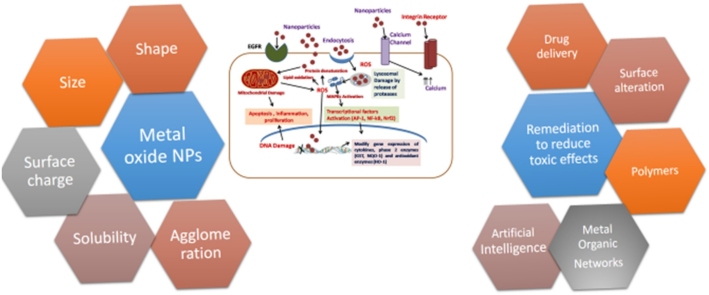
Graphical abstract
Keywords: Metal oxide nanoparticles, Metallic nanoparticles, Toxicity, Physicochemical properties, Delivery, Artificial intelligence, Metal-organic frameworks
Highlights
-
•
Physiochemical interactions and molecular mechanism of metal/metal oxides’toxicity.
-
•
Remediation to reduce the toxicity of metal nanoparticles.
-
•
Delivery approaches through polymers, responsive surface alteration, metal oraganic frameworks.
-
•
Artificial intelligence/Computational approach for predicting toxicity based on physicochemical characteristics.
Abstract
Metal/metal oxide nanoparticles show promise for various applications, including diagnosis, treatment, theranostics, sensors, cosmetics, etc. Their altered chemical, optical, magnetic, and structural properties have differential toxicity profiles. Depending upon their physical state, these NPs can also change their properties due to alteration in pH, interaction with proteins, lipids, blood cells, and genetic material. Metallic nanomaterials (comprised of a single metal element) tend to be relatively stable and do not readily undergo dissolution. Contrarily, metal oxide and metal alloy-based nanomaterials tend to exhibit a lower degree of stability and are more susceptible to dissolution and ion release when introduced to a biological milieu, leading to reactive oxygen species production and oxidative stress to cells. Since NPs have considerable mobility in various biological tissues, the investigation related to their adverse effects is a critical issue and required to be appropriately addressed before their biomedical applications. Short and long-term toxicity assessment of metal/metal oxide nanoparticles or their nano-formulations is of paramount importance to ensure the global biome's safety; otherwise, to face a fiasco. This article provides a comprehensive introspection regarding the effects of metal/metal oxides’ physical state, their surface properties, the possible mechanism of actions along with the potential future strategy for remediation of their toxic effects.
1. Introduction
Nano-intervention has turned out to be inherent to numerous state-of-the-art technological developments in diverse scientific avenues, including biomedical and pharmaceutical perspectives. Metals/metal oxide nanoparticles display their ability for various applications. Various researchers have used gold nanoparticles (NPs), iron/iron oxide NPs, zinc oxide NPs, silver NPs, copper oxide NPs, titanium oxide NPs, cobalt oxide NPs, aluminum oxide NPs for diagnostics, including imaging, drug delivery, therapy, and theranostics [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]]. Factors that offer the highest promise of technological advancement also pose a threat to humans, animals, and the environment. Despite stringent regulations towards nano-intervention in biomedical applications, nano-science has achieved considerable improvement in 'bench to bedside' conversion in recent times, augmenting the probability of human exposure with myriads of metallic nano-formulations. The enhanced production of nanomaterials leads to an increased probability of environmental release, either deliberately in discharge or accidentally in spillages, and poses a higher risk of adverse effects. Understanding the nano-bio interactions, associations of physicochemical characteristics of nanoparticles or formulations with biological setup, and their kinetics are of keen interest in explicating the basic relationship of NPs with biological systems. Usually, the toxicity of NPs is related to their nano size and large surface area and is theoretically expected to be more toxic than their bulk counterparts. Many of these inherent characteristics of NPs, such as size, surface area, shape, charge, crystal structure, and solubility, have possible implications in their toxicity [[13], [14], [15], [16]].
The toxicity of different NPs has been extensively explored [[17], [18], [19], [20], [21]]. Due to their nano size, the particles can access the circulatory/lymphatic systems and subsequently to the tissues and organs [22]. Metallic nanomaterials (comprised of a single metal element) tend to be relatively stable and do not readily undergo dissolution. Contrarily, metal oxide and metal alloy-based nanomaterials tend to exhibit a lower degree of stability and are more susceptible to dissolution and ion release when introduced to a biological milieu, leading to reactive oxygen species (ROS) production and oxidative stress to cells [[23], [24], [25], [26], [27], [28]]. The potential toxic effects of NP on a host can occur due to accidental or intentional exposure via ingestion, dermal absorption, inhalation, or parenteral administration. The type of NPs, their route of entry, and quantum are essential factors that affect different organs and tissues with significant health issues. It is due to different pharmacological behavior, their inherent properties and interaction with different biological fluids or environments like plasmic proteins which create a "corona" upon entering the blood; "Corona" refers to a layer of organic/inorganic molecules (which can include biological macromolecules like proteins, lipids, and nucleic acids) that are adsorbed to the nanomaterial surface upon entry into biological systems. Hence NPs may or may not pursue the predictable absorption/distribution/metabolism/excretion i.e ADME model in the host. For example, nanomaterials generally have longer maximal half-lives than traditional counterparts extending to years in some tissues [29]. These NPs can change their properties due to alteration in pH, interaction with proteins, lipids, blood cells, and genetic material. Since NPs have considerable mobility in various biological tissues, the investigation related to their adverse effects is a significant issue and needs to be appropriately addressed before their specific applications. This article focuses on the physicochemical properties of metal/metal oxide nanoparticles, their toxic effects, and computational approaches to predict their toxicity, further strategies for remediation, and a framework to ameliorate the toxic effects of nanoparticles.
2. Physicochemical interactions and molecular mechanism of toxicity
Various distinct properties of NPs like size, shape, charge, crystal structure, surface area, dose, mass, susceptibility for particular cell type, determine not only the mechanism of action for suitable biological application but also determine their mechanism of toxicity. Numerous in-vitro and in-vivo introspection has also confirmed that certain categories of NPs are more frequently toxic at the molecular, cellular, or tissue level [30]. Brand et al. [31] identified a set of specific deleterious effects specific to NP-based drug formulations compared to products with similar active pharmaceutical ingredients (API). Various physicochemical characteristics impacting the toxicological profiles of the NPs are discussed below:
2.1. Size
The particle size can also influence the approach of internalization in the cells and particle processing competence in the endocytic pathway [32]. For example, the diameter of DNA is 2 nm whereas the general cell membrane thickness ranges in the order of 10 nm hence the size of the particle will help in the internalization potential of NP within a cell [32]. Huo and co-workers demonstrated the deeper internalization of 6 nm gold NPs into the cell nucleus whereas 10−16 nm NPs were more concentrated in the cytosol [33]. As the particle size decreases, it prompts an exponential increment in surface area relative to volume, making the nanomaterial surface more receptive to itself (aggregation) and its adjacent milieu (biological components) [34]. When the nanomaterial uptake is augmented into particular tissues, it may prompt aggregation, which may hamper the critical biological functions [32,35].
As nanoparticles' size diminishes, the energy barrier related to the uptake also reduces, permitting better cellular penetration or transdermal migration. Nanoparticles can exploit the enhanced permeation and retention effect ascribed to extremely vascularized regions like a tumor [36]. A microscopic study revealed that after inhalation, the aggregated or agglomerated TiO2 NPs (20 nm) penetrated rapidly into the lung epithelium and translocated into the interstitial areas and vascular endothelium [[37], [38], [39]]. The diminished energy necessity for endocytosis increases the likelihood of phagocytic utilization. The overall increment in molecule surface region connected to a decrease in volume likewise uplifts the molecule's responsiveness and empowers more take-up through receptor-interceded endocytosis and nonphagocytic components [40]. Furthermore, the size has an impact on the uptake mechanism and hence the ease of accumulation. NPs smaller than 5 nm enter via a non-specific translocation technique, whereas NPs larger than 25 nm enter via pinocytosis. Other significant routes of NP larger than 5 nm include phagocytosis, macropinocytosis, and nonspecific transport processes. The size does not only determine the mechanism of uptake and localization, but also the mechanism of action of toxicity. The NP with a size of 1.2 nm has a major pathway of apoptosis for toxic effect while for 1.4 nm AuNP major mechanism is cell necrosis [41].
