Abstract
Obstructive uropathy is known to be associated with acute kidney injury (AKI). This study aimed to investigate the etiologies, clinical characteristics, consequences and also assess the impact of AKI on long-term outcomes. This multicenter, retrospective study of 1683 patients with obstructive uropathy who underwent percutaneous nephrostomy (PCN) analyzed clinical characteristics, outcomes including progression to end-stage kidney disease (ESKD), overall mortality, and the impact of AKI on long-term outcomes. Obstructive uropathy in adults was most commonly caused by malignancy, urolithiasis, and other causes. AKI was present in 78% of the patients and was independently associated with preexisting chronic kidney disease (CKD). Short-term recovery was achieved in 56.78% after the relief of obstruction. ESKD progression rate was 4.4% in urolithiasis and 6.8% in other causes and older age, preexisting CKD, and stage 3 AKI were independent factors of progression. The mortality rate (34%) was highly attributed to malignant obstruction (52%) stage 3 AKI was also an independent predictor of mortality in non-malignant obstruction. AKI is a frequent complication of adult obstructive uropathy. AKI negatively affects long-term kidney outcomes and survival in non-malignant obstructions. A better understanding of the epidemiology and prognostic factors is needed for adult obstructive uropathy.
Subject terms: Nephrology, Risk factors, Urology
Introduction
Obstructive uropathy is a common clinical condition that leads to acute or chronic kidney failure. Obstruction to urine flow can occur in any location in the urinary tract, and may be acute or chronic, unilateral or bilateral, and partial or complete. While obstructive uropathy is known to be a frequent cause of CKD/ESKD in children, it is known to be associated with AKI in adults1,2.
Obstruction to urine flow increases the pressure proximal to the obstruction, consequently decreasing the intraglomerular pressure gradient, which leads to a decreased glomerular filtration rate (GFR)3. Activation of the renin–angiotensin–aldosterone system mediated by renal vasoconstriction further reduces the renal blood flow and GFR4. In addition to the hemodynamic effects, abnormalities in the cellular paradigm, including the development of interstitial inflammation, tubular atrophy, and fibrosis, also contribute to kidney injury5.
The incidence, etiologies, and clinical manifestations of obstructive uropathy are likely to vary according to the patients’ age, sex, or underlying disease conditions. For example, urolithiasis is known to be a common cause in young adults, whereas benign prostatic hypertrophy and retroperitoneal or pelvic malignancies are more common in the elderly6. Although early recognition and prompt relief of obstruction can reverse the injury and restore function completely, delayed diagnosis due to the absence of specific symptoms, superimposed infection, or underlying comorbidities might affect the short- and long-term kidney outcomes.
In contrast to pediatric obstructive uropathy, which is mainly caused by congenital abnormalities of the urinary tract, there have been no extensive epidemiological data regarding the clinical characteristics and long-term outcomes of obstructive uropathy in adults. In this study, we conducted a multicenter retrospective analysis of 1683 consecutive patients who underwent PCN for obstructive uropathy in Korea. This study aimed to determine the etiologies, clinical manifestations, outcomes, and impact of superimposed AKI on these outcomes.
Results
Study subjects
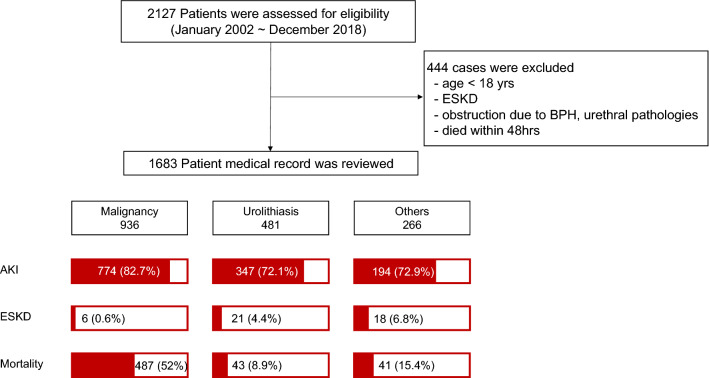
Among the 2127 patients who underwent PCN, 444 who were under 18 years, previously diagnosed with ESKD, died within 48 h of admission or with lower urinary tract obstruction were excluded and 1683 patients were finally analyzed. Multiple episodes of PCN insertion were counted as one. A total of 1683 patients were analyzed (Fig. 1).
Figure 1.
Enrollment and outcomes. Among 2127 patients who underwent PCN for obstructive uropathy between January 1, 2002 and December 31, 2018, in three tertiary hospitals were assessed for the inclusions, patients were excluded and finally 1683 patients were enrolled for assessment. According to PCN divided into three etiologies, the short-term renal outcome, long term renal outcome and all-cause mortality were schematized.
Etiologies of obstructive uropathy in adults
The patients were divided into three groups according to the etiology of obstruction. The most common cause was malignancy (n = 936, 55.6%), followed by urolithiasis (n = 481, 28.6%), and other causes (n = 266, 15.8%) (Table 1). Other causes included ureteral stricture, intra-abdominal abscess, retroperitoneal fibrosis, vesicoureteral reflux, and renal tuberculosis. Among the malignancies, metastatic colorectal cancer (n = 245, 26.2%) was the most common, followed by gastric cancer (n = 188, 20.1%), bladder cancer (n = 143, 15.3%), uterine cervical cancer (n = 97, 10.4%), and prostate cancer (n = 68, 7.3%) (Supplementary Table S1).
