Abstract
Objective
To describe the rationale and application of triggered EMG (T‐EMG) in intraoperative neurophysiological monitoring, and to explore the efficacy and safety of posterior percutaneous endoscopic cervical discectomy (PPECD) in the treatment of cervical spondylotic radiculopathy (CSR) under multimodal intraoperative neurophysiological monitoring (IOM).
Methods
This study was a retrospective cohort control study. The clinical data of 74 patients with single‐segment CSR from June 2015 to August 2018 were analyzed retrospectively, of whom 35 underwent IOM‐assisted PPECD with triggered EMG (T‐EMG group), while 39 were subjected to IOM‐assisted PPECD alone (IOM group). Operation time, hospital stay, and complications were recorded for both groups. The curative effect was evaluated according to the Visual Analog Scale (VAS) of neck and arm pain, Japanese Orthopaedic Association (JOA) score, and modified MacNab scale.
Results
Operations were successful and all patients were followed up for at least 24 (average 31.77 ± 9.51) months with no patient lost to follow‐up. No significant difference was found in preoperative baseline data between the T‐EMG and the IOM group (P > 0.05). Also, no significant difference was found in the operation time between the T‐EMG (108.29 ± 11.44 min) and the IOM (110.13 ± 12.70 min) (P > 0.05) group, but the difference in hospital stay (T‐EMG: 5.66 ± 0.99 days; IOM: 7.10 ± 1.43 days) was statistically significant (P < 0.05). The VAS for the neck and upper limbs in the two groups at 1 month post‐operation (T‐EMG: 2.09 ± 1.07, 2.26 ± 0.92; IOM:2.18 ± 1.05, 2.31 ± 0.77) and the last follow‐up (T‐EMG: 0.83 ± 0.62, 0.86 ± 0.55; IOM: 0.90 ± 0.50, 0.87 ± 0.61) were significantly different from the preoperative scores (T‐EMG: 6.14 ± 1.09, 7.17 ± 1.04; IOM: 6.18 ± 1.28, 7.15 ± 1.23) (P < 0.05). However, no significant difference was found between the two groups (P > 0.05). The 1‐month postoperative JOA scores for the two groups (12.69 ± 0.76; 12.59 ± 0.82) and those at the last follow‐up (14.60 ± 0.77; 14.36 ± 0.78) were significantly different from the preoperative scores (11.09 ± 0.98; 11.05 ± 0.89) (P < 0.05), but the difference between the two groups was not significant (P > 0.05). One patient in the T‐EMG group developed a transient aggravation of symptoms on the first day after surgery. In the IOM group, three patients had intraoperative cerebrospinal fluid leakage, and symptoms of C5 nerve root paralysis were presented in four patients following surgery. Compared with the IOM group, the T‐EMG group had fewer complications (1/35; 7/39, P < 0.05). At the last follow‐up, the modified MacNab criteria were 91.43% (32/35) and 89.7% (35/39) for the T‐EMG group and IOM group, respectively.
Conclusions
Triggered EMG prevents the occurrence of neurological complications, which not only aids PPECD for CSR treatment in achieving satisfactory results, but also reduces average hospital stay and complication rates.
Keywords: Cervical endoscopy, Cervical spondylotic radiculopathy, Intraoperative monitoring, Percutaneous endoscopic cervical discectomy, Triggered EMG
Introduction
Cervical spondylotic radiculopathy (CSR) is often caused by nerve compression due to cervical disc herniation, intervertebral foramen stenosis, and hyperplastic osteophytes 1 , 2 . Its main symptoms include pain in the neck and upper limbs, with or without numbness, and with surgical treatment needed in severe cases 3 . Since its first proposal by Smith and Robinson in 1955 4 , anterior cervical discectomy and fusion (ACDF) has been regarded as the gold standard for CSR treatment. However, ACDF is complicated by cage subsidence, adjacent stage degeneration, and loss of intervertebral height 5 , 6 . Adequate decompression while retaining as much of the spinal structure and stability as possible has become a major concern of orthopaedic spine surgeons.
With the rapid development of minimally invasive spinal technology and instruments, endoscopy has found wide applications in spinal surgery 7 , 8 , 9 . At present, the endoscopic treatment of CSR includes an anterior approach and a posterior approach. Endoscopic decompression via the anterior approach has been found to effectively relieve nerve compression and achieve satisfactory clinical results 10 . However, the characteristics of this approach increase the possibility of vertebral arterial injury, and the removal of soft and bony compression behind the vertebral body proves difficult. Posterior percutaneous endoscopic cervical discectomy (PPECD), which was first reported by Ruetten et al. 11 in 2007, is one of the minimally invasive surgical methods for CSR treatment. Zhang et al. 1 reported the treatment of 42 patients with CSR by O‐Arm‐assisted PPECD, with a satisfaction rate of 83.33% during a 15‐month follow‐up. In a randomized controlled trial of PPECD and ACDF applied for the treatment of CSR, 175 patients were followed up for 2 years with an excellent and good operative success rate of 87.4%, proving that PPECD is a safe and effective alternative to traditional surgery 12 . The resection of lateral protruding lesions or vegetations in the intervertebral foramen through the posterior approach has the advantages of less trauma and better maintenance of stability 13 . Nevertheless, high efficacy often comes with risks and complications. It is inevitable, for example, to pull the nerve and spinal cord during PPECD, hence the risk of subsequent nerve and spinal cord injury undoubtedly increases.
