Abstract
Objective
Publications on hidden blood loss (HBL) after posterior lumbar interbody fusion (PLIF) for lumbar spine stenosis syndrome (LSS) have been reported, but the modified HBL (mHBL) was different from HBL obtained by classical formula and there are few studies on lumbar spine hemorrhage with rheumatoid arthritis (RA). Therefore, the aim of our study is to respectively evaluate the importance of hidden blood loss (HBL) and modified HBL (mHBL) after posterior lumbar interbody fusion (PLIF) in patients diagnosed with LSS and RA, to explore the correlation between RA activity and HBL as well as mHBL.
Methods
A total of 61 patients (nine males and 52 females) diagnosed with LSS and RA who underwent PLIF were included. Data contained demographics, RA‐related parameters such as duration of RA, Steinbrocker classification (used to evaluated RA activity), the disease‐modifying anti‐rheumatic drugs (DMARDs), osteoporosis and total knee arthroplasty; operation and hemorrhage parameters. Then HBL and mHBL were calculated by Gross formula and modified formula, respectively. Subgroup analysis on HBL and mHBL was performed based on gender, age (≤60 years and ˃60 years), different number of surgical segments (single segment, double segment, and ≥3 segments), and taking DMARDs or not. ANOVA analysis was performed on HBL and mHBL in different surgery segment number and Steinbrocker classification of RA. Independent sample t‐test was used in comparison of gender and age, as well as in comparison between HBL and mHBL based on whether the patient took DMARDs or not. Furthermore, paired t‐test was used to compare the volume between HBL and mHBL.
Results
The mean age and duration of RA was 65.2 ± 9.3 years and 14.3 ± 10.7 years, respectively. There were 13 grade I cases, 34 grade II cases, and 14 grade III cases as assessed by Steinbrocker classification and the most common anti‐RA drugs were DMARDs (57.4%). The mean intraoperative bleeding, drainage, and blood loss in drainage (DBL) was 453.3 ± 377.8 mL, 489.1 ± 253.8 mL, and 304.6 ± 156.3 mL, respectively. There was no difference on HBL and mHBL in gender. HBL and mHBL was larger in patients over 60 years (P = 0.040 and P = 0.023). There were differences in intraoperative blood loss, drainage, and DBL based on different number of segments but not in HBL and mHBL, or on Steinbrocker classification. DBL was lower in DMARDs group than non‐drugged group (P = 0.03), while HBL and mHBL were both of no significance. The comparison of HBL and mHBL showed statistical difference (P < 0.001), suggesting that mHBL volume is larger than HBL.
Conclusions
Patients diagnosed as LSS with RA have amounts of HBL or mHBL after PLIF. HBL or mHBL is not associated with RA activity, which may not increase in RA patients compared with common ones. Taking DMARDs may reduce postoperative DBL. The fact that mHBL is larger than HBL provides an all‐round basis for measuring factual HBL.
Keywords: Hidden blood loss, Lumbar stenosis syndrome, Modified hidden blood loss, Posterior lumbar interbody fusion, Rheumatoid arthritis
1. No statistical difference both in HBL and mHBL according to Steinbroker classification of three groups, which proposed no correlation between RA activity and HBL or mHBL. 2. DBL was of statistical difference (P = 0.03) between DMARDs‐taking group and non‐drug group with the mean volume of 415.8 and 261.8 mL, respectively, but yield no difference of HBL and mHBL between the two groups.

Introduction
Posterior approach on lumbar spine, especially with multiple segments, was usually accompanied by massive bleeding 1 , 2 . However, most surgeons paid more attention to intraoperative blood loss and postoperative drainage, while neglecting the component of interstitial oozing, the blood accumulation in surgical site, hemolysis, or other reasons for hemoglobin (Hb) reduction, which exist but can be overlooked easily and were defined as hidden blood loss (HBL) 3 , 4 . Excessive hemorrhage will lead to perioperative insufficient blood perfusion of organ and blood coagulation dysfunction and the condition was usually remedied by blood transfusion. However, the volume of blood transfusion mainly depended on clinical experiences and visible blood loss. Consequently, postoperative anemia would sill happen in some patients even if receiving so‐called corresponding blood transfusion. Therefore, it was critical to focus on the actual blood loss and HBL during perioperation period.
Publications referring to HBL after orthopaedic surgery such as hip and knee arthroplasty and posterior lumbar interbody fusion (PLIF) have showed concern about the issue3–7 . As was reported, the calculation of HBL was based on a formula for patient blood volume (PBV) 8 and total blood loss (TBL) 9 . Wen et al. 7 reviewed 169 consecutive patients who underwent PLIF with the HBL occupying 39% of the TBL, meanwhile they addressed the fact that significant HBL may have a correlation with postoperative mortality. Genarally, the concept of HBL attracted much attention on management of perioperative blood loss as well as the relative adverse effects.
