induced complications [86]. Although myocarditis was observed in COVID-19 patients, the appearance of this illness in individuals post-COVID-19 is associated with depressed cardiac performance and merits further investigations [87], [88].
The detrimental effect exerted by the SARS-CoV-2 on the myocardium also extends to the electrocardiographic abnormalities. For example, both atrial and ventricular arrhythmias were observed [89], thus indicating unlocalized damage of the myocardium probably due to the myocarditis, or direct cardiomyocyte damage. Inflammation of the myocardium, particularly parts of the conduction system (SA node, AV node, or Purkinje fibers) infrequently generate arrhythmias that could be deadly. The arrhythmic recordings observed in COVID-19 patients are mostly ST-elevation myocardial infarction (STEMI), or NSTEMI [88], [90], inverted T wave (NSTEMI), and inverted P wave [91].
In addition to these cardiac injuries, hypertension was noticed in COVID-19 patients which increased their death rate [92]. The diffuse endotheliitis and vascular injury observed in patients with COVID-19 may have lasting effects on autonomic regulatory mechanisms namely the baroreceptor mechanism. The proposed COVID-19-associated dysautonomia may cause impairment to blood pressure and heart responses and hypertension in these patients [93]. Among hospitalized patients with COVID-19, the prevalence of acute kidney injury (AKI) is as high as 46% [94]. It is conceivable that the vascular injury and the kidney injury in COVID-19 patients might lead to hypertension in these patients and to more severe hypertension when the hypertension is a pre-existing condition to COVID-19.
One of the major concerns is the possible ability of the SARS-CoV-2 to adopt dormancy since it infects immune privileged sites in the body (i.e. brain, eye, and testis). Reports indicated reoccurrence of SARS-CoV-2 after clinical remission [95], [96], which implies latency of the virus since it is accessible to the testes and the brain [97], [98]. In this case, a viral wave may reoccur in these subjects yet sparking another vexing insult to the heart. This adds to the long-term sequelae of the SARS-CoV-2 attack seen in COVID-19 patients after remission.
4. Potential cardiac side effects of COVID-19 treatment
In addition to potential injury associated with the illness itself, some of the pharmacotherapies used to treat patients with COVID-19 may also have potential side effects specific for the heart. To date, there is no specific treatment that can eradicate SARS-CoV-2 infection. However, several classes of anti-viral medications and others have been used to improve outcomes of patients with COVID-19. An overview of medications with potential benefits in this population is provided in Table 1.
Table 1.
List of currently approved and investigational treatments for COVID-19 and their potential cardiac side effects.
| Class | Drug | Mechanism | Efficacy and Dosing | Potential cardiac side effects |
|---|---|---|---|---|
| Antiviral | Remdesivir | Nucleotide analog and inhibits viral RNA-dependent RNA polymerase | In hospitalized patients not requiring mechanical ventilation; 200 mg IV initially and then 100 mg once daily for 4–9 days (total 5–10 days) [103] | QT prolongation, Bradycardia. |
| Favipiravir | RNA polymerase inhibitor | Limited data; high-quality trials are ongoing. | QTc prolongation (maybe due to combination with other antivirals) [104], [105] | |
| Lopinavir/Ritonavir | Lopinavir is a protease inhibitor. Ritonavir increases bioavailability of lopinavir via CYP3A4 inhibition. | Role in COVID-19 treatment is controversial. | Low-density Lipoprotein (LDL) elevation leading to cardiovascular disease (CVD), QT prolongation [106], [107] | |
| Molnupiravir | Enhances viral RNA mutations and impairs virus replication [108] | Phase 3 clinical trials still ongoing – 200/400/800 mg twice daily for 5 days [109] | No significant side effects | |
| Oseltamivir | Neuraminidase inhibitor [110] | Limited data on efficacy against COVID-19 | No significant side effects | |
| Umifenovir | Inhibition of viral membrane fusion [111] | Limited data on efficacy against COVID-19. 200 mg PO (1 capsule is 100 mg) thrice a day for up to 14 days [112] | No significant side effects | |
| Glucocorticoids | Dexamethasone | Immunosuppressive and an anti-inflammatory agent | Reduces all-cause mortality in hospitalized patients with severe COVID-19 disease. Administered as 6 mg IV daily for 10 days or until discharge. | Decreased resting heart rate [113] |
| Immunosuppressants | Tocilizumab | IL-6 receptor blocker | Reduces all-cause mortality in hospitalized patients requiring supplemental oxygen within 24 h of admission to ICU. 400 mg IV – one dose only [114] | Increased risk of secondary infections. Avoid use in conjunction with JK inhibitors. |
| Barcitinib | Janus-kinase inhibitor | May reduce all-cause mortality in hospitalized COVID-19 patients requiring supplemental oxygen or non-invasive ventilation. 4 mg PO once daily for up to 14 days [115] | Do not use in conjunction with IL-6 inhibitors. |
5. The cardiac adverse effects of the currently approved COVID-19 vaccines
At the time of this writing, the WHO reported that around 7.0 billion doses of vaccines have been administered worldwide. The currently approved vaccines by the WHO are five; AZD1222 (AstraZeneca), BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), BBIBP-CorV (Sinopharm), CoronaVac (Sinovac), and Ad26. COV2. S (Johnson & Johnson). While there is a remarkable variation in the technologies used for immunization as well as the respective efficacies between the different vaccines, a significant protection was notice in primed individuals who were administered any of these WHO-approved vaccines [99].
