Abstract
This review summarizes the literature of preclinical studies and clinical trials on the use of mesenchymal stem cells (MSCs) to treat meniscus injury and promote its repair and regeneration and provide guidance for future clinical research. Due to the special anatomical features of the meniscus, conservative or surgical treatment can hardly achieve complete physiological and histological repair. As a new method, stem cells promote meniscus regeneration in preclinical research and human preliminary research. We expect that, in the near future, in vivo injection of stem cells to promote meniscus repair can be used as a new treatment model in clinical treatment. The treatment of animal meniscus injury, and the clinical trial of human meniscus injury has begun preliminary exploration. As for the animal experiments, most models of meniscus injury are too simple, which can hardly simulate the complexity of actual meniscal tears, and since the follow‐up often lasts for only 4–12 weeks, long‐term results could not be observed. Lastly, animal models failed to simulate the actual stress environment faced by the meniscus, so it needs to be further studied if regenerated meniscus has similar anti‐stress or anti‐twist features. Despite these limitations, repair of the meniscus by MSCs has great potential in clinics. MSCs can differentiate into fibrous chondrocytes, which can possibly repair the meniscus and provide a new strategy for repairing meniscus injury.
Keywords: Cytology treatment, Meniscus injury, MSCs
The purpose of this review is to summarize the literature of pre‐clinical studies and clinical trials on the use of mesenchymal stem cells to treat meniscus injury and promote its repair and regeneration and provide guidance for future clinical research.
Introduction
The meniscus of the knee is a wedge‐shaped fibrocartilaginous tissue with a thick peripheral rim and a thin inner rim. The menisci are attached between the femoral condyle and tibial surface, with the medial meniscus covering 60% of the tibiofemoral surface and lateral meniscus covering 90% of the articular surface 1 . Water and collagen tissues constitute 72% and 22%, respectively, of the composition of the meniscus. Out of the 22% collagen tissue, 80% is type I collagen and 20% is type II collagen 2 . Notably, the distribution of collagen types differs, as type I collagen fibers form a network structure filled with interstitial cells, glycosaminoglycans, glycoproteins, and elastin 3 . The phenotype of interstitial cells has more fibroblast‐like cells in the peripheral and more chondrocyte‐like cells in the inner rim 4 . The main functions of the meniscus are to spread load, reduce contact stress, absorb impact, lubricate joints, and maintain proprioception 5 .
Annular capillaries formed by upper and lower arteries can provide limited blood flow for the tissue in 10%–25% of the peripheral meniscus rim, 10%–30% of the inner meniscus rim, and in the anterior and posterior horns of the meniscus 6 . When these capillaries enter the meniscus, they are normally arranged into three layers—upper, middle, and lower layers—and this forms the basis for self‐repair after meniscus injury. Tears are located at the peripheral attachment sites. However, in the remaining parts of the meniscus, there is no clear vascular nourishment, yet a membranous synovial layer covers the surface of the meniscus and provides synovial fluid, which is a major source for the limited growth repair of the tissue 6 . Anderson et al. 7 recommended a classification system of zone 1 (rim width <3 mm), zone 2 (rim width 3 to <5 mm), and zone 3 (rim width ≥5 mm). Meniscofemoral and meniscotibial, or zone 1, are also commonly referred to as outer third, or red‐red (R/R), tears. Tears located in the middle third (zone 2) are classified as red‐white (R/W) tears, and tears in the inner third (zone 3) are termed white‐white (W/W) tears. R/W tears occur at the junction of the outer and middle third regions, approximately 4 mm from the meniscal attachment, with a vascular supply predominately in the outer third of the tear. W/W tears are located in the region in which no blood supply exists 8 (Fig. 1).
Fig 1.
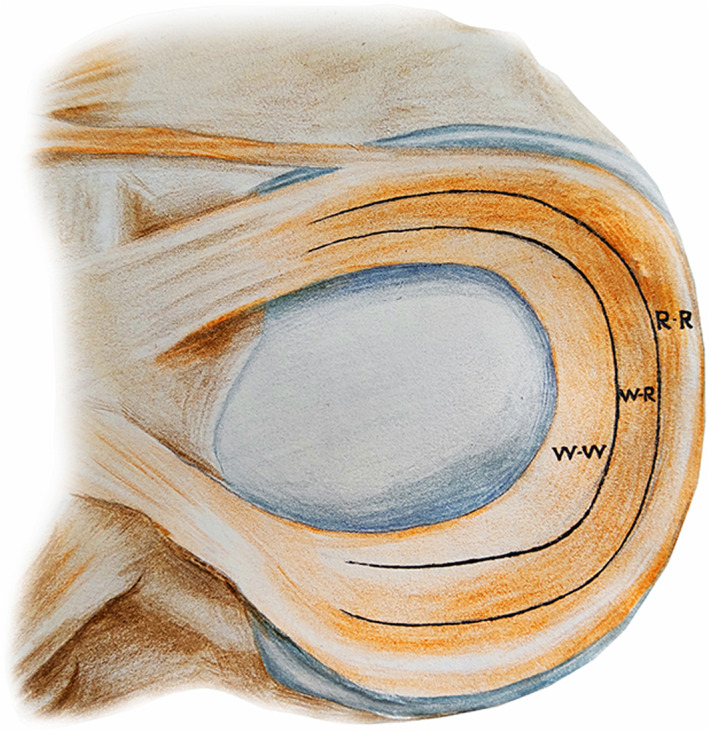
Illustration of meniscus demonstrating the classic three zones according to the reported vascularity. R‐R, red‐red; R‐W, red‐white; W‐W, white‐white. The R‐R zone has a good blood supply, if the tear is very small, sometimes it can heal on its own and may not necessarily require surgical treatment. The R‐W zone has partial blood supply and its self‐healing potential is low. There is almost no blood supply in the W‐W zone, and a tear in this area will not heal by itself.
Meniscus injuries are associated with the shape of injury and state of stress during injury. The types of meniscus injury include oblique (parrot‐beak), horizontal (spallation), radial, vertical (longitudinal), and bucket handle‐like tears (Fig. 2). An oblique (parrot‐beak) tear is a kind of minor meniscus injury, which tears from the free edge of the meniscus to the middle of the meniscus, affecting the superior and inferior surfaces. If the injury tears throughout the whole meniscus, it should be called a radial tear. The meniscus with a horizontal (spallation) tear is just like puff pastry, tears across the central layer, but may not affect the surface, while a vertical (longitudinal) tear is parallel to the edge of the meniscus. The type of injury can exist either in the red or white zone, and if accompanied by a flip of the free edge of the meniscus, it should be called bucket handle‐like tear. As mentioned earlier, there is a relationship between the ability of meniscus repair and the type of injury. Some injuries, such as oblique (parrot‐beak) tears and radial tears, normally cross the white zone, and they do not cure easily, even when stitching the meniscus with sutures or Fast‐fix. The bucket handle‐like tear is a severe type of meniscus injury.
Fig 2.