According to Gross formula, , the classical HBL was usually obtained by subtracting intraoperative blood loss and drainage from TBL 9 . However, the validity of HBL was also problematic because the calculation of HBL was reported incomplete where the volume of blood loss in drainage was overestimated. Xu et al. 10 proposed that the drainage was not totally equal to the postoperative hemorrhage and postoperative hemorrhage was not the amount of postoperative fluid loss but the real blood component loss in drainage (DBL). Based on this theory, the modified HBL (mHBL) based on the DBL should be statistically different from HBL. However, this viewpoint has never been verified by spine procedure.
Spinal disease combined with rheumatoid arthritis (RA) was widely observed on cervical spine involvement but seldom reported on lumbar spine disease 11 . Lumbar stenosis syndrome (LSS) was the most prevalent spinal disease and severe cases would have surgery performed using posterior approach, while we know only of limited management on perioperative blood loss for LSS cases involved by RA, contrasted with ones without RA. Actually, the prevalence of LSS combined with RA was much higher than reported while there was little evidence for clinicians to refer to. So, there was an ambiguous plan for treatment and rehabilitation for cases with RA 12 . RA patients involving the spine would suffer from inflammation of the synovium which can aggravate the lesion of the vertebrae, facet, and ligament, as well as abnormal angiogenesis proliferation 13 ; thus the interstitial oozing of blood after surgery was probably more violent. Hence, the situation may cause more blood loss, especially on LSS attributed to the impact of both degeneration and RA.
Therefore, based on RA patients with LSS reaching surgery indication, the purpose of the present study is: (i) to evaluate the importance of monitoring TBL, HBL, and mHBL in the perioperation period of PLIF; (ii) to explore whether the operated number of levels, RA activity, and anti‐RA drugs could put impact on HBL or mHBL; and (iii) to identify the difference between HBL and mHBL for LSS patients with RA.
Methods
Participants and Exclusion Criteria
This study was a single‐center prospective cohort series study. A total of 61 patients with RA were included in this study who underwent PLIF surgery from January 2018 to December 2019 by the same surgeon. The study was approved by the ethics committee of our institution (protocol number: 2018PHC076) and all patients provided signed informed consent. Criteria for selecting the subjects were as follows: (i) all participants diagnosed with LSS and RA reaching surgical indications who completed this study with stable intra‐ and postoperative fluid shift and hemodynamics; (ii) the intervene for participants were PLIF or PLIF combined with posterior‐lateral fusion and all surgeries were performed by the same senior surgeon; (iii) the comparisons on HBL and mHBL could be performed for all participants and extracted data on perioperative blood‐associated information as well as RA‐related information was intact and identified; (iv) the outcomes of all TBL, HBL, and mHBL could be acquired; and (v) this study was a single‐center retrospective cohort study. Exclusion criteria were patients: (i) with spinal tumors and spinal infections; (ii) with blood diseases, severe anemia, and coagulopathy before surgery; (iii) using anti‐platelet drugs or anticoagulants; (iv) with dural rupture; and (v) with systemic infection. In addition, as HBL was calculated by formula, the statistical error enlargers with more excessive blood loss, and consequentially patients with total blood loss greater than 2.5 L have been excluded from this study 6 .
Data Extraction
Data extraction included (i) demographic characteristics such as gender, age, height, weight, body mass index (BMI), and American Society of Anesthesiologists physical status (ASA) classification; (ii) RA‐related parameters such as duration of RA, Steinbrocker classification, disease‐modifying anti‐rheumatic drugs (DMARDs), osteoporosis, and total knee arthroplasty; (iii) surgery information such as operation time, the number of surgical segments, and pedicle screws; and (iv) blood loss‐related indicators such as hematocrit (Hct) and Hb, intraoperative bleeding, postoperative drainage, and blood transfusion.
Management of Blood Loss
Intraoperative blood loss is usually collected by suction canister equipment and then returned into patients. All the patients underwent autologous blood transfusion and 31 of them received intra‐ or postoperative allogeneic blood transfusion. The blood loss is determined according to the amount of blood in equipment and infiltrated into the gauze by anesthesiologist, nurses, and the suction canisters manager.
All patients had the deep fascial drainage tube routinely placed and perioperative fluid shift and hemodynamics were kept stable. The drainage tube was removed 48–72 h after operation and Hct and Hb in the blood were measured preoperatively and after drainage removal (on second or third day after surgery). In addition, to obtain the accurate calculation of blood loss in the drainage fluid, the Hct in drainage (Hctd) was measured daily, 2 or 3 days before they were removed.
The Definition and Calculation of HBL and mHBL
PBV
PBV means the patient total blood volume, it was unfixed in various patients. According to Nadler formula 8 , , k1 = 0.3669, k2 = 0.03219, k3 = 0.6041 in male and k1 = 0.3561, k2 = 0.03308, k3 = 0.1833 in female.