Nonetheless, a report by the US Centers for Disease Control and Prevention’s (CDC) safety committee indicated a “likely association” between Moderna and Pfizer-BioNTech COVID-19 vaccines and myocarditis and pericarditis in some young adults [100], manifested with chest discomfort/pain, increase in Troponin release and abnormal ECG tracings that resolved with or without treatment [101]. Although the CDC’s Advisory Committee on Immunization Practices expected more cases in individuals aged 16–24 who got vaccinated with Moderna and Pfizer-BioNTech COVID-19 vaccines, the CDC continues to recommend the vaccine for individuals above 12 years since the protective effect of the vaccine outweighs the resolving myocarditis in the noticed vaccinated individuals. The exact cause of these side effects is still unclear, but they could be attributed to the molecular mimicry of the spike proteins of the SARS-CoV-2 and self-antigens, thus triggering similar immune responses and complications to those of the SARS-CoV-2 infection [101]. In addition, thrombotic thrombocytopenia is associated with immunizations with the AZD1222 and Ad26. COV2. S vaccines were noticed [102] which could have consequences in the near and distant future.
6. Translational outlook
Since COVID-19 may result in cardiac injury in susceptible individuals, clarifying mechanisms underlying the interaction between the SARS-CoV2 virus and myocardial cells is critical. Several mechanisms have been identified, including direct infection of cardiomyocytes by viral particles, myocardial injury during cytokine storms, or infection of cardiac pericytes. Additional molecular pathways responsible for cardiac injury during COVID-19 illness may be elucidated utilizing available cardiac and molecular imaging techniques. In addition to advancing our knowledge about COVID-19, this search may identify therapeutic targets that can be used in high-risk patients in whom cardiac dysfunction during COVID-19 may have serious implications.
7. Conclusion
There is a clear implication of the SARS-CoV-2 in cardiac complications either through direct infection, or as a long-term injurious sequelae post-remission. The likely route of access of the SARS-CoV-2 to the myocardium is via extracellular vesicles and/or circulating blood cells as a Trojan horse for viral particles. SARS-CoV-2 infects mainly the pericytes (coronary arteries) and cardiomyocytes via the ACE2/TMPRSS2 proteins, NRP-1, CD147, and integrin α5β1, or through the endosomal pathway involving CTSB/CTSL. The current adopted treatment must be monitored closely in COVID-19 subjects due to some side effects on the heart. Nonetheless, the approved vaccines may potentially have additional cardiac side effects, albeit rare. More studies are warranted to decipher the molecular interactions of SARS-CoV-2 with cardiomyocytes to identify pharmaco-protective strategies against cardiac adverse effects of this infectious disease.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Data availability
No data was used for the research described in the article.