Table 1.
Demographic and clinical characteristics of the study cohort according to etiologies.
| Malignancy (N = 936, 55.6%) | Urolithiasis (N = 481, 28.6%) | Others (N = 266, 15.8%) | p-value | ||
|---|---|---|---|---|---|
| Gender (male) | 496 (53%) | 236 (49.1%) | 92 (34.6%)§† | < 0.001 | |
| Age [years] | 62.99 [24, 95] | 62.37 [23, 95] | 59.06 [21, 93]§† | < 0.001 | |
| Comorbidities | |||||
| DM | 224 (23.9%) | 210 (43.7%)* | 98 (36.8%)§ | < 0.001 | |
| HTN | 376 (40.2%) | 296 (61.5%)* | 125 (47%)† | < 0.001 | |
| Chronic liver disease | 79 (8.4%) | 53 (11.0%) | 21 (7.9%) | 0.204 | |
| IHD | 39 (4.2%) | 41 (8.5%)* | 16 (6.0%) | 0.003 | |
| PAOD | 39 (4.2%) | 28 (5.8%) | 14 (5.3%) | 0.320 | |
| Heart failure | 25 (2.7%) | 19 (4.0%) | 14 (5.3%) | 0.098 | |
| Dementia | 13 (1.4%) | 22 (4.6%)* | 6 (2.3%) | 0.001 | |
| Hemiparesia | 6 (0.6%) | 19 (4.0%)* | 3 (1.1%)† | < 0.001 | |
| COPD | 59 (6.3%) | 31 (6.4%) | 11 (4.1%) | 0.350 | |
| CKD | 204 (21.8%) | 110 (22.9%) | 86 (32.3%)§† | 0.002 | |
| Laboratory test | |||||
| Hb [g/dL] | 10.02 [3.40, 16.10] | 11.7 [4.40, 19.10]* | 10.74 [4.50, 16.80]§† | < 0.001 | |
| WBC [× 103/µL] | 8.74 [0.35, 45.50] | 12.56 [0.89, 56.00]* | 10.23 [1.56, 35.55]§† | < 0.001 | |
| Platelet [× 103/µL] | 239 [7.4, 844.0] | 205 [13.0, 649.0]* | 223.5 [17.0, 609.0]† | < 0.001 | |
| Na [mmol/L] | 136.4 ± 4.99 | 137.06 ± 4.82 | 136.6 ± 5.67 | 0.176 | |
| K [mmol/L] | 4.48 [2.02, 9.74] | 4.27 [2.60, 8.39]* | 4.40 [2.67, 7.38] | < 0.001 | |
| Cl [mmol/L] | 101.86 [68.0, 123.0] | 103.40 [73.0, 134.0]* | 103.17 [75.0, 117.4]§ | < 0.001 | |
| Total CO2 [mmol/L] | 22.4 ± 5.05 | 20.98 ± 5.02* | 20.5 ± 5.3§ | < 0.001 | |
| Baseline Cr [mg/dL] | 0.87 ± 0.55 | 0.93 ± 0.64 | 1.06 ± 0.69§ | 0.001 | |
| Baseline eGFR | 88.25 [1.59, 188.96] | 84.08 [7.99, 184.42] | 79.10 [10.98, 144.70]§ | 0.002 | |
| CRP [mg/L] | 66.04 [0.11, 444.57] | 108.41 [0.13, 435.50]* | 81.82 [0.10, 348.68]§† | < 0.001 | |
| Procalcitonin[ng/mL] | 6.03 [0.02, 100.00] | 26.68 [0.04, 200.00]* | 24.65 [0.03, 100.00]§ | < 0.001 | |
| Glucose [mg/dL] | 127.4 [31.0, 581.0] | 150.8 [30.0, 743.0]* | 147.1 [62.0, 824.0]§ | < 0.001 | |
| Protein [g/dL] | 6.59 ± 0.86 | 6.58 ± 0.92 | 6.67 ± 0.95 | 0.326 | |
| Albumin [g/dL] | 3.52 [1.50, 6.00] | 3.58 [1.10, 5.50] | 3.52 [1.80, 4.90] | 0.175 | |
| Uric acid [mg/dL] | 6.3 ± 3.3 | 6.3 ± 3.1 | 6.4 ± 2.9 | 0.994 | |
| AKI | 774 (82.7%) | 347 (72.1%)* | 194 (72.9%)§ | < 0.001 | |
| Stage 1 | 283 (36.6%) | 131 (37.8%) | 76 (39.2%) | ||
| Stage 2 | 176 (22.7%) | 103 (29.7%) | 41 (21.1%) | ||
| Stage 3 | 315 (40.7%) | 113 (32.6%) | 77 (39.7%) | ||
DM diabetes mellitus, HTN hypertension, IHD ischemic heart disease, PAOD peripheral arterial occlusive disease, COPD chronic obstructive pulmonary disease, CKD chronic kidney disease, Hb hemoglobin, eGFR estimated glomerular filtration rate, CRP C-reactive protein, AKI acute kidney injury.