In spinal surgery, the application of intraoperative neurophysiological monitoring (IOM) allows to keep track of the neural function system in real time, thus providing clear advantages in reducing nerve injury and operative complications 14 . The IOM method measures the changes of membrane potential, conduction velocity, and ion channel activity of nerve and cell ion channels by electrophysiological instruments, microelectrodes, and other techniques. It is commonly used in spinal surgery and includes the measurement of somatosensory‐evoked potentials (SEP), motor‐evoked potentials (MEP), and electromyography (EMG). The latter includes spontaneous free‐running (S‐EMG) or triggered (T‐EMG) electromyography. The ascending conduction function of the lateral posterior funiculus and the posterior funiculus of the spinal cord is reflected by SEP. When an electrical stimulation is applied to the peripheral nerve pathway, the corresponding potential waveforms are recorded in the central nervous system or nerve trunk. Conversely, MEP monitors spinal cord movement at any time by stimulating the motor area of the cortex to generate excitement and signals through the descending conduction pathway 15 , 16 , 17 , 18 . Furthermore, F‐EMG detects the action potential produced by single nerve traction and other stimuli, reflecting whether the operation provokes or damages the corresponding nerve roots or nerve fibers. At the same time, T‐EMG judges nerve root function by monitoring the feedback waveform generated by direct stimulation.
In spinal surgery, IOM is used to monitor the neurological system in real time to lower the risk of neurological complications. However, with each monitoring method having its own advantages and defects, a single application is often disturbed by external factors resulting in false positive detection events.
Multimode IOM combined with SEP and MEP is a reliable and effective method for evaluating spinal cord function 17 . EMG is suitable for monitoring the decompression of a single nerve root during PPECD. More specifically, T‐EMG is used to evaluate the proximity of motor nerve, and S‐EMG is applied to detect secondary nerve stimulation caused by tension or compression 19 . This is particularly important when dealing with nerve roots that have severe adhesions in PPECD. Through the stimulation of the adhesion area, there is direct feedback of the distance from the nerve root in order to avoid the injury of the spinal cord or nerve root. Although the application of multimodal IOM can effectively reduce the occurrence of intraoperative false positive and false negative events, few reports on the application of T‐EMG in PPECD exist and its clinical application value and safety are still uncertain.
The purpose of this retrospective study is to: (i) explore the efficacy of IOM‐assisted PPECD in the treatment of CSR; (ii) evaluate the safety of multi‐mode IOM in PPECD; and (iii) explore the application value of T‐EMG in PPECD.
Materials and Methods
Inclusion and Exclusion Criteria
Inclusion criteria: (i) a total of 74 patients with unilateral and single segment CSR were admitted to Henan Provincial People's Hospital from June 2015 to August 2018; (ii) patients were treated with IOM‐assisted PPECD with or without triggered EMG; (iii) visual analog scale (VAS), Japanese Orthopaedic Association (JOA), and MacNab scores were compared; and (iv) all cases were part of a retrospective cohort study.
Exclusion criteria: (i) patients with cervical spondylotic myelopathy, multi‐segmental or central protrusion; (ii) patients with a severe ossification of the posterior longitudinal ligament and ligamentum flavum; (iii) patients with obvious instability or spondylolisthesis of diseased segments; (iv) patients with lumbar trauma, cancer, and other serious systemic diseases; or (v) those lost to follow‐up.
Group Allocations
T‐EMG group: 35 patients (22 men and 13 women); IOM group: 39 patients (23 men and 16 women). Both surgeries were performed by the same orthopaedic spine surgeon.
Preoperative Preparation
A 32‐channel XLTEK Protektor32 (Canada) monitor was used for continuous IOM. The extremities were monitored by SEP, the upper limb recording electrodes were placed at the C3’ and C4’ points, and the reference electrodes were positioned at the Fz points. The recording electrode of the lower limb was attached at the Cz point, and the reference electrode was placed at the Fz point. The stimulation electrodes were the median nerve of the bilateral wrist and the posterior tibial nerve of the bilateral medial malleolus. The EMG monitoring of muscles was performed differently in separate segments of lesions. The deltoid muscle and biceps brachii muscle were monitored at the C4/5 segment, the extensor carpi radialis muscle was monitored at the C5/6 segment, whereas the total extensor muscle of finger was monitored at the C6/7 segment (Fig. 1).
Fig. 1.
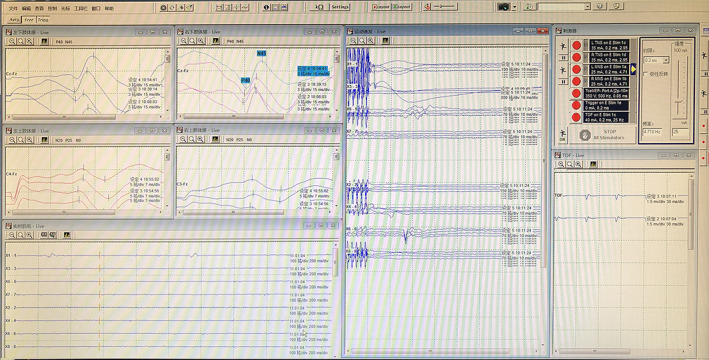
Real‐time monitoring of multimode IOM during PPECD.
Surgical Procedure
IOM Group
Anesthesia and Position
The surgeries were performed under intravenous inhalation combined anesthesia and in prone position, with the cervical vertebrae and head fixed with a Mayfield skull clamp (USA). Arms were placed parallel to the body and secured with duct tape. Intravenous anesthesia was maintained by propofol combined with remifentanil. Sevoflurane was used in inhalation anesthesia and the MacNab value was controlled as <0.5.