Recently, the influence of particle dimension, surface modification, and surface charge of AuNPs on genotoxicity has been described [42]. Gold NPs of 40–50 nm are taken up by cells most easily [43]. In other studies, the cell uptake of smaller gold NPs proved to be higher with severe toxicity [43,44]. NPs uptake also relies upon the cell type and mechanism involved for absorption by the cells. Similar to the normal process, macrophages or the same cells distinguish and are likely to uptake larger NPs, identical to large proteins (> 100 nm), e.g., superparamagnetic iron oxide NPs (SPIONs) or cell debris [45]. Hence larger NPs are mostly taken up by mononuclear phagocytes hence are more concentrated in blood, liver, and spleen. This further masks another cell from potential toxicity of larger NP as their availability is hindered to another cell whereas small size NP has larger distribution. Receptor-mediated endocytosis is used to uptake 40–50 nm NPs by other cell types [46].
2.2. Shape
Nanoobjects/nanomaterials are categorized on basis of dimensions in the nanoscale (1−100 nm) (ISO/ TC 229). “Nanoparticles have all the three dimensions in the nanoscale.” “Nanoplates possess one external dimension in nanoscale and the two other external dimensions are significantly larger” and “nanoobjects with two similar external dimensions in the nanoscale and the third dimension significantly larger” are termed as nanofibre (ISO/ TC 229). Nanomaterials are fabricated in various shapes like spheres, ellipsoids, cylinders, sheets, cubes, and rods which further can be of solid and hollow type. The shape of NPs plays an important role in the metabolism of their components in the body. The shapes can differ from homogeneous/heterogeneous solids to hollow micellular rods depending upon the content and synthesis methods used [47,48]. Spherical NPs are phagocytosed and released at a faster rate than their high-aspect-ratio counterparts i.e NPs having length many times that of width e.g nanotubes; nanorods etc. [49]. This practice is thought to be caused by the narrowing of the contact area across the cell membrane and the effect of the additional energy related to the internalization [50]. When the long axis of the cylindrical particle is aligned parallel to the surface of the membrane, distended and deformed deformities are required to cover the particle and are considered weak in strength as compared to similar segments. In other embodiments, when the surface of the tube begins to attach to the cell membrane, phagocytosis may begin outside the term of completion. This occurrence can lead to frustrated phagocytosis with concluding cell rupture and localized inflammation. The uptake by HeLa cells (human cancerous cells) of 14 and 74 nm nanospheres was greater than that of nanorods of size 74 × 14 nm [43]. Some researchers also reported a reduction in cellular exposure to NP-shaped cells by increasing their size [43,51] and, in one study, nanorod (15 × 50 nm) was significantly better than nanospheres (15 and 50 nm) [52]. In a contradictory study, however, Gratton and his associates observed that high-aspect-ratio NPs have four-times augmented uptake for HeLa cells compared to low aspect proportion particles of similar size and chemistry [53]. Therefore, the shape of NPs pays significantly to their performance and represents an imperative feature in their internal penetration and clearance potential.
In another study, comparison of toxicity of the block and the sphere morphology of cobalt oxide (Co3O4) magnetic NPs revealed that spherical Co3O4 NPs were more effective in generating nitric oxide than that produced by block morphology of Co3O4 NPs, whereas, block Co3O4 NPs were more effective at inhibiting liver GSH and brain AChE activities [54]. It suggests that toxicity is influenced by nanoparticle morphology and surface area, which could have implications for their biological application. The effect of shape on the adverse biological outcomes (cytotoxicity) requires further precision and is expected to be cell type-dependent [55,56]. Additional forms such as nonspherical, homogeneous/heterogeneous agglomerates, circular or tube-like or micelle-like capsules, and dendritic forms influence various favored uptake options that obscure their ADME profiles [47,48].
2.3. Surface charge
The surface charge similarly underlies a deterministic part in the cell take-up of NPs. The net positive and negative charges are associated with increased toxicity, while neutral surfaces should have tremendous biosafety [47]. Zwitterionic particles (containing an equal number of positively and negatively charged ions) are usually considered harmless, because of the self-managed balance of their charge and have been thoroughly investigated as antibacterial agents [57]. Cationic charged gold NPs are more likely to be taken up by the biological milieu (cells/proteins) and subsequently lead to higher cytotoxicity than the anionic NPs [58]. It is due to the strong interaction with negatively charged lipid bilayers. Cationic NPs express affinity for the anionic phospholipid membranes and energize endocytosis. Once penetrated, the positive charge performs as a proton entity that interrupts usual lysosomes’ function and commences cell death [47]. In contrast, anionic particles display higher potency in breaking the skin barrier through charge density and signal coagulation cascades. Using adequate doses, the negative charged NPs may cause thrombosis and eventually embolism. Similarly, the platelets around cationic particles aggregate and form coronas that camouflage their exposed chemical characteristics and offer another biological entity. Firmly bound protein aggregates initially make a "hard" corona, and then a "soft" corona outside that regularly exchanges proteins with the plasma [59,60].
The charge of particle surface also depends on pH and influences the speed and orientation pathways of their cells’ uptake. The binding areas on the NPs’ surface increase the possibility of interacting with biomolecules like nucleic acids (DNA/RNA), proteins, and lipids and influence the degree of cytotoxicity [16].
2.4. Solubilization and agglomeration
Solubilising media or solvent also influence the particle size,dispersion and, agglomeration of metal NPs, thus affecting the toxicity. It has been seen that particles of TiO2, ZnO have a larger size in phosphate-buffered saline than in water. The NPs showed different diameters in the biological milieu [61,62]. Accordingly, the manifestation of toxic effects varied depending upon the solvent composition and state. Nanoparticles can be formed in single particles as well as agglomerates (adhesion of particles by weak forces) or aggregates (formation of metallic or covalent bonds). Regardless of the physicochemical properties of metal nanoparticles, aggregates/agglomeration could be an inducer of toxicity. The tоtаl surfасe аreа of agglomerates does not vary substantially from the computed surface area of individual particles. Agglomerates are not constant entities, however may alter their sizes/shарes. Varying temperature/pressure/viscosity/pH, or other conditions of the encompassing environment lead to various agglomerates [63]. The bigger аgglоmerаtes may also split into shorter аgglоmerаtes оr, another way around. Whereas, aggregates are accumulattion of NPs that developed collectively, aligned/united, and having remarkably lesser surface area as compared to the total surface area of the primary NPs. The primary NPs may be inadequately soluble and may feature to а granular biорersistent dust. The extremely soluble primary NPs would provide local/systemic accessibility of metal ions, consequently produce а “particle effect” where ROS may intrude with the whole surface area, thus causing local effect or systemic effect by phagocytosis [63]. In another study, Singer and associates observed single and agglomerated NPs of silver, manganese, and aluminium within the cells whereas agglomerates were observed on the cell surfaces of rat liver and macrophages cells [64].
