Introduction
Amyloids are depositions of insoluble proteinaceous fibrils with β-sheet secondary structure. Many proteins can form amyloids, including immunoglobulin light chain (AL amyloid) and less frequently, immunoglobulin heavy chain (AH amyloid). AL amyloid deposition is typically associated with AL amyloidosis, a systemic condition often associated with multiple myeloma.1 AL amyloidosis can result in tumor-like accumulations of amyloid called “amyloidomas.” These occur rarely but are most frequent in the respiratory, urinary, and gastrointestinal tracts.2 Systemic amyloidosis can also result in accumulation in the central nervous system, with typical localization in regions with absent or leaky blood-brain barrier, including the choroid plexus.3 Although most amyloidomas are thought to be associated with systemic processes such as B-cell lineage malignancies, amyloidomas can occur without evidence of an underlying systemic process and in this case are known as “primary amyloidomas.” Primary cerebral amyloidoma (PCA) describes amyloidoma of the brain without evidence of an underlying systemic process. Only about 40 cases of PCA have been reported.4 Although the presence of heavy chains (AH amyloid) has infrequently been reported for peripheral amyloidomas, to our knowledge all previously reported PCAs have had only light chains (AL amyloid).5 The overall limited clinical experience with PCAs has resulted in questions regarding optimal treatment. Although surgical resection is the definitive option, radiation therapy has also been used, particularly for anatomically challenging locations. Here, we report the first known amyloid light and heavy chain (ALH) PCAs and their management with radiation therapy.
Patient Cases
Case 1
A 58-year-old left-handed woman presented with a 2-year history of progressive paresthesias in the left hand and weakness in the left arm and leg. Medical history was notable for migraine without aura occurring approximately once monthly for decades. Physical examination was notable for 4/5 strength throughout the left arm and leg and 3 + reflexes at the patellar and Achilles tendons on the left. Electromyography and nerve conduction studies of the left arm were normal. Magnetic resonance imaging (MRI) of the brain demonstrated a T2-hyperintense lesion with associated heterogeneous enhancement on T1 postgadolinium sequences in the right parietal lobe extending to the frontal lobe along the motor strip (Fig 1).
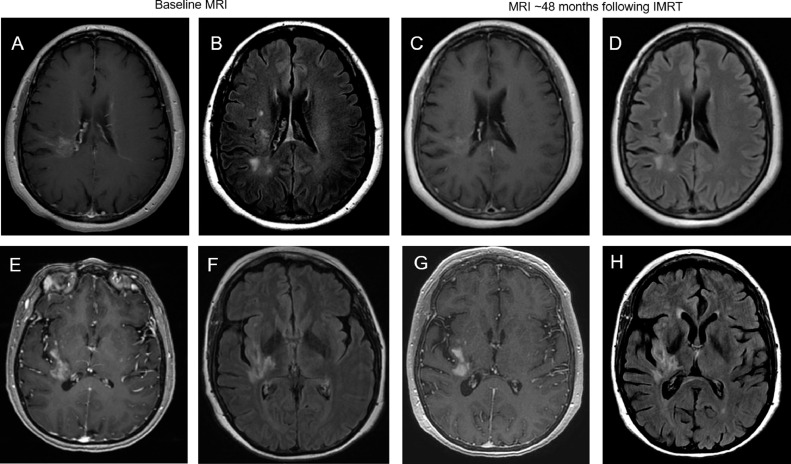
Fig. 1.
Patient case 1 – top row. (A) Initial magnetic resonance imaging (MRI) scan with periventricular radial pattern of enhancement of T1-weighted postgadolinium sequence. (B) Associated T2 fluid-attenuated inversion recovery (FLAIR) abnormality on initial MRI. (C) Stable appearance of enhancement on T1-weighted postgadolinium sequence at 48 months after intensity modulated radiation therapy (IMRT). (D) Stable appearance of T2 FLAIR abnormality at 48 months after IMRT. Patient case 2 – bottom row. (E) Initial MRI scan with serpiginous pattern of enhancement on T1-weighted postgadolinium sequence. (F) Associated T2 FLAIR abnormality on initial MRI. (G) Stable appearance of enhancement on T1-weighted postgadolinium sequence at 48 months after IMRT. (H) Stable appearance of T2 FLAIR abnormality at 48 months after IMRT.