TBL
TBL is total perioperative blood loss. It mainly contains intraoperative blood loss and consecutive loss after surgery and actually reflects the whole blood loss and the direct signal of patients whether with anemia or not. According to Gross formula 9 , . Hctpre is preoperative Hct, Hctpost is Hct on the second or third postoperative day, Hctave is the average of Hctpre and Hctpost.
Intraoperative Blood Loss
Intraoperative blood loss generally equals visible blood loss during operation. Intraoperative blood loss = suction canisters collection volume + blood loss in gauze – flushing saline amount, which is usually paid more attention to and represented as the “total blood loss” by most surgeons instead of the actual TBL.
HBL
HBL is hidden blood loss mainly caused by tissue interstitial extravasation, blood accumulated in the surgical site, and hemolysis, which is often overlooked by surgeons. According to Gross formula, HBL = TBL – intraoperative blood loss – postoperative drainage, if there was blood transfusion, then HBL = TBL + autologous blood transfusion + allogeneic blood transfusion – intraoperative blood loss – postoperative drainage 1 , 8 . HBL was seen as one of the primary outcomes in this study.
mHBL
However, the algorithm mentioned by Gross et al. has obvious bias when regarding drainage as direct visible postoperative blood loss, while Xu et al. 10 mentioned many non‐blood components contained in the drainage fluid and the true blood should be removed in the drainage fluid for more accuracy. Therefore, the formula for estimating mHBL is proposed, mHBL = TBL + autologous blood transfusion + allogeneic blood transfusion – intraoperative blood loss – postoperative drainage × Hctd/Hctave (DBL was the sum of daily blood loss in drainage fluid after surgery), which were also regarded as the primary outcomes (Fig. 1).
Fig. 1.
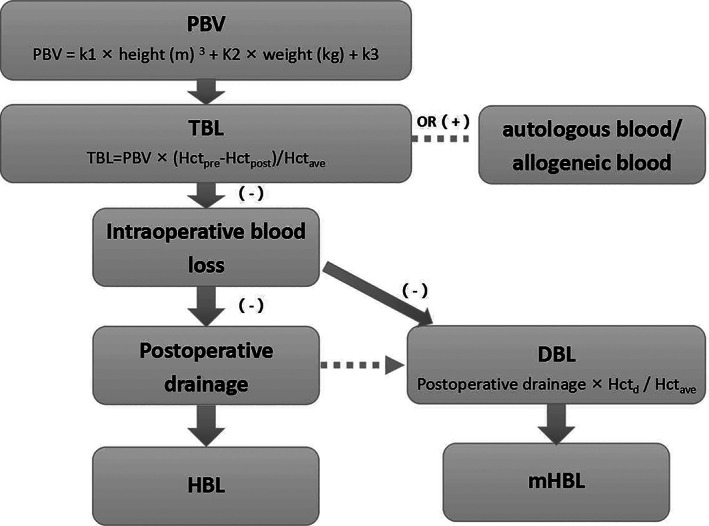
The diagram and definition of HBL and mHBL. DBL, real blood component loss in drainage; HBL, hidden blood loss; mHBL, modified hidden blood loss; Hctpre, preoperative Hct; Hctpost, Hct on the 2nd or 3rd postoperative day; Hctave, the average of Hctpre and Hctpost; PBV, patient blood volume; TBL, total blood loss.
Subgroup Analysis
It was reported that the age and gender would make a difference in perioperative hemorrhage 7 , and the cut‐off value of age was 60 years, so a subgroup analysis on stratification by age (≤60 years and ˃60 years) and gender would be performed. In addition, the different number of surgical segments (single segment, double segment, and ≥3 segments) and taking DMARDs or not would effect HBL and mHBL, thus the subgroups were formed.
Statistical Analysis
Data analysis was performed by SPSS 22.0 software (International Business Machines Corporation, Armonk, NY, USA). The subgroup analysis would be performed with independent sample t‐test. ANOVA analysis was performed in comparison to TBL, HBL, and mHBL in different number of surgical segments and in comparison to HBL and mHBL in different Steinbrocker classification. Independent sample t‐test was used in comparison to drainage, HBL, and mHBL based on whether the patient took DMARDs or not. Furthermore, paired t‐test was used to compare the volume between HBL and mHBL. P < 0.05 was considered statistically significant.
Results
Demographics Characteristics and RA Information
There were nine male and 52 female patients with RA, with mean age was 65.2 ± 9.3 years, and average BMI was 25.8 ± 3.7 kg/m2. The mean duration of RA was 14.3 ± 10.7 years (0.67–40 years) and the Steinbrocker classification (grade I–IV, used to evaluated RA activity and a higher grade represents a more serious condition) showed 13 people in grade I, 34 in grade II, and 14 in grade III. The most common anti‐RA drugs are single or combined administration of DMARDs (57.4%), followed by glucocorticoid hormone therapy, Chinese medicine treatment, NSAIDs pain relief treatment, and untreated. The average conservative treatment time was 2.8 years and the most common ASA classification was ASA II (49 patients). There were 11 patients who underwent total knee arthroplasty (four cases in single knee and seven cases in double knees), 37 patients had osteoporosis.