Approach and Exposure
The puncture point was located 1.5 cm adjacent to the affected side of the responsible intervertebral space. The 18 gauge needle was punctured to the outer edge of the lamina, and was removed after positioning the guide wire. Thereafter, a 10 mm skin incision was made at the guide wire, and the working casing was expanded step‐by‐step. The spinal endoscopic system was then connected and the operation was performed under continuous saline irrigation.
Decompression
The soft tissue on the facet joint and lamina surface was removed by bipolar radiofrequency, revealing the “V” point at the contacts of the lower edge of the superior lamina, the upper edge of the inferior lamina, the medial side of the facet joint, and the lateral junction of the lamina. The lateral bone of the inferior edge of the superior lamina, the medial edge of the facet joint, and the lateral bone of the upper edge of the inferior lamina were removed by endoscopic drilling. The lateral portion of the ligamentum flavum was removed to expose the lateral margin of the dural sac and the nerve root. After full decompression, the upper shoulder and axilla of the nerve root were explored.
Closure
Following hemostasis, the endoscope and working cannula were removed, and the incision was finally sutured.
T‐EMG Group
The surgical methods and postoperative management for the T‐EMG group were the same as those for the IOM group.
When the amplitude of SEP decreased by 50% or the latency extended by more than 10%, and when the amplitude of MEP decreased by 80%, the monitors were programmed to remind the operator to find the cause and avoid spinal cord or nerve injury. When the adhesion between spinal nerve and lesion was severe, a current of 1‐2 mA at 1‐2 Hz frequency and 0.2 mS wave width was directly generated by a stimulator. If the EMG response persisted, monitors immediately reminded the operator to avoid spinal cord or nerve root injury (Fig. 2).
Fig. 2.
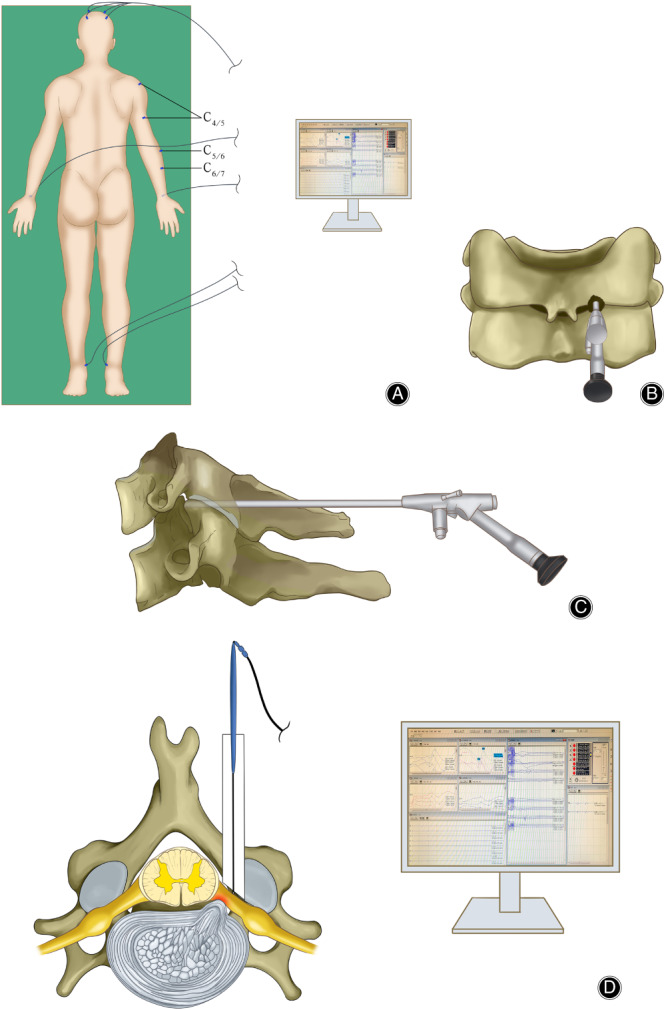
(A) The PPECD procedure was done under real‐time monitoring by IOM. T‐EMG monitors muscles differently in different lesions. C4/5: deltoid with biceps; C5/6: extensor carpi radialis; C6/7: extensor digitorum communis. (B, C) Expose the “V” point and decompress endoscopically. (D) The stimulator directly stimulates the tissue adherent to the spinal nerve and observes the latency time and amplitude to determine the neurological function.
Outcome Measures
The operation time, hospital stay, and complications in the two groups were recorded and compared. The Visual Analog Scale (VAS) and the Japanese Orthopaedic Association (JOA) score were recorded preoperatively, 1 month after operation, and at the last follow‐up, when the modified MacNab criteria score was also determined to evaluate surgical efficacy.
Visual Analog Scale (VAS)
The VAS is the most used questionnaire for quantification of pain. It is a continuous scale comprised of a horizontal or vertical line, usually 10 cm in length. For pain intensity, the scale is most commonly anchored by “no pain” (score of 0) and “pain as bad as it could be” (score of 10). A score of 0 is considered as no pain, 1–3 mild pain, 4–6 moderate pain, and 7–10 severe pain.
Japanese Orthopaedic Association (JOA)
JOA is one of the most frequently used clinical outcome measures to quantify functional status in patients with cervical myelopathy. A score of 16–17 indicates normal function (best possible outcome), a score of 12–15 is grade 1, a score of 8–11 is grade 2, and a score of 0–7 is the most severe deficits. The recovery rate for the JOA score = [(postoperative score – preoperative score)/(17‐preoperative score)] × 100%.