Stereotactic biopsy revealed focal amyloid deposition (Congo Red positivity with apple green birefringence) in the brain parenchyma and blood vessels (Fig 2) without beta-amyloid on immunohistochemistry (IHC). Liquid chromatography tandem mass spectrometry detected a peptide profile consistent with ALH (lambda light chain and alpha heavy chain) type amyloid deposition. CD138 IHC revealed sparse plasma cells. Systemic workup, including bone marrow biopsy, serum protein electrophoresis, serum immunofixation, free light chains, transthoracic echocardiogram, and skeletal survey, was unremarkable. The diagnosis of PCA was made with the paucity of plasma cells not allowing for a diagnosis of a plasma cell dyscrasia or plasmacytoid marginal zone lymphoma despite the residual plasma cell population.
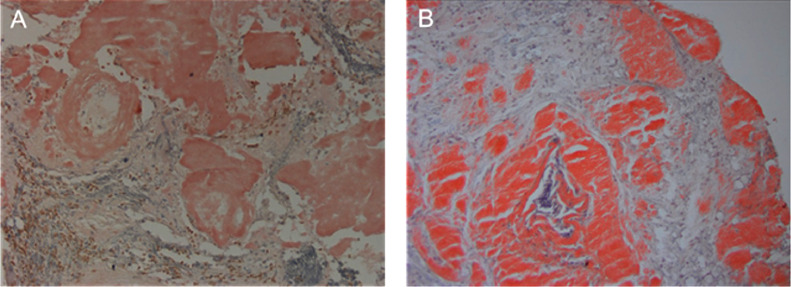
Fig. 2.
Congo red stain of biopsy tissue (20 ×) demonstrated parenchymal deposits of congophilic (pink) amyloid. (A) Patient case 1. (B) Patient case 2.
Owing to the location of the lesion, surgical resection was not feasible. Thus, treatment was with intensity modulated radiation therapy (IMRT) to 24 Gy in 12 fractions to the T2 fluid-attenuated inversion recovery (FLAIR) abnormality to designate the clinical target volume followed by an expansion of 1 cm to the planning target volume (Fig 3). Treatment was well tolerated with some radiation-induced alopecia. Weakness and paresthesias, her presenting symptoms, remained stable. At 48 months after treatment there has been no evidence of clinical or radiographic progression, nor has there been emergence of systemic disease.
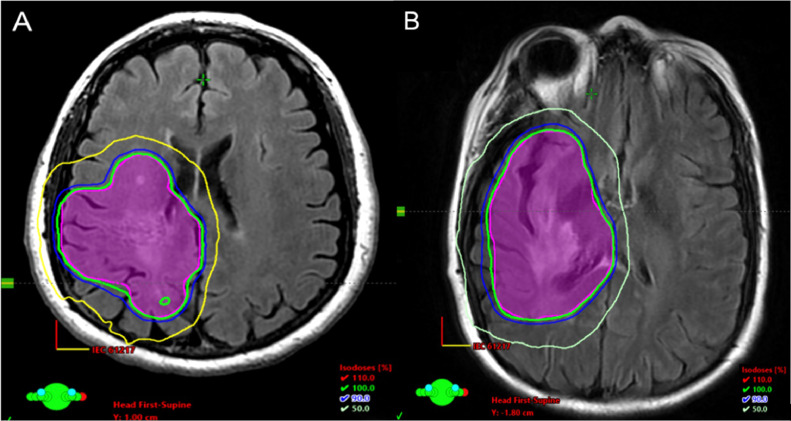
Fig. 3.
Planning computed tomography (CT) of the brain fused with magnetic resonance imaging (MRI) T2 fluid-attenuated inversion recovery (FLAIR) in the axial plane. Planning target volume (pink) covered at 24 Gy (green – 100% isodose line) with photon energy of 6 MV using 3 arcs in an intensity modulated radiation therapy (IMRT) approach. (A) Patient case 1. (B) Patient case 2.
Case 2
A 54-year-old right-handed woman presented with a 4-year history of left hand numbness that progressed to numbness of the entire left arm and then was followed by progressive left leg weakness and numbness. Medical history was notable for cervical spine fusion (C5-C7) without complication 15 years before presentation. Physical examination was notable for decreased sensation in the left face and left arm and leg, 4+/5 strength in the left arm, and 4/5 strength in the left leg. There was spasticity in the left leg. Electromyography and nerve conduction studies of the left arm were normal. MRI of the brain (Fig 1) demonstrated a T2 hyperintense lesion involving the right corona radiata with irregular enhancement on T1 postgadolinium sequences.