DBL, HBL, and mHBL in Patients with RA
The average TBL and intraoperative blood loss were 912.3 ± 332.3 mL and 453.3 ± 377.8 mL and the mean drainage and DBL were 489.1 ± 253.8 mL and 304.6 ± 156.3 mL, respectively. Daily mean drainage volume and DBL‐related information from first to third day postoperatively were showed in Table 1, from which the Hctd at postoperative day 3 was 24.1%, 15.8%, and 6.3%, respectively, and the mean Hct was gradually decreased. Besides, Hctd was just slightly lower than normal Hct on the first day while the blood components were few before drainage removal.
TABLE 1.
Drainage and blood loss in drainage at postoperative day 3
| Time | Drainage, mL | Hct of drainage, % | RBC in drainage, mL | DBL, mL |
|---|---|---|---|---|
| 1st day post‐op | 312.3 ± 107.4 | 24.1 ± 3.2 | 74.4 ± 37.8 | 209.6 ± 133.0 |
| 2nd day post‐op | 137.4 ± 89.3 | 15.8 ± 2.5 | 22.0 ± 15.8 | 72.5 ± 59.3 |
| 3rd day post‐op | 115.1 ± 70.5 | 6.3 ± 2.7 | 3.1 ± 3.7 | 21.4 ± 11.8 |
DBL, drainage blood loss; Hct: hematocrit; post‐op, post‐operation; RBC: red blood cell.
DBL, HBL, and mHBL in Different Genders and Ages
The subgroup analysis showed there was no statistical difference in terms of gender (P ˃ 0.05). HBL was larger in the group of more than 60 years than the other group (464.5 ± 291.8 vs 287.9 ± 240.0 mL, P = 0.040), so was mHBL (660.7 ± 208.5 vs 511.9 ± 224.6 mL, P = 0.023) (Table 2).
TABLE 2.
Calculation of HBL and mHBL according to Gross formula and modified formula in different age groups and genders
| Indexes | PBV, mL | Intraoperative blood loss, mL | TBL, mL | Drainage, mL | DBL, mL | HBL, mL | HBL/TBL, % | mHBL, mL | mHBL/TBL, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Male (9 cases) | 4749.0 ± 521.6 | 567.1 ± 360.6 | 943.1 ± 322.3 | 411.1 ± 294.2 | 219.9 ± 135.2 | 342.3 ± 310.6 | 31.2 ± 27.8 | 572.6 ± 255.8 | 59.9 ± 15.1 |
| Female (52 cases) | 3728.0 ± 381.8 | 435.3 ± 391.1 | 948.3 ± 337.2 | 511.9 ± 270.3 | 313.5 ± 182.1 | 431.1 ± 278.6 | 43.3 ± 22.4 | 630.2 ± 216.5 | 68.5 ± 16.0 | |
| P | <0.001 | 0.350 | 0.968 | 0.312 | 0.170 | 0.422 | 0.178 | 0.498 | 0.158 | |
| Age | ≤60 y (17 cases) | 4122.6 ± 553.5 | 498.9 ± 312.5 | 815.2 ± 395.0 | 481.2 ± 301.1 | 285.6 ± 210.2 | 287.9 ± 240.0 | 32.4 ± 20.1 | 511.9 ± 224.6 | 65.8 ± 18.5 |
| ˃60 y (44 cases) | 3784.4 ± 512.7 | 43.1 ± 413.8 | 993.7 ± 299.3 | 503.2 ± 265.9 | 305.8 ± 168.3 | 464.5 ± 291.8 | 44.8 ± 23.7 | 660.7 ± 208.5 | 67.9 ± 15.3 | |
| P | 0.028 | 0.577 | 0.073 | 0.781 | 0.708 | 0.040 | 0.075 | 0.023 | 0.666 |
DBL, drainage blood loss; HBL, hidden blood loss; mHBL, modified hidden blood loss; PBV, patient blood volume; TBL, total blood loss.
DBL, HBL, and mHBL among Different Number of Levels
The mean operation time was 152.4 ± 49.1 min and the average number of surgical segments was 2.8 ± 1.6 levels, by which three subgroups including 15 cases in single segment, 16 cases in double segment, and 30 cases in ≥3 segments group were separated. Statistical significance of intraoperative blood loss (P = 0.001), postoperative drainage (P < 0.0001), and DBL (P = 0.005) showed in Table 3 and Fig. 2 suggested the parameters above gradually enlarged with the increased surgical levels while HBL, mHBL, and the proportion of TBL obtained no difference in various segment number.
TABLE 3.
