Abstract
It has been discovered that both inflammation and platelet aggregation could cause crucial effect on the occurrence and development of cardiovascular diseases. As a combination of platelet and lymphocyte, platelet-lymphocyte ratio (PLR) was proved to be correlated with the severity as well as prognosis of cardiovascular diseases. Exploring the relationship between PLR and in-hospital mortality in cardiac intensive care unit (CICU) patients was the purpose of this study. PLR was calculated by dividing platelet count by lymphocyte count. All patients were grouped by PLR quartiles and the primary outcome was in-hospital mortality. The independent effect of PLR was determined by binary logistic regression analysis. The curve in line with overall trend was drawn by local weighted regression (Lowess). Subgroup analysis was used to determine the relationship between PLR and in-hospital mortality in different subgroups. We included 5577 CICU patients. As PLR quartiles increased, in-hospital mortality increased significantly (Quartile 4 vs. Quartile 1: 13.9 vs. 8.3, P < 0.001). After adjusting for confounding variables, PLR was proved to be independently associated with increased risk of in-hospital mortality (Quartile 4 vs. Quartile 1: OR 95% CI 1.55, 1.08–2.21, P = 0.016, P for trend < 0.001). The Lowess curves showed a positive relationship between PLR and in-hospital mortality. The subgroup analysis revealed that patients with low Acute Physiology and Chronic Health Evaluation IV (APACHE IV) or with less comorbidities had higher risk of mortality for PLR. Further, PLR quartiles had positive relation with length of CICU stay (Quartile 4 vs. Quartile 1: 2.7, 1.6–5.2 vs. 2.1, 1.3–3.9, P < 0.001), and the length of hospital stay (Quartile 4 vs. Quartile 1: 7.9, 4.6–13.1 vs. 5.8, 3.3–9.8, P < 0.001). PLR was independently associated with in-hospital mortality in CICU patients.
Subject terms: Biomarkers, Diseases, Risk factors
Introduction
The concept of the Coronary Artery Care Unit (CCU) was first proposed in the early 1960s and quickly gained widespread support, which benefitted from rapid technological advances in cardiovascular medicine1. At the beginning, the primary purpose of these specialized wards which developer in coronary care established was to reduce mortality in patients with acute myocardial infarction (MI)2. Nevertheless, over the past few decades, the CCU's landscape has changed. Mortality of acute coronary syndromes decreased steadily over time3,4, while the occurrence of other severe cardiovascular diseases appeared to increase5. Because of the greater diversity of diseases among patients admitted to CCU units, the concept of cardiac intensive care unit (CICU) had been used to represent this complex care environment more accurately. It has emerged to provide more targeted services for patients with critical heart diseases at present6. Nowadays, CICU has taken on a more important position. And easily accessible prognostic indicators for CICU patients are always welcomed by clinicians.
Previous studies have demonstrated that increased peripheral blood platelet count caused the rise of adverse cardiovascular outcomes, in patients with acute myocardial infarction (AMI), higher platelet count was proved to be associated with mortality and reinfarction within the first year after primary percutaneous intervention (PCI)7–10. Low amount of peripheral blood lymphocyte count which reflects inflammatory state, was also confirmed to increase adverse clinical outcomes in patients with cardiovascular diseases, such as congestive heart failure, advanced heart failure, coronary artery disease and unstable angina pectoris11–15. As a new prognostic marker, platelet-lymphocyte ratio (PLR) was the combination of the two indexes which provides the concept of platelet aggregation and inflammatory pathways. In clinical practice, elevated PLR was shown to be associated with adverse outcomes. In the area of non-cardiovascular disease, PLR was proved to be an important inflammatory marker that predicted mortality in cancer population16–18, critical limb ischemia in peripheral artery disease19 and neonate early-onset sepsis20. In cardiovascular disease, PLR was proved to be positively correlated with the occurrence of no-reflow after PCI21. Moreover, PLR has been proven to be independently associated with long-range survival rate in patients with STEMI and NSTEMI respectively22,23. However, no research has demonstrated that how PLR affects patients with severe cardiovascular disease. Thus, investigating the relationship between PLR and in-hospital mortality of patients in CICU was the target of this research.
Method
Population selection criteria
Patients admitted to CICU were included. Adult patients (≥ 18 years) hospitalized for more than 2 days at their first admission were available. Patients meeting the following criteria were excluded: (1) hospital admission for non-heart disease; (2) lymphocyte and platelet data missing; (3) individual data missing ≥ 5%; (4) hematologic malignancy such as: leukemia and lymphoma. A total of 5577 patients were included (Fig. 1).
Figure 1.

Flow chart of study population. CICU: cardiac intensive care unit.
Data extraction
The data used in this study was from eICU Collaborative Research Database24, which collected information on 20,859 admissions from 208 hospitals in the United States between 2014 and 2015. The eICU database was made available by Philips Healthcare in partnership with the MIT Laboratory for Computational Physiology. This database is available at: https://doi.org/10.13026/C2WM1R. And the author was approved to access to the database through Protecting Human Research Participants exam (certificate number: 9728458).
Following data were collected: demographics (age, gender and race), vital signs (blood pressure, heart rate, respiration rate, oxygen saturation), body mass index, diagnoses and comorbidities (congestive heart failure, coronary artery disease, acute coronary syndrome, ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI), arrhythmias, cardiac arrest, bradycardia, atrial fibrillation, ventricular arrhythmias, atrioventricular block, cardiomyopathy, valve disease, shock, pulmonary embolism, pulmonary hypertension, hypertension, diabetes, hypercholesterolemia, chronic obstructive pulmonary disease (COPD), respiratory failure, chronic kidney disease, acute kidney injury, malignancy, stroke, sepsis), laboratory parameters (white blood cell, lymphocyte, monocyte and neutrophil percentage, red blood cell platelet, hemoglobin, hematocrit, glucose, creatinine, blood nitrogen urea, sodium, potassium, neutrophil to lymphocyte ratio (NLR)), and medication use (antiplatelet, oral anticoagulants, beta-blockers, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB), statin), transfusion (including red blood cell, plasma, platelet), acute physiology score (APS) and Acute Physiology and Chronic Health Evaluation IV (APACHE IV)25.
PLR was obtained by dividing platelet count by lymphocyte count. NLR was obtained by dividing neutrophil percentage by lymphocyte percentage. All of the hematological parameters were obtained by the first blood test after admission to CICU within 48 h and measured at the same time.
Grouping and outcomes
According to PLR quartiles, all enrolled patients were divided into four groups. The primary outcome was in-hospital mortality. Secondary outcomes were length of CICU stay and length of hospital stay.
Statistical analysis
Normally distributed continuous variables were expressed as mean ± standard deviation (SD) and compared between groups using analysis of variance. Skewed data were expressed as median and interquartile range (IQR) and compared using Kruskal–Wallis test. Categorical variables were expressed as number (percentage) and compared between groups using Chi-square test.
The relationship between PLR and in-hospital mortality was identified by binary logistic regression analysis and the results were expressed as odds ratio (OR) and 95% confidence interval (CI). P for trend was calculated. Covariates were selected by statistical analysis and clinical doubt to modulate the outcome. The curves that conformed to the general trend were plotted through local weighted regression (Lowess). Subgroup analysis was used to determine the relationship between PLR and in-hospital mortality in different subgroups, P for interaction was calculated. All tests were two-sided, P < 0.05 was considered statistically significant. All methods were carried out in accordance with relevant guidelines and regulations. All data analysis were performed by Stata V.15.1.
Ethical approval
The eICU database was made available by Philips Healthcare in partnership with the MIT Laboratory for Computational Physiology. This study was exempted from institutional review Board approval for the following reasons: (1) retrospective design, which was lack of direct patient intervention; (2) Private certification of reidentification risk conforming to safe harbor standards for security protocols (Cambridge, MA) (HIPAA Certification no. 1031219-2).
Method statement
All methods were carried out in accordance with relevant guidelines and regulations.