Modified MacNab Criteria
The modified MacNab criteria were used to evaluate the efficacy of surgery. The modified MacNab criteria include four grades: excellent, good, fair, and poor. Excellent: symptoms disappear completely, return to original work and life; good: mild symptoms, activity is slightly limited, no impact on work and life; fair: symptoms are relieved, activities are limited, affecting normal work and life; poor: there is no difference before and after treatment, even aggravated.
Statistical Analysis
Data were subjected to statistical analyses using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Statistical methods were employed to compare patient demographic data and clinical outcomes for both study groups. Continuous and categorical parameters were analyzed using a t‐test and a chi‐squared test, respectively. A P value of <0.05 was considered statistically significant.
Results
Follow‐Up
Surgeries were successfully performed for patients in both groups. All patients were followed up for an average of 31.77 ± 9.51 months.
General Results
The mean age of patients was 44.23 ± 8.02 years in T‐EMG group and 46.92 ± 9.72 years in IOM group, and there was no significant difference between the two groups (P = 0.121).
The sex ratio (male: female ratio of 22:13 in T‐EMG vs 23:16 in IOM) showed significant difference between the two groups (P = 0.733).
The follow‐up time (T‐EMG: 31.80 ± 8.42 months and IOM: 31.74 ± 10.60 months) showed no significant difference between the two groups (P = 0.681).
The operation time between the two groups (T‐EMG: 108.29 ± 11.44 min and IOM: 110.13 ± 12.70 min) showed no significant difference (P = 0.413).
The hospital stay in the T‐EMG group (5.66 ± 0.99 days) was significantly shorter than that in the IOM group (7.10 ± 1.43 days) (P = 0.048) (Table 1).
TABLE 1.
Comparison of the baseline data between the NM group and PECD group
| Item | T‐EMG group (n = 34) | IOM group (n = 39) | P‐value |
|---|---|---|---|
| Sex (M/F, n) | 22/13 | 23/16 | 0.733 |
| Age (mean ± SD, years) | 44.23 ± 8.02 | 46.92 ± 9.72 | 0.121 |
| Operation time (mean ± SD, min) | 108.29 ± 11.44 | 110.13 ± 12.70 | 0.413 |
| Hospital stay (mean ± SD, day) | 5.66 ± 0.99 | 7.10 ± 1.43 | 0.048 |
| Follow‐up (mean ± SD, months) | 31.80 ± 8.42 | 31.74 ± 10.60 | 0.681 |
| Segment (n) | |||
| C4/5 | 5 | 4 | ‐ |
| C5/6 | 16 | 18 | ‐ |
| C6/7 | 13 | 17 | ‐ |
A case of a CSR patient treated with PPECD assisted by T‐EMG was shown in Figs 3, 4, 5, 6, 7.
Fig. 3.
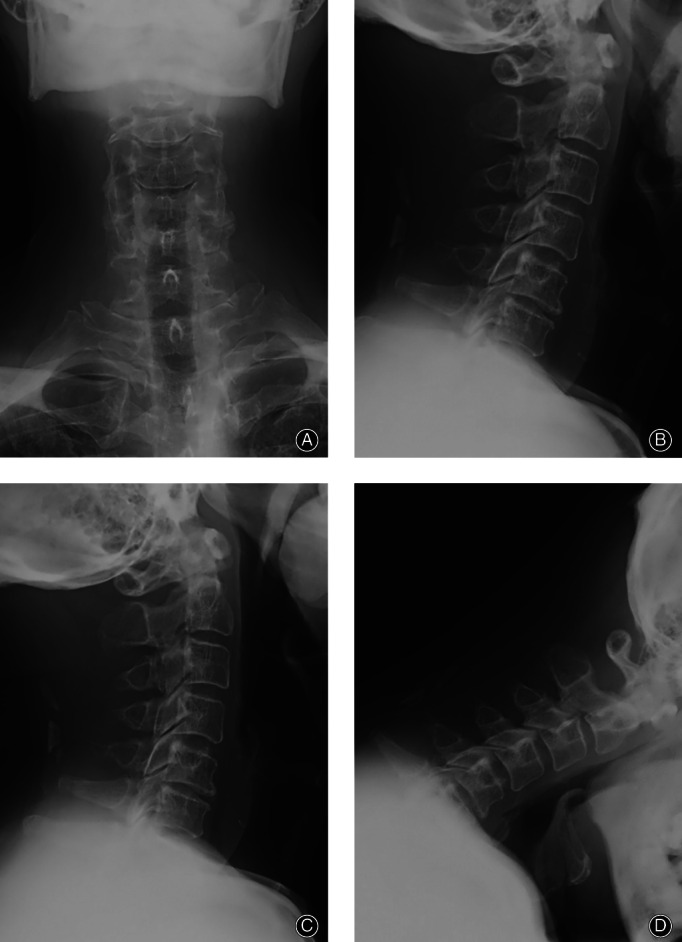
A 36‐year‐old male patient with neck discomfort presented right upper limb pain and numbness for 5 months. (A–D) Preoperative anteroposterior and lateral X‐ray of cervical vertebra, hyperextension, and flexion X‐ray.
Fig. 4.