References
- 1.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Y.M., Xu G., Wang B., Liu B.C. Cytokine storm syndrome in coronavirus disease 2019: a narrative review. J. Intern. Med. 2021;289(2):147–161. doi: 10.1111/joim.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cappy P., Candotti D., Sauvage V., Lucas Q., Boizeau L., Gomez J., Enouf V., Chabli L., Pillonel J., Tiberghien P., Morel P., Laperche S. No evidence of SARS-CoV-2 transfusion transmission despite RNA detection in blood donors showing symptoms after donation. Blood. 2020;136(16):1888–1891. doi: 10.1182/blood.2020008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Järhult J.D., Hultström M., Bergqvist A., Frithiof R., Lipcsey M. The impact of viremia on organ failure, biomarkers and mortality in a Swedish cohort of critically ill COVID-19 patients. Sci. Rep. 2021;11(1):7163. doi: 10.1038/s41598-021-86500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prebensen C., Myhre P.L., Jonassen C., Rangberg A., Blomfeldt A., Svensson M., Omland T., Berdal J.E. Severe acute respiratory syndrome coronavirus 2 RNA in plasma is associated with intensive care unit admission and mortality in patients hospitalized with coronavirus disease 2019. Clin. Infect. Dis. 2021;73(3):e799–e802. doi: 10.1093/cid/ciaa1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi W.Y., Gemberling M., Wang J., Holdway J.E., Shen M.C., Karlstrom R.O., Poss K.D. In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration. Development. 2013;140(3):660–666. doi: 10.1242/dev.088526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqi H.K., Weber B., Zhou G., Regan J., Fajnzylber J., Coxen K., Corry H., Yu X.G., DiCarli M., Li J.Z., Bhatt D.L. Increased prevalence of myocardial injury in patients with SARS-CoV-2 viremia. Am. J. Med. 2021;134(4):542–546. doi: 10.1016/j.amjmed.2020.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pérez-Bermejo J.A., Kang S., Rockwood S.J., Simoneau C.R., Joy D.A., Ramadoss G.N., Silva A.C., Flanigan W.R., Li H., Nakamura K., Whitman J.D., Ott M., Conklin B.R., McDevitt T.C. SARS-CoV-2 infection of human iPSC-derived cardiac cells predicts novel cytopathic features in hearts of COVID-19 patients. bioRxiv. 2020 doi: 10.1126/scitranslmed.abf7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv. Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J., Petitjean S.J.L., Koehler M., Zhang Q., Dumitru A.C., Chen W., Derclaye S., Vincent S.P., Soumillion P., Alsteens D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun. 2020;11(1):4541. doi: 10.1038/s41467-020-18319-6. 4541-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson E.L., Alkass K., Bergmann O., Maguire J.J., Roderick H.L., Davenport A.P. Genes encoding ACE2, TMPRSS2 and related proteins mediating SARS-CoV-2 viral entry are upregulated with age in human cardiomyocytes. J. Mol. Cell. Cardiol. 2020;147:88–91. doi: 10.1016/j.yjmcc.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bristow M.R., Zisman L.S., Altman N.L., Gilbert E.M., Lowes B.D., Minobe W.A., Slavov D., Schwisow J.A., Rodriguez E.M., Carroll I.A., Keuer T.A., Buttrick P.M., Kao D.P. Dynamic regulation of SARS-Cov-2 binding and cell entry mechanisms in remodeled human ventricular myocardium. JACC Basic Transl. Sci. 2020;5(9):871–883. doi: 10.1016/j.jacbts.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Österlund P., Joensuu M., Meunier F.A., Butcher S.J., Winkler M.S., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang F.C., Wang H.Y., Ma M.M., Guan T.W., Pan L., Yao D.C., Chen Y.L., Chen W.B., Tu Y.S., Fu X.D. CyPA-CD147-ERK1/2-cyclin D2 signaling pathway is upregulated during rat left ventricular hypertrophy. Sheng Li Xue Bao. 2015;67(4):393–400. [PubMed] [Google Scholar]
- 20.Beddingfield B.J., Iwanaga N., Chapagain P.P., Zheng W., Roy C.J., Hu T.Y., Kolls J.K., Bix G.J. The integrin binding peptide, ATN-161, as a novel therapy for SARS-CoV-2 infection, JACC. Basic Transl. Sci. 2021;6(1):1–8. doi: 10.1016/j.jacbts.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker N.R., Chaffin M., Bedi K.C., Jr., Papangeli I., Akkad A.D., Arduini A., Hayat S., Eraslan G., Muus C., Bhattacharyya R.P., Stegmann C.M., Margulies K.B., Ellinor P.T. Myocyte-specific upregulation of ACE2 in cardiovascular disease: implications for SARS-CoV-2-mediated myocarditis. Circulation. 2020;142(7):708–710. doi: 10.1161/CIRCULATIONAHA.120.047911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicin L., Abplanalp W.T., Mellentin H., Kattih B., Tombor L., John D., Schmitto J.D., Heineke J., Emrich F., Arsalan M., Holubec T., Walther T., Zeiher A.M., Dimmeler S. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur. Heart J. 2020;41(19):1804–1806. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao M.M., Yang W.L., Yang F.Y., Zhang L., Huang W.J., Hou W., Fan C.F., Jin R.H., Feng Y.M., Wang Y.C., Yang J.K. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target Ther. 2021;6(1):134. doi: 10.1038/s41392-021-00558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scudellari M. How the coronavirus infects cells - and why Delta is so dangerous. Nature. 2021;595(7869):640–644. doi: 10.1038/d41586-021-02039-y. [DOI] [PubMed] [Google Scholar]