Stereotactic biopsy revealed focal amyloid deposition (Congo Red positivity with apple green birefringence) in the brain parenchyma and blood vessels (Fig 2) with peptide profile identified via mass spectrometry as ALH (lambda light chain and alpha heavy chain). In situ hybridization demonstrated lambda light chain positive cells somewhat mirroring a separate CD20+ cell population as labeled with IHC and demonstrating mild cytologic atypia. Taken together, findings were suspicious for mild burden of marginal zone lymphoma with plasmacytoid differentiation. A monoclonal band was identified in the serum and characterized by immunofixation electrophoresis as a faint lambda light chain; this resolved at time of follow-up. Other systemic workup, including bone marrow biopsy, transthoracic echocardiogram, cardiac MRI, skull to thigh fluorodeoxyglucose positron emission tomography, and skeletal survey, was unremarkable. The diagnosis of PCA was made.
Owing to the location of the lesion, surgical resection was not feasible. Thus, similar to the first patient discussed, treatment was with IMRT to 24 Gy in 12 fractions to the T2 FLAIR abnormality with an expansion of 0.5 cm to create the planning target volume (Fig 3). Treatment was well-tolerated with stable neurologic symptoms. At 48 months after treatment there has been no evidence of clinical or radiographic progression nor has there been emergence of systemic disease.
Discussion
Although the presence of heavy chains has infrequently been reported for peripheral amyloidomas, to our knowledge all previously reported PCAs have had only light chain deposition (AL amyloid).5 In addition, although PCAs typically have a benign course, progression can occur with worsening of neurologic morbidity over time. Therefore, definitive treatment was considered after biopsy. Tissue sampling with histologic confirmation remains the only way to prove the diagnosis, as on MRI PCA may mimic inflammatory lesions (particularly granulomatous, such as sarcoidosis), malignant lesions (such as primary brain tumors), or even vascular lesions. In addition to obtaining tissue for definitive diagnosis, surgical resection has the advantage of alleviating any mass effect, with radiation therapy considered as an alternative if surgical resection is not feasible due to the anatomic regions involved. An additional benefit of radiation therapy is limiting the interventional risk associated with resection, such as hemorrhage or infection. These cases represent the first known ALH PCAs and their successful management with radiation therapy.
The combination of radiographic findings and slowly progressive symptoms described in these cases is typical of the clinical course for PCAs described in the literature. Presentation is typically at about 50 years of age and symptoms include seizures, hemiparesis, and headaches.4,5 PCAs are typically supratentorial and adjacent to the ventricular system with radial involvement of the white matter and perivascular areas. On MRI, PCAs have varied and often mixed signal on T2-weighted images, with the most consistent feature being heterogeneous enhancement on T1-weighted postgadolinium sequences.4 This irregular enhancement is considered a result of perivascular deposition of amyloid resulting in blood-brain barrier dysfunction. Due to the radiographic appearance and progressive symptoms, PCA may be misdiagnosed as vascular anomalies, inflammatory processes, or other types of tumors. Diagnosis is ultimately made on biopsy, although advanced imaging (such as dedicated positron emission tomography techniques) is under investigation.
It is prudent to rule out a systemic process contributing to any amyloidoma due to a presumed underlying clonal process in the B-cell lineage driving amyloid deposition. That said, there are no known cases of a PCA later developing systemic involvement; however, clinical experience in terms of number of cases and duration of follow-up remains limited.4,5 Indeed, there is one case report of a scapular amyloidoma with initial negative systemic workup for malignancy but subsequent development of diffuse large B-cell lymphoma and systemic amyloid deposition within 1 year.6 Although the clinical course of PCA is typically benign, persistence of abnormal cells may lead to progressive amyloid deposition and worsening of symptoms. It has been suggested that the contributory clonal process may “burn-out” due to cytotoxicity from the accumulation of amyloids.7 Due to the suggestion of persistent underlying cell populations given the pathologic appearance in the patients discussed herein, treatment was offered.