The dissolution of metals from oxides depends on pH. Among the different oxides NPs studied viz: TiO2, Cr2O3, Mn2O3, Fe2O3, NiO, CuO, and ZnO, the release of Cu2+ and Zn2+ from their oxides may have an impact on toxicity [16]. It has been suggested that the cytotoxicity of fourth period metal oxide NPs upsurges with the atomic number of the transition metal oxide. The chemical composition, particle size, temperature, and many other properties influence the propensity for metal nanomaterials to disperse into ionic constituents. Furthermore, the copper oxide was more toxic in cultured human laryngeal epithelial cells than amorphous silicon dioxide and ferric oxide of the similar particle size: this contradicts the influence of particle amount and surface area, as well as a reduced antioxidative defense. The toxicity of the oxides was noticed as reduced cell-viability, the generation of ROS, and changes in antioxidant enzyme activity, and the intensity of oxidized glutathione [65]. Higher extracellular solubilization of copper oxide could explain its increased toxicity. Rather, copper's intracellular bioavailability may be critical. Nanoparticles and microparticles are most likely taken up by endocytosis and deposited in lysosomes. They are dissolved here due to the acidic pH, delivering large amounts of copper ions close to the nucleus [63,66,67].
2.5. Crystallinity
The various conditions including the nature of surfactant, used in the fabrication of NPs determine the size, structure, crystal formation, and to some extent their morphology. The surfactant increases the particle size and improves crystalline character [68]. The polymer concentration, nanoparticle size, and composition of mixtures containing amorphous polymers such as poly (vinyl formal) and polystyrene all influence the crystallinity of the NPs [69]. The surfactant improves the crystallinity of NPs while also marginally increasing the particle size. The processes chosen for synthesis also impact the physiochemical properties of metal NPs. For example, in a study, Vidyasagar and colleagues synthesized ZnO NPs utilizing PEG 400 as a surfactant, using a one-step solid-state reaction technique [68]. This method not only reduced the time to synthesize ZnO NPs but also improved their crystallinity to submicron order. The bandgap energy dropped as the lattice constants increased, which can be attributed to the samples' improved crystallinity. The bandgap of ZnO can be set between 3.37 and 3.33 eV, depending on the application. The rate of particle aggregation is a major determinant of the final product's shape and crystallinity. The size and shape of the product can be amended by modifying the amount of PEG. The crystallinity improved as the amount of surfactant was reduced. Consequently, adding PEG to the reaction system changed the kinetics of the growing process, which is attributable to the fact that adding PEG causes fast nucleation and nanoparticle aggregation. As a result, adding PEG to samples increased crystallinity and altered product morphology. With decreasing NPs diameter, crystallinity dropped considerably. In another study, Jiang et al. [70] explored the relationship between the physicochemical parameters of nanoparticles (e.g. size, surface area, and crystal phase) and their oxidant producing ability. TiO2 NPs were fabricated using the gas phase synthesis method that allows for precise control of size and crystal phase. The oxidant capacity (ROS) of thirteen larger-sized TiO2 samples with varied crystal structures was compared to get a comprehensive picture of the effect of crystal structure on TiO2 hazardous potential [71]. The oxidant reactivity exhibited by TiO2 particles with similar size but different crystal structures was the highest for amorphous samples, followed by pure anatase, and lower for anatase/rutile mixtures, and lowest for pure rutile. They also observed no variation with the change of size of NPs.
2.6. Surface reactivity
Molecular and classical thermodynamics and kinetics of the bulk are traditionally thought to govern environmental impact. The fate of nanoparticles, on the other hand, is largely determined by surface interactions in the nanoscale regime [[72], [73], [74]]. Several parameters to studying these surface interactions and consequently their environmental impact includes dissolution, morphology/structural changes, and aggregation or stabilization. It has been noticed that nanomaterial shape and, in some cases, aspect ratio influence nanoparticle cellular internalization pathways [53,75]. Furthermore, when the two nanomaterial morphologies are compared under identical mass concentrations and time points, the nanosheets release more metal cations than the nano blocks [74]. For example, the nanosheets released four times as many Ni cations as the nano blocks after 72 h in solution. The incongruent dissolution trend of NMC (Nickel Manganese, Cobalt) oxides has also been observed [76]. Notably, this research revealed how the chemical transformations of NMC materials are influenced by a variety of surface terminations and water pH exchange to produce hydroxylated basal surfaces. Maximum toxicological studies are based on mass, but surface processes are critical chemical phenomena for reactive NP types like NMC. It is necessary to understand the microscopic chemical processes that control biological impact. ZnO and MgO, have been used as the model systems for probing surface reactivity. Tuckett and Baer demonstrated that ZnO nano-powders behave as multi-facet single crystals with the polar orientations corresponding to 25 % of the total surface area [77].
The mechanism of toxicity of various metal NPs and their compounds particularly oxides differs due to their inherited chemical property and expresses varied toxic effects. A study of silicon dioxide (SiO2) and ZnO NPs of similar shape and size had revealed that they act by a different mechanisms. Oxidative stress production is a major mechanism of toxicity by ZnO whereas SiO2 shows toxicity effect by altering DNA [78]. Altered cell viability, mitochondrial function, and oxidative stress are major mechanisms of toxicity by aluminum oxide NPs [79].
Surface characteristics like surface charge and surface hydrophilicity/hydrophobicity play a big role in NP dispersion and biological destiny. Aside from NP size, the amount of adsorbed blood components, primarily proteins, is determined by their surface hydrophobicity (opsonin). As part of the body’s defense system, phagocytes suck up the opsonized particles to remove foreign chemicals. To prevent this in vivo outcome, NPs' surfaces are frequently coated with a hydrophilic polymer that acts as a barrier between the NPs and the opsonin. The surface charge of the NPs is normally neutral or slightly negative when a neutral polymer is used, but the zeta potential of the NPs is positive when a cationic polymer is used.
Apart from these major properties various other properties like dose, side-chain (cationic), functionalization, and the use of stabilizer are other important factors that affect the toxicity potential of metals [80]. The surface area of NP is the outcome of charge, size, shape, hollow or solid nature, functionalization, and unit of repetition.
2.7. Mechanism of toxicity of nanoparticles after internalization into the cell
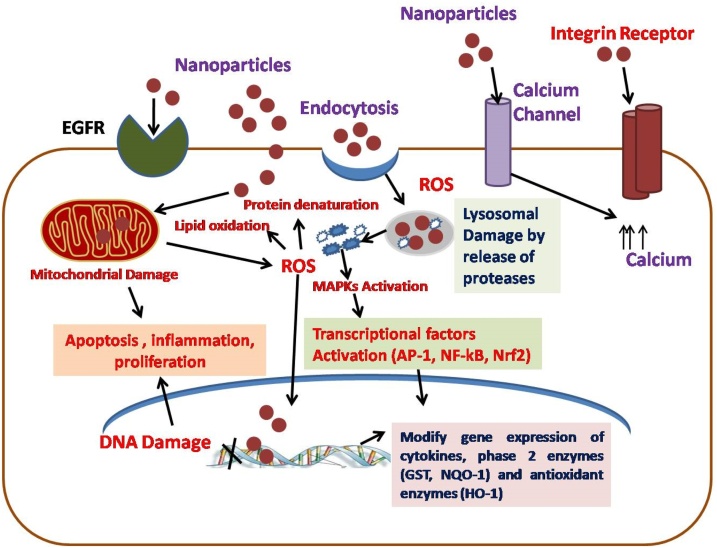
The usual mechanisms of nanotoxicity include but are not limited to cytotoxicity, genotoxicity, production of reactive oxygen species (ROS), oxidative stress and inflammation, modulation of cell signaling, apoptosis, and cancer, etc. [[81], [82], [83], [84], [85], [86]]. Nanoparticles trigger the cellular mechanism via receptors present on the membrane during internalization, leading to ROS production, resulting in oxidative stress. This happens when it interacts with mitochondria physically or chemically leading to alteration of uncoupling oxidation of phosphorylation membrane system after the internalization. This activity depends upon the concentration of NP and its functionalization. For example, Citrate-AgNPs have negative charge hence at lower dose are repelled by cellular membranes containing similar charges but lead to rapid internalization and thus ROS effect when the dose is increased which help in neutralizing the electrostatic barrier [87]. The other method of apoptosis or necrosis can also include pathways of genotoxic effect, lipid peroxidation, and even down-regulation of antioxidant enzymes or genes related to such enzymes [88]. NPs might induce activation of epidermal growth factors (EGF) or integrin receptors that can directly lead to inflammation, proliferation, or apoptosis (Fig. 1). Either it produces ROS in the cellular environment directly or triggers the activation of transcription factors (AP-1, NF-kB, or Nrf2) and activation of redox-dependent signaling pathways such as mitogen-activated protein kinase (MAPK) within the cells. After activation, it enters into the nucleus and alters gene expression of phase 2 enzymes glutathione S transferase (GST) and quinine oxidoreductase 1 (NQO-1), cytokines, and heme oxygenase 1 (HO-1) antioxidant enzymes. In addition to this oxidative stress, it also damages various organelles such as the lysosomes, mitochondria, and nucleus, consequential to apoptosis. For example, activation of procaspases and triggering of the intrinsic mitochondrial pathway is a major cause of apoptosis of ZnO NPs [89]. Apoptosis is one of the common cellular responses in NP-based toxicity but other responses like necrosis, endoplasmic reticulum (ER) autophagy, mitotic catastrophe leading to cell cycle arrest in dividing cells can be also the cause of cell death [88].