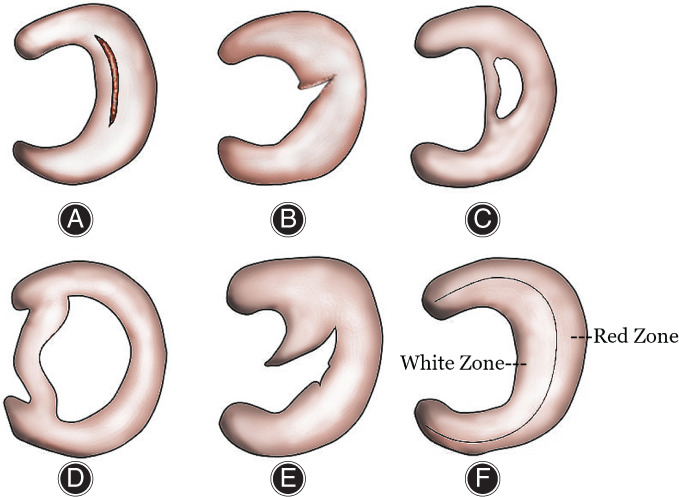
Anatomical structure of meniscus and type of injury. The types of meniscus injury include vertical (A), radial (B), horizontal (C), bucket handle‐like tears (D), and oblique (E). Annular capillaries formed by the upper and lower arteries can provide limited blood flow for the tissue in 10%–25% of the lateral meniscus rim, 10%–30% of the medial meniscus rim, and in the anterior and posterior horns of the meniscus (Red Zone, F). The tears in the white zone have limited ability to self‐repair; patients may suffer persistent and repeated symptoms, as well as aggravated injuries, due to the lack of self‐healing.
As are characteristics of our description of the meniscus blood supply, these tears have limited ability to self‐repair. Patients may suffer persistent and repeated symptoms, as well as aggravated injuries due to the lack of self‐healing. Besides, there is a high failure rate of 18% (16% for single longitudinal type and 28% for double longitudinal type) for surgery to repair meniscal avascular zones 8 , and thus partial resection of the meniscus has become the treatment of choice in clinics. Studies have shown that even in cases where meniscus injury is combined with anterior cruciate ligament injury, partial resection of the meniscus can lead to a 10–20‐fold increase in the incidence of lateral knee osteoarthritis 9 , 10 , 11 . The incidence of osteoarthritis is even higher among patients with complete meniscus resection. Long‐term follow‐up showed that the knee force line of patients had changed, and their joint space had varying degrees of stenosis. This was particularly prominent for patients with a body mass index (BMI) >30 12 , 13 , 14 . It has been suggested that the femorotibial joint is inherently a completely mismatched surface where the stress of the joint is focused on a very small area, and this is extremely unfavorable for the transfer of load 15 . However, the presence of menisci makes the joint a mildly mismatched surface through self‐filling, and partial or complete resection of the meniscus would inevitably lead to reduced stress‐sharing, resulting in further degenerative changes of articular cartilage due to the focused stress.
Therefore, our systematic review compiled multiple studies with the aim to: (i) review in vivo experiments for the treatment of meniscus injury (particularly in the avascular zones) using MSCs; (ii) evaluate the efficacy of MSCs from different sources in the treatment of meniscus injury in animals and humans; and (iii) find the evidence to support that the intra‐articular injection of stem cells can be used as a new method for the treatment of meniscus injury.
Data and Methods
A literature search was performed in PubMed including the following keywords: meniscal repair, meniscal injury, biomaterials, and stem cells. A literature search from 1997 to 2017 was conducted and all studies evaluating development and application were included in the review. The inclusion criteria were: (i) article published recently in an authoritative magazine, including periodical paper, academic paper, and review; (ii) content of article is closely related to meniscus injury and stem cells and is highly recognized by joint surgeons; (iii) study design is a prospective or retrospective comparative study; (iv) experiment objects, injections, histological findings, and clinical outcomes were compared; and (v) humans or animals with meniscus injury. The exclusion criteria were as follows: (i) unable to obtain full text; (ii) repeat studies; and (iii) low evidence level. If the article was in line with the topic mentioned above, the full text was accessed, and the article was read in detail and included in this review.
There were 694 records identified through database searching; 398 records were excluded and only 34 articles were included in the qualitative synthesis. In all, 32 articles were on animal research and the others were on human research (Fig. 3).
Fig 3.
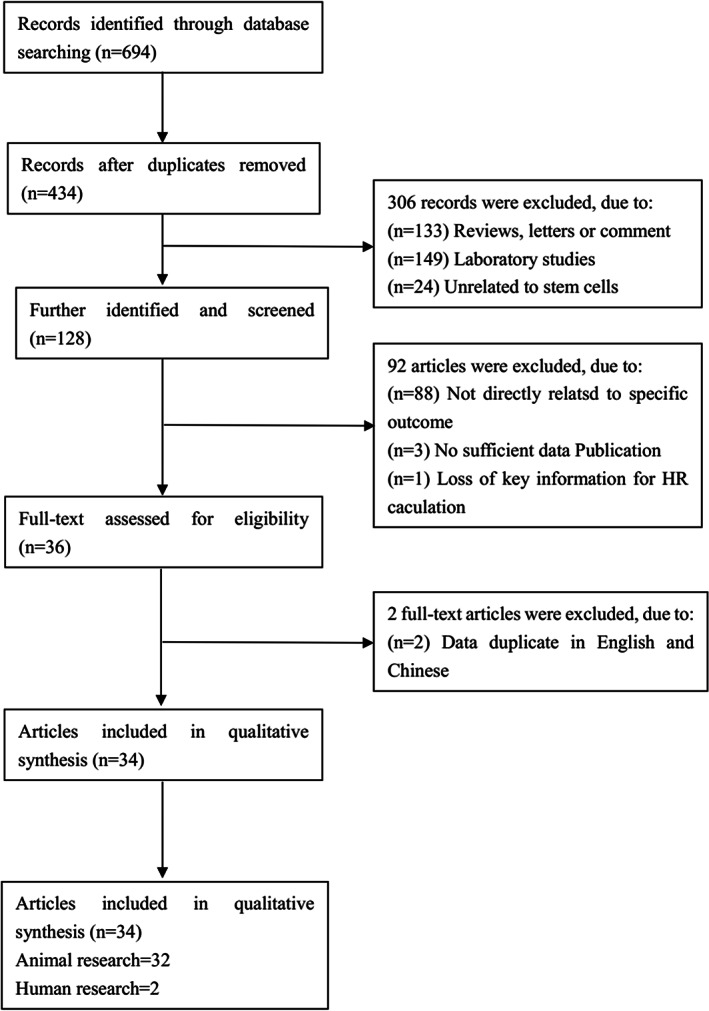
Retrieval process for literature for this article includes five inclusion criteria and three exclusion criteria. A literature search from 1997 to 2017 was conducted and all studies evaluating development and application were included in the review. There were 694 records identified through database searching; 398 records were excluded and only 34 articles were included in the qualitative synthesis. In all, 32 articles were animal research, and the others were human research.
Current Status of Cytological Treatment of Meniscus Injury
Cytological treatment of the meniscus induces meniscus fibroblasts to differentiate and mature. Recent technologies have induced differentiation of MSCs (Fig. 4). MSCs are pluripotent stem cells that are distributed in many human organs, including skeletal muscles, pancreas, adipose tissues, placenta, bones, and bone marrow. Regardless of the source, all MSCs have similar abilities in osteogenesis, adipogenesis, and chondrogenesis 16 , 17 . MSCs normally used in clinics are derived from bone marrow, adipose tissue, umbilical cord blood, and dental pulp.