In these cases, radiation therapy was chosen owing to the lesions not being amenable to surgical resection and the presence of cell populations on biopsy suggesting possible underlying marginal zone lymphoma (for which 24 Gy is a standard definitive treatment dose). In addition to marginal zone lymphoma, PCA has been reported in association with primary central nervous system lymphoplasmacytic lymphoma.8 As mentioned, peripheral lymphoproliferative processes can also result in abnormal immunoglobulin production including light and/or heavy chains, which may lead to amyloid deposition. Overall, the high rate of responsiveness of indolent B-cell lymphomas, solitary plasmacytomas, and benign lymphoid infiltrates to radiation therapy demonstrates the radiosensitivity of differentiated B-cells. IMRT for the treatment of peripheral amyloidomas has been used with doses ranging from 20 to 50 Gy.9, 10, 11 One case has described local control of an AL PCA using IMRT, 30.6 Gy in 1.8 Gy per fraction, with follow-up to 18 months.12 Whole brain radiation therapy (30 Gy in 10 fractions) has been used effectively for AL PCA but is associated with long-term neurocognitive effects and a more conformal radiation therapy approach may be more ideal.13 Table 1 summarizes literature where radiation therapy was used for control of amyloidomas. Therapeutic efficacy may be different for ALH amyloidoma, which may have a more benign course based on limited experience with renal lesions.14 Both patients were treated with localized radiation therapy to 24 Gy and have achieved durable control at 4 years of follow-up with minimal acute and no treatment-related long-term side effects.
Table 1.
Prior published work using radiation therapy for local control of amyloidomas
| Author (year) | Disease site | Number of cases | Dose (Gy)/fractions (fx) |
|---|---|---|---|
| Bertelsen et al (2021)15 | Larynx | 5 | 45 Gy/25 fx |
| Cooper et al (2018)16 | Bladder | 1 | 24 Gy/12 fx |
| Copperman et al (2019)17 | Periocular | 4 | 20-30/10-20 fx |
| Gallivan and Gallivan (2010)18 | Larynx | 1 | 20 Gy/10 fx |
| Kalra et al (2001)19 | Tracheobronchial | 1 | 20 Gy/10 fx |
| Kapoor et al (2014)9 | Lung | 1 | 30 Gy/10 fx |
| Lee et al (1995)13 | Whole brain | 1 | 30 Gy |
| Meier et al (2017)12 | Cerebral | 1 | 30.6 Gy/17 fx |
| Monroe et al (2004)20 | Tracheobronchial | 1 | 24 Gy/12 fx |
| Neben-Wittich et al (2007)21 | Tracheobronchial | 7 | 20 Gy/10 fx |
| Neuner et al (2012)11 | Larynx | 1 | 45 Gy/25 fx |
| Poovaneswaran et al (2008)22 | Bronchial | 1 | 24 Gy/12 fx |
| Ren and Ren (2012)23 | Pulmonary | 3 | 24 Gy/12 fx |
| Truong et al (2012)10 | Airway | 10 | 18-20 Gy/10 fx |
Limitations of this study include its small sample size and retrospective nature of data collection. That said, this presentation is rare, and our case series demonstrates the effectiveness of radiation for this diagnosis.
Conclusions
Here, 2 cases of novel ALH PCA treated with IMRT remain controlled at 48-months follow-up with minimal acute and no long-term side effects. Our experience suggests that a dose of 24 Gy treated in conventional fraction to the T2 FLAIR lesion is adequate for disease control of ALH PCA. Ongoing experience will be valuable to further characterize responsiveness to radiation therapy and the clinical course of ALH PCA.
Acknowledgments
The authors would like to thank the 2 patients included in this study, who both provided written consent before publication.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Paccalin M, Hachulla E, Cazalet C, et al. Localized amyloidosis: A survey of 35 French cases. Amyloid. 2005;12:239–245. doi: 10.1080/13506120500351174. [DOI] [PubMed] [Google Scholar]
- 2.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 3.Schröder R, Linke RP. Cerebrovascular involvement in systemic AA and AL amyloidosis: A clear haematogenic pattern. Virchows Arch. 1999;434:551–560. doi: 10.1007/s004280050383. [DOI] [PubMed] [Google Scholar]
- 4.Bray DP, Rich CW, Ellis JA, et al. Minimally invasive resection of intracerebral amyloidoma: Case report and systematic review of the literature. World Neurosurg. 2020;138:205–213. doi: 10.1016/j.wneu.2020.02.072. [DOI] [PubMed] [Google Scholar]