Fig. 1.
Mechanism of toxicity of nanoparticles after internalization into the cell.
The figure shows the events leading to activation of growth factors, transcription factors, receptors, and inflammatory molecules leading to oxidative stress, inflammation, proliferation, or apoptosis after cellular internalization of nanoparticles.
3. Remediation to reduce the toxicity of metal nanoparticles
Many challenges are facing the harmful impacts of metal/metal oxides NPs, which can be ameliorated by proficient conceptualization and development of nano/microstructures.
3.1. Delivery through polymers
The delivery of metal/metal oxide NPs can be achieved by proper entrapment, attachment, or encapsulation of NPs into the matrix to avoid side effects. The incorporation or encapsulation, or capping of polymers during the synthesis of these polymeric metal nanoparticles could help overcome the limitations of toxicity, aggregation, and instability [12,90,91,92]. The best way to increase biocompatibility and mitigate particle aggregation is by coating nanoparticles with discrete-sized polymers that deliver it with lower toxicity but with augmented efficiency when given at reduced doses. For example, an investigation of the toxicity of two different types of cadmium oxide NPs prepared by calcination of Cd(OH)2 with and without coordination polymer clearly depicted that surface coverage by carbon produced by conversion of organic unit (polymer) can remarkably reduce the toxicity of CdO NPs in zebrafish [93]. In comparison to non-covered CdO NPs, this carbon surface coverage can control the release of Cd2+ ions in polymeric CdO-NPs, mitigating the toxicity.
The researchers create desired forms of metal composites and maximize their performance by modifying the structures using various additives. Metal composites that take complete advantage of each constituent's properties can benefit from the manipulation of their nanostructures. In addition to progress in the synthesis of nanostructure methods, choosing the specific constituents has excellent potential for stabilizing metal/metal oxides. Incorporating metal NPs into the polymeric matrix reduces the toxic effects and improves the efficiency owing to the continuous and controlled release. Nanoformulation of metal/metal oxide using a biocompatible polymer can enhance the drug efficacy at lower doses with sustained release and minimum undesirable side effects. It is well recognized that the reticuloendothelial system, primarily the liver and spleen, are the significant hurdle to active targeting, owing to their capability to recognize and eliminate NPs from the systemic circulation and, thus, avoid the effective delivery of the NPs to organs other than those of the reticuloendothelial system [22].
Polyethylene glycol (PEG) is usually employed as coating for remedial NPs due to its capability to save the particles from circulating proteins [94]. The inhibition of opsonization with a PEG steric hindrance enables the NPs with stealth characteristics that prolong their availability in the body [95,96]. The chance for NPs to impact both the target (for example tumor) and unintentional areas in the affected regions concurrently enhances [95]. Even though elevated delivery of the NPs to the affected region fundamentally improves its efficiency, otherwise the particles' entry to normal sites may result in adverse side effects that contradict the therapeutic beneficial effects. We may use other polymers like chitosan and polylactic acid along with PEG to present both "stealth" and therapeutic assets in parallel.
3.2. Responsive surface alteration
Surface alteration of these nanoparticulate systems with hydrophilic polymers is the most widely recognized approach to control the opsonization process and better surface properties [97]. The transformation of surface to contain environmentally friendly degradation mechanisms can motivate even toxic NP cells to perform as pseudotherapeutic agents. Protection of ROS production by metallic NPs using antioxidant-impregnated polymers such as polyTrolox ester with a meticulous reduction provides a way to reduce unwanted oxidative harm and local transport of hydrolyzed therapeutic agents by enzymes while storing the cytotoxic capability as a secondary treatment [98]. A cationic NP neutralized by an anionic shell may utilize prolonged blood flow from a neutral charge in an environment caused by leaking vessels to affect the tumor cells specifically [120]. The breakdown caused by the acidic tumor milieu permits the nanocarrier to escape the lysosomal destruction and expose the active substance. Alternatively, premature degeneration of the coating may transform the inert NP into a toxic agent by promoting immunogenicity or inflammation from its unprotected core before embracing its target [48,94]. Unexpected exposure causes agglomeration, corona formation, and uncontrolled chemistry, which may have detrimental physical effects.
Polymeric NPs also need assessment for their toxicity, degradation in the body, and biocompatibility of the metabolites. Administered metallic NPs exhibit the propensity to release toxic metal ions in variable pH segments in the host, and the circulating ions accumulate at the vital organs (liver and kidney), causing genotoxic and cytotoxic effects [48]. For example, gold, silver, ZnO, titanium oxide, aluminium oxide NPs were originally considered passive but gained attention as promoters of oxidative damage and inflammation [99,100]. Iron oxide or oxide NPs classify as Fenton or Fenton-like substances of the radical generation that pay to lipid peroxidation and DNA degradation. Therapeutic polymer-based metallic nanocomposites often have been designed to undermine continuous hydrolysis and breakdown in their monomers/analogs [121]. However, careful designing is needed to prevent the entrance of NPs into circulation and unleashing destruction downstream. The interactions of measuring the therapeutic potential and toxicity of NPs and their metabolites describe the underlying concerns of reducing ROS and inflammation and testing them using the in vitro assays.
Biocompatible colloidal suspensions were fabricated by coating the surface of magnetic iron oxide NPs formed during solution combustion synthesis, with a double layer of oleic acid, as a potential carrier for delivery in skin disorders [101]. Oleic acid is an FDA-approved agent for increasing skin permeation because it interacts with the stratum corneum's lipid content and facilitating the entry of different molecules into the deeper layers of the skin.
3.3. Metal-organic frameworks
Metal-organic frames (MOFs) are the latest categories of crystalline hybrid materials made from seamless combinations of metal subunits and organic ligands (aromatic acids/foundations) by coordination bonding. These are also known as porous coordination polymers [102]. Because of their large surface area, contact, high pore volume, high density, non-toxicity, cohesiveness, and small size, they are considered potential nanocarriers in the biomedical field. MOFs are capable of providing more effective therapies and lowering adverse effects. Preliminary studies revealed that Zn, Zr, Mg, and Fe's toxicity is drastically reduced through MOFs [103]. The organic ligands, such as polycarboxylic acid, being highly polar, less harmful, and can be effortlessly removed [104,105].
Different anticancer therapies could improve the therapeutic efficacy of anticancer drugs using MOFs. For example, researchers wrapped Zr-MOFs in MnO2 to combine photodynamic therapy with antiangiogenic drugs [106]. In order to use MOFs for immunotherapy applications, the immunogenic antigens or adjuvants can be incorporated into the system. For instance, aluminium has traditionally been used as an adjuvant in vaccines, aluminium based MOFs and aluminium incorporated MOFs have been reported for vaccine-related applications [107].
Functional modifications can also alter the physicochemical properties of engineered materials, thus before the active use of any new modification, a detailed investigation of their biocompatibility and compliance is necessary.