Result
Subjects and baseline characteristics
5577 patients admitted to CICU were analyzed (Fig. 1). According to PLR quartiles, all patients were divided into four groups: PLR < 104.8 (n = 1392), 104.8 ≤ PLR < 167.0 (n = 1399), 167.0 ≤ PLR < 271.0 (n = 1392), PLR ≥ 271.0 (n = 1394). The characteristics of different PLR groups were summarized in Table 1, patients with higher PLR had the following characteristics: elder, male, Caucasian, lower systolic, mean and diastolic blood pressure, lower oxygen saturation, and higher heart rate, respiration rate, body mass index. Patients in higher PLR quartiles also tended to present more diagnoses and comorbidities of congestive heart failure, arrhythmias, atrial fibrillation, valve disease, shock, COPD, respiratory failure, chronic kidney disease, acute kidney injury, malignancy, sepsis and less coronary artery disease, acute coronary syndrome, STEMI, NSTEMI, cardiac arrest, ventricular arrhythmias, atrioventricular block, hypertension. Moreover, patients in higher PLR quartiles presented higher white blood cell, neutrophil percentage, platelet, glucose, creatinine, blood nitrogen urea, potassium values and lower lymphocyte, monocyte percentage, red blood cell, hemoglobin, hematocrit, sodium values. Patients in higher PLR quartiles received more oral anticoagulant and less antiplatelet, ACEI/ARB, Beta-blockers, statin treatment. Last but not the least, patients in the highest PLR quartile had highest APS and APACHE IV scores which were used to evaluate the severity of ICU patients and predict their prognosis.
Table 1.
Characteristics of patients stratified by PLR quartiles.
| Characteristics | Total (n = 5577) | Quartiles of PLR | P Value | |||
|---|---|---|---|---|---|---|
| Quartile 1 (n = 1392) PLR < 104.8 | Quartile 2 (n = 1399) 104.8 ≤ PLR < 167.0 | Quartile 3 (n = 1392) 167.0 ≤ PLR < 271.0 | Quartile 4 (n = 1394) PLR ≥ 271.0 | |||
| Age (years) | 66.1 ± 15.3 | 63.2 ± 15.3 | 66.0 ± 15.3 | 67.1 ± 15.2 | 68.3 ± 15.0 | < 0.001 |
| Gender, n (%) | 0.002 | |||||
| Male | 3027 (54.3) | 817 (58.7) | 739 (52.8) | 723 (51.9) | 748 (53.7) | |
| Female | 2550 (45.7) | 575 (41.3) | 660 (47.2) | 669 (48.1) | 646 (46.3) | |
| Ethnicity, n (%) | < 0.001 | |||||
| Caucasian | 3958 (71.0) | 937 (67.3) | 952 (68.1) | 999 (71.8) | 1070 (76.8) | |
| African American | 914 (16.4) | 272 (19.5) | 241 (17.2) | 233 (16.7) | 168 (12.0) | |
| Other | 705 (12.6) | 183 (13.2) | 206 (14.7) | 160 (11.5) | 156 (11.2) | |
| Vital signs | ||||||
| Systolic blood pressure (mmHg) | 122.6 ± 19.0 | 123.2 ± 19.1 | 123.5 ± 19.7 | 123.7 ± 19.2 | 120.1 ± 17.9 | < 0.001 |
| Diastolic blood pressure (mmHg) | 66.0 ± 11.3 | 67.3 ± 11.4 | 66.7 ± 11.6 | 65.7 ± 11.2 | 64.2 ± 10.7 | < 0.001 |
| Mean blood pressure (mmHg) | 82.2 ± 12.9 | 83.6 ± 12.9 | 82.8 ± 13.1 | 82.1 ± 13.2 | 80.1 ± 12.0 | < 0.001 |
| Heart rate (beats/min) | 89.2 ± 22.3 | 85.7 ± 21.8 | 86.6 ± 21.5 | 90.5 ± 22.8 | 94.0 ± 22.1 | < 0.001 |
| Respiration rate (beats/min) | 21.1 ± 6.3 | 20.0 ± 6.0 | 20.8 ± 6.0 | 21.2 ± 6.2 | 22.4 ± 6.8 | < 0.001 |
| Oxygen saturation (%) | 97 (95, 99) | 98 (96, 100) | 97 (95, 99) | 97 (95, 99) | 97 (94, 99) | < 0.001 |
| Body mass index (kg/m2) | 29.3 ± 8.3 | 29.9 ± 7.9 | 29.5 ± 8.1 | 29.6 ± 8.9 | 28.1 ± 8.3 | < 0.001 |
| Diagnoses and comorbidities, n (%) | ||||||
| Congestive heart failure | 1256 (22.5) | 261 (18.8) | 308 (22.0) | 343 (24.6) | 344 (24.7) | < 0.001 |
| Coronary artery disease | 1853 (33.2) | 551 (39.6) | 539 (38.5) | 407 (29.2) | 356 (25.5) | < 0.001 |
| Acute coronary syndrome | 1085 (19.5) | 362 (26.0) | 316 (22.6) | 232 (16.7) | 175 (12.6) | < 0.001 |
| STEMI | 384 (6.9) | 149 (10.7) | 115 (8.2) | 73 (5.2) | 47 (3.4) | < 0.001 |
| NSTEMI | 403 (7.2) | 110 (7.9) | 120 (8.6) | 89 (6.4) | 84 (6.0) | 0.027 |
| Arrhythmias | 1865 (33.4) | 411 (29.5) | 444 (31.7) | 503 (36.1) | 507 (36.4) | < 0.001 |
| Cardiac arrest | 407 (7.3) | 153 (11.0) | 88 (6.3) | 93 (6.7) | 73 (5.2) | < 0.001 |
| Bradycardia | 224 (4.0) | 64 (4.6) | 59 (4.2) | 61 (4.4) | 40 (2.9) | 0.086 |
| Atrial fibrillation | 1117 (20.0) | 208 (14.9) | 259 (18.5) | 320 (23.0) | 330 (23.7) | < 0.001 |
| Ventricular arrhythmias | 241 (4.3) | 82 (5.9) | 62 (4.4) | 58 (4.2) | 39 (2.8) | 0.001 |
| Atrioventricular block | 119 (2.1) | 47 (3.4) | 31 (2.2) | 23 (1.7) | 18 (1.3) | 0.001 |
| Cardiomyopathy | 365 (6.5) | 95 (6.8) | 100 (7.2) | 96 (6.9) | 74 (5.3) | 0.189 |
| Valve disease | 164 (2.9) | 34 (2.4) | 38 (2.7) | 42 (3.0) | 50 (3.6) | 0.318 |
| Shock | 1718 (30.8) | 331 (23.8) | 351 (25.1) | 425 (30.5) | 611 (43.8) | < 0.001 |
| Pulmonary embolism | 129 (2.3) | 39 (2.8) | 30 (2.1) | 31 (2.2) | 29 (2.1) | 0.567 |
| Pulmonary hypertension | 69 (1.2) | 13 (0.9) | 20 (1.4) | 17 (1.2) | 19 (1.4) | 0.647 |
| Hypertension | 1689 (30.3) | 407 (29.2) | 458 (32.7) | 441 (31.7) | 383 (27.5) | 0.011 |
| Diabetes | 1133 (20.3) | 257 (18.5) | 302 (21.6) | 297 (21.3) | 277 (19.9) | 0.144 |
| COPD | 633 (11.4) | 105 (7.5) | 144 (10.3) | 170 (12.2) | 214 (15.4) | < 0.001 |
| Respiratory failure | 1669 (300) | 365 (26.2) | 360 (25.7) | 416 (29.9) | 528 (37.9) | < 0.001 |
| Chronic kidney disease | 856 (15.4) | 163 (11.7) | 203 (14.5) | 236 (17.0) | 254 (18.2) | < 0.001 |