- 25.Yang J., Chen T., Zhou Y. Mediators of SARS-CoV-2 entry are preferentially enriched in cardiomyocytes. Hereditas. 2021;158(1):4. doi: 10.1186/s41065-020-00168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., Sepe P.A., Resasco T., Camporotondo R., Bruno R., Baldanti F., Paolucci S., Pelenghi S., Iotti G.A., Mojoli F., Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escher F., Pietsch H., Aleshcheva G., Bock T., Baumeier C., Elsaesser A., Wenzel P., Hamm C., Westenfeld R., Schultheiss M., Gross U., Morawietz L., Schultheiss H.-P. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020;7(5):2440–2447. doi: 10.1002/ehf2.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindner D., Fitzek A., Bräuninger H., Aleshcheva G., Edler C., Meissner K., Scherschel K., Kirchhof P., Escher F., Schultheiss H.-P., Blankenberg S., Püschel K., Westermann D. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietsch H., Escher F., Aleshcheva G., Baumeier C., Morawietz L., Elsaesser A., Schultheiss H.-P. Proof of SARS-CoV-2 genomes in endomyocardial biopsy with latency after acute infection. Int. J. Infect. Dis. 2021;102:70–72. doi: 10.1016/j.ijid.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunez Lopez Y.O., Casu A., Pratley R.E. Investigation of extracellular vesicles from SARS-CoV-2 infected specimens: a safety perspective. Front. Immunol. 2021;12(1209) doi: 10.3389/fimmu.2021.617042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elrashdy F., Aljaddawi A.A., Redwan E.M., Uversky V.N. On the potential role of exosomes in the COVID-19 reinfection/reactivation opportunity. J. Biomol. Struct. Dyn. 2021;39(15):5831–5842. doi: 10.1080/07391102.2020.1790426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassanpour M., Rezaie J., Nouri M., Panahi Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104422. 104422-104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawasaki T., Kitsukawa T., Bekku Y., Matsuda Y., Sanbo M., Yagi T., Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126(21):4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 34.Soker S., Takashima S., Miao H.Q., Neufeld G., Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Cao Y., Yamada S., Thirunavukkarasu M., Nin V., Joshi M., Rishi M.T., Bhattacharya S., Camacho-Pereira J., Sharma A.K., Shameer K., Kocher J.-P.A., Sanchez J.A., Wang E., Hoeppner L.H., Dutta S.K., Leof E.B., Shah V., Claffey K.P., Chini E.N., Simons M., Terzic A., Maulik N., Mukhopadhyay D. Cardiomyopathy and worsened ischemic heart failure in SM22-α Cre-mediated neuropilin-1 null mice: dysregulation of PGC1α and mitochondrial homeostasis. Arterioscler. Thromb. Vasc. Biol. 2015;35(6):1401–1412. doi: 10.1161/ATVBAHA.115.305566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P., Wei D., Zhang Y., Sun X.X., Gong L., Yang X., He L., Zhang L., Yang Z., Geng J.J., Chen R., Zhang H., Wang B., Zhu Y.M., Nan G., Jiang J.L., Li L., Wu J., Lin P., Huang W., Xie L., Zheng Z.H., Zhang K., Miao J.L., Cui H.Y., Huang M., Zhang J., Fu L., Yang X.M., Zhao Z., Sun S., Gu H., Wang Z., Wang C.F., Lu Y., Liu Y.Y., Wang Q.Y., Bian H., Zhu P., Chen Z.N. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target Ther. 2020;5(1):283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan P., Zhao H., Zhang X., Li Q., Qu J., Zuo S., Yang F., Liang G., Zhang J.H., Liu X., He H., Feng H., Chen Y. Cyclophilin a signaling induces pericyte-associated blood-brain barrier disruption after subarachnoid hemorrhage. J. Neuroinflamm. 2020;17(1):16. doi: 10.1186/s12974-020-1699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner C.J., Badu-Nkansah K., Crowley D., van der Flier A., Hynes R.O. Integrin-α5β1 is not required for mural cell functions during development of blood vessels but is required for lymphatic-blood vessel separation and lymphovenous valve formation. Dev. Biol. 2014;392(2):381–392. doi: 10.1016/j.ydbio.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valencik M.L., McDonald J.A. Cardiac expression of a gain-of-function alpha(5)-integrin results in perinatal lethality. Am. J. Physiol. Heart Circ. Physiol. 2001;280(1):H361–H367. doi: 10.1152/ajpheart.2001.280.1.H361. [DOI] [PubMed] [Google Scholar]