*Malignancy vs. urolithiasis, §malignancy vs. others, †urolithiasis vs. others, p-value < 0.05.
Patients with malignancy-associated obstruction were more likely to be older, male, have lower hemoglobin levels, and have AKI. In contrast, patients with urolithiasis had a higher prevalence of diabetes, hypertension, ischemic heart disease, hemiparesis, and dementia. The prevalence of underlying CKD was the highest in the other causes group, with a significantly lower baseline eGFR. Inflammatory markers, including levels of C-reactive protein (CRP), procalcitonin, and leukocyte counts, were also significantly higher in patients with urolithiasis or other causes, suggesting that these patients might have superimposed urinary tract infection (Table 1).
Obstructive uropathy associated with AKI
Among the 1683 patients who underwent PCN, 1313 (78.0%) initially presented with AKI. A total of 216 patients (16.4%) required temporary dialysis. Patients with AKI were more likely to be older, male, and have preexisting CKD with a significantly lower baseline eGFR, and lower hemoglobin, albumin, and total CO2 levels. However, patients with AKI had a significantly higher leukocyte count, CRP, procalcitonin, and uric acid levels than those without. Furthermore, the percentage of malignancy as the etiology of obstruction was significantly higher in patients with AKI (Table 2).
Table 2.
Demographic and clinical characteristics of the study cohort according to the presence of AKI.
| No AKI (n = 368, 21.9%) | AKI (n = 1315, 78.1%) | p-value | |
|---|---|---|---|
| Gender (Male) | 159 (43.2%) | 665 (50.6%) | 0.013 |
| Age (year) | 58.90 ± 14.82 | 63.17 ± 14.10 | < 0.001 |
| Comorbidities | |||
| DM | 110 (30.2%) | 421 (32.0%) | 0.526 |
| HTN | 158 (42.9%) | 639 (48.6%) | 0.059 |
| Chronic liver disease | 35 (9.5%) | 118 (9.0%) | 0.759 |
| IHD | 16 (4.3%) | 80 (6.1%) | 0.252 |
| PAOD | 17 (4.6%) | 64 (4.9%) | 0.486 |
| HF | 9 (2.4%) | 49 (3.7%) | 0.262 |
| Dementia | 4 (1.1%) | 37 (2.8%) | 0.057 |
| Hemiparesia | 6 (1.6%) | 22 (1.7%) | 0.585 |
| COPD | 16 (4.3%) | 85 (6.5%) | 0.138 |
| Connective tissue disease | 15 (4.1%) | 49 (3.7%) | 0.758 |
| CKD | 37 (10.1%) | 363 (27.6%) | < 0.001 |
| Laboratory test | |||
| Hb [g/dL] | 11.4 ± 2.26 | 10.3 ± 2.23 | < 0.001 |
| WBC [× 103/µL] | 8.55 [0.35, 32.03] | 10.39 [0.46, 46.70] | < 0.001 |
| Platelet [× 103/µL] | 249.1 [9.5, 837.0] | 241.3 [7.4, 844.0] | 0.117 |
| Na [mmol/L] | 138.4 [114.0, 147.0] | 136.2 [107.2, 158.0] | < 0.001 |
| K [mmol/L] | 4.09 [2.02, 6.20] | 4.49 [2.50, 9.74] | < 0.001 |
| Cl [mmol/L] | 103.1 [81.0, 115.0] | 102.3 [68.0, 134.0] | 0.041 |
| Total CO2 [mmol/L] | 24.5 [10.3, 48.1] | 21.3 [4.1, 41.4] | < 0.001 |
| Baseline Cr [mg/dL] | 0.8 [0.17, 5.93] | 1.0 [0.10, 6.40] | < 0.001 |
| Baseline eGFR | 94.73 [9.40, 157.14] | 83.80 [7.99, 188.96] | < 0.001 |
| CRP [mg/L] | 57.25 [0.23, 383.39] | 87.39 [0.10, 444.57] | < 0.001 |
| Procalcitonin [ng/mL] | 7.64 [0.03, 87.90] | 20.64 [0.02, 200.0] | < 0.001 |
| Glucose [mg/dL] | 133.9 ± 64.5 | 138.4 ± 72.5 | 0.379 |
| HbA1c [% of THb] | 6.96 ± 1.75 | 6.57 ± 1.86 | 0.064 |
| Protein [g/dL] | 6.79 [3.30, 9.20] | 6.56 [3.20, 9.70] | < 0.001 |
| Albumin [g/dL] | 3.72 ± 0.59 | 3.49 ± 0.60 | < 0.001 |
| Uric acid [mg/dL] | 4.8 [1.3, 14.8] | 6.7 [1.1, 29.1] | < 0.001 |
| Etiology of obstruction | < 0.001 | ||
| Malignancy | 162 (44.0%) | 774 (58.9%) | |
| Stone | 134 (36.4%) | 347 (26.4%) | |
| Others | 72 (19.3%) | 194 (14.8%) | |
DM diabetes mellitus, HTN hypertension, IHD ischemic heart disease, PAOD peripheral arterial occlusive disease, COPD chronic obstructive pulmonary disease, CKD chronic kidney disease, Hb hemoglobin, eGFR estimated glomerular filtration rate, CRP C-reactive protein, AKI acute kidney injury.