| Acute kidney injury | 1031 (18.5) | 216 (15.5) | 226 (16.2) | 276 (19.8) | 313 (22.5) | < 0.001 |
| Malignancy | 272 (4.9) | 38 (2.7) | 53 (3.8) | 67 (4.8) | 114 (8.2) | < 0.001 |
| Stroke | 223 (4.0) | 58 (4.2) | 71 (5.1) | 48 (3.5) | 46 (3.3) | 0.066 |
| Sepsis | 1301 (23.3) | 207 (14.9) | 236 (16.9) | 316 (22.7) | 542 (38.9) | < 0.001 |
| Laboratory parameters | ||||||
| White blood cell (109/L) | 11.8 ± 6.5 | 12.0 ± 7.6 | 11.1 ± 5.7 | 11.7 ± 6.1 | 12.3 ± 6.4 | < 0.001 |
| Lymphocyte (109/L) | 1.6 ± 1.3 | 2.9 ± 1.7 | 1.6 ± 0.6 | 1.2 ± 0.5 | 0.7 ± 0.3 | < 0.001 |
| Monocyte percentage (%) | 7.5 ± 3.7 | 7.8 ± 3.2 | 8.2 ± 3.4 | 7.6 ± 3.5 | 6.6 ± 4.3 | < 0.001 |
| Neutrophil percentage (%) | 74.7 ± 12.6 | 64.4 ± 13.2 | 72.5 ± 9.8 | 78.2 ± 8.5 | 83.7 ± 9.6 | < 0.001 |
| Red blood cell (109/L) | 4.1 ± 0.8 | 4.2 ± 0.9 | 4.1 ± 0.8 | 4.0 ± 0.8 | 4.0 ± 0.8 | < 0.001 |
| Platelet (109/L) | 231 ± 102 | 187 ± 81 | 214 ± 74 | 242 ± 93 | 282 ± 125 | < 0.001 |
| Hemoglobin (g/dL) | 12.0 ± 2.5 | 12.7 ± 2.5 | 12.2 ± 2.4 | 11.8 ± 2.4 | 11.2 ± 2.4 | < 0.001 |
| Hematocrit (%) | 36.4 ± 7.1 | 38.3 ± 7.2 | 37.0 ± 6.8 | 36.0 ± 7.0 | 34.4 ± 6.8 | < 0.001 |
| Glucose (mg/dL) | 162.4 ± 98.3 | 167.7 ± 98.5 | 156.3 ± 92.3 | 161.1 ± 95.1 | 164.9 ± 106.4 | 0.014 |
| Creatinine (mg/dL) | 1.80 ± 1.76 | 1.65 ± 1.62 | 1.72 ± 1.71 | 1.90 ± 1.89 | 1.93 ± 1.80 | < 0.001 |
| Blood nitrogen urea (mg/dL) | 30.1 ± 23.4 | 25.7 ± 19.4 | 28.0 ± 19.8 | 32.0 ± 24.9 | 34.8 ± 27.3 | < 0.001 |
| Sodium (mmol/L) | 136.9 ± 6.0 | 137.8 ± 5.3 | 137.4 ± 5.9 | 136.8 ± 6.3 | 135.8 ± 6.4 | < 0.001 |
| Potassium (mmol/L) | 4.2 ± 0.8 | 4.2 ± 0.7 | 4.2 ± 0.8 | 4.2 ± 0.8 | 4.3 ± 0 | < 0.001 |
| PLR | 167.0 (104.8, 271.0) | 76.4 (57.0, 90.7) | 134.2 (119.3, 148.7) | 208.6 (185.9, 236.6) | 416.5 (326.8, 584.0) | < 0.001 |
| NLR | 6.0 (3.3, 11.7) | 2.6 (1.7, 4.0) | 4.7 (3.1, 7.2) | 7.5 (5.1, 11.3) | 15.6 (9.7, 25.5) | < 0.001 |
| Medication use, n (%) | ||||||
| Antiplatelet | 2659 (47.7) | 743 (53.4) | 711 (50.8) | 635 (45.6) | 570 (40.9) | < 0.001 |
| Oral anticoagulants | 687 (12.3) | 141 (10.1) | 167 (11.9) | 186 (13.4) | 193 (13.9) | 0.013 |
| Beta-blockers | 2446 (43.9) | 647 (46.5) | 651 (46.5) | 591 (42.5) | 557 (40.0) | 0.001 |
| ACEI/ARB | 1490 (26.7) | 387 (27.8) | 407 (29.1) | 385 (27.7) | 311 (22.3) | < 0.001 |
| Statin | 1717 (30.8) | 487 (35.0) | 460 (32.9) | 399 (28.7) | 371 (26.6) | < 0.001 |
| Transfusion | 105 (1.9) | 23 (1.7) | 25 (1.8) | 20 (1.4) | 37 (2.7) | 0.091 |
| APS | 41 (28, 57) | 36 (25, 56) | 38 (26, 53) | 42 (30, 56) | 46 (34, 61) | < 0.001 |
| APACHE IV | 55 (40, 72) | 50 (35, 70) | 52 (38, 67) | 57 (42, 72) | 61 (47, 77) | < 0.001 |
Continuous variables were presented as mean ± SD or median (IQR). Categorical variables were presented as number (percentage).
PLR platelet-lymphocyte ratio, STEMI ST-elevation myocardial infarction, NSTEMI non-ST-elevation myocardial infarction, COPD chronic obstructive pulmonary disease, PLR platelet-lymphocyte ratio, NLR neutrophil to lymphocyte ratio, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, APS acute physiology score, APACHE IV Acute Physiology and Chronic Health Evaluation IV.
Association between PLR and outcomes
Overall, in-hospital mortality rate was 10.7%. As PLR quartiles increased, in-hospital mortality increased significantly (Quartile 4 vs. Quartile 1: 13.9 vs. 8.3, P < 0.001) (Table 2). In unadjusted logistic regression analysis, there was a positive correlation between PLR and in-hospital mortality (Quartile 4 vs. Quartile 1: OR, 95% CI: 1.77, 1.39–2.25, P < 0.001, P for trend < 0.001). In model 2, after adjusting for age, gender and ethnicity, higher PLR quartiles were still associated with increased risk of in-hospital mortality (Quartile 4 vs. Quartile 1: OR, 95% CI: 1.63, 1.28–2.09, P < 0.001, P for trend < 0.001). In model 3, adjusted for more confounding variables, PLR was still independently related to the increased risk of in-hospital mortality (Quartile 4 vs. Quartile 1: OR, 95% CI: 1.55, 1.08–2.21, P = 0.016, P for trend < 0.001). (Table 3).
Table 2.
Outcomes of patients stratified by PLR quartiles.
| Outcomes | Total (n = 5577) | Quartiles of PLR | P Value | |||
|---|---|---|---|---|---|---|
| Quartile 1 (n = 1392) PLR < 104.8 | Quartile 2 (n = 1399) 104.8 ≤ PLR < 167.0 | Quartile 3 (n = 1392) 167.0 ≤ PLR < 271.0 | Quartile 4 (n = 1394) PLR ≥ 271.0 | |||
| In-hospital mortality, n (%) | 597 (10.7) | 116 (8.3) | 120 (8.6) | 168 (12.1) | 193 (13.9) | < 0.001 |
| Length of CICU stay (days) | 2.3 (1.4, 4.2) | 2.1 (1.3, 3.9) | 2.2 (1.3, 3.9) | 2.5 (1.4, 4.4) | 2.7 (1.6, 5.2) | < 0.001 |
| Length of hospital stay (days) | 6.3 (3.9, 11.1) | 5.8 (3.3, 9.8) | 5.8 (3.5, 10.3) | 6.4 (4.0, 11.1) | 7.9 (4.6, 13.1) | < 0.001 |
Continuous variables were presented as median (IQR). Categorical variables were presented as number (percentage). P values were calculated using Kruskal–Wallis test or Chi-square test to compare differences in outcomes between different PLR quartiles.
PLR platelet-lymphocyte ratio, CICU cardiac intensive care unit.
Table 3.
The association between PLR and in-hospital mortality.