Fig 4.
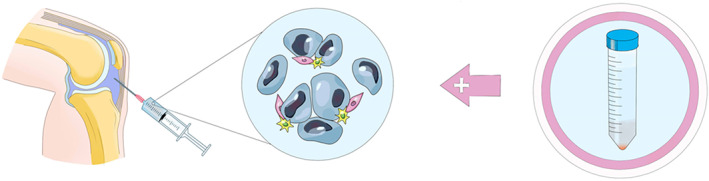
Cytological treatment of meniscus injury using MSCs. As the structural characteristics of the joint capsule, the knee joint is a relatively closed space. The prepared stem cell preparations can be injected directly into the joint cavity through the skin.
Researchers 18 have compared the ability of different MSCs (derived from the bone marrow, meniscus, synovium, adipose tissue, muscles, ligaments, and periosteum) used most commonly for the meniscus and found that marrow‐derived MSCs had the highest differentiation potential followed by synovium‐derived MSCs. However, ease of retrieval, size of the trauma, and the range of selectable materials should also be considered when deciding on the source of cells in the clinics. Due to these factors, adipose MSCs have irreplaceable advantages 19 .
Animal Experiments
More and more researchers began to focus on the ability of MSCs to repair meniscus injury in vitro (Table 1).
TABLE 1.
Animal experiments
| Time | Animal | Injection | Observation time | Histological findings |
|---|---|---|---|---|
| Mizuno et al. 20 , 2007 | Rats | 1 × 107 synovial MSCs | 12 weeks | Chondrocytes matured morphologically and gaps around the outside of the defect improved. |
| Zellner et al. 21 , 2013 | New Zealand white rabbits | 1 × 105 autologous MSCs‐hyaluronic acid‐collagen complex implantation | 12 weeks | The repair tissue was meniscus‐like with a low cell number, which showed typical meniscal pericellular cavities, and extensive amounts of extracellular matrix. |
| Al Faqeh H et al. 21 , 2011 | Sheep | 2 × 106 bone marrow MSCs | 6 weeks | The H&E staining showed presence of cells and fibrous tissue in the meniscus‐like tissue of the CM group and this feature resembles fibrocartilage as in native meniscus even though the cell looks immature with large nucleus. |
| Horie et al. 26 , 27 , 2011 | Rats | 5 × 106 synovium‐MSCs | 12 weeks | The contour of the regenerated menisci sharpened, and the ultimate forms were closer to the normal meniscal shape. |
| Zellner et al. 28 , 2009 | New Zealand white rabbits | 1.5 × 106 synovial MSCs | 12 weeks | The reconstitution of meniscus architecture with typically radial‐orientated collagen fibers could be observed. |
| Kondo et al. 29 , 2015 | Primates | 2.5 × 105 synovial MSCs | 16 weeks | The regenerated meniscus was positively stained with safranin‐O in the MSC group. |
| Katagiri 30 , 2012 | Rats | 2.5 × 104 synovial MSCs | 12 weeks | The contours of the regenerated menisci were sharper, and the stainability of type II collagen in the matrices was high. |
| Ruiz et al. 37 , 2010 | Rabbits | 1 × 105 adipose MSCs | 12 weeks | The healed areas presented a slight cellularity increase compared with the normal tissue. |
| Qi et al. 38 , 2015 | New Zealand white rabbits | 2 × 106 adipose MSCs | 12 weeks | The anterior portion of the meniscus was regenerated, with a greater mass of hypercellular fibrocartilaginous tissue and extracellular matrix (ECM). |
| Pabbruwe et al. 39 , 2009 | Sheep | 3.5 × 105 human bone marrow MSC‐absorbable collagen complex implantation | 40 days | Extensive integration at one of the meniscal surfaces when using undifferentiated stem cells but revealed only limited integration when using differentiated cells. |
| Tatsuhiro et al. 40 , 2015 | Japanese rabbits | 5 × 104 adipose MSCs | 12 weeks | Safranin‐O staining was noted at 2 weeks and increased gradually over time until 12 weeks |
| Dutton et al. 44 , 2009 | Pigs | 1 × 106 bone marrow MSCs | 12 weeks | MSC‐treated menisci with complete cellularity, fibrochondrocytes being the predominant cells. |
| Shen et al., 46 2013 | Rats | 6 × 106 human meniscus progenitor cells | 12 weeks | Intra‐articular injection of hMeSPCs induced significantly more neo‐tissue formation and extracellular matrix (ECM) deposition. |
| Okuro et al. 47 , 2013 | Rats | 5 × 106 syngeneic MSCs, the minor mismatched MSCs and the major mismatched MSCs | 8 weeks | The regenerated meniscus was different from the normal meniscus from the viewpoints of contour, cellularity, matrix staining, and type II collagen immunostaining. |
| Hong et al. 48 , 2010 | Rabbits | 2 × 106 human bone marrow MSCs | 12 weeks | MSCs appeared to enhance regeneration of the meniscus, although the MSCs observed in the meniscus were too few to account for the regeneration. |
Synovial MSCs in Meniscus Repair
A study conducted by Mizuno et al. 20 injected synovial MSCs into the joint cavity of mice with meniscus and cartilage injuries and found the cylindrical defect of a meniscus filled with fibrous cartilage‐like tissues. The control group was untreated, and its size of meniscal defects was significantly larger than that of the experimental group. Similar filling of the defect was also seen in the cartilage modeling group. This indicates that the injection of uninduced synovial MSCs into the joint cavity could promote meniscus and cartilage repair in mice, and the migration of stem cells is possibly driven by inflammatory reactions. Such pro‐inflammatory migration has been confirmed in many animal experiments 21 , 22 , 23 , 24 , 25 .
Immunofluorescence‐labeled synovial MSCs were injected into mice and rat models with meniscus resection, and the area of meniscus defects in the experimental group was found to be significantly smaller than that in the control group. Labeled stem cells were identified in regenerated meniscal tissues with a morphology similar to that of fibrochondrocytes 26 , 27 . However, no similar stem cells were found in other tissues or organs.
A study conducted by Zellner et al. divided New Zealand white rabbits into four groups and resected the anterior horn and body of the meniscus 28 . Experimental groups 1, 2, and 3 were given injections of synovial MSCs, autologous platelet‐rich plasma, and autologous bone marrow into the joint cavity. After 12 weeks of growth, the four groups showed varying degrees of healing, and the one treated with MSCs had the largest area of meniscus when compared with the others. This indicated that MSCs could promote the healing of meniscus avascular zones in rabbits. Rabbits treated with bone marrow or platelet‐rich plasma showed no significant difference when compared with the control.