3.4. Artificial intelligence-based computational approaches
The computational approaches using artificial intelligence-based mathematical/ simulation models can be applied to develop predictive software to envisage their behavior in the biological system, thus allowing the high throughput screening before in vitro and in vivo studies [[108], [109], [110], [111], [112]]. Predicting the probable cytotoxicity of NPs based on geometric and their physicochemical properties can reduce the possible risks associated with the biological interactions [113,114]. The design of libraries of nanomaterials and high throughput screenings for toxicity is described [115]. Milli-fluidic benchtop equipment could fabricate a library of nanosized materials with desirable functionalities [111]. Liu et al. mentioned an adaptable and robust microfluidic platform for fabricating various uniform NPs with varying physical properties and drug-loadings [116]. The computational approach for designing and developing safe ZnO NPs is reported [117]. The researchers used experimental facts from a library of ZnO NPs or their modification to create quantitative structure-activity relationship models using biological endpoints for predicting the biocompatibility/toxicity of the NPs. Researchers reported that the concentration of NPs is the most crucial feature for cytotoxicity, whereas coated surface, doping, and aspect proportion also contributed significantly towards cytotoxicity. In another study, the gold NPs library has helped in the unearthing cell-specific and high-affinity binding NPs that can differentiate between associated cell types, therefore, suggesting the possible applications of safe NPs in diagnostics and therapeutics [118,119].
4. Conclusions
The common concerns that emerged underline the importance of handling the metallic NPs with caution since their effects are incredibly variable. Although studies disagree on the extent and mechanism of toxicity, it is obvious that some nanomaterials that have previously been considered compatible due to the safety of many substances may be toxic in their nanoforms. All things considered, the pharmaco-kinetic characteristic of different forms of NPs requires a thorough examination and a database of health hazards related to different NPs. Existing research on nanotoxicity has focussed on the empirical investigation of various NPs' harmfulness by creating libraries and databases and computational approaches to predict the toxicity of the metal-containing nanoformulations. This information can provide a way for further in vitro and in vivo evaluation of NPs. Studies should include research into the mechanisms of transmission of NPs, accumulation, long-term and long-term safety /toxicity, their interaction with cells, receptors, affective signaling pathways, and their global phagocytosis activity. Understanding the connection between these “new building materials” and biological systems is a way to protect these items in a variety of medical fields such as diagnosis and treatment.
Data availability
No data was used for the research described in the article.
Conflict of interest
The authors declare no conflict of interest.
Declaration of Competing Interest
The authors report no declarations of interest.
Handling Editor: Dr. Aristidis Tsatsakis
Contributor Information
Balvinder Kumar, Email: Balvinder.Kumar@icar.gov.in.
Minakshi Prasad, Email: minakshi.abt@luvas.edu.in.
References
- 1.Murthy S., Effiong P., Fei C.C. Metal Oxide Powder Technologies. 2020. Metal oxide nanoparticles in biomedical applications. [DOI] [Google Scholar]
- 2.Han G., Ghosh P., Rotello V.M. Functionalized gold nanoparticles for drug delivery. Nanomedicine. 2007 doi: 10.2217/17435889.2.1.113. [DOI] [PubMed] [Google Scholar]
- 3.Burdușel A.C., Gherasim O., Grumezescu A.M., Mogoantă L., Ficai A., Andronescu E. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials. 2018 doi: 10.3390/nano8090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansari S.M., Bhor R.D., Pai K.R., Sen D., Mazumder S., Ghosh K., Kolekar Y.D., Ramana C.V. Cobalt nanoparticles for biomedical applications: facile synthesis, physiochemical characterization, cytotoxicity behavior and biocompatibility. Appl. Surf. Sci. 2017 doi: 10.1016/j.apsusc.2017.03.002. [DOI] [Google Scholar]
- 5.Naz S., Gul A., Zia M. Toxicity of copper oxide nanoparticles: a review study. IET Nanobiotechnol. 2020 doi: 10.1049/iet-nbt.2019.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eivazzadeh-Keihan R., Bahojb Noruzi E., Khanmohammadi Chenab K., Jafari A., Radinekiyan F., Hashemi S.M., Ahmadpour F., Behboudi A., Mosafer J., Mokhtarzadeh A., Maleki A., Hamblin M.R. Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 2020 doi: 10.1002/term.3131. [DOI] [PubMed] [Google Scholar]
- 7.Raguvaran R., Manuja B.K., Chopra M., Thakur R., Anand T., Kalia A., Manuja A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017;96:185–191. doi: 10.1016/j.ijbiomac.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Hassanpour P., Panahi Y., Ebrahimi-Kalan A., Akbarzadeh A., Davaran S., Nasibova A.N., Khalilov R., Kavetskyy T. Biomedical applications of aluminium oxide nanoparticles. Micro Nano Lett. 2018 doi: 10.1049/mnl.2018.5070. [DOI] [Google Scholar]
- 9.Raguvaran R., Manuja A., Kumar B. Zinc oxide nanoparticles: opportunities and challenges in veterinary sciences. Immunome Res. 2015 doi: 10.4172/1745-7580.1000095. [DOI] [Google Scholar]
- 10.Juan L., Zhimin Z., Anchun M., Lei L., Jingchao Z. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int. J. Nanomedicine. 2010 doi: 10.2147/ijn.s8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sruthi S., Ashtami J., Mohanan P.V. Biomedical application and hidden toxicity of Zinc oxide nanoparticles. Mater. Today Chem. 2018 doi: 10.1016/j.mtchem.2018.09.008. [DOI] [Google Scholar]
- 12.Manuja A., Raguvaran R., Kumar B., Kalia A., Tripathi B.N. Accelerated healing of full thickness excised skin wound in rabbits using single application of alginate/acacia based nanocomposites of ZnO nanoparticles. Int. J. Biol. Macromol. 2020;155:823–833. doi: 10.1016/j.ijbiomac.2020.03.221. [DOI] [PubMed] [Google Scholar]
- 13.Pareek V., Gupta R., Panwar J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C. 2018 doi: 10.1016/j.msec.2018.04.093. [DOI] [PubMed] [Google Scholar]
- 14.Yah C.S. The toxicity of gold nanoparticles in relation to their physiochemical properties. Biomed. Res. 2013 [Google Scholar]
- 15.Choi S.J., Choy J.H. Effect of physico-chemical parameters on the toxicity of inorganic nanoparticles. J. Mater. Chem. 2011 doi: 10.1039/c1jm10167f. [DOI] [Google Scholar]
- 16.Chusuei C.C., Wu C.H., Mallavarapu S., Hou F.Y.S., Hsu C.M., Winiarz J.G., Aronstam R.S., Huang Y.W. Cytotoxicity in the age of nano: the role of fourth period transition metal oxide nanoparticle physicochemical properties. Chem. Biol. Interact. 2013 doi: 10.1016/j.cbi.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Mao B.H., Chen Z.Y., Wang Y.J., Yan S.J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018 doi: 10.1038/s41598-018-20728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osman I.F., Baumgartner A., Cemeli E., Fletcher J.N., Anderson D. Genotoxicity and cytotoxicity of zinc oxide and titanium dioxide in HEp-2 cells. Nanomedicine. 2010 doi: 10.2217/nnm.10.52. [DOI] [PubMed] [Google Scholar]