| OR (95% CI) | P Value | P for trend | OR (95% CI) | P Value | P for trend | ||
|---|---|---|---|---|---|---|---|
| Model 1 | < 0.001 | Model 1 | < 0.001 | ||||
| Quartile 1: PLR < 104.8 | Reference | Quintile 1: PLR < 95.5 | Reference | ||||
| Quartile 2:104.8 ≤ PLR < 167.0 | 1.03 (0.80–1.35) | 0.817 | Quintile 2: 95.5 ≤ PLR < 139.0 | 0.86 (0.64–1.16) | 0.320 | ||
| Quartile 3:167.0 ≤ PLR < 271.0 | 1.51 (1.18–1.94) | 0.001 | Quintile 3:139.0 ≤ PLR < 198.9 | 1.11 (0.84–1.47) | 0.466 | ||
| Quartile 4: PLR ≥ 271.0 | 1.77 (1.39–2.25) | < 0.001 | Quintile 4:198.9 ≤ PLR < 314.7 | 1.35 (1.02–1.77) | 0.033 | ||
| Quintile 5: PLR ≥ 314.7 | 1.74 (1.34–2.27) | < 0.001 | |||||
| Model 2 | < 0.001 | Model 2 | < 0.001 | ||||
| Quartile 1: PLR < 104.8 | Reference | Quintile 1: PLR < 95.5 | Reference | ||||
| Quartile 2:104.8 ≤ PLR < 167.0 | 1.00 (0.76–1.31) | 0.999 | Quintile 2: 95.5 ≤ PLR < 139.0 | 0.85 (0.63–1.15) | 0.285 | ||
| Quartile 3:167.0 ≤ PLR < 271.0 | 1.43 (1.11–1.84) | 0.005 | Quintile 3:139.0 ≤ PLR < 198.9 | 1.06 (0.80–1.41) | 0.677 | ||
| Quartile 4: PLR ≥ 271.0 | 1.63 (1.28–2.09) | < 0.001 | Quintile 4:198.9 ≤ PLR < 314.7 | 1.27 (0.97–1.68) | 0.086 | ||
| Quintile 5: PLR ≥ 314.7 | 1.60 (1.23–2.09) | 0.001 | |||||
| Model 3 | < 0.001 | Model 3 | < 0.001 | ||||
| Quartile 1: PLR < 104.8 | Reference | Quintile 1: PLR < 95.5 | Reference | ||||
| Quartile 2:104.8 ≤ PLR < 167.0 | 1.49 (1.07–2.07) | 0.017 | Quintile 2: 95.5 ≤ PLR < 139.0 | 1.26 (0.87–1.82) | 0.214 | ||
| Quartile 3:167.0 ≤ PLR < 271.0 | 1.99 (1.45–2.73) | < 0.001 | Quintile 3:139.0 ≤ PLR < 198.9 | 1.70 (1.19–2.42) | 0.003 | ||
| Quartile 4: PLR ≥ 271.0 | 1.55 (1.08–2.21) | 0.016 | Quintile 4:198.9 ≤ PLR < 314.7 | 1.62 (1.15–2.30) | 0.006 | ||
| Quintile 5: PLR ≥ 314.7 | 1.47 (1.00–2.17) | 0.052 | |||||
Models were derived from binary logistic regression analysis. P for trend was calculated using binary logistic analysis to determine whether there was a trend when PLR was included as a grouping variable in the model (Quartile 1–4 or Quintile1-5). When PLR was included as a grouping variable in the model, P values were calculated using binary logistic analysis to determine whether there was a relationship between PLR quartiles (quintiles) and in-hospital mortality with Quartile1 (Quintile 1) serving as the reference group. When PLR was included as a continuous variable in the model, P values were calculated using binary logistic analysis to determine whether there was a relationship between PLR and in-hospital mortality. Model 1: unadjusted. Model 2: adjusted for age, gender, ethnicity. Model 3: adjusted for age, gender, ethnicity, systolic blood pressure, diastolic blood pressure, mean blood pressure, heart rate, body mass index, respiration, coronary artery disease, acute coronary syndrome, congestive heart failure, NSTEMI, cardiac arrest, arrhythmias, atrial fibrillation, ventricular arrhythmias, atrioventricular block, respiratory failure, stroke, malignancy, cardiomyopathy, hypertension, diabetes, white blood cell, red blood cell, hematocrit, blood nitrogen urea, creatinine, sodium, potassium, oral anticoagulants, ACEI/ARB, beta-blockers, statin, transfusion, NLR, APS and APACHE IV.
PLR platelet-lymphocyte ratio, NLR neutrophil–lymphocyte ratio, OR odds ratio, CI confidence interval.
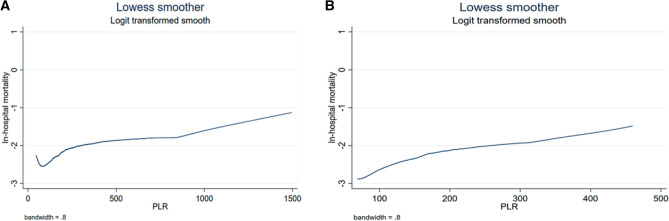
From Lowess curve in Fig. 2A, we found that mortality was lowest when PLR was about equal to 60. Specifically, when PLR was less than 60, PLR was inversely associated with mortality, while when the PLR was greater than 60, in-hospital mortality increased with the increase of the PLR. Besides, as shown in Fig. 2B, as PLR increased, in-hospital mortality increased with a range of 10 to 90 percent of PLR.
Figure 2.
(A) Association between PLR and in-hospital mortality presented through Lowess smoothing. (B) Association between a range of 10 to 90 percent of PLR and in-hospital mortality presented through Lowess smoothing. Abbreviation: PLR: platelet-lymphocyte ratio.
In addition, increased PLR quartiles were associated with prolonged length of CICU stay (Quartile 4 vs. Quartile 1: 2.7, 1.6–5.2 vs. 2.1, 1.3–3.9, P < 0.001) and hospital stay (Quartile 4 vs. Quartile 1: 7.9, 4.6–13.1 vs. 5.8, 3.3–9.8, P < 0.001) (Table 2).
Subgroup analysis
No significant interactions were observed in most subgroups. The risk of in-hospital mortality decreased in patients with higher heart rate. Patients with more comorbidities such as coronary artery disease, acute coronary syndrome, hypertension had higher risk of in-hospital mortality for PLR. But patients with cardiac arrest, shock, acute kidney injury had lower risk of in-hospital mortality. Increased risk of in-hospital mortality for PLR was also confirmed in patients with high red blood cell and low glucose, APS, APACHE IV. Besides, patients who received antiplatelet, oral anticoagulants, beta-blocker, ACEI/ARB therapy had higher risk of mortality for PLR (Table 4).
Table 4.
Subgroup analysis of associations between in-hospital mortality and PLR.
| Subgroups | N | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for interaction |
|---|---|---|---|---|---|---|
| Age (years) | 0.932 | |||||
| < 67 | 2687 | Reference | 0.96 (0.64–1.45) | 1.47 (1.00–2.15) | 1.78 (1.23–2.60) | |
| ≥ 67 | 2890 | Reference | 1.02 (0.72–1.46) | 1.43 (1.03–2.00) | 1.60 (1.16–2.22) | |
| Gender | 0.519 | |||||
| Male | 3027 | Reference | 1.12 (0.79–1.59) | 1.59 (1.14–2.21) | 1.94 (1.41–2.67) | |
| Female | 2550 | Reference | 0.94 (0.62–1.41) | 1.43 (0.98–2.09) | 1.57 (1.08–2.29) | |
| Ethnicity | 0.677 | |||||
| Caucasian | 3958 | Reference | 1.11 (0.80–1.52) | 1.47 (1.09–1.98) | 1.84 (1.38–2.45) | |
| African American | 914 | Reference | 0.64 (0.34–1.19) | 1.58 (0.94–2.66) | 1.60 (0.91–2.82) | |