Rhesus macaques at 12–13 years old were used to simulate meniscus injury in aged primates 29 . First, they cut the transverse ligament at the anterior horn of the meniscus, freeing the anterior horn and the body of the meniscus. Then, they removed half of the meniscus by cutting it at the level of the medial collateral ligament. Subsequently, the autologous synovial MSCs were cultured into aggregates and transplanted onto the meniscus defect of one knee (average number of cells: 3 × 106), while the other knee was left untreated. After the surgery, the rhesus macaques were allowed free activity to simulate human activity as much as possible. Morphological observation, magnetic resonance imaging (MRI), and histological scoring were performed after 8 and 16 weeks post‐surgery. Both knees showed healing of the defects, but the size of meniscus in the MSC group was significantly larger than that in the control group. Safranin staining was positive in the experimental group and negative in the control group, indicating rich mucopolysaccharide structures in the regenerated meniscus. This was consistent with the morphology of the fibrous cartilage meniscus. MRI T1rho mapping showed that the T1rho values of the menisci in the experimental group were closer to the normal meniscus, suggesting that regenerated menisci in the experimental group have more similar components with the normal meniscus than those in the control group. For both the experimental and control groups, degeneration of articular cartilage was found in the load‐bearing parts of the femoral condyle. This degeneration was not significantly different between two groups 8 weeks after transplantation, but the area of cartilage degeneration in the experimental group was significantly smaller than the control after 16 weeks. This further revealed that meniscus resection could aggravate cartilage degeneration at related sites, and MSC repair of the meniscus could delay such degeneration. Also, stem cells may play a direct role in cartilage repair.
In addition to revealing the possibility of using synovial MSCs to repair menisci, Kondo et al. 29 also proposed the advantages of tissue engineering technologies to traditional cytology treatment. In Kondo's study, articular cartilage was degenerated in the MSC and control groups in all four primates at 16 weeks, while Mankin's score for histology was better in the MSC group compared to that in the control group in all four primates at 16 weeks. Katagiri 30 used synovial MSCs to treat meniscal defects in rats and stated that stem cell‐material complexes are more advantageous than joint cavity injection due to the following reasons: (i) they possess higher potential in cartilage formation; (ii) they do not require an adhesive, as they can adhere to the meniscal surface by tension and gravity; and (iii) they can delay stem cell degeneration. Koch et al. 31 showed that an accelerated repair of a combined avascular and vascular meniscus defect can be achieved by using a mesenchymal stromal cell‐loaded porous polyurethane scaffold in a New Zealand white rabbit model, in which white rabbits were anesthetized using a combined intramuscular application of 0.6 mL/kg of ketamine 10% and xylazine 2% beforehand. Arthroscopic meniscectomy is still the main treatment at present, but it also increases the risk of osteoarthritis 32 . So, tissue engineering offers new treatment modalities for meniscus repair or even for meniscus replacement 33 .
Bone Marrow MSCs in Meniscus Repair
Bone marrow MSCs were also used in early studies of mice, rabbit, goat, and other animals 34 , 35 , 36 . These studies further confirmed that MSCs help to repair meniscus injuries. This repair can also restore the histological, mechanical, and morphological characteristics of defective menisci. Synovial and bone marrow MSCs can be obtained easily in animal experiments, but the sampling of these cells may be difficult in clinics, leading to significant trauma. Thus, more and more studies are focusing on adipose MSCs that are of similar activity but can be isolated and sampled more easily with a relatively high survival rate. Ruiz et al. performed suture repair of the meniscus avascular zone tear in rabbits 37 . After surgery, one side of the joint cavity was injected with allogeneic adipose MSCs and the possibility of meniscal repair increased 32‐fold compared with the other cavity without injection. Qi et al. performed meniscus anterior horn resections in 18 New Zealand rabbits and randomly injected adipose MSCs (superparamagnetic iron oxide‐labeled and non‐labeled) and saline into their joint cavities 38 . After the surgery, a magnetic field with the same intensity was placed around the rabbit knee to induce the labeled MSCs to migrate toward the area of meniscal defect. At the end of the study, pathological results showed that regenerated meniscus tissues of rabbits injected with adipose MSCs were similar to the normal rabbits, regardless of the labelling. The newly formed tissues showed the morphology of fibrous chondrocytes and were wrapped in massive extracellular matrix. There was no significant difference between the labeled and non‐labeled groups. Prussian staining of the iron oxide revealed that the labeled stem cells were tightly adhered to the regenerated meniscus tissues. Apart from these findings, varying levels of cartilage degeneration were observed in all three groups. But, in the MSC group, the level of degeneration was significantly lower than that in the control group. Although labeled‐migration induction seemed superfluous in this study, it did show that the low signals developed by magnetic materials can be detected by MRI, which provides new possibilities of chemotactic migration of MSCs.
With the development of tissue engineering, the choice of stem cell carriers has become a new concern. Pabbruwe et al. implanted human bone marrow MSC‐absorbable collagen complex into the menisci of sheep and found that most uninduced MSCs showed good integration with meniscal fibrous chondrocytes. Biomechanical testing demonstrated a significant 2‐fold increase in tensile strength in all constructs using the stem cell/collagen‐scaffold compared to the control groups after 40 days in culture 39 . However, MSCs induced by transforming growth factor‐β1 (TGF‐β1) into chondrocytes rarely aggregated at the fibrocartilage surface. This could be due to the fact that TGF‐β1 induces stem cells to differentiate into hyaline cartilage. Al Faqeh et al. injected stem cells cultured in chondrogenic media and basal media into the joint cavity of sheep with osteoarthritis (model established by resecting the medial meniscus and the anterior cruciate ligament) 22 . They showed that, regardless of the induction, histological repair of the articular cartilage was observed in all the sheep injected with MSCs, and the joint was covered by cartilage tissue with similar thickness and quality compared to that in normal knee cartilage tissue. However, when MSCs cultured in chondrogenic media were injected, they differentiated into fibrous cartilage tissues that covered the defects, whereas MSCs cultured in the basal media only formed scar tissue.
Adipose MSCs in Meniscus Repair
In an animal study, 40 Japanese rabbits have been divided into two groups, and 20 rabbits in the experimental group had an injury of 1.5 mm in diameter at the anterior horn of the meniscus 40 . A cylindrical complex made up of allogeneic adipose MSCs (derived from the subscapular fat pad of rabbits) and steroid matrix was transplanted at the defects. The control group was left untreated after injury. Meniscal defects in the experimental group were significantly smaller after 4 weeks, while there was no change in the control group. All the rabbits were killed 12 weeks after modeling, and the defects in both groups generally disappeared. However, the morphology of the regenerated meniscus in the experimental group was more similar to that of the normal meniscus. Histological analysis showed that the experimental group was positive for Safranin‐O, type I collagen, and DiI‐labeled HE staining, whereas the control group was negative for all these tests. These results showed that, histologically, the regenerated meniscus in the experimental group was more similar to normal meniscus fibrous cartilage, but in the control group, it was more similar to scar tissue.
A full‐thickness cartilage defect rabbit model with a height of more than 5 mm has been established by Ishihara et al. 41 . Three‐dimensional complexes containing bone marrow MSCs were transplanted to the defect, and it was found that uninduced MSCs differentiated into bone and cartilage simultaneously after 52 weeks of observation. Also, a clear boundary was found between the bone and cartilage. Thus, this technique might be effective for clinical treatment of bone and cartilage injuries. Neither Tatsuhiro nor Ishihara used a common absorbable scaffold as the carrier for MSCs. They developed a novel method for fusing spheroids or cylinders derived from BMMSCs to make a large construct without using a scaffold. It has been shown that cylindrical or spherical structures are more suitable for the internal microenvironment of the meniscus. MSCs simultaneously forming into a spherical structure can also increase the possibility of cell survival 42 . Degradable complexes can reduce the interference of scaffolds on the meniscal microenvironment, cell differentiation, and physiological characteristics of signal factors, such as cytokines 43 .