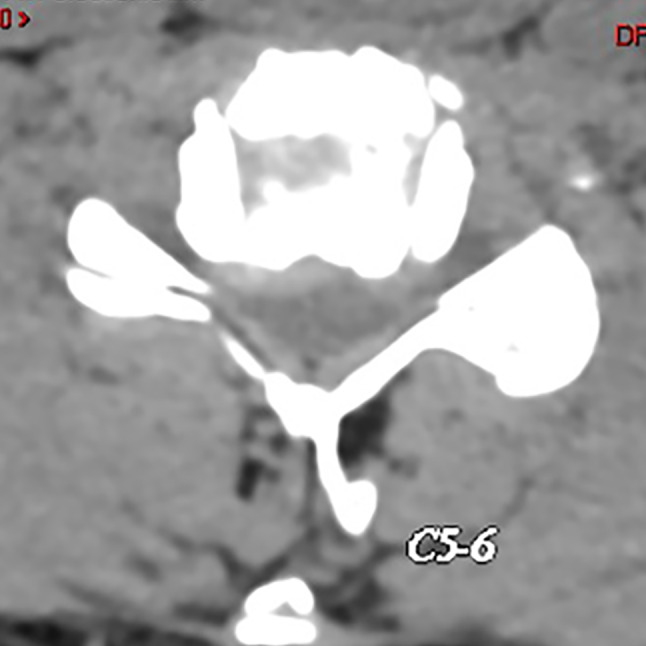
A 36‐year‐old male patient with neck discomfort presented right upper limb pain and numbness for 5 months. Preoperative CT showed right disc herniation and foraminal stenosis.
Fig. 5.
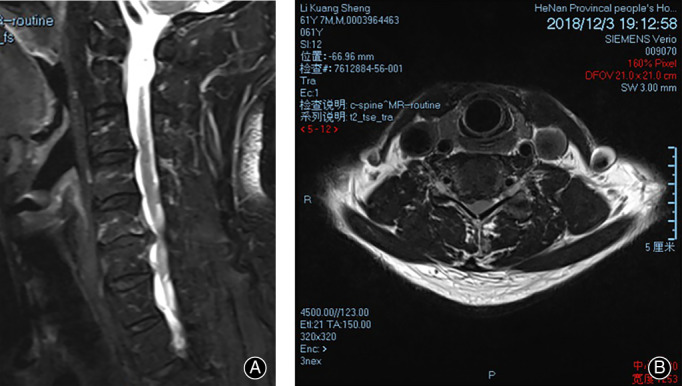
A 36‐year‐old male patient with neck discomfort presented right upper limb pain and numbness for 5 months. (A, B) Preoperative MRI showed a herniated right intervertebral disc and compressed nerve root.
Fig. 6.
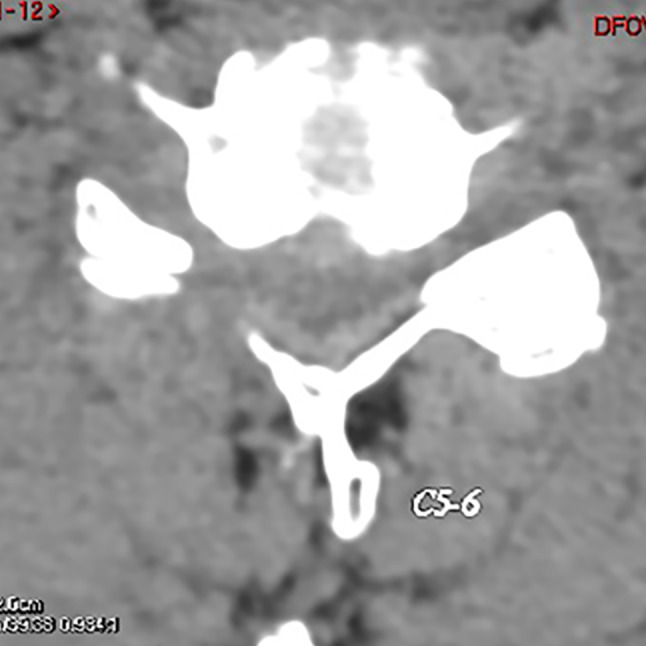
One month after the operation, CT showed that the right intervertebral foramen was enlarged.
Fig. 7.
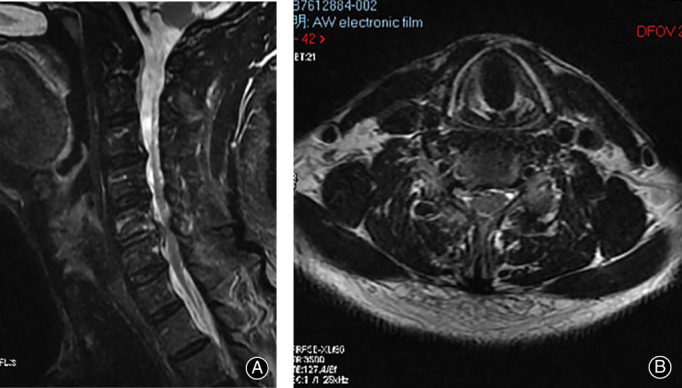
(A, B) Postoperative MRI showed that the right nerve root was no longer compressed.
Visual Analog Scale (VAS)
In the T‐EMG group, the neck and arm VAS scores at 1 month (2.09 ± 1.07; 2.26 ± 0.92) and the last follow‐up (0.83 ± 0.62; 0.86 ± 0.55) were significantly lower than those before surgery (6.14 ± 1.09; 7.17 ± 1.04) (P < 0.05). Meanwhile, the VAS scores in the IOM group at 1 month (2.18 ± 1.05; 2.31 ± 0.77) and the last follow‐up (0.90 ± 0.50; 0.87 ± 0.61) were significantly lower than those before surgery (6.18 ± 1.28; 7.15 ± 1.23) (P < 0.05). The 1‐month postoperative VAS scores and those at the last follow‐up were not significantly different between the two groups (P > 0.05) (Table 2, Fig. 8).