| Other | 705 | Reference | 1.72 (0.71–4.15) | 1.77 (0.71–4.45) | 1.66 (0.65–4.24) | |
| Body mass index (kg/m2) | 0.447 | |||||
| < 27.9 | 2793 | Reference | 1.43 (0.97–2.12) | 1.76 (1.21–2.57) | 1.95 (1.36–2.81) | |
| ≥ 27.9 | 2784 | Reference | 0.76 (0.53–1.11) | 1.35 (0.96–1.88) | 1.69 (1.21–2.36) | |
| Systolic blood pressure (mmHg) | 0.257 | |||||
| < 120 | 2734 | Reference | 0.99 (0.71–1.38) | 1.53 (1.12–2.10) | 1.54 (1.14–2.08) | |
| ≥ 120 | 2843 | Reference | 1.11 (0.71–1.74) | 1.52 (1.00–2.32) | 1.96 (1.29–2.99) | |
| Diastolic blood pressure (mmHg) | 0.496 | |||||
| < 65 | 2768 | Reference | 0.95 (0.68–1.34) | 1.32 (0.96–1.80) | 1.53 (1.13–2.07) | |
| ≥ 65 | 2809 | Reference | 1.14 (0.73–1.77) | 1.70 (1.12–2.58) | 1.87 (1.23–2.84) | |
| Mean blood pressure (mmHg) | 0.907 | |||||
| < 81 | 2859 | Reference | 1.10 (0.79–1.55) | 1.59 (1.16–2.17) | 1.64 (1.21–2.23) | |
| ≥ 81 | 2718 | Reference | 0.89 (0.57–1.38) | 1.22 (0.80–1.86) | 1.57 (1.04–2.39) | |
| Heart rate (beats/min) | 0.038 | |||||
| < 87 | 2744 | Reference | 0.98 (0.64–1.49) | 1.58 (1.06–2.34) | 2.08 (1.40–3.08) | |
| ≥ 87 | 2833 | Reference | 1.07 (0.75–1.51) | 1.36 (0.98–1.88) | 1.42 (1.04–1.94) | |
| Respiration rate (beats/min) | 0.053 | |||||
| < 20 | 2313 | Reference | 1.22 (0.78–1.89) | 1.79 (1.17–2.73) | 2.13 (1.40–3.24) | |
| ≥ 20 | 3264 | Reference | 0.93 (0.67–1.30) | 1.33 (0.98–1.81) | 1.53 (1.13–2.06) | |
| Oxygen saturation (%) | 0.563 | |||||
| < 97 | 2174 | Reference | 1.20 (0.78–1.85) | 1.69 (1.13–2.53) | 2.04 (1.38–3.02) | |
| ≥ 97 | 3403 | Reference | 0.94 (0.67–1.32) | 1.39 (1.01–1.92) | 1.58 (1.15–2.16) | |
| Congestive heart failure | 0.661 | |||||
| Yes | 1256 | Reference | 1.39 (0.79–2.44) | 1.94 (1.15–3.29) | 2.07 (1.23–3.49) | |
| No | 4321 | Reference | 0.93 (0.69–1.27) | 1.37 (1.03–1.83) | 1.67 (1.27–2.20) | |
| Coronary artery disease | 0.030 | |||||
| Yes | 1853 | Reference | 1.54 (0.94–2.53) | 2.26 (1.38–3.70) | 2.77 (1.70–4.53) | |
| No | 3724 | Reference | 0.87 (0.63–1.19) | 1.23 (0.92–1.65) | 1.41 (1.07–1.87) | |
| Acute coronary syndrome | 0.036 | |||||
| Yes | 1085 | Reference | 1.96 (1.09–3.55) | 2.58 (1.41–4.72) | 3.44 (1.86–6.35) | |
| No | 4492 | Reference | 0.86 (0.64–1.16) | 1.31 (1.00–1.72) | 1.51 (1.15–1.96) | |
| STEMI | 0.526 | |||||
| Yes | 384 | Reference | 1.33 (0.55–3.18) | 1.76 (0.70–4.47) | 2.57 (0.97–6.84) | |
| No | 5193 | Reference | 1.01 (0.76–1.33) | 1.49 (1.15–1.93) | 1.73 (1.34–2.22) | |
| NSTEMI | 0.081 | |||||
| Yes | 403 | Reference | 4.37 (1.42–13.44) | 5.37 (1.71–16.83) | 6.24 (2.00–19.44) | |
| No | 5174 | Reference | 0.91 (0.69–1.21) | 1.39 (1.07–1.80) | 1.63 (1.27–2.10) | |
| Arrhythmias | 0.089 | |||||
| Yes | 1865 | Reference | 1.41 (0.85–2.34) | 2.19 (1.37–3.49) | 2.51 (1.58–3.97) | |
| No | 3712 | Reference | 0.92 (0.67–1.26) | 1.28 (0.95–1.74) | 1.52 (1.14–2.04) | |
| Cardiac arrest | 0.004 | |||||
| Yes | 407 | Reference | 0.89 (0.52–1.52) | 1.44 (0.86–2.42) | 1.12 (0.64–1.96) | |
| No | 5170 | Reference | 1.62 (1.13–2.31) | 2.37 (1.69–3.32) | 3.21 (2.32–4.44) | |
| Bradycardia | 0.162 | |||||
| Yes | 224 | Reference | 7.13 (0.83–61.1) | 6.87 (0.80–58.9) | 11.12 (1.29–96.18) | |
| No | 5353 | Reference | 0.98 (0.75–1.29) | 1.46 (1.14–1.88) | 1.69 (1.32–2.16) | |
| Atrial fibrillation | 0.281 | |||||
| Yes | 1117 | Reference | 1.03 (0.54–1.96) | 1.91 (1.08–3.38) | 2.11 (1.20–3.71) | |
| No | 4460 | Reference | 1.03 (0.77–1.38) | 1.38 (1.04–1.83) | 1.65 (1.26–2.17) | |
| Ventricular arrhythmias | 0.516 | |||||
| Yes | 241 | Reference | 1.14 (0.47–2.76) | 1.85 (0.80–4.27) | 1.16 (0.42–3.19) | |
| No | 5336 | Reference | 1.04 (0.79–1.38) | 1.52 (1.17–1.97) | 1.86 (1.45–2.40) | |
| Atrioventricular block | 0.711 | |||||
| Yes | 119 | Reference | 3.33 (0.57–19.4) | 3.38 (0.52–21.79) | 2.81 (0.37–21.66) | |
| No | 5458 | Reference | 1.00 (0.76–1.31) | 1.48 (1.15–1.90) | 1.74 (1.36–2.22) | |
| Cardiomyopathy | 0.464 | |||||
| Yes | 365 | Reference | 1.24 (0.44–3.48) | 2.15 (0.83–5.58) | 2.43 (0.91–6.53) | |
| No | 5212 | Reference | 1.02 (0.77–1.34) | 1.47 (1.14–1.90) | 1.73 (1.35–2.23) | |
| Valve disease | 0.425 | |||||
| Yes | 164 | Reference | 0.28 (0.28–2.82) | 3.67 (0.93–14.43) | 1.97 (0.48–8.03) | |
| No | 5413 | Reference | 1.06 (0.81–1.38) | 1.45 (1.12–1.87) | 1.76 (1.37–2.25) | |
| Shock | 0.005 | |||||
| Yes | 1718 | Reference | 1.02 (0.67–1.56) | 1.29 (0.88–1.91) | 1.10 (0.76–1.60) | |
| No | 3859 | Reference | 1.01 (0.71–1.44) | 1.52 (1.10–2.12) | 2.05 (1.48–2.84) | |
| Pulmonary embolism | 0.746 | |||||
| Yes | 129 | Reference | 3.70 (0.66–20.59) | 1.98 (0.31–12.67) | 2.13 (0.33–13.69) | |
| No | 5448 | Reference | 1.00 (0.76–1.31) | 1.50 (1.17–1.93) | 1.76 (1.37–2.25) | |
| Pulmonary hypertension | 0.805 | |||||
| Yes | 69 | Reference | 0.61 (0.75–4.98) | 1.18 (0.17–8.33) | 0.65 (0.79–5.29) | |
| No | 5508 | Reference | 1.04 (0.79–1.36) | 1.51 (1.18–1.95) | 1.79 (1.40–2.29) | |
| Hypertension | 0.021 | |||||
| Yes | 1689 | Reference | 2.26 (1.10–4.61) | 2.91 (1.45–5.84) | 4.08 (2.06–8.09) | |
| No | 3888 | Reference | 0.92 (0.68–1.23) | 1.39 (1.06–1.82) | 1.51 (1.16–1.96) | |
| Diabetes | 0.415 | |||||
| Yes | 1133 | Reference | 1.10 (0.61–2.00) | 1.45 (0.82–2.57) | 2.18 (1.26–3.77) | |
| No | 4444 | Reference | 1.01 (0.75–1.37) | 1.53 (1.16–2.01) | 1.67 (1.27–2.19) | |
| Hypercholesterolemia | 0.369 | |||||
| Yes | 411 | Reference | 0.26 (0.53–1.28) | 2.07 (0.79–5.42) | 1.83 (0.68–4.94) | |
| No | 5166 | Reference | 1.09 (0.83–1.43) | 1.47 (1.14–1.91) | 1.76 (1.37–2.26) | |
| COPD | 0.447 | |||||
| Yes | 633 | Reference | 0.63 (0.25–1.62) | 1.87 (0.87–4.04) | 1.79 (0.85–3.79) | |
| No | 4944 | Reference | 1.08 (0.82–1.43) | 1.44 (1.11–1.88) | 1.74 (1.34–2.25) | |