Studies have also compared the effect of stem cell treatment to meniscus suturing. Dutton et al. suggested that meniscus and cartilage morphology and repair ability, as well as load bearing in pigs, are similar to humans 44 . Thus, the team assigned 28 pigs with radial meniscus tears into three groups: group 1 was the control, group 2 was given meniscus stitching with suture and protein glue, and group 3 was given joint cavity injection of simple MSCs. Groups 2 and 3 had improved treatment outcomes compared to those in the control group, and group 3 showed better meniscus healing but insignificant mechanical improvement when compared with group 2. Zellner et al. made a 4‐mm longitudinal tear at the lateral meniscus of New Zealand rabbits and divided them into five groups based on the following treatments: 5–0 suture group, hyaluronic acid injection group, platelet‐rich plasma injection group, autologous MSCs‐hyaluronic acid‐collagen complex implantation group, and the blank control 21 . Suture augmentation is an important research field in the application of MSCs. The stem cells could be the repair cells themselves and differentiate into fibrochondrocytes to promote meniscal healing by synthesizing. The developed repair tissue was meniscus‐like and showed biomechanical properties that can withstand physiological compressive and tensile forces toward the menisci.
Using biological methods to induce differentiation of adipose MSCs may not gain satisfactory results 45 . Thus, Meier et al. used a custom‐built device to simulate constant cyclical and uniaxial strain on cells. They showed that, under mechanical strain, adipose MSCs showed simultaneous fibrogenesis (increased expression of type I collagen and versican) and chondrogenesis (increased expression of type II collagen, Sox 9, and aggrecan). They also show that mechanical stimulation promotes fibrogenesis prior to chondrogenesis, indicating that stretching stress may be an important factor for fibrogenesis. Since simple mechanical stimulation failed to induce protein expression, both mechanical and biochemical stimulations may be required to induce cell differentiation. This may explain why, in the above experiments, the mechanical properties of some regenerated menisci could not be restored to the previous levels. Although most animals were allowed unlimited activity, knee surgery would inevitably lead to changes in joint stress. The simulation of normal joint stress of menisci in animal experiments has become a new problem for scientists to solve.
Human Meniscus Progenitor Cells in Meniscus Repair
Synovium, bone marrow, or adipose tissue in some small animals cannot provide enough MSCs, which poses a limitation. Since MSCs have low immunogenicity, some studies have suggested the use of purified human MSCs for animal experiments. Shen et al. built a mice meniscus injury model and injected human meniscus progenitor cells into the defects 46 . The study not only confirmed the effectiveness of the treatment to promote meniscus regeneration but also identified a regulatory pathway by which the progenitor cells decreased the expression of both type I and type X collagen and increased the expression of type II collagen. These findings indicate that progenitor cells have the potential to prevent osteoarthritis and repair hyaline cartilage.
The use of non‐homologous MSCs significantly compensated for the disadvantage that small animals, such as mice and rabbits, only provide a shortage of the cells 46 , but some studies questioned if non‐homologous MSCs could trigger autoimmune attacks. Okuro et al. performed syngeneic, minor, and major mismatched transplantation of synovial MSCs into the joint cavity of rats with meniscal defects and found that the repair of defects in groups injected with syngeneic and minor mismatched MSCs was superior to the major mismatched group 47 . Huge amounts of macrophages and CD8 T cells were aggregated around the defects in the latter. However, Hong et al. used human bone marrow MSCs to treat rabbit meniscus injury and found no significant difference between the control and experimental groups 48 .
Currently, artificial induction of MSCs has entered the gene level. The CRISPR/Cas9 system is one of the hottest topics discussed lately due to its robustness and effectiveness in genome editing. The technology has been widely used in life science research, including microbial, plant, animal, and human cell studies 49 , 50 , 51 . CRISPR/Cas9 genome editing technology can also be used to prompt a particular phenotype in stem cell‐derived target cells. With the advent of CRISPR/Cas9 technology, efficient and robust genome engineering of stem cells has raised the possibilities for more meaningful disease modeling in the relevant molecular, cellular, and anatomical contexts. CRISPR/Cas9 technology can be employed to knock‐in tissue‐specific selectable markers to track stem cell differentiation and reporters without disrupting the expression and function of targeted genes. In addition to real‐time tracking of cell lineage and fate, tissue‐specific reporters are also useful in the selection of specific cell populations during stem cell differentiation 49 . It was found that only 11 genes had overlapping expression for hyaline cartilage and fibrous cartilage 52 . Only nerves and bone marrow, of all interstitial tissues, can be used as cell markers for chondrocyte differentiation and express similar gene sequences. Expression of specific genes may influence the direction of stem cell differentiation. Yi et al. 53 found that DI‐NPs are good tracers for gene delivery and could potentially be used for imaging and tracing using various tools in vitro and in vivo, and it was also a good gene delivery carrier and induces chondrogenic differentiation of hMSCs. This is particularly meaningful for the meniscus, because it can simultaneously develop two morphologies, hyaline cartilage and fibrous cartilage. TGF‐β1 or insulin‐like growth factor‐1 (IGF‐1) has been previously reported to modify gene expression in bone marrow MSCs and thus repair huge meniscus injuries in vitro 54 , 55 . Although modification at the gene level makes the differentiation of MSCs more controllable, more evidence is needed. Also, uncontrolled expression of TGF‐β1 might result in significant joint fiber hyperplasia or serious systemic damage 55 .
In fact, MSCs will lose a portion of their functions after isolation and cultivation in vitro as the surrounding microenvironments are changed and not the same as the environment in vivo. Preconditioning of MSCs by hypoxia, pharmacological agents, chemical agents, trophic factors, cytokines, and physical factors prior to their application is capable of initiating survival signaling to counter the rigorous harsh microenvironment for the application of MSC transplantation 56 .
Human Experiments
There have been only two clinical experiments of MSCs for meniscus repair (Table 2). One was a case report of a 32‐year‐old female, and the other was a multicenter, randomized, double‐blind control study, which recruited 55 subjects with medial meniscus damage.
TABLE 2.