TABLE 2.
Comparison of preoperative and postoperative visual analog scale scores (VAS) between the two groups (Mean ± SD)
| Groups | Neck‐VAS score | Arm‐VAS score | ||||
|---|---|---|---|---|---|---|
| Pre‐op | Post‐op 1 m | Last F‐up | Pre‐op | Post‐op 1 m | Last F‐up | |
| T‐EMG group | 6.14 ± 1.09 | 2.09 ± 1.07* | 0.83 ± 0.62* | 7.17 ± 1.04 | 2.26 ± 0.92* | 0.86 ± 0.55* |
| IOM group | 6.18 ± 1.28 | 2.18 ± 1.05* | 0.90 ± 0.50* | 7.15 ± 1.23 | 2.31 ± 0.77* | 0.87 ± 0.61* |
| P‐value | 0.403 | 0.840 | 0.096 | 0.895 | 0.279 | 0.915 |
Note: Compared with preoperative scores * P < 0.05
Fig. 8.
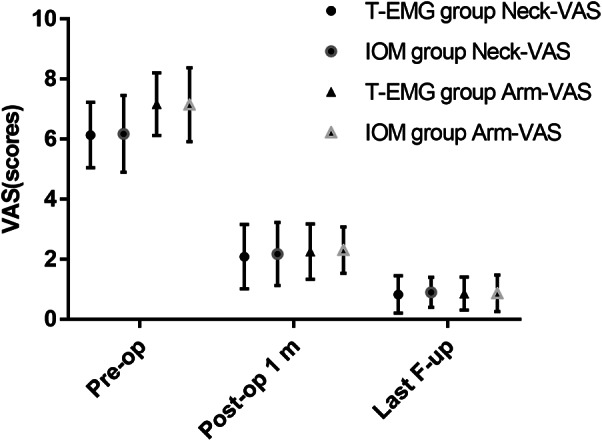
The dynamic changes of neck and arm pain in the two groups were assessed by Visual Analog Scale (VAS).
Japanese Orthopaedic Association (JOA) Score
In the T‐EMG group, JOA scores at 1 month (12.69 ± 0.76) and the last follow‐up (14.60 ± 0.77) were significantly improved compared with those before surgery (11.09 ± 0.98) (P < 0.05). In the IOM group, the JOA scores at 1 month (12.59 ± 0.82) and the last follow‐up (14.36 ± 0.78) were significantly improved compared with those before surgery (11.05 ± 0.89) (P < 0.05). The 1‐month postoperative JOA scores and those at the last follow‐up were not significantly different between the two groups (P > 0.05) (Table 3, Fig. 9).
TABLE 3.
Comparison of preoperative and postoperative Japanese Orthopaedic Association (JOA) scores and complication between the two groups (Mean ± SD)
| Groups | JOA | Complication | ||
|---|---|---|---|---|
| Pre‐op | Post‐op 1 m | Last F‐up | ||
| T‐EMG group | 11.09 ± 0.98 | 12.69 ± 0.76* | 14.60 ± 0.77* | 1/35 |
| IOM group | 11.05 ± 0.89 | 12.59 ± 0.82* | 14.36 ± 0.78* | 7/39 |
| P‐value | 0.650 | 0.567 | 0.987 | 0.040 |
Note: Compared with preoperative scores * P < 0.05
Fig. 9.
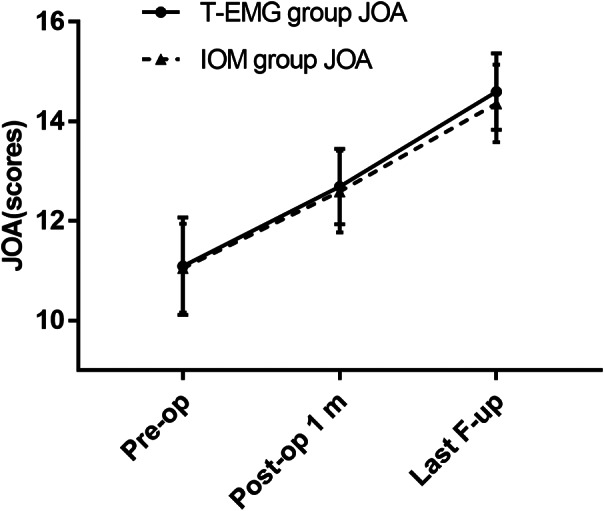
The dynamic changes process of cervical spine function in the two groups were assessed by Japanese Orthopaedic Association (JOA) score.
Modified MacNab Criteria
At the last follow‐up, modified MacNab was used to evaluate the curative effect. The satisfaction rate of modified MacNab was 91.43% in the T‐EMG group, and 89.7% in the IOM group.
Complications
In the T‐EMG group, one patient presented a transient aggravation of symptoms on the first day after operation, which gradually improved within 1 month. In the IOM group, three cases of intraoperative cerebrospinal fluid leakage occurred, and the drainage tube was removed after 1 week of head‐down and high‐posture drainage. The symptoms of C5 nerve root paralysis occurred postoperatively in four cases, while symptoms disappeared completely after 3 months of symptomatic treatment. Compared with the IOM group, fewer complications occurred in the T‐EMG group (P < 0.05).