Calculation of HBL and mHBL according to Gross formula and modified formula in different number of levels
| Indexes | Intraoperative blood loss, mL | TBL, mL | Drainage, mL | DBL, mL | HBL, mL | HBL/TBL, % | mHBL, mL | mHBL/TBL, % |
|---|---|---|---|---|---|---|---|---|
| 1 level (15 cases) | 189.0 ± 145.2 | 841.7 ± 208.5 | 318.3 ± 248.5 | 218.4 ± 177.1 | 487.8 ± 213.7 | 55.9 ± 16.7 | 603.0 ± 162.4 | 70.9 ± 12.6 |
| 2 levels (15 cases) | 347.3 ± 138.9 | 1008.3 ± 384.2 | 427.5 ± 201.8 | 251.1 ± 98.4 | 473.3 ± 324.5 | 43.8 ± 17.9 | 652.7 ± 249.9 | 62.6 ± 10.1 |
| ≥3 levels (31 cases) | 634.2 ± 459.1 | 917.5 ± 348.4 | 617.9 ± 221.1 | 373.1 ± 169.0 | 305.2 ± 243.0 | 31.3 ± 19.6 | 561.0 ± 209.8 | 63.3 ± 15.6 |
| Total (61 cases) | 453.3 ± 377.8 | 912.3 ± 332.3 | 489.1 ± 253.8 | 304.6 ± 156.3 | 408.8 ± 268.3 | 41.4 ± 21.4 | 612.2 ± 217.5 | 66.7 ± 14.3 |
| P | 0.001 | 0.672 | <0.001 | 0.005 | 0.112 | 0.173 | 0.062 | 0.074 |
DBL, drainage blood loss; HBL, hidden blood loss; mHBL, modified hidden blood loss; TBL, total blood loss.
Fig. 2.
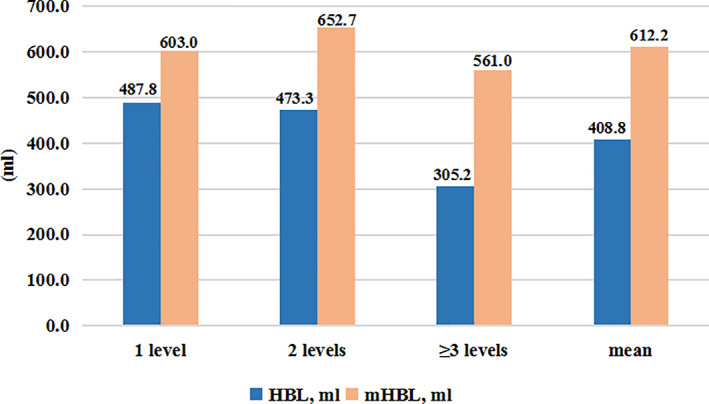
HBL and mHBL in different number of levels. HBL: hidden blood loss; mHBL: modified hiden blood loss.
HBL and mHBL between DMARDs and non‐DMARDs Groups
Table 4 showed no statistical difference both in HBL and mHBL according to Steinbrocker classification of the three groups, which proposed no correlation between RA activity and HBL or mHBL. Figure 3 showed DBL was of statistical difference (P = 0.03) between DMARDs‐taking group and non‐drug group with the mean volume of 415.8 ± 297.5 mL and 261.8 ± 256.4 mL, respectively, but yield no difference of HBL and mHBL between the two groups.
TABLE 4.
Calculation of HBL and mHBL according to Gross formula and modified formula in different Steinbrocker classification
| Classification | HBL, mL | HBL/TBL, % | mHBL, mL | mHBL/TBL, % |
|---|---|---|---|---|
| Grade I (13 cases) | 422.8 ± 246.9 | 42.9 ± 18.8 | 589.1 ± 231.1 | 66.2 ± 11.4 |
| Grade II (34 cases) | 391.3 ± 281.2 | 39.3 ± 21.9 | 600.9 ± 215.4 | 68.7 ± 17.4 |
| Grade III (14 cases) | 447.0 ± 271.1 | 42.1 ± 19.2 | 661.4 ± 179.5 | 66.4 ± 14.1 |
| Total (61 cases) | 408.8 ± 268.3 | 41.4 ± 21.4 | 612.2 ± 217.5 | 66.7 ± 14.3 |
| P | 0.411 | 0.961 | 0.878 | 0.812 |
HBL, hidden blood loss; mHBL, modified hidden blood loss; TBL, total blood loss.
Fig. 3.
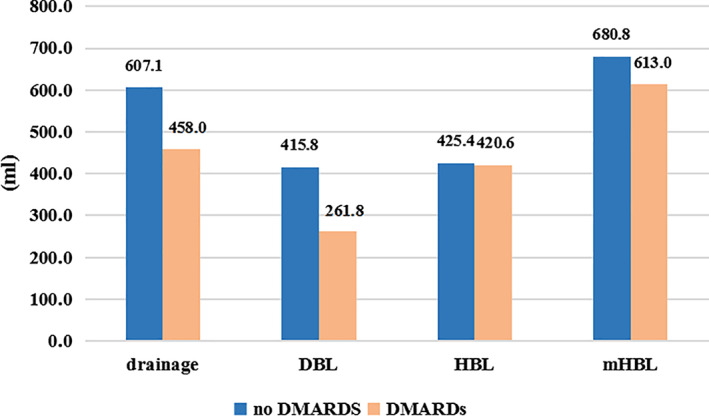
Drainage, DBL, HBL and mHBL based on whether taking DMARDs or not. DBL, drainage blood loss; DMARDs, disease‐modifying anti‐rheumatic drugs; HBL, hidden blood loss; mHBL, modified hiden blood loss.