| Respiratory failure | 0.061 | |||||
| Yes | 1669 | Reference | 0.98 (0.67–1.43) | 1.28 (0.90–1.82) | 1.25 (0.90–1.75) | |
| No | 3908 | Reference | 1.12 (0.75–1.66) | 1.66 (1.15–2.41) | 1.95 (1.35–2.82) | |
| Chronic kidney disease | 0.807 | |||||
| Yes | 856 | Reference | 1.11 (0.59–2.09) | 1.15 (0.62–2.11) | 1.72 (0.97–3.05) | |
| No | 4721 | Reference | 1.00 (0.74–1.34) | 1.57 (1.19–2.06) | 1.71 (1.31–2.25) | |
| Acute kidney injury | 0.005 | |||||
| Yes | 1031 | Reference | 0.95 (0.59–1.53) | 1.06 (0.68–1.67) | 1.03 (0.67–1.60) | |
| No | 4546 | Reference | 1.06 (0.76–1.47) | 1.65 (1.22–2.24) | 2.04 (1.52–2.75) | |
| Malignancy | 0.017 | |||||
| Yes | 272 | Reference | 0.50 (0.18–1.41) | 0.67 (0.26–1.73) | 0.56 (0.23–1.34) | |
| No | 5305 | Reference | 1.07 (0.81–1.41) | 1.56 (1.20–2.02) | 1.85 (1.44–2.39) | |
| Sepsis | 0.063 | |||||
| Yes | 1301 | Reference | 1.20 (0.70–2.06) | 1.56 (0.95–2.56) | 1.19 (0.74–1.90) | |
| No | 4276 | Reference | 0.96 (0.70–1.31) | 1.37 (1.02–1.84) | 1.84 (1.37–2.48) | |
| Stroke | 0.882 | |||||
| Yes | 223 | Reference | 2.38 (0.79–7.11) | 3.15 (1.01–9.83) | 1.90 (0.56–6.44) | |
| No | 5354 | Reference | 0.97 (0.73–1.27) | 1.46 (1.13–1.88) | 1.76 (1.38–2.26) | |
| Antiplatelet | 0.011 | |||||
| Yes | 2659 | Reference | 1.28 (0.85–1.94) | 1.97 (1.33–2.92) | 2.52 (1.71–3.71) | |
| No | 2918 | Reference | 0.86 (0.61–1.23) | 1.19 (0.86–1.65) | 1.30 (0.95–1.78) | |
| Oral anticoagulants | < 0.001 | |||||
| Yes | 687 | Reference | 5.22 (0.62–43.86) | 9.66 (1.24–75.16) | 26.79 (3.61–75.16) | |
| No | 4890 | Reference | 1.01 (0.77–1.32) | 1.47 (1.14–1.89) | 1.54 (1.20–1.98) | |
| Beta-blockers | 0.001 | |||||
| Yes | 2446 | Reference | 2.16 (1.31–3.56) | 2.50 (1.52–4.12) | 3.73 (2.31–6.02) | |
| No | 3131 | Reference | 0.73 (0.53–1.02) | 1.20 (0.90–1.61) | 1.22 (0.91–1.63) | |
| ACEI/ARB | 0.001 | |||||
| Yes | 1490 | Reference | 2.32 (1.00–5.36) | 3.43 (1.53–7.68) | 6.01 (2.74–13.15) | |
| No | 4087 | Reference | 0.94 (0.71–1.25) | 1.36 (1.04–1.78) | 1.42 (1.09–1.84) | |
| Statin | 0.091 | |||||
| Yes | 1717 | Reference | 1.77 (1.01–3.10) | 2.00 (1.14–3.52) | 2.91 (1.69–4.99) | |
| No | 3860 | Reference | 0.86 (0.63–1.17) | 1.34 (1.01–1.77) | 1.47 (1.11–1.93) | |
| White blood cell (109/L) | 0.232 | |||||
| < 10.3 | 2761 | Reference | 0.91 (0.58–1.44) | 1.80 (1.19–2.71) | 2.49 (1.67–3.73) | |
| ≥ 10.3 | 2816 | Reference | 1.15 (0.82–1.60) | 1.35 (0.98–1.85) | 1.36 (1.00–1.85) | |
| Neutrophil percentage (%) | 0.671 | |||||
| < 76 | 2736 | Reference | 0.77 (0.52–1.12) | 1.25 (0.84–1.86) | 1.97 (1.26–3.09) | |
| ≥ 76 | 2841 | Reference | 0.88 (0.59–1.33) | 0.90 (0.62–1.32) | 0.89 (0.62–1.29) | |
| Red blood cell (109/L) | 0.049 | |||||
| < 4.1 | 2796 | Reference | 0.98 (0.68–1.41) | 1.20 (0.85–1.69) | 1.40 (1.01–1.94) | |
| ≥ 4.1 | 2781 | Reference | 1.02 (0.69–1.51) | 1.79 (1.25–2.57) | 2.01 (1.38–2.92) | |
| Platelet (109/L) | 0.151 | |||||
| < 217 | 2770 | Reference | 1.18 (0.84–1.66) | 1.73 (1.24–2.42) | 2.09 (1.48–2.96) | |
| ≥ 217 | 2807 | Reference | 0.84 (0.54–1.30) | 1.28 (0.87–1.89) | 1.51 (1.04–2.20) | |
| Hemoglobin (g/dL) | 0.021 | |||||
| < 12.1 | 2753 | Reference | 0.81 (0.56–1.18) | 1.08 (0.76–1.53) | 1.24 (0.89–1.72) | |
| ≥ 12.1 | 2824 | Reference | 1.20 (0.82–1.75) | 1.90 (1.33–2.72) | 2.20 (1.52–3.20) | |
| Hematocrit (%) | 0.119 | |||||
| < 36.9 | 2781 | Reference | 0.86 (0.58–1.26) | 1.14 (0.80–1.62) | 1.34 (0.96–1.87) | |
| ≥ 36.9 | 2796 | Reference | 1.16 (0.80–1.69) | 1.85 (1.30–2.64) | 2.12 (1.47–3.08) | |
| Glucose (mg/dL) | < 0.001 | |||||
| < 132 | 2739 | Reference | 1.31 (0.84–2.04) | 2.44 (1.62–3.68) | 2.21 (1.45–3.37) | |
| ≥ 132 | 2838 | Reference | 0.94 (0.67–1.33) | 1.11 (0.80–1.53) | 1.56 (1.16–2.11) | |
| Creatinine (mg/dL) | 0.321 | |||||
| < 1.18 | 2780 | Reference | 1.29 (0.81–2.07) | 1.99 (1.28–3.10) | 2.13 (1.37–3.32) | |
| ≥ 1.18 | 2797 | Reference | 0.92 (0.66–1.27) | 1.25 (0.92–1.69) | 1.48 (1.10–1.99) | |
| Blood nitrogen urea (mg/dL) | 0.060 | |||||
| < 23 | 2762 | Reference | 0.97 (0.65–1.49) | 1.55 (1.04–2.29) | 1.57 (1.05–2.36) | |
| ≥ 23 | 2815 | Reference | 1.00 (0.70–1.42) | 1.32 (0.95–1.83) | 1.57 (1.15–2.15) | |
| Sodium (mmol/L) | 0.632 | |||||
| < 138 | 2758 | Reference | 1.11 (0.75–1.67) | 1.65 (1.14–2.39) | 1.75 (1.22–2.52) | |
| ≥ 138 | 2819 | Reference | 0.97 (0.68–1.39) | 1.40 (0.99–1.96) | 1.83 (1.31–2.57) | |
| Potassium (mmol/L) | 0.295 | |||||
| < 4.1 | 2500 | Reference | 0.88 (0.58–1.34) | 1.33 (0.91–1.95) | 1.46 (0.99–2.13) | |
| ≥ 4.1 | 3077 | Reference | 1.13 (0.79–1.60) | 1.62 (1.16–2.25) | 1.94 (1.41–2.67) | |
| Transfusion | 0.128 | |||||
| Yes | 105 | Reference | 4.19 (0.43–40.62) | 2.44 (0.20–29.19) | 9.31 (1.11–77.88) | |
| No | 5472 | Reference | 1.01 (0.77–1.32) | 1.50 (1.17–1.93) | 1.69 (1.32–2.16) | |
| APS | < 0.001 | |||||
| < 41 | 2729 | Reference | 2.52 (1.15–5.51) | 4.61 (2.19–9.70) | 6.16 (2.93–12.92) | |
| ≥ 41 | 2848 | Reference | 0.87 (0.65–1.18) | 1.04 (0.79–1.38) | 1.04 (0.79–1.36) | |
| APACHE IV | < 0.001 | |||||
| < 55 | 2747 | Reference | 2.55 (1.17–5.57) | 3.96 (1.85–8.48) | 6.61 (3.16–13.81) | |
| ≥ 55 | 2830 | Reference | 0.85 (0.63–1.15) | 1.02 (0.77–1.35) | 0.99 (0.76–1.30) | |
Binary logistic regression analysis was used and results were presented as OR (odds ratio) and 95% CI (confidence interval). P for interaction was calculated using binary logistic analysis to determine whether there is interaction between different subgroups and PLR quartiles.
STEMI ST-elevation myocardial infarction, NSTEMI non-ST-elevation myocardial infarction, COPD chronic obstructive pulmonary disease, PLR platelet-lymphocyte ratio, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, APS acute physiology score, APACHE IV Acute Physiology and Chronic Health Evaluation IV.