Discussion
Efficacy of IOM‐Assisted PPECD in CSR Treatment
The posterior approach of PPECD was used to partly open the window on the outside of the interlaminar space and the medial articular process in order to form a small hole similar to a “keyhole.” Once the nerve root and lateral dural sac were exposed, the nerve root was decompressed under endoscope. The success of the operation is determined by whether the intraoperative operation is precise enough or not. The technology of IOM can timely, objectively, and effectively evaluate the structural and functional integrity of the nervous system through the real‐time changes of its electrophysiological signals. In our study, the scores of VAS and JOA for the T‐EMG group and the IOM group at each follow‐up time point after operation were significantly improved as compared with those before operation (P < 0.05), with no significant difference between the two groups (P > 0.05). This demonstrates that IOM‐assisted PPECD with triggered EMG is effective in the treatment of CSR. There was no significant difference in operation time between the two groups; however, hospital stay was significantly lower for the T‐EMG group. The T‐EMG technique effectively reduced the unnecessary traction stimulation of spinal cord and nerve during the operation, which accelerated postoperative recovery and significantly reduced hospitalization time in the T‐EMG group.
Safety of Multi‐Mode IOM in PPECD
Haijun et al. 20 reported a 94.0% excellent and good operative success rate for 50 cases of CSR treated with PPECD. Their study, however, included one case of aggravation of pain and numbness, two cases of dural tear, one case of decreased muscle strength and one case of recurrence of intervertebral disc herniation. Even though PPECD has achieved satisfactory results, it is possible to injure the spinal cord and nerves during operation because of the difficulty of the procedure and poor tolerance of the cervical section of the spinal cord 13 , 21 . Although IOM can reduce the risk of nerve injury during spinal surgery, each monitoring method has its own advantages and drawbacks, and a single application is often disturbed by external factors resulting in false positive alarm events 22 . When the IOM index is abnormal, the anesthetic, blood pressure, temperature, and other interference factors should be eliminated first, and further close communication with the operator is necessary to avoid iatrogenic spinal cord injury. During the PPECD operations in our study, MEP was the main mode of multi‐mode IOM, whereas SEP and EMG were auxiliary modes. The technique anesthetics have the least effect on is SEP, but it is easily affected by temperature, blood pressure, radio frequency, and other factors. At the same time, MEP has highly sensitivity to spinal cord ischemia and traction, and the ability to feedback spinal cord motor function in real time, but is greatly affected by anesthetics, especially muscle relaxants, and has a high false positive rate. Therefore, muscle relaxants should be avoided during MEP monitoring during PPECD; propofol and remifentanil should be used instead for the maintenance of anesthesia. When the amplitude of MEP decreases by 80%, the operation has to be temporarily stopped to find the cause and avoid traction injury to the spinal cord during decompression. Close attention must be paid to SEP when operating under a microscope. According to the changes of waveform, it is possible to judge whether the injury is ischemic or mechanical, which can effectively prevent irreversible injury to the spinal cord. With the combined characteristics of each monitoring method, the multi‐mode combined application of NM can effectively reduce the occurrence of false positive and false negative events. In the present study, the incidence of complications in the T‐EMG group was significantly lower than that in the IOM group (P < 0.05), suggesting that multi‐mode IOM can effectively reduce the incidence of complications and improve the safety of the surgical procedure.
Application Value of t‐EMG in PPECD
Cervical spondylotic radiculopathy often involves a single nerve root, thus EMG has its unique application value in PPECD. The F‐EMG method is usually used to detect secondary nerve stimulation caused by stretch or compression, but is easily disturbed by an electric knife, bipolar techniques, and other factors. If the nerve is mechanically stimulated such as traction, the muscle has an action potential burst. However, it is difficult to judge the degree of injury and whether the neurological function is still intact by this burst waveform. Meanwhile, T‐EMG is commonly used to judge the position of pedicle screw, and has the advantage that it can monitor the condition of nerve root in real time. Its drawback, however, is that the degree of proximity to the motor nerve is not too accurate. In this study, T‐EMG was used to judge the degree of proximity between the decompression area and the nerve root. In cases with long lesion time and severe nerve root adhesion, direct stimulation with 1‐2 mA current at 1‐2 Hz frequency and 0.2 mS wave width was applied. Waveforms were recorded in the corresponding muscles to determine nerve root function based on latency time and amplitude. Based on our observations, a continuous EMG response means that the nerves innervating the muscle are involved. Therefore, it is helpful to identify and judge the presence of nerve root segmental function, so that the surgeon can find the cause in time and avoid further stimulation and injury to the associated nerve root.
Limitations
Our study is a retrospective study with a limited number of cases, which disadvantages can be reduced by future long‐term follow‐up and prospective studies.
Conclusion
Multi‐mode IOM‐assisted PPECD has achieved satisfactory results in CSR treatment, and has the advantages of short hospital stay and high safety. Moreover, T‐EMG has a unique value in the treatment of severe adhesion of nerve roots in PPECD.
Ethical Information
The study protocol adheres to the principles set forth by the 1964 Declaration of Helsinki and its later amendments.
Grant Sources: This study was supported by the National Natural Science Foundation of China (Grant Nos. 81874017, 81960403, and 82060405); Natural Science Foundation of Gansu Province of China (20JR5RA320); Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2017‐ZD02); Joint Project of Medical Science and Technology of Henan Province (LHGJ20190859); Overseas Research and Training Project of Health Science and Technology Talents in Henan Province (HWYX 2019159).
Disclosure: The authors declare that they have no competing interests.
Contributor Information
Ya‐yi Xia, Email: xiayy@lzu.edu.cn.
Shu‐lian Chen, Email: chshl3896@163.com.