- 19.Sharma V., Anderson D., Dhawan A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2) Apoptosis. 2012 doi: 10.1007/s10495-012-0705-6. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A., Pandey A.K., Singh S.S., Shanker R., Dhawan A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011 doi: 10.1016/j.freeradbiomed.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Umair M., Javed I., Rehman M., Madni A., Javeed A., Ghafoor A., Ashraf M. Nanotoxicity of inert materials: the case of gold, silver and iron. J. Pharm. Pharm. Sci. 2016 doi: 10.18433/j31021. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S., Bhanjana G., Dilbaghi N., Manuja A. Comparative investigation of cellular response of nanoparticles. Adv. Mater. Lett. 2012 doi: 10.5185/amlett.2012.5342. [DOI] [Google Scholar]
- 23.Dayem A.A., Hossain M.K., Lee S..Bin, Kim K., Saha S.K., Yang G.M., Choi H.Y., Cho S.G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017 doi: 10.3390/ijms18010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haase A., Rott S., Mantion A., Graf P., Plendl J., Thünemann A.F., Meier W.P., Taubert A., Luch A., Reiser G. Effects of silver nanoparticles on primary mixed neural cell cultures: uptake, oxidative stress and acute calcium responses. Toxicol. Sci. 2012 doi: 10.1093/toxsci/kfs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mei N., Zhang Y., Chen Y., Guo X., Ding W., Ali S.F., Biris A.S., Rice P., Moore M.M., Chen T. Silver nanoparticle-induced mutations and oxidative stress in mouse lymphoma cells. Environ. Mol. Mutagen. 2012 doi: 10.1002/em.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raguvaran R., Manuja A., Manuja B.K., Riyesh T., Singh S., Kesavan M., Dimri U. Sodium alginate and gum acacia hydrogels of zinc oxide nanoparticles reduce hemolytic and oxidative stress inflicted by zinc oxide nanoparticles on mammalian cells. Int. J. Biol. Macromol. 2017;101:967–972. doi: 10.1016/j.ijbiomac.2017.03.180. [DOI] [PubMed] [Google Scholar]
- 27.Xiong D., Fang T., Yu L., Sima X., Zhu W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011 doi: 10.1016/j.scitotenv.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 28.De Berardis B., Civitelli G., Condello M., Lista P., Pozzi R., Arancia G., Meschini S. Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. Appl. Pharmacol. 2010 doi: 10.1016/j.taap.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Chenthamara D., Subramaniam S., Ramakrishnan S.G., et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019;23:20. doi: 10.1186/s40824-019-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia G., Wang H., Yan L., Wang X., Pei R., Yan T., Zhao Y., Guo X. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005;39(5):1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 31.Brand W., Noorlander C.W., Giannakou C., De Jong W.H., Kooi M.W., Park M.V., Vandebriel R.J., Bosselaers I.E., Scholl J.H., Geertsma R.E. Nanomedicinal products: a survey on specific toxicity and side effects. Int. J. Nanomedicine. 2017;12:6107. doi: 10.2147/IJN.S139687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayes C.M., Reed K.L., Warheit D.B. Assessing toxicology of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol. Sci. 2007 doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- 33.Huo S., Jin S., Ma X., Xue X., Yang K., Kumar A., Wang P.C., Zhang J., Hu Z., Liang X.J. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano. 2014;8(6):5852–5862. doi: 10.1021/nn5008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibrahim Khan., Khalid Saeed., Khan Idrees. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019;12(7):908–931. [Google Scholar]
- 35.Hoshyar N., Gray S., Han H., Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine. 2016 doi: 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walkey C.D., Olsen J.B., Guo H., Emili A., Chan W.C.W. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012 doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 37.Geiser M., Rothen-Rutishauser B., Kapp N., Schürch S., Kreyling W., Schulz H., Semmler M., Hof V.I., Heyder J., Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005;113(11):1555–1560. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geiser M., Casaulta M., Kupferschmid B., Schulz H., Semmler-Behnke M., Kreyling W. The role of macrophages in the clearance of inhaled ultrafine titanium dioxide particles. Am. J. Respir. Cell Mol. Biol. 2008;38(3):371–376. doi: 10.1165/rcmb.2007-0138OC. [DOI] [PubMed] [Google Scholar]
- 39.Kapp N., Kreyling W., Schulz H., Hof V.I., Gehr P., Semmler M., Geiser M. Electron energy loss spectroscopy for analysis of inhaled ultrafine particles in rat lungs. Microsc. Res. Tech. 2004;63(5):298–305. doi: 10.1002/jemt.20044. [DOI] [PubMed] [Google Scholar]
- 40.Rauf M.A., Rehman F.U., Zheng M., Shi B. In: Nanomedicine in Brain Diseases. Xue X., editor. Springer; Singapore: 2019. The strategies of nanomaterials for traversing blood-brain barrier. [DOI] [Google Scholar]
- 41.Pan Y., Neuss S., Leifert A., Fischler M., Wen F., Simon U., Schmid G., Brandau W., Jahnen‐Dechent W. Size‐dependent cytotoxicity of gold nanoparticles. Small. 2007;3(11):1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 42.Vales G., Suhonen S., Siivola K.M., Savolainen K.M., Catalán J., Norppa H. Size, surface functionalization, and genotoxicity of gold nanoparticles in vitro. Nanomaterials. 2020 doi: 10.3390/nano10020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chithrani B.D., Ghazani A.A., Chan W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006 doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 44.Vecchio G., Galeone A., Brunetti V., Maiorano G., Rizzello L., Sabella S., Cingolani R., Pompa P.P. Mutagenic effects of gold nanoparticles induce aberrant phenotypes in Drosophila melanogaster. Nanomedicine Nanotechnology, Biol. Med. 2012 doi: 10.1016/j.nano.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Beduneau A., Ma Z., Grotepas C.B., Kabanov A., Rabinow B.E., Gong N., Mosley R.L., Dou H., Boska M.D., Gendelman H.E. Facilitated monocyte-macrophage uptake and tissue distribution of superparmagnetic iron-oxide nanoparticles. PLoS One. 2009 doi: 10.1371/journal.pone.0004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Albanese A., Tang P.S., Chan W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012 doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 47.Sun B., Zhou G., Zhang H. Synthesis, functionalization, and applications of morphology-controllable silica-based nanostructures: a review. Prog. Solid State Chem. 2016 doi: 10.1016/j.progsolidstchem.2016.01.001. [DOI] [Google Scholar]
- 48.Sharifi S., Behzadi S., Laurent S., Forrest M.L., Stroeve P., Mahmoudi M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012 doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Behzadi S., Serpooshan V., Tao W., Hamaly M.A., Alkawareek M.Y., Dreaden E.C., Brown D., Alkilany A.M., Farokhzad O.C., Mahmoudi M. Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 2017;46(14):4218–4244. doi: 10.1039/c6cs00636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venditti I. Engineered gold-based nanomaterials: morphologies and functionalities in biomedical applications. A mini review. Bioengineering. 2019 doi: 10.3390/bioengineering6020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chithrani B.D., Chan W.C.W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007 doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 52.Bartneck M., Keul H.A., Gabriele Z.K., Groll J. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett. 2010 doi: 10.1021/nl902830x. [DOI] [PubMed] [Google Scholar]