Human experiments
| Time | Research type | Inclusion criteria | Injection | Observation time and evaluation indicators | Outcome |
|---|---|---|---|---|---|
| Pak et al. 57 , 2013 | Case reports | 32‐year‐old female |
Mixed injection of platelet‐rich plasma, calcium chloride, and low‐dose autologous adipose MSCs (14 days after op); Platelet‐rich plasma and calcium chloride (28 days after op) |
Visual analogue scale (VAS) score and MRI (3 months after op) | The patient's symptoms improved, and repeated MRI showed almost complete disappearance of the torn meniscus |
| Vangsness et al. 58 , 2012 | Multicenter, randomized, double‐blind control study | 55 subjects with medial meniscus damage |
Group A: bone marrow MSCs (5 × 107), human serum albumin, sodium hyaluronate, and plasma; Group B: bone marrow MSCs (1.5 × 108); Group C: sodium hyaluronate |
VAS score and MRI (12 months after op) | A higher proportion of those with osteoarthritic changes experienced a reduction in pain following the treatment with mesenchymal stem cells. |
Autologous Adipose MSCs in Meniscus Repair
A 32‐year‐old female who had a grade 2 tear in her medial meniscus and had received treatment with autologous adipose MSCs has been reported 57 . Joint cavity injection was performed on the day of liposuction and 3 days and 7 days after surgery, and another dose of PRP with CaCl2 and hyaluronic acid (1 mL) was injected in the same fashion as the first day. A mixed injection of platelet‐rich plasma, calcium chloride, and low‐dose dexamethasone was performed 14 days after the surgery. Injection of platelet‐rich plasma and calcium chloride was performed 28 days after the surgery. After 3 months of follow‐up, the patient reported significantly alleviated pain. Her visual analogue scale (VAS) score was significantly lower, and MRI showed “almost fully repaired” meniscus. The team combined multiple agents for the treatment, and the conclusion was based on the premise that “platelet‐rich plasma and hyaluronic acid do not improve the healing of the meniscus.” However, injection of dexamethasone into the cavity after surgery would influence VAS scoring, and thus using MRI to evaluate the level of meniscus injury makes this study lacking and in need of improvement.
Bone Marrow MSCs in Meniscus Repair
A multicenter, randomized, double‐blind control study has been conducted 58 . A total of 55 subjects with medial meniscus damage were recruited from seven medical centres and randomly assigned into three groups. Group A received a cavity injection of bone marrow MSCs complex containing 5 × 107 bone marrow MSCs, human serum albumin, sodium hyaluronate, and plasma; group B received a cavity injection of bone marrow MSCs complex containing 1.5 × 108 MSCs; and group C received a cavity injection of sodium hyaluronate as control 7 days after the meniscus resection. Bone marrow MSCs were from the bone marrow bank. Meniscal volume was analyzed by semi‐quantitative MRI 12 months after the surgery, and a growth greater than 15% was considered positive. The results showed that 24% of patients in group A and 6% of patients in B showed positive growth, whereas no patients had such growth in group C. This indicated that cavity injection of bone marrow MSCs could promote meniscal regeneration. In addition, patients who also had cartilage degeneration reported significantly alleviated pain after treatment with MSCs; the symptoms of patients in the control group were not significantly relieved. This is regarded as level‐1 evidence of evidence‐based medicine. Previous in vitro studies have confirmed that simply injecting human serum albumin, sodium hyaluronate, or plasma failed to promote meniscal healing, but cytokines contained in human serum albumin and plasma may induce the differentiation of bone marrow MSCs 59 . Thus, there is a need to eliminate certain interference factors to evaluate if bone marrow MSCs can improve the need for meniscal regeneration.
Discussion
It is undeniable that the ability of animals to repair is different from humans, and meniscus injuries in some animals could be completely healed without any treatment in due course of time 60 . Thus, if a gene modification could lead to an animal model that has a higher resemblance to pathological changes in humans, it could become an important research direction.
At present, although arthroscopic partial meniscectomy is the main method for the treatment of meniscus injury, tissue engineering and mesenchymal stem cells in vivo treatment of meniscus injury as a new technique has also begun a preliminary clinical exploration 61 . But there are ethical limitations to human experiments. This makes the progress in human experiments very slow. In addition, since different areas of the meniscus possess different healing abilities, the selection of patients is more difficult in human experiments.
In conclusion, principles for MSCs to repair meniscus injuries include the following: (i) MSCs can directly differentiate into fibrous chondrocytes; (ii) MSCs can provide massive amounts of cytokines and signal factors by themselves that enhance cellular viability and cellular proliferation and reduce cell apoptosis; and (iii) MSCs can migrate to the site of injury to extensive proliferative and anti‐inflammatory. Several studies have shown that MSCs derived from a variety of connective tissues demonstrated remarkable efficacy in promoting meniscus regeneration and repair 62 . It has been found that, in addition to acting as seed cells that possess the ability of multi‐directional differentiation, MSCs can secrete cytokines or growth factors by autocrine or paracrine signaling 63 . Under the regulation of stem cells, the level of cytokines, including TGF‐β, IGF‐1, bone morphogenetic protein (BMP), and fibroblast growth factor (FGF) increased several hundred‐fold. Meanwhile, MSCs can suppress damage‐induced inflammation by regulating multiple anti‐inflammatory cytokines, such as interleukin (IL)‐10, TGF‐β, and prostaglandin E2 by paracrine regulation, thereby providing the proper microenvironment for meniscus healing 64 . MSCs can generate synovial tissue during meniscal repair, which provides more nutrition for the meniscus. This was proposed by Nakagawa 65 who injected synovial MSCs in to minipigs with meniscus injury and found that synovial MSCs promoted meniscal healing.
Currently, a large number of preclinical studies have shown that in vivo injection of MSCs to treat meniscus injury is a safe, reliable, and efficient method to repair meniscus injury. Although human clinical research has been carried out, it still has great limitations. First, human clinical research involves ethical restrictions, so it is difficult to carry out it on a large scale, which leads to the slow progress of the research. Second, these few clinical studies lack a good control group as a reference, lack long‐term follow‐up, and lack an end point of objective results after in vivo injection. Third, the complexity of the human meniscus tissue itself, partial vascularity, multiple cell types in the tissue, and interspecific variation of the meniscus may add some difficulties to the experiment. Fourth, compared with repairing cartilage, meniscus repaired by MSCs lack of postoperative histological examination, imaging examination, and arthroscopic examination left researchers unable to identify the type of new‐born tissue, in addition to a lack of reliable experimental results. Lastly, there is no consensus on the ideal cell source and scaffold for the treatment of meniscus injury by in vivo injection of MSCs 66 .
The treatment of meniscus repair and regeneration is very limited as a new method, and stem cells promote meniscus regeneration in preclinical research and human preliminary research. We expect that, in the near future, in vivo injection of stem cells to promote meniscus repair can be used as a new treatment model in clinical treatment.
Conclusion
Due to the special anatomical features of the meniscus, conservative or surgical treatment can hardly achieve complete physiological and histological repair. Cytology treatment is a very promising field for post‐damage meniscus repair. Many animal experiments and a few human experiments indicate that MSCs can promote meniscal regeneration, but the lack of human evidence and defects of animal experiments have left many problems unsolved. As for the animal experiments, most models of meniscus injury are too simple, which can hardly simulate the complexity of actual meniscal tears, and since the follow‐up often lasts for only 4–12 weeks, long‐term results could not be observed. Lastly, animal models failed to simulate the actual stress environment faced by the meniscus, so it needs to be further studied if regenerated meniscus has similar anti‐stress or anti‐twist features. Despite these limitations, repair of the meniscus by MSCs has great potential in clinics.
Disclosure: The authors declare no conflict of interest. No benefits in any form have been, or will be, received from a commercial party related directly or indirectly to the subject of this manuscript. Supported by Major Program of Development Fund for Shanghai Zhangjiang National Innovtaion Demonstration Zone<Stem Cell Strategic Biobank and Stem Cell Clinical Technology Transformation Platform> (ZJ2018‐ZD‐004).