References
- 1. Zhang C, Wu J, Xu C, et al. Minimally invasive full‐endoscopic posterior cervical foraminotomy assisted by O‐arm‐based navigation. Pain Physician, 2018, 21: E215–E223. [PubMed] [Google Scholar]
- 2. Ke W, Zhi J, Hua W, et al. Percutaneous posterior full‐endoscopic cervical foraminotomy and discectomy: a finite element analysis and radiological assessment. Comput Methods Biomech Biomed Engin, 2020, 23: 805–814. [DOI] [PubMed] [Google Scholar]
- 3. Yao S, Ouyang B, Lu T, Chen Q, Luo C. Treatment of cervical spondylotic radiculopathy with posterior percutaneous endoscopic cervical discectomy: short‐term outcomes of 24 cases. Medicine (Baltimore), 2020, 99: e20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith GW, Robinson RA. The treatment of certain cervical‐spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am, 1958, 40: 607–624. [PubMed] [Google Scholar]
- 5. Chen C, Yuchi CX, Gao Z, et al. Comparative analysis of the biomechanics of the adjacent segments after minimally invasive cervical surgeries versus anterior cervical discectomy and fusion: a finite element study. J Orthop Translat, 2020, 23: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eck JC, Humphreys SC, Lim TH, et al. Biomechanical study on the effect of cervical spine fusion on adjacent‐level intradiscal pressure and segmental motion. Spine (Phila Pa 1976), 2002, 27: 2431–2434. [DOI] [PubMed] [Google Scholar]
- 7. Wang YB, Chen SL, Cao C, Zhang K, Liu LM, Gao YZ. Percutaneous transforaminal endoscopic discectomy and fenestration discectomy to treat posterior ring apophyseal fractures: a retrospective cohort study. Orthop Surg, 2020, 12: 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang JQ, Chen C, Zhao H. Revision surgery after percutaneous endoscopic transforaminal discectomy compared with primary open surgery for symptomatic lumbar degenerative disease. Orthop Surg, 2019, 11: 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu PF, Li YW, Wang B, Jiang B, Tu ZM, Lv GH. Posterior cervical foraminotomy via full‐endoscopic versus microendoscopic approach for radiculopathy: a systematic review and meta‐analysis. Pain Physician, 2019, 22: 41–52. [PubMed] [Google Scholar]
- 10. Deng ZL, Chu L, Chen L, Yang JS. Anterior transcorporeal approach of percutaneous endoscopic cervical discectomy for disc herniation at the C4‐C5 levels: a technical note. Spine J, 2016, 16: 659–666. [DOI] [PubMed] [Google Scholar]
- 11. Ruetten S, Komp M, Merk H, Godolias G. A new full‐endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9‐mm endoscopes: prospective 2‐year results of 87 patients. Minim Invasive Neurosurg, 2007, 50: 219–226. [DOI] [PubMed] [Google Scholar]
- 12. Ruetten S, Komp M, Merk H, et al. Full‐endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9‐mm endoscopes: a prospective, randomized, controlled study. Spine (Phila Pa 1976), 2008, 33: 940–948. [DOI] [PubMed] [Google Scholar]
- 13. Xiao CM, Yu KX, Deng R, et al. Modified K‐hole percutaneous endoscopic surgery for cervical foraminal stenosis: partial pediculectomy approach. Pain Physician, 2019, 22: E407–E416. [PubMed] [Google Scholar]
- 14. Nunes RR, Bersot CDA, Garritano JG. Intraoperative neurophysiological monitoring in neuroanesthesia. Curr Opin Anaesthesiol, 2018, 31: 532–538. [DOI] [PubMed] [Google Scholar]
- 15. Bhagat S, Durst A, Grover H, et al. An evaluation of multimodal spinal cord monitoring in scoliosis surgery: a single centre experience of 354 operations. Eur Spine J, 2015, 24: 1399–1407. [DOI] [PubMed] [Google Scholar]
- 16. Cofano F, Zenga F, Mammi M, et al. Intraoperative neurophysiological monitoring during spinal surgery: technical review in open and minimally invasive approaches. Neurosurg Rev, 2019, 42: 297–307. [DOI] [PubMed] [Google Scholar]
- 17. Walker CT, Kim HJ, Park P, et al. Neuroanesthesia guidelines for optimizing transcranial motor evoked potential neuromonitoring during deformity and complex spinal surgery: a Delphi consensus study. Spine (Phila Pa 1976), 2020, 45: 911–920. [DOI] [PubMed] [Google Scholar]
- 18. MacDonald DB, Dong C, Quatrale R, et al. Recommendations of the International Society of Intraoperative Neurophysiology for intraoperative somatosensory evoked potentials. Clin Neurophysiol, 2019, 130: 161–179. [DOI] [PubMed] [Google Scholar]
- 19. Riley MR, Doan AT, Vogel RW, Aguirre AO, Pieri KS, Scheid EH. Use of motor evoked potentials during lateral lumbar interbody fusion reduces postoperative deficits. Spine J, 2018, 18: 1763–1778. [DOI] [PubMed] [Google Scholar]
- 20. Haijun M, Xiaobing Z, Bin G, et al. Trans‐interlamina percutaneous endoscopic cervical discectomy for symptomatic cervical spondylotic radiculopathy using the new Delta system. Sci Rep, 2020, 10: 10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quillo‐Olvera J, Lin GX, Kim JS. Percutaneous endoscopic cervical discectomy: a technical review. Ann Transl Med, 2018, 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macdonald DB, Skinner S, Shils J, Yingling C, American Society of Neurophysiological Monitoring . Intraoperative motor evoked potential monitoring ‐ a position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol, 2013, 124: 2291–2316. [DOI] [PubMed] [Google Scholar]