- 40.Yang L., Nilsson-Payant B.E., Han Y., Jaffré F., Zhu J., Wang P., Zhang T., Redmond D., Houghton S., Møller R., Hoagland D., Carrau L., Horiuchi S., Goff M., Lim J.K., Bram Y., Richardson C., Chandar V., Borczuk A., Huang Y., Xiang J., Ho D.D., Schwartz R.E., enOever B.R. t, Evans T., Chen S. Cardiomyocytes recruit monocytes upon SARS-CoV-2 infection by secreting CCL2. Stem Cell Rep. 2021;16:2274–2288. doi: 10.1016/j.stemcr.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navaratnarajah C.K., Pease D.R., Halfmann P., Taye B., Barkhymer A., Howell K.G., Charlesworth J.E., Christensen T.A., Kawaoka Y., Cattaneo R., Schneider J.W. Highly efficient SARS-CoV-2 infection of human cardiomyocytes: spike protein-mediated cell fusion and its inhibition. J. Virol. 2021;95 doi: 10.1128/JVI.01368-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel V.B., Zhong J.-C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280(34):30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wakui H., Tamura K., Tanaka Y., Matsuda M., Bai Y., Dejima T., Masuda S., Shigenaga A., Maeda A., Mogi M., Ichihara N., Kobayashi Y., Hirawa N., Ishigami T., Toya Y., Yabana M., Horiuchi M., Minamisawa S., Umemura S. Cardiac-specific activation of angiotensin II type 1 receptor-associated protein completely suppresses cardiac hypertrophy in chronic angiotensin II-infused mice. Hypertension. 2010;55(5):1157–1164. doi: 10.1161/HYPERTENSIONAHA.109.147207. [DOI] [PubMed] [Google Scholar]
- 46.Paradis P., Dali-Youcef N., Paradis F.W., Thibault G., Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc. Natl. Acad. Sci. USA. 2000;97(2):931–936. doi: 10.1073/pnas.97.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos R.A., Castro C.H., Gava E., Pinheiro S.V., Almeida A.P., Paula R.D., Cruz J.S., Ramos A.S., Rosa K.T., Irigoyen M.C., Bader M., Alenina N., Kitten G.T., Ferreira A.J. Impairment of in vitro and in vivo heart function in angiotensin-(1-7) receptor MAS knockout mice. Hypertension. 2006;47(5):996–1002. doi: 10.1161/01.HYP.0000215289.51180.5c. [DOI] [PubMed] [Google Scholar]
- 48.Vecchione C., Patrucco E., Marino G., Barberis L., Poulet R., Aretini A., Maffei A., Gentile M.T., Storto M., Azzolino O., Brancaccio M., Colussi G.L., Bettarini U., Altruda F., Silengo L., Tarone G., Wymann M.P., Hirsch E., Lembo G. Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3Kgamma. J. Exp. Med. 2005;201(8):1217–1228. doi: 10.1084/jem.20040995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomes E.R.M., Santos R.A.S., Guatimosim S. Angiotensin-(1-7)-mediated signaling in cardiomyocytes. Int. J. Hypertens. 2012;2012 doi: 10.1155/2012/493129. 493129-493129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomes E.R., Lara A.A., Almeida P.W., Guimarães D., Resende R.R., Campagnole-Santos M.J., Bader M., Santos R.A., Guatimosim S. Angiotensin-(1-7) prevents cardiomyocyte pathological remodeling through a nitric oxide/guanosine 3′,5′-cyclic monophosphate-dependent pathway. Hypertension. 2010;55(1):153–160. doi: 10.1161/HYPERTENSIONAHA.109.143255. [DOI] [PubMed] [Google Scholar]
- 51.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc. Natl. Acad. Sci. USA. 2008;105(22):7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuba K., Imai Y., Rao S., Jiang C., Penninger J.M. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J. Mol. Med. 2006;84(10):814–820. doi: 10.1007/s00109-006-0094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizutani T. Signal transduction in SARS-CoV-infected cells. Ann. N. Y. Acad. Sci. 2007;1102(1):86–95. doi: 10.1196/annals.1408.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;202(5):756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magadum A., Kishore R. Cardiovascular manifestations of COVID-19 infection. Cells. 2020;9(11):2508. doi: 10.3390/cells9112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen S., Yang L., Nilsson-Payant B., Han Y., Jaffré F., Zhu J., Wang P., Zhang T., Redmond D., Houghton S., Møller R., Hoagland D., Horiuchi S., Acklin J., Lim J., Bram Y., Richardson C., Chandar V., Borczuk A., Huang Y., Xiang J., Ho D., Schwartz R., tenOever B., Evans T. SARS-CoV-2 infected cardiomyocytes recruit monocytes by secreting CCL2. Res. Sq. 2020 [Google Scholar]
- 57.Morimoto H., Takahashi M. Role of monocyte chemoattractant protein-1 in myocardial infarction. Int. J. Biomed. Sci. 2007;3(3):159–167. [PMC free article] [PubMed] [Google Scholar]
- 58.Mellado M., Rodríguez-Frade J.M., Vila-Coro A.J., Fernández S., Martín de Ana A., Jones D.R., Torán J.L., Martínez A.C. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. Embo J. 2001;20(10):2497–2507. doi: 10.1093/emboj/20.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arai H., Charo I.F. Differential regulation of G-protein-mediated signaling by chemokine receptors. J. Biol. Chem. 1996;271(36):21814–21819. doi: 10.1074/jbc.271.36.21814. [DOI] [PubMed] [Google Scholar]