References
- 1. Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am, 1983, 65: 538–547. [PubMed] [Google Scholar]
- 2. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure‐function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials, 2011, 32: 7411–7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herwig J, Egner E, Buddecke E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann Rheum Dis, 1984, 43: 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abraham AC, Donahue TL. From meniscus to bone: a quantitative evaluation of structure and function of the human meniscal attachments. Acta Biomater, 2013, 9: 6322–6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fox AJ, Wanivenhaus F, Burge AJ, Warren RF, Rodeo SA. The human meniscus: a review of anatomy, function, injury, and advances in treatment. Clin Anat, 2015, 28: 269–287. [DOI] [PubMed] [Google Scholar]
- 6. Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med, 1982, 10: 90–95. [DOI] [PubMed] [Google Scholar]
- 7. Anderson AF, Irrgang JJ, Dunn W, et al. Interobserver reliability of the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) classification of meniscal tears. Am J Sports Med, 2011, 39: 926–932. [DOI] [PubMed] [Google Scholar]
- 8. Barber‐Westin SD, Noyes FR. Clinical healing rates of meniscus repairs of tears in the central‐third (red‐white) zone. Arthroscopy, 2014, 30: 134–146. [DOI] [PubMed] [Google Scholar]
- 9. Roos H, Lauren M, Adalberth T, et al. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty‐one years, compared with matched controls. Arthritis Rheum, 1998, 41: 687–693. [DOI] [PubMed] [Google Scholar]
- 10. Mansson O, Sernert N, Rostgard‐Christensen L, Kartus J. Long‐term clinical and radiographic results after delayed anterior cruciate ligament reconstruction in adolescents. Am J Sports Med, 2015, 43: 138–145. [DOI] [PubMed] [Google Scholar]
- 11. Paradowski PT, Keska R, Witonski D. Does concomitant meniscectomy affect medium‐term outcome of anterior cruciate ligament reconstruction? A preliminary report. Arch Med Sci, 2014, 10: 992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuraishi J, Akizuki S, Takizawa T. Arthroscopic lateral meniscectomy in knees with lateral compartment osteoarthritis: a case series study. Arthroscopy, 2006, 22: 878–883. [DOI] [PubMed] [Google Scholar]
- 13. Okazaki K, Miura H, Matsuda S, et al. Arthroscopic resection of the discoid lateral meniscus: long‐term follow‐up for 16 years. Arthroscopy, 2006, 22: 967–971. [DOI] [PubMed] [Google Scholar]
- 14. Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty‐two years after meniscectomy. Arthritis Rheum, 2004, 50: 2811–2819. [DOI] [PubMed] [Google Scholar]
- 15. Goodfellow JW. Closed meniscectomy. J Bone Joint Surg Br, 1983, 65: 373–374. [DOI] [PubMed] [Google Scholar]
- 16. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell, 2008, 3: 301–313. [DOI] [PubMed] [Google Scholar]
- 17. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 2005, 105: 1815–1822. [DOI] [PubMed] [Google Scholar]
- 18. Yu H, Adesida AB, Jomha NM. Meniscus repair using mesenchymal stem cells ‐ a comprehensive review. Stem Cell Res Ther, 2015, 6: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell‐based therapies. Tissue Eng, 2001, 7: 211–228. [DOI] [PubMed] [Google Scholar]
- 20. Mizuno K, Muneta T, Morito T, et al. Exogenous synovial stem cells adhere to defect of meniscus and differentiate into cartilage cells. J Med Dent Sci, 2008, 55: 101–111. [PubMed] [Google Scholar]
- 21. Zellner J, Hierl K, Mueller M, et al. Stem cell‐based tissue‐engineering for treatment of meniscal tears in the avascular zone. J Biomed Mater Res B Appl Biomater, 2013, 101: 1133–1142. [DOI] [PubMed] [Google Scholar]
- 22. Al Faqeh H, Nor Hamdan BM, Chen HC, Aminuddin BS, Ruszymah BH. The potential of intra‐articular injection of chondrogenic‐induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol, 2012, 47: 458–464. [DOI] [PubMed] [Google Scholar]
- 23. Caminal M, Fonseca C, Peris D, et al. Use of a chronic model of articular cartilage and meniscal injury for the assessment of long‐term effects after autologous mesenchymal stromal cell treatment in sheep. N Biotechnol, 2014, 31: 492–498. [DOI] [PubMed] [Google Scholar]
- 24. Moriguchi Y, Tateishi K, Ando W, et al. Repair of meniscal lesions using a scaffold‐free tissue‐engineered construct derived from allogenic synovial MSCs in a miniature swine model. Biomaterials, 2013, 34: 2185–2193. [DOI] [PubMed] [Google Scholar]
- 25. Hatsushika D, Muneta T, Nakamura T, et al. Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthritis Cartilage, 2014, 22: 941–950. [DOI] [PubMed] [Google Scholar]
- 26. Horie M, Sekiya I, Muneta T, et al. Intra‐articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells, 2009, 27: 878–887. [DOI] [PubMed] [Google Scholar]
- 27. Horie M, Driscoll MD, Sampson HW, et al. Implantation of allogenic synovial stem cells promotes meniscal regeneration in a rabbit meniscal defect model. J Bone Joint Surg Am, 2012, 94: 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zellner J, Mueller M, Berner A, et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J Biomed Mater Res A, 2010, 94: 1150–1161. [DOI] [PubMed] [Google Scholar]
- 29. Kondo S, Muneta T, Nakagawa Y, et al. Transplantation of autologous synovial mesenchymal stem cells promotes meniscus regeneration in aged primates. J Orthop Res, 2017, 35: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 30. Katagiri H, Muneta T, Tsuji K, et al. Transplantation of aggregates of synovial mesenchymal stem cells regenerates meniscus more effectively in a rat massive meniscal defect. Biochem Biophys Res Commun, 2013, 435: 603–609. [DOI] [PubMed] [Google Scholar]
- 31. Koch M, Achatz FP, Lang S, et al. Tissue engineering of large full‐size meniscus defects by a polyurethane scaffold: accelerated regeneration by mesenchymal stromal cells. Stem Cells Int, 2018, 2018: 8207071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petty CA, Lubowitz JH. Does arthroscopic partial meniscectomy result in knee osteoarthritis? A systematic review with a minimum of 8 years' follow‐up. Arthroscopy, 2011, 27: 419–424. [DOI] [PubMed] [Google Scholar]
- 33. Buma P, Ramrattan NN, van Tienen TG, Veth RP. Tissue engineering of the meniscus. Biomaterials, 2004, 25: 1523–1532. [DOI] [PubMed] [Google Scholar]
- 34. Yamasaki T, Deie M, Shinomiya R, et al. Meniscal regeneration using tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. J Biomed Mater Res A, 2005, 75: 23–30. [DOI] [PubMed] [Google Scholar]
- 35. Yamasaki T, Deie M, Shinomiya R, Yasunaga Y, Yanada S, Ochi M. Transplantation of meniscus regenerated by tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. Artif Organs, 2008, 32: 519–524. [DOI] [PubMed] [Google Scholar]
- 36. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum, 2003, 48: 3464–3474. [DOI] [PubMed] [Google Scholar]