Using the decision tree model, we identified some relevant risk factors for AKI and performed a logistic regression analysis. Preexisting CKD, presence of hyponatremia (serum Na ≤ 135 mEq/L), and higher uric acid level at initial presentation were found to be independent risk factors for AKI in patients with obstructive uropathy (Table 3).
Table 3.
Logistic regression analysis of factors associated with AKI.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age | 1.02 | 1.01–1.03 | < 0.001 | |||
| Sex | 0.76 | 0.60–0.96 | 0.018 | |||
| HTN | 1.22 | 0.97–1.54 | 0.093 | |||
| DM | 1.08 | 0.84–1.39 | 0.567 | |||
| CKD | 3.37 | 2.34–4.85 | < 0.001 | 2.03 | 1.01–4.0 | 0.04 |
| IHD | 1.43 | 0.82–2.47 | 0.21 | |||
| Chronic liver disease | 1.07 | 0.72–1.59 | 0.75 | |||
| Hb | 0.82 | 0.77–0.86 | < 0.001 | |||
| CRP | 1.04 | 1.01–1.07 | 0.02 | 1.68 | 0.96–2.95 | 0.07 |
| Hyponatremia (serum Na ≤ 135 mEq/L) | 2.97 | 2.1–4.12 | < 0.001 | 2.28 | 1.23–4.21 | 0.01 |
| Albumin | 0.52 | 0.41–0.65 | < 0.001 | |||
| Hyperuricemia (serum uric acid ≥ 6.0 mg/dL) | 1.32 | 1.23–1.42 | < 0.001 | 2.15 | 1.21–3.84 | 0.01 |
Multiple logistic regression analysis was conducted after adjustment for sex, age, hypertension, diabetes mellitus, ischemic heart disease, chronic liver disease, chronic kidney disease (eGFR ≤ 60 ml/min/1.73 m2), Hb, hyponatremia (serum Na ≤ 135 mg/d), CRP ≥ 6 mg/dL, albumin, and hyperuricemia (serum uric acid ≥ 6.0 mg/dL).
HTN hypertension, DM diabetes mellitus, CKD chronic kidney disease, IHD ischemic heart disease, Hb hemoglobin, CRP C-reactive protein.
Kidney outcomes of obstructive uropathy
Of the 1313 patients who developed AKI, 746 (56.8%) showed short-term functional recovery within 7 days after PCN, and there was no difference in the recovery rates across the different etiologies of obstruction (Table 4). Logistic regression analysis showed that only the presence of preexisting CKD was an independent factor for the failure of short-term recovery (Supplementary Table S2).
Table 4.
Kidney outcomes of obstructive uropathy.
| Malignancy (N = 936) | Stone (N = 481) | Others (N = 266) | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AKI | 774 (82.3%) | 347 (72.1%) | 194 (72.9%) | 0.001 | ||||||
| AKI Recovery | 419 (54.1%) | 223 (64.3%) | 104 (53.6%) | 0.84 | ||||||
| Progression to ESKD | 6 (0.6%) | 21 (4.4%) | 18 (6.8%) | 0.02 | ||||||
| No AKI (N = 162) | AKI (N = 774) | p-value | No AKI (N = 134) | AKI (N = 347) | p-value | No AKI (N = 72) | AKI (N = 194) | p-value | ||
| 1 (0.6%) | 5 (0.7%) | 0.91 | 0 (0%) | 21 (6.1%) | 0.003 | 2 (2.8%) | 16 (8.2%) | 0.01 | ||
AKI acute kidney injury, ESKD end-stage kidney disease.
Regarding long-term kidney outcomes, 45 patients (2.67%) progressed to ESKD. The progression rates in patients with urolithiasis and other causes were 4.4% and 6.8%, respectively, while that of malignant obstruction was only 0.6% (Table 4). This may be related to a higher cancer-related death rate. Due to huge differences, we separately analyzed the risk factors of ESKD progression between malignant and non-malignant causes of obstruction.
Logistic regression analysis revealed that older age, preexisting CKD, and stage 3 AKI were independent factors leading to the progression to ESKD. The presence of CKD and stage 3 AKI showed a 19-fold and fivefold increased risk of ESKD, respectively (Table 5).
Table 5.
Logistic regression analysis of factors associated with ESKD progression (non-malignant obstruction).
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age | 4.61 | 1.1–19.32 | 0.03 | 5.05 | 2.24–11.35 | < 0.001 |
| Sex | 1.11 | 0.81–1.51 | 0.52 | 0.77 | 0.37–1.6 | 0.48 |
| HTN | 1.03 | 0.75–1.40 | 0.86 | 1.52 | 0.64–3.5 | 0.32 |
| DM | 2.68 | 1.37–5.25 | 0.004 | 1.19 | 0.97–4.09 | 0.11 |
| CKD | 15.16 | 6.57–34.99 | < 0.001 | 19.17 | 7.5–48.99 | < 0.001 |
| AKI stage 3 | 9.17 | 2.05–40.93 | 0.01 | 5.31 | 1.05–26.78 | 0.04 |
Multiple logistic regression analysis was conducted after adjustment for sex, age over 65 years, hypertension, diabetes mellitus, ischemic heart disease, underlying liver disease, chronic kidney disease (eGFR ≤ 60 ml/min/1.73 m2), and AKI stage.