Discussion
No studies have been conducted on CICU patients who are more in need of a simple prognostic factor such as PLR. This is the first study to explore the role of PLR in patients with severe cardiovascular disease, which will provide a clinical basis for the application of PLR in CICU patients. This study confirmed the relationship between PLR and in-hospital mortality in CICU patients. (1) As PLR quartiles increased, in-hospital mortality increased significantly. And after adjusting for possible confounding variables, PLR was still independently associated with in-hospital mortality. (2) The Lowess curves presented a positive relationship between PLR and in-hospital mortality. (3) Significant interactions were observed in several subgroups. (4) Length of CICU and hospital stay were prolonged as PLR increased.
In the previous study, inflammation has been proven to be strongly associated with development and prognosis of cardiovascular disease26. And there was evidence that lymphocytes played a key role in the regulation of inflammatory response at all levels during atherosclerosis. During the systemic inflammatory response, the lymphocyte count was proved to decrease because of increased lymphocyte apoptosis27. This may explain the underlying mechanism for the diagnostic and prognostic validity of low lymphocyte count in patients with acute coronary syndrome and stable coronary artery disease (CAD), respectively14,28. The prethrombotic state is caused by increased megakaryocyte series proliferation and relative thrombocytosis, which reflects the body's persistent inflammatory state7. Moreover, some studies have demonstrated an increase in the incidence of adverse events as the platelet count increased7–10. Patients with higher platelet count corresponded to worse outcomes in ACS29. The reason may be that elevated levels of platelet mononuclear aggregation (PMA) in the blood of patients with coronary heart disease, which correlated with plaque stability30,31. And high PMA levels in patients with NSTEMI have been proven to increase the risk of adverse outcomes32. As a member of systemic inflammatory response family, PLR is the combination of platelet and lymphocyte, which represents the situation of aggregation and inflammatory pathways, and is able to amplify changes in these two indicators, especially in cases where some clinicians tend to overlook such changes, such as when the indicator values are near the upper or lower limits of normal. Paying attention to PLR in clinical practice and improving the level of nursing and monitoring may improve the prognosis and reduce mortality.
As an available indicator, PLR has already been proven to be associated with severity and prognosis of cardiovascular disease. An observational study which enrolled 619 patients with NSTEMI confirmed that high PLR could independently predict the increased of long-term mortality22. And previous study included 636 patients with ST-elevated acute myocardial infarction showed that PLR was an independent predictor of cardiovascular mortality23. Moreover, PLR was proved to be a conventional risk factor in predicting severe atherosclerosis, and independently associated with increased Gensini score33. Besides, it was showed that high preoperative PLR level increased the incidence of no-reflow in patients after PCI20.
Our research reached a similar conclusion that increased PLR was independently associated with in-hospital mortality in CICU patients, providing evidence for the use of PLR in patients with severe cardiovascular disease. In the subgroups of congestive heart failure, coronary artery disease, valvular disease, cardiomyopathy, arrhythmias, and shock, the same conclusion could be reached. These diseases almost covered most diseases in CICU, which confirmed the reliability of PLR application in CICU patients.
Through the Lowess curves, it has been demonstrated that when PLR was lower than 60, the mortality rate decreased with the increase of PLR, which suggested that we should be flexible when using PLR to judge the disease condition of CICU patients. When the PLR is very small, consideration should be given that whether the patient has other comorbidities that may increase mortality, such as diseases of the blood system. In patients with idiopathic thrombocytopenia, the platelet count is less than 100*109/L, and even less than 10*109/L in severe cases, resulting in an extremely small PLR value. In this study, we only excluded patients with hematologic malignancies from hematologic diseases. And due to retrospective studies, we could not rule out the possibility of missed diagnosis.
Therefore, when applying PLR in clinical judgment, it is unreasonable to think that the smaller the PLR, the better, and it is necessary to define a threshold value. The value of 10–90%PLR was set as the reference range, and the Lowess curve showed that the mortality rate increased with the increase of PLR, that is, there was no inflection point. In this way, a more reasonable reference range for PLR was 69–460. When the PLR is below 69, the patients should be considered for other comorbidities that may increase mortality. When the PLR is greater than 460, we need to be aware that the patients' condition may be severe with a higher mortality rate.
In addition, as PLR quartiles increased, the length of hospital stay and the length of hospital stay significantly increased, which brought the psychological, physical, and financial burden on patients. Therefore, more attention should be paid to inexpensive, easily accessible indicators like PLR, which is more cost-effective, especially in some cases that more complex score could not be calculated, for example, the patient is unable to undergo complex examination or the patient is in a remote area without the condition to do so.
Other classic prognostic markers, such as neutrophil–lymphocyte ratio (NLR), were also proved to be associated with the prognosis of patients with severe cardiovascular disease34. In contrast, few studies on PLR were conducted. In our study, we explored the relationship between PLR and in-hospital mortality in CICU patients and used the receiver operating characteristic (ROC) analysis to assess the ability of NLR and PLR in predicting the incidence of death. In comparison with PLR, the area under the ROC curve (AUC) of NLR was larger (NLR vs. PLR: 0.63 vs. 0.60), which suggested that NLR has a better ability to indicate the risk of in -hospital mortality. Meanwhile, we added NLR to Model 3, and the results indicated that PLR was related to in-hospital mortality independently of NLR. The purpose of our study was not to prove that PLR was the best predictor, but to improve the theoretical basis of PLR application in CICU patients. In clinical practice, the judgment of a patient's condition cannot only rely on a single indicator. We can make a clearer judgment of the patient's condition by using all the indicators we have already known, and through the exploration of these simple prognostic indicators, we will be able to use these indicators to build a prognostic model of CICU patients and confirm it in prospective studies.
Independent association between PLR and in-hospital mortality in CICU patients was proved in this study, which is convenient for clinical use. The multi-center and large sample size made the conclusion more reliable. However, this study also had some limitations. First of all, bias is inevitable due to the retrospective study. Secondly some important indexes can’t be collected such as left ventricular ejection fraction, C-reactive protein, cholesterol. Generally speaking, the accuracy of the model is determined by the variables in the model, and the accuracy of the model in this study is affected to a certain extent due to the lack of the above variables. This will be improved in further research. The inability to dynamically analyze PLR was also the limitation.
Conclusion
To sum up, the results indicated that PLR was an independent predictor of CICU patient mortality in hospital. The in-hospital mortality rate increased significantly as PLR quartiles increased. Further, high PLR was related to prolonged CICU and hospital stay length. And patients with low APACHE IV or with less comorbidities had higher risk of mortality for PLR.
Supplementary Information
Acknowledgements
Thanks to Biyang Zhang for his great contribution to the first edition of this study.
Author contributions
G.Z. and Y.Z. contributed to the design, data collection and article writing. J.W. and Y.L. contributed to the data analysis. G.Z., Y.Z., J.W. and Y.L. contributed to manuscript revising.
Funding
This study was supported by grants from Beijing Municipal Health Commission (Grant No. PXM2020_026272_000002 and Grant No. PXM2020_026272_000014) and Natural Science Foundation of Beijing, China (Grant No. 7212027) to Yujie Zhou.
Data availability
The data used in this study was from eICU Collaborative Research Database24, which is available at: https://doi.org/10.13026/C2WM1R. The author was approved to access to the database through Protecting Human Research Participants exam (certificate number: 9728458).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-02686-1.