- 37. Ruiz‐Iban MA, Diaz‐Heredia J, Garcia‐Gomez I, et al. The effect of the addition of adipose‐derived mesenchymal stem cells to a meniscal repair in the avascular zone: an experimental study in rabbits. Arthroscopy, 2011, 27: 1688–1696. [DOI] [PubMed] [Google Scholar]
- 38. Qi Y, Yang Z, Ding Q, et al. Targeted transplantation of iron oxide‐labeled, adipose‐derived mesenchymal stem cells in promoting meniscus regeneration following a rabbit massive meniscal defect. Exp Ther Med, 2016, 11: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pabbruwe MB, Kafienah W, Tarlton JF, Mistry S, Fox DJ, Hollander AP. Repair of meniscal cartilage white zone tears using a stem cell/collagen‐scaffold implant. Biomaterials, 2010, 31: 2583–2591. [DOI] [PubMed] [Google Scholar]
- 40. Toratani T, Nakase J, Numata H, et al. Scaffold‐free tissue‐engineered allogenic adipose‐derived stem cells promote meniscus healing. Arthroscopy, 2017, 33: 346–354. [DOI] [PubMed] [Google Scholar]
- 41. Ishihara K, Nakayama K, Akieda S, Matsuda S, Iwamoto Y. Simultaneous regeneration of full‐thickness cartilage and subchondral bone defects in vivo using a three‐dimensional scaffold‐free autologous construct derived from high‐density bone marrow‐derived mesenchymal stem cells. J Orthop Surg Res, 2014, 9: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salamon A, van Vlierberghe S, van Nieuwenhove I, et al. Gelatin‐based hydrogels promote chondrogenic differentiation of human adipose tissue‐derived mesenchymal stem cells in vitro. Materials (Basel), 2014, 7: 1342–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koga H, Muneta T, Ju YJ, et al. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells, 2007, 25: 689–696. [DOI] [PubMed] [Google Scholar]
- 44. Dutton AQ, Choong PF, Goh JC. Enhancement of meniscal repair in the avascular zone using mesenchymal stem cells in a porcine model. J Bone Joint Surg Br, 2010, 92: 169–175. [DOI] [PubMed] [Google Scholar]
- 45. Meier EM, Wu B, Siddiqui A, Tepper DG, Longaker MT, Lam MT. Mechanical stimulation increases knee meniscus gene RNA‐level expression in adipose‐derived stromal cells. Plast Reconstr Surg Glob Open, 2016, 4: e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shen W, Chen J, Zhu T, et al. Intra‐articular injection of human meniscus stem/progenitor cells promotes meniscus regeneration and ameliorates osteoarthritis through stromal cell‐derived factor‐1/CXCR4‐mediated homing. Stem Cells Transl Med, 2014, 3: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Okuno M, Muneta T, Koga H, et al. Meniscus regeneration by syngeneic, minor mismatched, and major mismatched transplantation of synovial mesenchymal stem cells in a rat model. J Orthop Res, 2014, 32: 928–936. [DOI] [PubMed] [Google Scholar]
- 48. Hong JH, Park JI, Kim KH, Kim YM, Joo YB, Jeon YS. Repair of the complete radial tear of the anterior horn of the medial meniscus in rabbits: a comparison between simple pullout repair and pullout repair with human bone marrow stem cell implantation. Knee Surg Relat Res, 2011, 23: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patmanathan SN, Gnanasegaran N, Lim MN, Husaini R, Fakiruddin KS, Zakaria Z. CRISPR/Cas9 in stem cell research: current application and future perspective. Curr Stem Cell Res Ther, 2018, 13: 632–644. [DOI] [PubMed] [Google Scholar]
- 50. Bak RO, Dever DP, Porteus MH. CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat Protoc, 2018, 13: 358–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hsu MN, Chang YH, Truong VA, Lai PL, Nguyen TKN, Hu YC. CRISPR technologies for stem cell engineering and regenerative medicine. Biotechnol Adv, 2019, 37: 107447. [DOI] [PubMed] [Google Scholar]
- 52. Ochi K, Daigo Y, Katagiri T, et al. Expression profiles of two types of human knee‐joint cartilage. J Hum Genet, 2003, 48: 177–182. [DOI] [PubMed] [Google Scholar]
- 53. Yi SW, Kim HJ, Oh HJ, et al. Gene expression profiling of chondrogenic differentiation by dexamethasone‐conjugated polyethyleneimine with SOX trio genes in stem cells. Stem Cell Res Ther, 2018, 9: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Steinert AF, Palmer GD, Capito R, et al. Genetically enhanced engineering of meniscus tissue using ex vivo delivery of transforming growth factor‐beta 1 complementary deoxyribonucleic acid. Tissue Eng, 2007, 13: 2227–2237. [DOI] [PubMed] [Google Scholar]
- 55. Zhang H, Leng P, Zhang J. Enhanced meniscal repair by overexpression of hIGF‐1 in a full‐thickness model. Clin Orthop Relat Res, 2009, 467: 3165–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med, 2018, 22: 1428–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pak J, Lee JH, Lee SH. Regenerative repair of damaged meniscus with autologous adipose tissue‐derived stem cells. Biomed Res Int, 2014, 2014: 436029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vangsness CT Jr, Farr J 2nd, Boyd J, et al. Adult human mesenchymal stem cells delivered via intra‐articular injection to the knee following partial medial meniscectomy: a randomized, double‐blind, controlled study. J Bone Joint Surg Am, 2014, 96: 90–98. [DOI] [PubMed] [Google Scholar]
- 59. Levy I, Sher I, Corem‐Salkmon E, et al. Bioactive magnetic near infra‐red fluorescent core‐shell iron oxide/human serum albumin nanoparticles for controlled release of growth factors for augmentation of human mesenchymal stem cell growth and differentiation. J Nanobiotechnology, 2015, 13: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hatsushika D, Muneta T, Horie M, Koga H, Tsuji K, Sekiya I. Intraarticular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. J Orthop Res, 2013, 31: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 61. Sekiya I, Koga H, Otabe K, et al. Additional use of synovial mesenchymal stem cell transplantation following surgical repair of a complex degenerative tear of the medial meniscus of the knee: a case report. Cell Transplant, 2019, 28: 1445–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guo W, Xu W, Wang Z, et al. Cell‐free strategies for repair and regeneration of meniscus injuries through the recruitment of endogenous stem/progenitor cells. Stem Cells Int, 2018, 2018: 5310471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Parekkadan B, van Poll D, Suganuma K, et al. Mesenchymal stem cell‐derived molecules reverse fulminant hepatic failure. PLoS One, 2007, 2: e941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Opitz CA, Litzenburger UM, Lutz C, et al. Toll‐like receptor engagement enhances the immunosuppressive properties of human bone marrow‐derived mesenchymal stem cells by inducing indoleamine‐2,3‐dioxygenase‐1 via interferon‐beta and protein kinase R. Stem Cells, 2009, 27: 909–919. [DOI] [PubMed] [Google Scholar]
- 65. Nakagawa Y, Muneta T, Kondo S, et al. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthritis Cartilage, 2015, 23: 1007–1017. [DOI] [PubMed] [Google Scholar]
- 66. Jacob G, Shimomura K, Krych AJ, Nakamura N. The meniscus tear: a review of stem cell therapies. Cells, 2019, 9: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]