AKI acute kidney injury, HTN hypertension, DM diabetes mellitus, CKD chronic kidney disease.
Mortality of obstructive uropathy
The overall mortality rate during the median follow-up period was 34%, with the highest rate observed in malignancy-associated obstruction (52%), followed by other causes (15.4%) and urolithiasis (8.9%) (p < 0.001). The mortality rate was significantly higher in patients with AKI, regardless of the etiology of the obstruction (Table 6).
Table 6.
Mortality of obstructive uropathy.
| Malignancy (n-936) | Urolithiasis (n = 481) | Others (n = 266) | p-value | ||||
|---|---|---|---|---|---|---|---|
| NonAKI (n = 162) | AKI (n = 774) | NonAKI (n = 134) | AKI (n = 347) | NonAKI (n = 72) | AKI (n = 194) | ||
| Mortality | 70 (43.2%) | 417 (53.9%) | 2 (1.5%) | 41 (11.8%) | 3 (4.2%) | 38 (19.6%) | < 0.001 |
AKI acute kidney injury.
We also separately analyzed malignancy vs. non-malignancy-associated obstructive uropathy to identify independent risk factors for mortality. Cox regression analysis showed that older age (OR, 3.49; 95% CI 1.71–7.1; p < 0.001), male sex (OR, 2.07; 95% CI 1.12–3.83; p = 0.02), lower albumin level (OR, 0.28; 95% CI 0.17–0.46; p < 0.001), and stage 3 AKI (OR, 2.28; 95% CI 1.26–4.14; p = 0.01) were found to be independent predictors of overall mortality in non-malignancy-associated obstructive uropathy (Table 7). The Kaplan–Meier survival curve also showed a stepwise decrease in the cumulative survival as the KDIGO AKI stage increased (79.6% with no AKI, 67.1% with stage 1 AKI, 65% with stage 2 AKI, and 55.8% with stage 3 AKI) (Fig. 2). In contrast, the cumulative survival did not differ significantly according to the KDIGO stage of AKI in patients with malignancy-associated obstructive uropathy. Only low albumin level (OR, 0.62; 95% CI 0.49–0.97; p = 0.03) was found to be an independent factor predicting mortality in these patients (Table 8).
Table 7.
Cox regression analysis of factors associates with mortality (non-malignant obstruction).
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age | 03.02 | 1.86–4.91 | 0.85 | 3.49 | 1.71–7.1 | < 0.001 |
| Sex | 1.81 | 1.15–2.84 | 0.01 | 2.07 | 1.12–3.83 | 0.02 |
| HTN | 1.17 | 0.75–1.86 | 0.50 | |||
| DM | 1.29 | 0.83–2.02 | 0.25 | |||
| CKD | 1.56 | 1.01–2.48 | 0.03 | 1.37 | 0.59–3.18 | 0.47 |
| Hb | 2.5 | 1.56–4.02 | < 0.001 | 0.96 | 0.82–1.11 | 0.57 |
| CRP | 1.00 | 0.92–1.01 | 0.13 | 1.00 | 0.99–1.00 | 0.07 |
| Albumin | 0.38 | 0.27–0.51 | < 0.001 | 0.28 | 0.17–0.46 | < 0.001 |
| Hyperuricemia (serum uric acid ≥ 6.0 mg/dL) | 1.09 | 1.09–3.23 | 0.02 | 1.02 | 0.56–2.14 | 0.79 |
| AKI stage 3 | 2.97 | 1.94–4.57 | < 0.001 | 2.28 | 1.26–4.14 | 0.01 |
Multivariate Cox regression analysis was conducted after adjusting for sex, age over 65 years, hypertension, diabetes mellitus, ischemic heart disease, underlying liver disease, chronic kidney disease (eGFR ≤ 60 ml/min/1.73 m2), KDIGO stage 3 AKI, Hb < 11 g/dL, CRP, albumin, and uric acid.
HTN hypertension, DM diabetes mellitus, CKD chronic kidney disease, Hb hemoglobin, CRP C-reactive protein.
Figure 2.
Cumulative survival of patients with obstructive uropathy according to AKI. The cumulative survival rates varied significantly with the AKI phase in non-malignancy related PCN. However, it was not different in malignancy related PCN.
Table 8.
Cox regression analysis of factors associated with mortality (malignant obstruction).
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age | 1.18 | 0.98–1.41 | 0.08 | 0.99 | 0.98–1.00 | 0.08 |
| Sex | 0.98 | 0.82–1.18 | 0.85 | |||
| HTN | 1.17 | 0.98–1.41 | 0.09 | |||
| DM | 1.01 | 0.82–1.24 | 0.95 | |||
| CKD | 1.20 | 0.96–1.51 | 0.11 | 1.23 | 0.83–1.83 | 0.29 |
| AKI stage 3 | 1.33 | 1.11–1.61 | 0.01 | 1.01 | 0.64–1.75 | 0.83 |
| Albumin | 0.61 | 0.51–0.74 | < 0.001 | 0.62 | 0.49–0.97 | 0.03 |
Multivariate Cox regression analysis was conducted after adjustment for sex, age over 65 years, hypertension, diabetes mellitus, ischemic heart disease, underlying liver disease, chronic kidney disease (eGFR ≤ 60 ml/min/1.73 m2), KDIGO stage 3 AKI, Hb < 11 g/dL, CRP, and albumin.