References
- 1.Julian DG. Treatment of cardiac arrest in acute myocardial ischaemia and infarction. Lancet. 1961;2(7207):840–844. doi: 10.1016/S0140-6736(61)90738-3. [DOI] [PubMed] [Google Scholar]
- 2.Killip T, 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit: A two year experience with 250 patients. Am J Cardiol. 1967;20(4):457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 3.Rogers WJ, Canto JG, Lambrew CT, Tiefenbrunn AJ, Kinkaid B, Shoultz DA, Frederick PD, Every N. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the US from 1990 through 1999: The National Registry of Myocardial Infarction 1, 2 and 3. J. Am. Coll. Cardiol. 2000;36(7):2056–2063. doi: 10.1016/S0735-1097(00)00996-7. [DOI] [PubMed] [Google Scholar]
- 4.Fox KA, Goodman SG, Klein W, Brieger D, Steg PG, Dabbous O, Avezum A. Management of acute coronary syndromes. Variations in practice and outcome; findings from the Global Registry of Acute Coronary Events (GRACE) Eur. Heart J. 2002;23(15):1177–1189. doi: 10.1053/euhj.2001.3081. [DOI] [PubMed] [Google Scholar]
- 5.Katz JN, Turer AT, Becker RC. Cardiology and the critical care crisis: A perspective. J. Am. Coll. Cardiol. 2007;49(12):1279–1282. doi: 10.1016/j.jacc.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 6.Katz JN, Minder M, Olenchock B, Price S, Goldfarb M, Washam JB, Barnett CF, Newby LK, van Diepen S. The genesis, maturation, and future of critical care cardiology. J. Am. Coll. Cardiol. 2016;68(1):67–79. doi: 10.1016/j.jacc.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 7.Thaulow E, Erikssen J, Sandvik L, Stormorken H, Cohn PF. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation. 1991;84(2):613–617. doi: 10.1161/01.CIR.84.2.613. [DOI] [PubMed] [Google Scholar]
- 8.Iijima R, Ndrepepa G, Mehilli J, Bruskina O, Schulz S, Schömig A, Kastrati A. Relationship between platelet count and 30-day clinical outcomes after percutaneous coronary interventions: Pooled analysis of four ISAR trials. Thromb. Haemost. 2007;98(4):852–857. [PubMed] [Google Scholar]
- 9.Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, Sadeghi M, Mehran R, Lansky AJ, Na Y, Stone GW. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial) Am. J. Cardiol. 2007;99(8):1055–1061. doi: 10.1016/j.amjcard.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 10.Vidwan P, Lee S, Rossi JS, Stouffer GA. Relation of platelet count to bleeding and vascular complications in patients undergoing coronary angiography. Am. J. Cardiol. 2010;105(9):1219–1222. doi: 10.1016/j.amjcard.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Ommen SR, Hammill SC, Gibbons RJ. The relative lymphocyte count predicts death in patients receiving implantable cardioverter defibrillators. Pacing Clin. Electrophysiol. 2002;25(10):1424–1428. doi: 10.1046/j.1460-9592.2002.01424.x. [DOI] [PubMed] [Google Scholar]
- 12.Acanfora D, Gheorghiade M, Trojano L, Furgi G, Pasini E, Picone C, Papa A, Iannuzzi GL, Bonow RO, Rengo F. Relative lymphocyte count: A prognostic indicator of mortality in elderly patients with congestive heart failure. Am. Heart J. 2001;142(1):167–173. doi: 10.1067/mhj.2001.115792. [DOI] [PubMed] [Google Scholar]
- 13.Zouridakis EG, Garcia-Moll X, Kaski JC. Usefulness of the blood lymphocyte count in predicting recurrent instability and death in patients with unstable angina pectoris. Am. J. Cardiol. 2000;86(4):449–451. doi: 10.1016/S0002-9149(00)00963-2. [DOI] [PubMed] [Google Scholar]
- 14.Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am. J. Cardiol. 1997;79(6):812–814. doi: 10.1016/S0002-9149(96)00878-8. [DOI] [PubMed] [Google Scholar]
- 15.Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97(1):19–22. doi: 10.1161/01.CIR.97.1.19. [DOI] [PubMed] [Google Scholar]
- 16.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DS, Foulis AK, Horgan PG, McMillan DC. A comparison of inflammation-based prognostic scores in patients with cancer: A Glasgow Inflammation Outcome Study. Eur. J. Cancer. 2011;47(17):2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am. J. Surg. 2009;197(4):466–472. doi: 10.1016/j.amjsurg.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 18.Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, Neoptolemos JP. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J. Gastrointest. Surg. 2008;12(8):1422–1428. doi: 10.1007/s11605-008-0554-3. [DOI] [PubMed] [Google Scholar]
- 19.Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, Eller P, Rief P, Hackl G, Pilger E, Brodmann M. Platelet-to-lymphocyte ratio: A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS ONE. 2013;8(7):e67688. doi: 10.1371/journal.pone.0067688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Can E, Hamilcikan Ş, Can C. The value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio for detecting early-onset neonatal sepsis. J. Pediatr. Hematol. Oncol. 2018;40(4):e229–e232. doi: 10.1097/MPH.0000000000001059. [DOI] [PubMed] [Google Scholar]
- 21.Yildiz A, Yuksel M, Oylumlu M, Polat N, Akyuz A, Acet H, Aydin M, Ülgen MS. The utility of the platelet-lymphocyte ratio for predicting no reflow in patients with ST-segment elevation myocardial infarction. Clin. Appl. Thromb. Hemost. 2015;21(3):223–228. doi: 10.1177/1076029613519851. [DOI] [PubMed] [Google Scholar]
- 22.Azab B, Shah N, Akerman M, McGinn JT., Jr Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J. Thromb. Thrombolysis. 2012;34(3):326–334. doi: 10.1007/s11239-012-0718-6. [DOI] [PubMed] [Google Scholar]
- 23.Temiz A, Gazi E, Güngör Ö, Barutçu A, Altun B, Bekler A, Binnetoğlu E, Şen H, Güneş F, Gazi S. Platelet/lymphocyte ratio and risk of in-hospital mortality in patients with ST-elevated myocardial infarction. Med. Sci. Monit. 2014;22(20):660–665. doi: 10.12659/MSM.890152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollard TJ, Johnson AEW, Raffa JD, Celi LA, Mark RG, Badawi O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci. Data. 2018;5:180178. doi: 10.1038/sdata.2018.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute physiology and chronic health evaluation (APACHE) IV: Hospital mortality assessment for today's critically ill patients. Crit. Care Med. 2006;34(5):1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 26.Carrillo-Salinas FJ, Ngwenyama N, Anastasiou M, Kaur K, Alcaide P. Heart inflammation: Immune cell roles and roads to the heart. Am. J. Pathol. 2019;189(8):1482–1494. doi: 10.1016/j.ajpath.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Núñez J, Miñana G, Bodí V, Núñez E, Sanchis J, Husser O, Llàcer A. Low lymphocyte count and cardiovascular diseases. Curr. Med. Chem. 2011;18(21):3226–3233. doi: 10.2174/092986711796391633. [DOI] [PubMed] [Google Scholar]
- 28.Thomson SP, Gibbons RJ, Smars PA, Suman VJ, Pierre RV, Santrach PJ, Jiang NS. Incremental value of the leukocyte differential and the rapid creatine kinase-MB isoenzyme for the early diagnosis of myocardial infarction. Ann. Int. Med. 1995;122(5):335–341. doi: 10.7326/0003-4819-122-5-199503010-00003. [DOI] [PubMed] [Google Scholar]
- 29.Mueller C, Neumann FJ, Hochholzer W, Trenk D, Zeller T, Perruchoud AP, Buettner HJ. The impact of platelet count on mortality in unstable angina/non-ST-segment elevation myocardial infarction. Am. Heart J. 2006;151(6):1214.e1–7. doi: 10.1016/j.ahj.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, Hechtman HB, Michelson AD. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 1998;31(2):352–358. doi: 10.1016/S0735-1097(97)00510-X. [DOI] [PubMed] [Google Scholar]
- 31.Linden MD, Furman MI, Frelinger AL, 3rd, Fox ML, Barnard MR, Li Y, Przyklenk K, Michelson AD. Indices of platelet activation and the stability of coronary artery disease. J. Thromb. Haemost. 2007;5(4):761–765. doi: 10.1111/j.1538-7836.2007.02462.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang SZ, Jin YP, Qin GM, Wang JH. Association of platelet-monocyte aggregates with platelet activation, systemic inflammation, and myocardial injury in patients with non-st elevation acute coronary syndromes. Clin. Cardiol. 2007;30(1):26–31. doi: 10.1002/clc.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yüksel M, Yıldız A, Oylumlu M, Akyüz A, Aydın M, Kaya H, Acet H, Polat N, Bilik MZ, Alan S. The association between platelet/lymphocyte ratio and coronary artery disease severity. Anatol. J. Cardiol. 2015;15(8):640–647. doi: 10.5152/akd.2014.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun H, Que J, Peng Y, Ye H, Xiang H, Han Y, Wang J, Ji K. The neutrophil-lymphocyte ratio: A promising predictor of mortality in coronary care unit patients: A cohort study. Int. Immunopharmacol. 2019;74:105692. doi: 10.1016/j.intimp.2019.105692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study was from eICU Collaborative Research Database24, which is available at: https://doi.org/10.13026/C2WM1R. The author was approved to access to the database through Protecting Human Research Participants exam (certificate number: 9728458).