- 60.Hossri S., Shadi M., Hamarsha Z., Schneider R., El-Sayegh D. Clinically significant anticardiolipin antibodies associated with COVID-19. J. Crit. Care. 2020;59:32–34. doi: 10.1016/j.jcrc.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H., Wang C., Zhao J., Sun X., Tian R., Wu W., Wu D., Ma J., Chen Y., Zhang D., Xie J., Yan X., Zhou X., Liu Z., Wang J., Du B., Qin Y., Gao P., Qin X., Xu Y., Zhang W., Li T., Zhang F., Zhao Y., Li Y., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jizzini M., Shah M., Zhou K. SARS-CoV-2 and anti-cardiolipin antibodies. Clin. Med. Insights Case Rep. 2020;13 doi: 10.1177/1179547620980381. 1179547620980381-1179547620980381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Groß S., Jahn C., Cushman S., Bär C., Thum T. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: from basic science to clinical implications. J. Mol. Cell. Cardiol. 2020;144:47–53. doi: 10.1016/j.yjmcc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearce L.R., Komander D., Alessi D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010;11(1):9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 65.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F., Zhao S., Somani S., Van Vleck T., Vaid A., Chaudhry F., De Freitas J.K., Fayad Z.A., Pinney S.P., Levin M., Charney A., Bagiella E., Narula J., Glicksberg B.S., Nadkarni G., Mancini D.M., Fuster V. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J. Am. Coll. Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buja L.M., Wolf D.A., Zhao B., Akkanti B., McDonald M., Lelenwa L., Reilly N., Ottaviani G., Elghetany M.T., Trujillo D.O., Aisenberg G.M., Madjid M., Kar B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020;48 doi: 10.1016/j.carpath.2020.107233. 107233-107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siddiq M.M., Chan A.T., Miorin L., Yadaw A.S., Beaumont K.G., Kehrer T., Cupic A., White K.M., Tolentino R.E., Hu B., Stern A.D., Tavassoly I., Hansen J., Sebra R., Martinez P., Prabha S., Dubois N., Schaniel C., Iyengar-Kapuganti R., Kukar N., Giustino G., Sud K., Nirenberg S., Kovatch P., Albrecht R.A., Goldfarb J., Croft L., McLaughlin M.A., Argulian E., Lerakis S., Narula J., García-Sastre A., Iyengar R. Functional effects of cardiomyocyte injury in COVID-19. J. Virol. 2021 doi: 10.1128/JVI.01063-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou L., Ma B., Han X. The role of autophagy in angiotensin II-induced pathological cardiac hypertrophy. J. Mol. Endocrinol. 2016;57(4):R143–r152. doi: 10.1530/JME-16-0086. [DOI] [PubMed] [Google Scholar]
- 69.Hayden M.S., Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 70.Gordon J.W., Shaw J.A., Kirshenbaum L.A. Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ. Res. 2011;108(9):1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 71.Hamid T., Gu Y., Ortines R.V., Bhattacharya C., Wang G., Xuan Y.T., Prabhu S.D. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119(10):1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Libermann T.A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell. Biol. 1990;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peng H., Sarwar Z., Yang X.-P., Peterson E.L., Xu J., Janic B., Rhaleb N., Carretero O.A., Rhaleb N.-E. Profibrotic role for interleukin-4 in cardiac remodeling and dysfunction. Hypertension. 2015;66(3):582–589. doi: 10.1161/HYPERTENSIONAHA.115.05627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. Jama. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eskandarani R., Sahli S., Sawan S., Alsaeed A. Simultaneous cardio-cerebral infarction in the coronavirus disease pandemic era: a case series. Medicine. 2021;100(4) doi: 10.1097/MD.0000000000024496. e24496-e24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.-R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Püschel K., Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iqbal P., Laswi B., Jamshaid M.B., Shahzad A., Chaudhry H.S., Khan D., Qamar M.S., Yousaf Z. The role of anticoagulation in post-COVID-19 concomitant stroke, myocardial infarction, and left ventricular thrombus: a case report. Am. J. Case Rep. 2021;22 doi: 10.12659/AJCR.928852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Agarwal S., Al Hashimi H., Agarwal S.K., Albastaki U. Association possible entre un infarctus du myocarde sans obstruction coronarienne et l′infection par le SRAS-CoV-2. Cmaj. 2021;193(5):E193–e196. doi: 10.1503/cmaj.202106-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai C., Guo Y., You Y., Hu K., Cai F., Xie M., Yang L., Ling K., Ye D., Misra S., Wang W., Li Y. Deep venous thrombosis in COVID-19 patients: a cohort analysis. Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029620982669. 1076029620982669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Page E.M., Ariëns R.A.S. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb. Res. 2021;200:1–8. doi: 10.1016/j.thromres.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McFadyen J.D., Stevens H., Peter K. The emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications. Circ. Res. 2020;127(4):571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tschöpe C., Sherif M., Anker M.S., Geisel D., Kuehne T., Kelle S. COVID-19-convalescence phase unmasks a silent myocardial infarction due to coronary plaque rupture. ESC Heart Fail. 2021;8(2):971–973. doi: 10.1002/ehf2.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., Shchendrygina A., Escher F., Vasa-Nicotera M., Zeiher A.M., Vehreschild M., Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rivera-Morales M.D., Pell R., Rubero J., Ganti L. Acute myopericarditis in the post COVID-19 recovery phase. Cureus. 2020;12(10):11247. doi: 10.7759/cureus.11247. e11247-e11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lakkireddy D.R., Chung M.K., Gopinathannair R., Patton K.K., Gluckman T.J., Turagam M., Cheung J.W., Patel P., Sotomonte J., Lampert R., Han J.K., Rajagopalan B., Eckhardt L., Joglar J., Sandau K.E., Olshansky B., Wan E., Noseworthy P.A., Leal M., Kaufman E., Gutierrez A., Marine J.E., Wang P.J., Russo A.M. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020;17(9):e233–e241. doi: 10.1016/j.hrthm.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stefanini G.G., Montorfano M., Trabattoni D., Andreini D., Ferrante G., Ancona M., Metra M., Curello S., Maffeo D., Pero G., Cacucci M., Assanelli E., Bellini B., Russo F., Ielasi A., Tespili M., Danzi G.B., Vandoni P., Bollati M., Barbieri L., Oreglia J., Lettieri C., Cremonesi A., Carugo S., Reimers B., Condorelli G., Chieffo A. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141(25):2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burkert F.R., Niederreiter L., Dichtl W., Mayr A., Virgolini I., Klauser A., Weiss G., Bellmann-Weiler R. Case report of a COVID-19-associated myocardial infarction with no obstructive coronary arteries: the mystery of the phantom embolus or local endothelitis. Eur. Heart J. Case Rep. 2021;5(2):ytaa521. doi: 10.1093/ehjcr/ytaa521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang T., Tang R., Ruan H., Chen R., Zhang Z., Sang L., Su X., Yi S., Ni Z., Hu Y., Liu L., Shan H., Lei C., Peng Y., Liu C., Li J., Hong C., Zhang N., Zhong N., Li S. Predictors of fatal outcomes among hospitalized COVID-19 patients with pre-existing hypertension in China. Clin. Respir. J. 2021;15(8):915–924. doi: 10.1111/crj.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Becker R.C. Anticipating the long-term cardiovascular effects of COVID-19. J. Thromb. Thrombolysis. 2020;50(3):512–524. doi: 10.1007/s11239-020-02266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Farouk S.S., Fiaccadori E., Cravedi P., Campbell K.N. COVID-19 and the kidney: what we think we know so far and what we don’t. J. Nephrol. 2020;33(6):1213–1218. doi: 10.1007/s40620-020-00789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gousseff M., Penot P., Gallay L., Batisse D., Benech N., Bouiller K., Collarino R., Conrad A., Slama D., Joseph C., Lemaignen A., Lescure F.-X., Levy B., Mahevas M., Pozzetto B., Vignier N., Wyplosz B., Salmon D., Goehringer F., Botelho-Nevers E. C.s.g. in behalf of the, Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J. Infect. 2020;81(5):816–846. doi: 10.1016/j.jinf.2020.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ravioli S., Ochsner H., Lindner G. Reactivation of COVID-19 pneumonia: a report of two cases. J. Infect. 2020;81(2):e72–e73. doi: 10.1016/j.jinf.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.López-Romero R., Nambo-Lucio M.J., Salcedo-Carrillo E., Hernández-Cueto M., Salcedo-Vargas M. The big challenge of SARS-CoV-2 latency: testes as reservoir. Gac. Med. Mex. 2020;156(4):328–333. doi: 10.24875/GMM.20000295. [DOI] [PubMed] [Google Scholar]
- 98.Tremblay M.-E., Madore C., Bordeleau M., Tian L., Verkhratsky A. Neuropathobiology of COVID-19: the role for glia. Front. Cell. Neurosci. 2020;14(363) doi: 10.3389/fncel.2020.592214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.I. National Center for, D.o.V.D. Respiratory Diseases, Science Brief: COVID-19 Vaccines and Vaccination, CDC COVID-19 Science Briefs, Centers for Disease Control and Prevention (US) Unless a copyright is indicated, information on CDC's sites, blogs, and applications is in the public domain and may be copied and distributed without permission., Atlanta (GA), 2020.