AKI acute kidney disease, HTN hypertension, DM diabetes mellitus, CKD chronic kidney disease.
Discussion
This multicenter, retrospective study demonstrated that the presence of AKI negatively affects the long-term outcomes of patients with who underwent PCN due to obstruction. The major findings of our study were as follows: (1) the most common cause of obstructive uropathy in adults was malignancy, followed by urolithiasis and other causes; (2) a substantial portion (78%) of patients presented with AKI, concomitant infection and short-term recovery after PCN insertion was achieved in 56.8% of these patients; (3) 4.4% of patients in the urolithiasis group and 6.8% in other causes group progressed to ESKD; (4) the overall mortality rate was 34%, with the highest mortality attributed to malignancy-associated obstruction (52%); and (5) stage 3 AKI was independently associated with both ESKD progression and mortality only in non-malignant causes of obstruction.
Obstruction to urine flow can occur at any site of the urinary tract. Clinical manifestations depend on the etiology, location, and duration of obstruction, as well as the presence of underlying comorbidities; thus, they can vary widely, ranging from asymptomatic cases to renal colic, urosepsis, and dialysis requiring AKI or ESKD. In the pediatric population, obstructive uropathy from congenital anomalies of the urinary tract is responsible for 30–50% of ESKD cases. However, data on the epidemiology of obstructive uropathy in the adult population are limited2.
This study aimed to investigate the clinical characteristics and outcomes of obstructive uropathy. PCN or pyelonephrostomy is an interventional procedure for the decompression of collecting systems and has become the primary option for temporary relief of obstruction in upper urinary tract obstruction since its first introduction in 19557. We first demonstrated that the most common causes of obstructive uropathy in adults undergoing PCN were malignancy (55.6%), followed by urolithiasis (28.7%), and other causes (15.2%). In contrast with a previous study that demonstrated that uterine cervical and bladder cancer are the most common malignancy types, metastatic colorectal and gastric cancers were responsible for 46.3% of cases in our study8. This might be due to the exclusion of patients with urogenital cancer-associated lower tract obstruction, which was likely to be relieved with bladder decompression, or due to geographical differences in prevalent cancer types. Obstructive uropathy from direct tumor invasion or metastatic retroperitoneal lymphadenopathy is usually considered an ominous sign, and as expected, the overall mortality from malignancy-associated obstructive uropathy was higher (52%) than that from urolithiasis or other causes.
Patient characteristics varied significantly according to the different etiologies of obstruction. Patients with malignant obstruction were significantly older, male, had lower hemoglobin levels, and were more likely to be associated with AKI despite a preserved baseline renal function. In contrast, patients with urolithiasis had a higher prevalence of diabetes, hypertension, ischemic heart disease, dementia, or hemiparesis, reflecting the close association between metabolic syndrome and urolithiasis. The prevalence of urolithiasis has been shown to have doubled in the United States in the recent years. Experimental studies have shown that type 2 diabetes and obesity facilitate lithogenesis by altering the proximal tubule handling of sodium and uric acid, and by lowering the urine pH9–11. The association between hypertension and urolithiasis has also been demonstrated in a population-based study12. Our study confirmed the association of urolithiasis not only with diabetes and hypertension but also with various cardiovascular and cerebrovascular diseases. Significantly higher CRP, procalcitonin levels, and leukocyte counts in this group suggested that stone-related obstructive uropathy might be associated with superimposed infection of the urinary tract. In contrast, obstructive uropathy from other causes was characterized by a female predominance, young age, and a higher prevalence of preexisting CKD.
The present study demonstrated that 78.1% of patients initially presented with AKI, suggesting that obstructive uropathy is frequently complicated by AKI. However, given that PCN is usually indicated upon renal functional deterioration or pyonephrosis, it could simply indicate that AKI is one of the most common indication of PCN in obstructive uropathy. In general, obstruction of a single kidney does not lead to AKI if the contralateral kidney function is normal. However, because we did not analyze the dual obstruction, it was difficult to classify the exact causes of AKI as predominantly postrenal or multifactorial. Given that obstructive uropathy is frequently complicated by urinary tract infection, sepsis, or combined volume depletion, the mechanisms underlying AKI development in this setting seem to be more complex. Among the many factors with significant differences, preexisting CKD, hyponatremia (≤ 135 mEq/L), and higher uric acid levels were independently associated with AKI.