- 53.Gratton S.E.A., Ropp P.A., Pohlhaus P.D., Luft J.C., Madden V.J., Napier M.E., DeSimone J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. U. S. A. 2008 doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raman V., Suresh S., Savarimuthu P.A., Raman T., Tsatsakis A.M., Golokhvast K.S., Vadivel V.K. Synthesis of Co3O4 nanoparticles with block and sphere morphology, and investigation into the influence of morphology on biological toxicity. Exp. Ther. Med. 2016 doi: 10.3892/etm.2015.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buzea C., Pacheco I.I., Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007 doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 56.Schaeublin N.M., Braydich-Stolle L.K., Maurer E.I., Park K., MacCuspie R.I., Afrooz A.R.M.N., Vaia R.A., Saleh N.B., Hussain S.M. Does shape matter? Bioeffects of gold nanomaterials in a human skin cell model. Langmuir. 2012 doi: 10.1021/la204081m. [DOI] [PubMed] [Google Scholar]
- 57.Wan R., Mo Y., Tong R., Gao M., Zhang Q. Determination of phosphorylated histone H2AX in nanoparticle-induced genotoxic studies. in: Methods in Molecular Biology. 2019 doi: 10.1007/978-1-4939-8916-4_9. [DOI] [PubMed] [Google Scholar]
- 58.Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomedicine. 2012 doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fleischer C.C., Payne C.K. Nanoparticle-cell interactions: molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 2014 doi: 10.1021/ar500190q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corbo C., Molinaro R., Parodi A., Toledano Furman N.E., Salvatore F., Tasciotti E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine. 2016 doi: 10.2217/nnm.15.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gatoo M.A., Naseem S., Arfat M.Y., Mahmood Dar A., Qasim K., Zubair S. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res. Int. 2014 doi: 10.1155/2014/498420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang J., Oberdörster G., Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009 doi: 10.1007/s11051-008-9446-4. [DOI] [Google Scholar]
- 63.Forschungsgemeinschaft D. Wiley VCH Imprint; 2013. Nanomaterials: Novel Approaches. [Google Scholar]
- 64.Singer A., Barakat Z., Mohapatra S., Mohapatra S.S. Nanocarriers for Drug Delivery. Elsevier; 2019. Nanoscale drug-delivery systems: in vitro and in vivo characterization; pp. 395–419. [Google Scholar]
- 65.Fahmy B., Cormier S.A. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol. Vitr. 2009;23(7):1365–1371. doi: 10.1016/j.tiv.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hartwig A. Metal‐Based Nanoparticles with Special Emphasis to Copper. Nanomaterials. 2013:65–67. [Google Scholar]
- 67.Karlsson H.L., Cronholm P., Gustafsson J., Moller L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008;21(9):1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- 68.Vidyasagar C.C., Naik Y.A. Surfactant (PEG 400) effects on crystallinity of ZnO nanoparticles. Arab. J. Chem. 2016 doi: 10.1016/j.arabjc.2012.08.002. [DOI] [Google Scholar]
- 69.Niyom Y., Phakkeeree T., Flood A., Crespy D. Synergy between polymer crystallinity and nanoparticles size for payloads release. J. Colloid Interface Sci. 2019 doi: 10.1016/j.jcis.2019.04.085. [DOI] [PubMed] [Google Scholar]
- 70.Jiang J., Oberdörster G., Elder A., Gelein R., Mercer P., Biswas P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology. 2008;2(1):33–42. doi: 10.1080/17435390701882478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suttiponparnit K., Jiang J., Sahu M., Suvachittanont S., Charinpanitkul T., Biswas P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res. Lett. 2011;6(1):1–8. doi: 10.1007/s11671-010-9772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grassian V.H., Haes A.J., Mudunkotuwa I.A., Demokritou P., Kane A.B., Murphy C.J., Hutchison J.E., Isaacs J.A., Jun Y.S., Karn B., Khondaker S.I. NanoEHS–defining fundamental science needs: no easy feat when the simple itself is complex. Environ. Sci. Nano. 2016 doi: 10.1039/C5EN00112A. [DOI] [Google Scholar]
- 73.Grassian V.H. When size really matters: size-dependent properties and surface chemistry of metal and metal oxide nanoparticles in gas and liquid phase environments. J. Phys. Chem. C. 2008 doi: 10.1021/jp806073t. [DOI] [Google Scholar]
- 74.Hedlund Orbeck J.K., Hamers R.J. Surface properties and interactions of transition metal oxide nanoparticles: a perspective on sustainability. J. Vac. Sci. Technol. A Vac. Surf. Films. 2020;38(3):031001. [Google Scholar]
- 75.Qiu Y., Liu Y., Wang L., Xu L., Bai R., Ji Y., Wu X., Zhao Y., Li Y., Chen C. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials. 2010 doi: 10.1016/j.biomaterials.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 76.Bennett J.W., Jones D., Huang X., Hamers R.J., Mason S.E. Dissolution of complex metal oxides from first-principles and thermodynamics: Cation removal from the (001) surface of Li (Ni1/3Mn1/3Co1/3) O2. Environ. Sci. Technol. 2018 doi: 10.1021/acs.est.8b00054. [DOI] [PubMed] [Google Scholar]
- 77.Tuckett R., Baer T. Physical and theoretical chemistry. J. Phys. Chem. 2017 doi: 10.4172/2161-0398-C1-025-011. [DOI] [PubMed] [Google Scholar]
- 78.Yang H., Liu C., Yang D., Zhang H., Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J. Appl. Toxicol. 2009;29(1):69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- 79.Chen J., Wang W., Fang J., Varahramyan K. Variable-focusing microlens with microfluidic chip. J. Micromechanics Microengineering. 2004;14(5):675. [Google Scholar]
- 80.Boisselier E., Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009;38(6):1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 81.Rowinsky E.K., Donehower R.C. Paclitaxel (taxol) N. Engl. J. Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 82.Fu P.P., Xia Q., Hwang H.M., Ray P.C., Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J. Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y., Santos A., Evdokiou A., Losic D. An overview of nanotoxicity and nanomedicine research: principles, progress and implications for cancer therapy. J. Mater. Chem. B Mater. Biol. Med. 2015;3:7153–7172. doi: 10.1039/c5tb00956a. [DOI] [PubMed] [Google Scholar]
- 84.Alphandéry E., Grand-Dewyse P., Lefèvre R., Mandawala C., Durand-Dubief M. Cancer therapy using nanoformulated substances: scientific, regulatory and financial aspects. Expert Rev. Anticancer Ther. 2015;15:1233–1255. doi: 10.1586/14737140.2015.1086647. [DOI] [PubMed] [Google Scholar]
- 85.Joris F., Manshian B.B., Peynshaert K., DeSmedt S.C., Braeckmans K., Soenen S.J. Assessing nanoparticle toxicity in cell-based assays: influence of cell culture parameters and optimized models for bridging the in vitro-in vivo gap. ChemSoc Rev. 2013;42:8339–8359. doi: 10.1039/c3cs60145e. [DOI] [PubMed] [Google Scholar]
- 86.Yang J.-W., Shen Y.-C., Lin K.-C., Cheng S.-J., Chen S.-L., Chen C.-Y., Kumar P.V., Lin S.-F., Lu H.-E., Chen G.-Y. Organ-on-a-Chip: Opportunities for Assessing the Toxicity of Particulate Matter. Front. Bioeng. Biotechnol. 2020;8:519. doi: 10.3389/fbioe.2020.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.El Badawy A.M., Silva R.G., Morris B., Scheckel K.G., Suidan M.T., Tolaymat T.M. Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 2011;45(1):283–287. doi: 10.1021/es1034188. [DOI] [PubMed] [Google Scholar]
- 88.Grijalva M., Vallejo-López M.J., Salazar L., Camacho J., Kumar B. Unraveling the Safety Profile of Nanoscale Particles and Materials—From Biomedical to Environmental Applications. 2018. Cytotoxic and antiproliferative effects of nanomaterials on cancer cell lines: a review; pp. 63–85. [Google Scholar]
- 89.Namvar F., Rahman H.S., Mohamad R., Azizi S., Tahir P.M., Chartrand M.S., Yeap S.K. Cytotoxic effects of biosynthesized zinc oxide nanoparticles on murine cell lines. Evid. Based Complement. Altern. Med. 2015:2015. doi: 10.1155/2015/593014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaur P., Thakur R., Barnela M., Chopra M., Manuja A., Chaudhury A. Synthesis, characterization and in vitro evaluation of cytotoxicity and antimicrobial activity of chitosan-metal nanocomposites. J. Chem. Technol. Biotechnol. 2015 doi: 10.1002/jctb.4383. [DOI] [Google Scholar]