Comparison between HBL and mHBL
Finally, comparisons between HBL and mHBL (408.8 ± 268.3 vs 612.2 ± 217.5 mL) together with their proportion of TBL (41.4% ± 21.4% vs 66.7 ± 14.3%) showed a statistical difference between the two measures (both P < 0.0001), suggesting that mHBL is larger than HBL by the modified formula.
Discussion
Studies on HBL and mHBL
A large amount of HBL accompanied with PLIF and PLF have been confirmed 1 , 2 , 3 , 14 . Yossi et al. 6 reported that innegligible HBL occupied 42% of TBL during perioperative period in addition to conventional intraoperative bleeding and postoperative drainage. Wen et al. 7 found the mean HBL was 588 mL, far exceeding the doctor's expectations, and Cha et al. 15 reported the mean TBL of posterior laminar decompression was 1256.9 mL, of which HBL accounted for 30% to 50%. This study found that the mean Hb decreased by 2.1 g/dL and Hct decreased by 9.8%, which was unparalleled to amount of visible blood loss and drainage, confirming the existence of HBL. Therefore, performing a blood transfusion, even if in accordance to intraoperative bleeding and postoperative drainage, would still induce a series of complications such as anemia, insufficient oxygen saturation of tissue, and defective coagulation function 16 . The average HBL on patients with RA was 408.8 mL, accounting for 41.4% of TBL, which was consistent with previous studies and showed that HBL of patients with RA may not increase after PLIF compared with non‐RA patients.
Gross's formula 9 used to estimate TBL and HBL, based on pre‐ and postoperative Hct in patient blood, has been widely accepted, so it was necessary to ensure stability of hemodynamics, which would be affected by fluid shift during perioperative stage by regulating the concentration and volume of RBC in circulation, and its influence of factors such as autologous or allogeneic blood profusion and postoperative drainage should be taken into account. Xu et al. 10 and Yossi et al. 6 pointed out a fact that was ignored, that drainage was not completely equivalent to postoperative visible blood loss due to the presence of non‐blood components. Xu et al. 10 firstly demonstrated that the average Hctd fluctuated from 15% to 25% in the first 24 h after surgery by measuring the drainage sample, and found Hctd gradually decreased on the second and third day, where the rest content was replaced by other components such as tissue fluid. Therefore, there would be a large bias in cases that drainage was equivalent to postoperative blood; consequentially a modified calculation method for mHBL was proposed. A sum of 61 drainage samples were collected and the average Hctd was 24.1%, 15.8%, and 6.3% on first postoperative 3 days, verifying the hypothesis proposed by Xu 10 .
Hidden Blood Loss in Terms of Gender and Age
A study conducted by Wen et al. 7 showed HBL was indifferent across genders (P = 0.019) but it was not an independent risk factor. Out data addressed whether DBL, HBL, and mHBL was comparable across genders. As was known, the size and PBV in females was less than in males, while the flaccidness of tissue was more severe, and the latter was the risk factor for HBL 5 , so it delivered overlaid outcomes. In that case, it was notable that the anemia had a more obvious appearance in females, which should be verified by larger sample studies.
Bai 17 and Wen et al. 7 both concluded that patients above 60 years were at risk factor for HBL in orthopaedic surgery. This study showed a similar result, that HBL and mHBL was larger in older patients, though there was no significance in DBL. A possible explanation was that the bleeding likely oozed more easier into the interstitial space due to muscle wastage and soft tissue relaxation in senile patients. In addition, a poor compensatory capacity due to angiosclerosis in senile patients may be another risk factor.
Hidden Blood Loss in Different Surgical Segments
Elgafy et al. 14 and Owens et al. 18 pointed out HBL was irrelevant to the number of surgical segments as the proportion of HBL is equivalent, depite the various volume of TBL and intraoperative blood loss in different levels. Three subgroups (PLIF on single segment, double segment, and ≥3 segments) were divided into in this study and there was no statistical difference in HBL among subgroups, which may be explained that the levels for decompressed were within 1 or 2 segment although the various number of segments for fusion. Our study concluded that there was no difference between HBL and mHBL in different surgical segments with the same grouping program, considering that the level number of decompression ranged from 1 to 3 although there was large level variation (1 to 7 levels) on fixation, consistent with the opinion of Elgafy et al. 14 . Hct and Hb were important parameters for HBL and it was associated with the change of Hct and Hb by correlation analysis. The larger reduction of Hb after surgery, greater destruction of RBC, and more loss of Hb and ensuing higher volume of HBL—which was identified by publications that showed HBL derived mainly from surrounding tissue intraoperative blood infiltrated into, hemolysis, and continuing loss after surgery 19 , 20 , 21 , 22 —suggesting that patients, with lower preoperative Hct and Hb, should be monitored more closely on HBL in case of more potential complications.