Kidney outcomes in obstructive uropathy primarily depend on the prompt relief of obstruction. Therefore, delayed diagnosis due to the absence of specific signs and symptoms or underlying comorbidities might affect recovery. An estimated GFR of ≥ 60 ml/min/1.73 m2, recovery to < 25% of baseline eGFR, or dialysis independence within 7 days after PCN were defined as short-term recovery. Functional recovery was achieved in 56.8% of the patients, and the underlying etiologies of obstruction did not affect the short-term recovery rates. The presence of CKD was an independent factor associated with the non-recovery of short-term renal function. This is comparable with data by Khalaf et al. that preoperative GFR is an independent factor affecting the functional recovery in a prospective study of 91-patients with unilateral obstructive uropathy13. However, the lack of information about the exact duration of obstruction, which is the single-most important factor for recovery, was one of the limitations of our study, resulting in a possibility that failed short-term recovery in 43.2% of patients is related to delayed timing of relief of obstruction. Failure to determine the regression of hydronephrosis was another limitation of this study.
Because only a few studies have determined the long-term outcome of obstructive uropathy in adults, we examined the ESKD progression and mortality. Using data from both the ESKD registry of the Korean Society of Nephrology and the hospitals, we found that 45 (2.67%) patients progressed to ESKD during the median follow-up period of 91 [range 12–219] months. The progression rate in patients with urolithiasis/other causes was 4.4% and 6.8%, respectively, while that from malignancy was only 0.6%. The very low progression rate might be due to the high premature death rate from malignancy-related complications. According to the United States Renal Data System (USRDS), urolithiasis accounts for only 0.2% of the incidents of ESRD14. However, El-Zoghby et al. demonstrated that the attributable risk of ESKD from symptomatic urolithiasis was 5.1% in a population-based study conducted in Olmsted County, Mn, USA, which is comparable with our data15. As patients with urolithiasis were more likely to be diabetic, hypertensive, or have various cardio-cerebro-vascular diseases in our study, the mechanisms underlying the progression of urolithiasis seem to be complex, involving multiple comorbidities, superimposed infection, or obstruction-related mechanisms. We found that older age, pre-existing CKD, and stage 3 AKI were independently associated with ESKD. Although AKI is known to contribute to the increased burden of progressive CKD/ESKD16,17, most of the data came from the study of patients with intrinsic causes of AKI, such as ischemia, sepsis, or nephrotoxicity, but not from obstructive uropathy. Therefore, this is the first study to demonstrate that superimposed AKI also plays an important role in progressive CKD in obstructive uropathy. In contrast, none of these factors, such as stage 3 AKI, affect the progression of malignant obstruction. Stage 3 AKI complicated by obstructive uropathy in our study was independently associated with overall mortality in patients with non-malignant obstruction. Older age, male sex, and hypoalbuminemia were found to predict mortality. However, similar to ESKD progression, none of these factors were associated with mortality in malignant obstruction, in which patients are likely to die from cancer-related complications.
Despite several novel findings, our study had some limitations. This was a retrospective study of patients with predominantly upper urinary tract obstruction who received PCN. Another limitation is the exclusion of common causes of obstructive uropathy, such as benign prostatic hypertrophy. In addition, the complex mechanisms that lead to AKI were not determined. However, to our knowledge, this is the first large-scale clinical study to analyze various etiologies, clinical manifestations, short- and long-term outcomes, and related risk factors in adult obstructive uropathy. With the increase in the aging population with multiple comorbidities, the incidence and outcome of adult obstructive uropathy are likely to change. A better understanding of the epidemiology and prognostic factors is needed for the development of proper management strategies for obstructive uropathy.
Methods
Study subjects
We retrospectively screened the medical records of 2127 patients who underwent PCN for obstructive uropathy between January 1, 2002 and December 31, 2018, in three tertiary hospitals in Korea. Four hundred forty four patients who were under age of 18 years, diagnosed with ESKD or died within 48 h of admission or obstruction due to BPH were excluded. Because lower urinary tract obstruction is frequently relieved by bladder decompression, we excluded those with obstruction due to BPH or other urethral pathologies. The study was approved by the Institutional Review Board of Korea University Anam Hospital (IRB number 2019AN0403) and conducted according to the criteria set by the Declaration of Helsinki. This study was a retrospective medical chart review, so informed consent was waived by Institutional Review Board of Korea University Anam Hospital.
Baseline demographics and clinical characteristics
Demographic features, and clinical and laboratory data of the patients at the time of admission were reviewed. The presence of comorbidities was identified using the International Classification of Disease-9 (ICD-9) code. Estimated GFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, and a prior history of eGFR < 60 ml/min/1.73 m2 for ≥ 3 months was considered as CKD. Baseline kidney function was defined as eGFR determined within 3 months before admission or the highest eGFR recorded during the follow-up period when baseline serum creatinine was not available. AKI was defined and staged according to the Kidney Disease Improving Global Outcomes (KDIGO)18.
Outcomes
Patients were followed up until December 2019. The median follow-up period for mortality and ESKD progression was 83 months (range 0–219 months) and 91 months (range 12–219 months), respectively. Short-term recovery was defined as the recovery of kidney function to either eGFR ≥ 60 ml/min/1.73 m2, ≤ 25% of baseline eGFR, or attainment of dialysis independence within 7 days after PCN insertion. Patients were classified as progressing to ESKD if they needed maintenance hemodialysis, peritoneal dialysis, or kidney transplantation. ESKD data were obtained from the Korean Society of Nephrology ESKD Registry. Mortality data were obtained from hospital records and the National Database of Statistics, Korea.