- 5.Fischer B, Palkovic S, Rickert C, et al. Cerebral AL lambda-amyloidoma: Clinical and pathomorphological characteristics. Amyloid. 2007;14:11–19. doi: 10.1080/13506120600960585. [DOI] [PubMed] [Google Scholar]
- 6.Pambuccian SE, Horyd ID, Cawte T, Huvos AG. Amyloidoma of bone, a plasma cell/plasmacytoid neoplasm. Report of three cases and review of the literature. Am J Surg Pathol. 1997;21:179–186. doi: 10.1097/00000478-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Westermark P. Localized AL amyloidosis: A suicidal neoplasm? Ups J Med Sci. 2012;117:244–250. doi: 10.3109/03009734.2012.654861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagannathan G, Uppal G, Judy K, Curtis MT. Cerebral amyloidoma resulting from central nervous system lymphoplasmacytic lymphoma: A case report and literature review. Case Rep Pathol. 2018;5083234 doi: 10.1155/2018/5083234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapoor R, Bhattacharyya T, Bahl A, et al. Primary amyloidoma of lung treated with radiation: A rare case report. Lung India. 2014;31:404–406. doi: 10.4103/0970-2113.142151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truong MT, Kachnic LA, Grillone GA, et al. Long-term results of conformal radiotherapy for progressive airway amyloidosis. Int J Radiat Oncol Biol Phys. 2012;83:734–739. doi: 10.1016/j.ijrobp.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 11.Neuner GA, Badros AA, Meyer TK, et al. Complete resolution of laryngeal amyloidosis with radiation treatment. Head Neck. 2012;34:748–752. doi: 10.1002/hed.21626. [DOI] [PubMed] [Google Scholar]
- 12.Meier T, Hazenfield JM, Girnius S, et al. A rare case of central nervous system amyloidoma treated with fractionated radiotherapy. J Neurosurg. 2017;127:338–341. doi: 10.3171/2016.7.JNS1690. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Krol G, Rosenblum M. Primary amyloidoma of the brain: CT and MR presentation. Am J Neuroradiol. 1995;16:712–714. [PMC free article] [PubMed] [Google Scholar]
- 14.Nasr SH, Said SM, Valeri AM, et al. The diagnosis and characteristics of renal heavy-chain and heavy/light-chain amyloidosis and their comparison with renal light-chain amyloidosis. Kidney Int. 2013;83:463–470. doi: 10.1038/ki.2012.414. [DOI] [PubMed] [Google Scholar]
- 15.Bertelsen C, Chadwick K, Holland J, Flint P, Schindler JS. Long-term follow-up after radiation therapy for laryngeal amyloidosis. Laryngoscope. 2021;131:1810–1815. doi: 10.1002/lary.29061. [DOI] [PubMed] [Google Scholar]
- 16.Cooper CT, Greene BD, Fegan JE, Rovira D, Gertz MA, Marcus DM. External beam radiation therapy for amyloidosis of the urinary bladder. Pract Radiat Oncol. 2018;8:25–27. doi: 10.1016/j.prro.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Copperman TS, Truong MT, Berk JL, Sobel RK. External beam radiation for localized periocular amyloidosis: A case series. Orbit. 2019;38:210–216. doi: 10.1080/01676830.2018.1483407. [DOI] [PubMed] [Google Scholar]
- 18.Gallivan GJ, Gallivan HK. Laryngeal amyloidosis causing hoarseness and airway obstruction. J Voice. 2010;24:235–239. doi: 10.1016/j.jvoice.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Kalra S, Utz JP, Edell ES, Foote RL. External-beam radiation therapy in the treatment of diffuse tracheobronchial amyloidosis. Mayo Clin Proc. 2001;76:853–856. doi: 10.1016/S0025-6196(11)63233-3. [DOI] [PubMed] [Google Scholar]
- 20.Monroe AT, Walia R, Zlotecki RA, Jantz MA. Tracheobronchial amyloidosis: A case report of successful treatment with external beam radiation therapy. Chest. 2004;125:784–789. doi: 10.1378/chest.125.2.784. [DOI] [PubMed] [Google Scholar]
- 21.Neben-Wittich MA, Foote RL, Kalra S. External beam radiation therapy for tracheobronchial amyloidosis. Chest. 2007;132:262–267. doi: 10.1378/chest.06-3118. [DOI] [PubMed] [Google Scholar]
- 22.Poovaneswaran SR, Hilmi Lockman H, Bone M, et al. Tracheobronchial amyloidosis: Utilization of radiotherapy as a treatment modality. Medscape J Med. 2008;10:42. [PMC free article] [PubMed] [Google Scholar]
- 23.Ren S, Ren G. External beam radiation therapy is safe and effective in treating primary pulmonary amyloidosis. Respir Med. 2012;106:1063–1069. doi: 10.1016/j.rmed.2012.02.011. [DOI] [PubMed] [Google Scholar]