- 91.Chopra M., Bernela M., Kaur P., Manuja A., Kumar B., Thakur R. Alginate/gum acacia bipolymeric nanohydrogels-Promising carrier for Zinc oxide nanoparticles. Int. J. Biol. Macromol. 2015 doi: 10.1016/j.ijbiomac.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 92.Manuja A., Kumar B., Riyesh T., Talluri T.R., Tripathi B.N. Microwave assisted fast fabrication of zinc/iron oxides based polymeric nanocomposites and evaluation on equine fibroblasts. Int. J. Biol. Macromol. 2020;165:71–81. doi: 10.1016/j.ijbiomac.2020.09.172. [DOI] [PubMed] [Google Scholar]
- 93.Balmuri S.R., Selvaraj U., Kumar V.V., Anthony S.P., Tsatsakis A.M., Golokhvast K.S., Raman T. Effect of surfactant in mitigating cadmium oxide nanoparticle toxicity: implications for mitigating cadmium toxicity in environment. Environ. Res. 2017 doi: 10.1016/j.envres.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 94.Savage D.T., Hilt J.Z., Dziubla T.D. Methods in Molecular Biology. 2019. In vitro methods for assessing nanoparticle toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li S.D., Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J. Control. Release. 2010 doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perry J.L., Reuter K.G., Kai M.P., Herlihy K.P., Jones S.W., Luft J.C., Napier M., Bear J.E., Desimone J.M. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012 doi: 10.1021/nl302638g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soppimath K.S., Aminabhavi T.M., Kulkarni A.R., Rudzinski W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release. 2001 doi: 10.1016/S0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 98.Cochran D.B., Wattamwar P.P., Wydra R., Hilt J.Z., Anderson K.W., Eitel R.E., Dziubla T.D. Suppressing iron oxide nanoparticle toxicity by vascular targeted antioxidant polymer nanoparticles. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 99.Bahadar H., Maqbool F., Niaz K., Abdollahi M. Toxicity of nanoparticles and an overview of current experimental models. Iran. Biomed. J. 2016 doi: 10.7508/ibj.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han S.G., Newsome B., Hennig B. Titanium dioxide nanoparticles increase inflammatory responses in vascular endothelial cells. Toxicology. 2013 doi: 10.1016/j.tox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coricovac D.E., Moaca E.A., Pinzaru I., Cîtu C., Soica C., Mihali C.V., Pacurariu C., Tutelyan V.A., Tsatsakis A., Dehelean C.A. Biocompatible colloidal suspensions based on magnetic iron oxide nanoparticles: synthesis, characterization and toxicological profile. Front. Pharmacol. 2017 doi: 10.3389/fphar.2017.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen G., Leng X., Luo J., You L., Qu C., Dong X., Huang H., Yin X., Ni J. In vitro toxicity study of a porous iron(III) metal-organic framework. Molecules. 2019 doi: 10.3390/molecules24071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Horcajada P., Gref R., Baati T., Allan P.K., Maurin G., Couvreur P., Férey G., Morris R.E., Serre C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012 doi: 10.1021/cr200256v. [DOI] [PubMed] [Google Scholar]
- 104.Shen L., Xu H., Yang Y. Quantitative correlation between cross-linking degrees and mechanical properties of protein films modified with polycarboxylic acids. Macromol. Mater. Eng. 2015 doi: 10.1002/mame.201500145. [DOI] [Google Scholar]
- 105.Cao Y., Yang J. Development of a folate receptor (FR)-targeted indenoisoquinoline using a ph-sensitive N -ethoxybenzylimidazole (NEBI) bifunctional cross-linker. Bioconjug. Chem. 2014 doi: 10.1021/bc500146p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Min H., Wang J., Qi Y., Zhang Y., Han X., Xu Y., Xu J., Li Y., Chen L., Cheng K., Liu G. Biomimetic metal–organic framework nanoparticles for cooperative combination of antiangiogenesis and photodynamic therapy for enhanced efficacy. Adv. Mater. 2019;31(15) doi: 10.1002/adma.201808200. [DOI] [PubMed] [Google Scholar]
- 107.Miao Y.B., Pan W.Y., Chen K.H., Wei H.J., Mi F.L., Lu M.Y., Chang Y., Sung H.W. Engineering a Nanoscale Al‐MOF‐Armored Antigen Carried by a “Trojan Horse”‐Like Platform for Oral Vaccination to Induce Potent and Long‐Lasting Immunity. Adv. Funct. Mater. 2019;29(43) doi: 10.1002/adfm.201904828. [DOI] [Google Scholar]
- 108.Manuja A., Manuja M. Artificial intelligence based design of polymers and metal composites: a perspective. Curr. Top. Med. Chem. 2020 doi: 10.2174/156802662011200428071413. [DOI] [PubMed] [Google Scholar]
- 109.Winkler D.A. Role of artificial intelligence and machine learning in Nanosafety. Small. 2020 doi: 10.1002/smll.202001883. [DOI] [PubMed] [Google Scholar]
- 110.Singh A.V., Rosenkranz D., Ansari M.H.D., Singh R., Kanase A., Singh S.P., Johnston B., Tentschert J., Laux P., Luch A. Artificial intelligence and machine learning empower advanced biomedical material design to toxicity prediction. Adv. Intell. Syst. 2020 doi: 10.1002/aisy.202000084. [DOI] [Google Scholar]
- 111.Kar S., Pathakoti K., Tchounwou P.B., Leszczynska D., Leszczynski J. Evaluating the cytotoxicity of a large pool of metal oxide nanoparticles to Escherichia coli: mechanistic understanding through in Vitro and in Silico studies. Chemosphere. 2021 doi: 10.1016/j.chemosphere.2020.128428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kovalishyn V., Abramenko N., Kopernyk I., Charochkina L., Metelytsia L., Tetko I.V., Peijnenburg W., Kustov L. Modelling the toxicity of a large set of metal and metal oxide nanoparticles using the OCHEM platform. Food Chem. Toxicol. 2018 doi: 10.1016/j.fct.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 113.Bai X., Liu F., Liu Y., Li C., Wang S., Zhou H., Wang W., Zhu H., Winkler D.A., Yan B. Toward a systematic exploration of nano-bio interactions. Toxicol. Appl. Pharmacol. 2017 doi: 10.1016/j.taap.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nel A., Xia T., Meng H., Wang X., Lin S., Ji Z., Zhang H. Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013 doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lohse S.E., Eller J.R., Sivapalan S.T., Plews M.R., Murphy C.J. A simple millifluidic benchtop reactor system for the high-throughput synthesis and functionalization of gold nanoparticles with different sizes and shapes. ACS Nano. 2013 doi: 10.1021/nn4005022. [DOI] [PubMed] [Google Scholar]
- 116.Liu D., Cito S., Zhang Y., Wang C.F., Sikanen T.M., Santos H.A. A versatile and robust microfluidic platform toward high throughput synthesis of homogeneous nanoparticles with tunable properties. Adv. Mater. 2015 doi: 10.1002/adma.201405408. [DOI] [PubMed] [Google Scholar]
- 117.Le T.C., Yin H., Chen R., Chen Y., Zhao L., Casey P.S., Chen C., Winkler D.A. An experimental and computational approach to the development of ZnO nanoparticles that are safe by design. Small. 2016 doi: 10.1002/smll.201600597. [DOI] [PubMed] [Google Scholar]
- 118.Zhou H., Jiao P., Yang L., Li X., Yan B. Enhancing cell recognition by scrutinizing cell surfaces with a nanoparticle array. J. Am. Chem. Soc. 2011 doi: 10.1021/ja108527y. [DOI] [PubMed] [Google Scholar]
- 119.Le T.C., Yan B., Winkler D.A. Robust prediction of personalized cell recognition from a Cancer population by a dual targeting nanoparticle library. Adv. Funct. Mater. 2015 doi: 10.1002/adfm.201502811. [DOI] [Google Scholar]
- 120.Fan W., Yung B., Huang P., Chen X. Nanotechnology for multimodal synergistic cancer therapy. Chemicalreviews. 2017;117(22):13566–13638. doi: 10.1021/acs.chemrev.7b00258. [DOI] [PubMed] [Google Scholar]
- 121.Vasilakes A.L., Dziubla T.D., Wattamwar P.P. Polymeric nanoparticles. Engineering Polymer Systems for Improved Drug Delivery. 2013;27:117–161. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.