Statistical analysis
Continuous variables with standard distributions were described as the mean ± standard deviation using Student’s t-test. If the variable distributions were skewed, they were described as medians (minimum–maximum). Categorical variables were analyzed using the Chi-square test. One-way analysis of variance with Bonferroni post-hoc analysis was used to compare two or more groups. A decision tree model was used to identify the risk factors for AKI and performed logistic regression analyses to assess the risk factors for AKI or recovery from AKI. Cox regression analysis was used to explore the risk factors of mortality. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 28.0. Statistical significance was set at p < 0.05.
Supplementary Information
Acknowledgements
This research was supported by a grant from the Korea University Anam Hospital, Seoul, Republic of Korea. (O2000701).
Author contributions
Research idea and study design: B.G.S., J.Y. and S.K.J. Data acquisition: B.G.S., J.Y., H.J.M., Y.B.S., T.B.K., J.H.L., S.W.O., M.G.K., S.Y.A., G.J.K., J.J.C., Y.S.K. Data analysis/interpretation: B.G.S., J.Y., S.W.O., S.Y.A., G.J.K., D.R.C. Statistical analysis: B.G.S., J.Y. Article draft and revision: S.K.J., B.G.S., J.Y. Supervision and mentorship: Y.J.K., D.R.C., W.Y.C. All authors were contributed to developing this manuscript as best as we can.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jihyun Yang and Bong Gyun Sun.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-03033-0.
References
- 1.Wang SJ, Mu XN, Zhang LY, Liu QY, Jin XB. The incidence and clinical features of acute kidney injury secondary to ureteral calculi. Urol. Res. 2012;40:345–348. doi: 10.1007/s00240-011-0414-6. [DOI] [PubMed] [Google Scholar]
- 2.Benfield MR, McDonald RA, Bartosh S, Ho PL, Harmon W. Changing trends in pediatric transplantation: 2001 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr. Transplant. 2003;7:321–335. doi: 10.1034/j.1399-3046.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 3.Selkurt EE, Deetjen P, Brechtelsbauer H. Tubular pressure gradients and filtration dynamics during urinary stop flow in the rat. Pflugers Arch. Gesamte Physiol. Menschen Tiere. 1965;286:19–35. doi: 10.1007/BF00362678. [DOI] [PubMed] [Google Scholar]
- 4.Klahr S, Pukerson ML. The pathophysiology of obstructive nephropathy: The role of vasoactive compounds in the hemodynamic and structural abnormalities of the obstructed kidney. Am. J. Kidney Dis. 1994;23:219–223. doi: 10.1016/S0272-6386(12)80975-9. [DOI] [PubMed] [Google Scholar]
- 5.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am. J. Physiol. Renal Physiol. 2002;283:F861–875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 6.Preminger, G.M. Obstructive uropathy. MSD Manual Professional Version https://www.msdmanuals.com/professional/genitourinary-disorders/obstructive-uropathy/obstructive-uropathy (2020).
- 7.Pabon-Ramos WM, et al. Quality improvement guidelines for percutaneous nephrostomy. J. Vasc. Interv. Radiol. 2016;27:410–414. doi: 10.1016/j.jvir.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 8.Pal DK, Sasmal S. Clinical profile and treatment outcome of obstructive uropathy in a tertiary care centre. ARC J. Urol. 2017;2:5–10. [Google Scholar]
- 9.Scales, C.D., Jr., Smith, A.C., Hanley, J.M., Saigal, C.S., & Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur. Urol.62, 160–165 (2012). [DOI] [PMC free article] [PubMed]
- 10.Poore W, et al. Obesity AND ITS IMPACT ON KIDNEY STONE FORMATION. Rev. Urol. 2020;22:17–23. [PMC free article] [PubMed] [Google Scholar]
- 11.Wong, Y.V., Cook, P. & Somani, B.K. The association of metabolic syndrome and urolithiasis. Int. J, Endocrinol.2015, 570674 (2015). [DOI] [PMC free article] [PubMed]
- 12.Cappuccio FP, Strazzullo P, Mancini M. Kidney stones and hypertension: Population based study of an independent clinical association. BMJ. 1990;300:1234–1236. doi: 10.1136/bmj.300.6734.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalaf IM, et al. Recoverability of renal function oafter treatment of adult patients with unilateral obstructive uropathy and normal contralateral kidney: a prospective study. Urology. 2004;64:664–668. doi: 10.1016/j.urology.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Collins AJ, et al. US renal data system 2010 annual data report. Am. J. Kidney Dis. 2011;57(A8):e1–526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 15.El-Zoghby ZM, et al. Urolithiasis and the risk of ESRD. Clin. J. Am. Soc. Nephrol. 2012;7:1409–1415. doi: 10.2215/CJN.03210312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanoff S, Okusa MD. Impact of acute kidney injury on chronic kidney disease and its progression. Contrib. Nephrol. 2011;171:213–217. doi: 10.1159/000327332. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CY. Linking the population epidemiology of acute renal failure, chronic kidney disease and end-stage renal disease. Curr. Opin. Nephrol. Hypertens. 2007;16:221–226. doi: 10.1097/MNH.0b013e3280895ad9. [DOI] [PubMed] [Google Scholar]
- 18.Palevsky PM, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 2013;61:649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.