HBL and mHBL between DMARDs and non‐DMARDs group
The main pathology of RA were synovial inflammation and RA patients are more prone to lumbar instability, osteoporosis, and stenosis compared with non‐RA patients 13 , 23 . Studies analyzing lumbar injuries such as fractures, spondylolisthesis, and scoliosis have found positive correlation between RA activity and the lumbar vertebrae injury or endplate degeneration 24 . It was shown in this study that both HBL and mHBL did not increase with a higher grade of Steinbrocker classification, and considering that RA was prone to bone destruction and vasospasm, consequentially dominant hemorrhage increased including more intraoperative blood loss and drainage when muscle exfoliation and laminar decompression was performed. While RA activity, which has not been confirmed to be related to blood tissue permeation and hemoiysis, proposed unidentified correlation with mHBL or HBL, which supported further indirect evidence of no statistical significance in HBL between RA and non‐RA patients.
It was indicated that early use of DMARDs can control the progression of RA, reduce complications and inflammatory response 25 , 26 , thus patients taking DMARDs in our study were believed to ameliorate vasospasm, muscle synovitis, and bone trabecular compactness, statistically reducing DBL. However, that there was no singnificance of mHBL or HBL between drug taking and non‐drug taking groups possibly because subtle statistical difference of DBL was compromised and masked when entering HBL or mHBL calculation with more volume or provided evidence that HBL was not associated with RA activity, as concluded above.
Comparisons between HBL and mHBL
This study confirmed the statistical difference between HBL and mHBL and their proportion of TBL in RA patients, consistent with the conclusion of Xu et al. 10 , which considered that mHBL was more than HBL through a modified scale of 67.3% of TBL. It proved that DBL need to be calculated rather than drained as blood loss directly, providing a better‐established basis for objective evaluation of TBL and perioperative blood transfusion, as well as reduction of potential complications, revealing the necessity of modified formula.
This study firstly reviewed LSS patients with RA and revealed that HBL or mHBL occupied half of TBL, which probably exceeded the surgeon's expectation and provided more evidence for timely blood transfusion. In clinical practice, both surgeons and RA patients were confused, since there were little data for reference; however, our study successfully eliminates the concern for preoperative communication and perioperative management on blood loss. In addition, mHBL was defined by taking the pure blood loss in drainage, instead of the mixture, into account, providing more reliable data for whether and when to transfuse blood. Meanwhile, the procedure emphasized the importance of minding the volume and color change of drainage. There were some limitations that need to be mentioned. Only 61 patients were included in this study, and such a small sample size may cause bias in mHBL calculation; thus, a larger population should be in consideration. Next, fluid shift and hemodynamics were assumed stable 2–3 days after PLIF and the reliability of conclusions will be affected with instabality. In addition, there may be twice Hct measures before surgery and the later one was extracted, thus the accuracy remains debatable.
In summary, patients with RA have a large noticeable amount of HBL or mHBL after PLIF. There was no difference on HBL and mHBL in gender. HBL and mHBL was larger in patients over 60 years. There were no significance on HBL and mHBL among different number of segments. HBL or mHBL is not associated with RA activity and the HBL of RA patients may not increase compared with other patients, while taking DMARDs may reduce postoperative DBL. The fact that mHBL by modified formula is larger than HBL provides an all‐round basis for measuring the real hidden blood loss.
Shuai Xu is the first author. Fanqi Meng and Chen Guo are co‐first authors.
Grant Sources: Government Procurement of National Health Commission of China (2127000218).
Disclosure: There is no conflict of interest. We have acquired approval by local ethics committee. All authors have signed patient consent forms.