- 100.C.f.D.C.a. Prevention, Selected adverse events reported after COVID-19 vaccination, 2021.
- 101.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ali M.J., Hanif M., Haider M.A., Ahmed M.U., Sundas F., Hirani A., Khan I.A., Anis K., Karim A.H. Treatment options for COVID-19: a review. Front. Med. 2020;7(480):480. doi: 10.3389/fmed.2020.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chinello P., Petrosillo N., Pittalis S., Biava G., Ippolito G., Nicastri E., Team I.E. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Negl. Trop. Dis. 2017;11(12) doi: 10.1371/journal.pntd.0006034. e0006034-e0006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Malvy D., Taburet A.-M., de Lamballerie X., Mentre F., Extramiana F. The safety profile of favipiravir should not be the first argument to suspend its evaluation in viral hemorrhagic fevers. PLoS Negl. Trop. Dis. 2020;14(6) doi: 10.1371/journal.pntd.0008259. e0008259-e0008259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ridjab D.A., Ivan I., Budiman F., Juliawati D.J. Current evidence for the risk of PR prolongation, QRS widening, QT prolongation, from lopinavir, ritonavir, atazanavir, and saquinavir: a systematic review. Medicine. 2021;100(31) doi: 10.1097/MD.0000000000026787. e26787-e26787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.K.R. Feingold, Lipid and lipoprotein levels in patients with COVID-19 infections, in: K.R. Feingold, B. Anawalt, A. Boyce, G. Chrousos, W.W. de Herder, K. Dhatariya, K. Dungan, A. Grossman, J.M. Hershman, J. Hofland, S. Kalra, G. Kaltsas, C. Koch, P. Kopp, M. Korbonits, C.S. Kovacs, W. Kuohung, B. Laferrère, E.A. McGee, R. McLachlan, J.E. Morley, M. New, J. Purnell, R. Sahay, F. Singer, C.A. Stratakis, D.L. Trence, D.P. Wilson (Eds.), Endotext, MDText.com, Inc. Copyright © 2000–2021, MDText.com, Inc., South Dartmouth (MA), 2000.
- 108.F. Kabinger, C. Stiller, J. Schmitzová, C. Dienemann, H.S. Hillen, C. Höbartner, P. Cramer, Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, bioRxiv (2021) 2021.05.11.443555. [DOI] [PMC free article] [PubMed]
- 109.Wahl A., Gralinski L.E., Johnson C.E., Yao W., Kovarova M., Dinnon K.H., Liu H., Madden V.J., Krzystek H.M., De C., White K.K., Gully K., Schäfer A., Zaman T., Leist S.R., Grant P.O., Bluemling G.R., Kolykhalov A.A., Natchus M.G., Askin F.B., Painter G., Browne E.P., Jones C.D., Pickles R.J., Baric R.S., Garcia J.V. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591(7850):451–457. doi: 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hama R., Bennett C.L. The mechanisms of sudden-onset type adverse reactions to oseltamivir. Acta Neurol. Scand. 2017;135(2):148–160. doi: 10.1111/ane.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leneva I.A., Russell R.J., Boriskin Y.S., Hay A.J. Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antivir. Res. 2009;81(2):132–140. doi: 10.1016/j.antiviral.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 112.Nojomi M., Yassin Z., Keyvani H., Makiani M.J., Roham M., Laali A., Dehghan N., Navaei M., Ranjbar M. Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial. BMC Infect. Dis. 2020;20(1):954. doi: 10.1186/s12879-020-05698-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brotman D.J., Girod J.P., Garcia M.J., Patel J.V., Gupta M., Posch A., Saunders S., Lip G.Y., Worley S., Reddy S. Effects of short-term glucocorticoids on cardiovascular biomarkers. J. Clin. Endocrinol. Metab. 2005;90(6):3202–3208. doi: 10.1210/jc.2004-2379. [DOI] [PubMed] [Google Scholar]
- 114.Naksuk N., Lazar S., Peeraphatdit T.B. Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol. Eur. Heart J. Acute Cardiovasc. Care. 2020;9(3):215–221. doi: 10.1177/2048872620922784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Titanji B.K., Farley M.M., Mehta A., Connor-Schuler R., Moanna A., Cribbs S.K., O’Shea J., DeSilva K., Chan B., Edwards A., Gavegnano C., Schinazi R.F., Marconi V.C. Use of Baricitinib in patients with moderate to severe coronavirus disease 2019. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021;72(7):1247–1250. doi: 10.1093/cid/ciaa879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.