References
- 1. Brecher ME, Monk T, Goodnough LT. A standardized method for calculating blood loss. Transfusion, 1997, 37: 1070–1074. [DOI] [PubMed] [Google Scholar]
- 2. Ogura Y, Dimar IJ, Gum JL, et al. Hidden blood loss following 2‐ to 3‐level posterior lumbar fusion. Spine J, 2019, 19: 2003–2006. [DOI] [PubMed] [Google Scholar]
- 3. Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br, 2004, 86: 561–565. [PubMed] [Google Scholar]
- 4. Korovessis P. Hidden blood loss in spine surgery for A1‐A3 thoracolumbar fractures. Comparison between three approaches. J Invest Surg, 2019, 32: 761–762. [DOI] [PubMed] [Google Scholar]
- 5. Yin MC, Chen GH, Yang J, et al. Hidden blood loss during perioperative period and the influential factors after surgery of thoracolumbar burst fracture: a retrospective case series. Medicine (Baltimore), 2019, 98: e14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smorgick Y, Baker KC, Bachison CC, Herkowitz HN, Montgomery DM, Fischgrund JS. Hidden blood loss during posterior spine fusion surgery. Spine J, 2013, 13: 877–881. [DOI] [PubMed] [Google Scholar]
- 7. Wen LF, Jin DX, Xie WX, et al. Hidden blood loss in posterior lumbar fusion surgery: an analysis of risk factors. Clin Spine Surg, 2018, 31: 180–184. [DOI] [PubMed] [Google Scholar]
- 8. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery, 1962, 51: 224–232. [PubMed] [Google Scholar]
- 9. Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology, 1983, 58: 277–280. [DOI] [PubMed] [Google Scholar]
- 10. Xu DR, Ren ZN, Chen X, et al. The further exploration of hidden blood loss in posterior lumbar fusion surgery. Orthop Traumatol Surg Res, 2017, 103: 527–530. [DOI] [PubMed] [Google Scholar]
- 11. Peter J, Laurence G, Addisu M. Surgical management of the lumbar spine in rheumatoid arthritis. Global Spine J, 2020, 10: 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shoji S, Norikazu H, Isao M, et al. Lumbar spine surgery in patients with rheumatoid arthritis (RA): what affects the outcomes?. Spine J, 2018, 18: 99–106. [DOI] [PubMed] [Google Scholar]
- 13. Krauss WE, Bledsoe JM, Clarke MJ, Nottmeier EW, Pichelmann MA. Rheumatoid arthritis of the craniovertebral junction. Neurosurgery, 2010, 66: 83–95. [DOI] [PubMed] [Google Scholar]
- 14. Elgafy H, Bransford RJ, McGuire RA, Dettori JR, Fischer D. Blood loss in major spine surgery: are there effective measures to decrease massive hemorrhage in major spine fusion surgery?. Spine (Phila Pa 1976), 2010, 35: S47–S56. [DOI] [PubMed] [Google Scholar]
- 15. Cha CW, Deible C, Muzzonigro T, Lopez‐Plaza I, Vogt M, Kang JD. Allogeneic transfusion requirements after autologous donations in posterior lumbar surgeries. Spine (Phila Pa 1976), 2002, 27: 99–104. [DOI] [PubMed] [Google Scholar]
- 16. Smith GH, Tsang J, Molyneux SG, White TO. The hidden blood loss after hip fracture. Injury, 2011, 42: 133–135. [DOI] [PubMed] [Google Scholar]
- 17. Bai B, Tian Y, Zhang YL, Ma MJ, Yu XR, Huang YG. Prediction of hidden blood loss during posterior spinal surgery. Chin Med Sci J, 2019, 34: 38–44. [DOI] [PubMed] [Google Scholar]
- 18. Owens RN, Crawford CR, Djurasovic M, et al. Predictive factors for the use of autologous cell saver transfusion in lumbar spinal surgery. Spine (Phila Pa 1976), 2013, 38: E217–E222. [DOI] [PubMed] [Google Scholar]
- 19. Erskine JG, Fraser C, Simpson R, Protheroe K, Walker ID. Blood loss with knee joint replacement. J R Coll Surg Edinb, 1981, 26: 295–297. [PubMed] [Google Scholar]
- 20. Zheng F, Jr CF, Sandhu HS, Girardi FP, Khan SN. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine (Phila Pa 1976), 2002, 27: 818–824. [DOI] [PubMed] [Google Scholar]
- 21. Pattison E, Protheroe K, Pringle RM, Kennedy AC, Dick WC. Reduction in haemoglobin after knee joint surgery. Ann Rheum Dis, 1973, 32: 582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faris PM, Ritter MA, Keating EM, Valeri CR. Unwashed filtered shed blood collected after knee and hip arthroplasties. A source of autologous red blood cells. J Bone Joint Surg Am, 1991, 73: 1169–1178. [PubMed] [Google Scholar]
- 23. Lawrence JS, Sharp J, Ball J, Bier F. Rheumatoid arthritis of the lumbar spine. Ann Rheum Dis, 1964, 23: 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ibrahim M, Suzuki A, Yamada K, et al. The relationship between cervical and lumbar spine lesions in rheumatoid arthritis with a focus on endplate erosion. J Spinal Disord Tech, 2015, 28: E154–E160. [DOI] [PubMed] [Google Scholar]
- 25. Atzeni F, Benucci M, Salli S, Bongiovanni S, Boccassini L, Sarzi‐Puttini P. Different effects of biological drugs in rheumatoid arthritis. Autoimmun Rev, 2013, 12: 575–579. [DOI] [PubMed] [Google Scholar]
- 26. Mojtaba A, Mohammad JM, Sirous J, et al. Strategies toward rheumatoid arthritis therapy; the old and the new. J Cell Physiol, 2019, 234: 10018–10031. [DOI] [PubMed] [Google Scholar]


