Abstract
Chronic obstructive pulmonary disease (COPD) may coexist with lung cancer, but the impact on prognosis is uncertain. Moreover, it is unclear whether pharmacological treatment for COPD improves the patient’s prognosis. We retrospectively investigated patients with advanced non-small-cell lung cancer (NSCLC) who had received chemotherapy at Kyoto University Hospital. Coexisting COPD was diagnosed by spirometry, and the association between pharmacological treatment for COPD and overall survival (OS) was assessed. Of the 550 patients who underwent chemotherapy for advanced NSCLC between 2007 and 2014, 347 patients who underwent spirometry were analyzed. Coexisting COPD was revealed in 103 patients (COPD group). The median OS was shorter in the COPD group than the non-COPD group (10.6 vs. 16.8 months). Thirty-seven patients had received COPD treatment, and they had a significantly longer median OS than those without treatment (16.7 vs. 8.2 months). Multivariate Cox regression analysis confirmed the positive prognostic impact of COPD treatment. Additional validation analysis revealed similar results in patients treated with immune checkpoint inhibitors (ICIs). Coexisting COPD had a significant association with poor prognosis in advanced NSCLC patients if they did not have pharmacological treatment for COPD. Treatment for coexisting COPD has the potential to salvage the prognosis.
Subject terms: Chronic obstructive pulmonary disease, Lung cancer
Introduction
Chronic obstructive pulmonary disease (COPD) is a major public health problem. In 2020, COPD was projected to rank fifth worldwide in terms of disease burden and third in terms of mortality1,2. Lung cancer is also one of the leading causes of death in many countries3. Several clinical reports have shown that the proportion of deaths from lung cancer in patients with COPD ranges from 4 to 33%1,4,5. The frequency of coexisting COPD has been reported to be 40–70% among lung cancer patients6–9.
Additionally, airflow limitations have been reported to be a significant risk factor for lung cancer, and share the same risk for the COPD pathogenesis, especially cigarette smoke exposure5,10–12. The pathological feature of COPD is the chronic inflammation in pulmonary alveoli caused by the activation of various cytokines by the stimulation of harmful substances within cigarette smoke or other environmental gases13–15. Consequently, chronic inflammation alters normal alveolar architecture, promotes emphysema, and stimulates cell proliferation and genetic mutation, affecting the development of lung cancer15,16. A hypothesis of a common mechanism of COPD and lung cancer17, which has recently attracted considerable attention, was proposed in 2008.
Coexisting COPD may cause severe dyspnea, exacerbation, and poor outcome, and it is possible that patients with lung cancer and coexisting COPD may have a much worse prognosis than those without COPD. In fact, some clinical studies have reported that patients with early-stage non-small-cell lung cancer (NSCLC) with COPD have a poor outcome relative to patients with lung cancer without COPD18,19. However, in patients with advanced lung cancer, the impact of coexisting COPD is uncertain. Moreover, it has not been fully examined whether the treatment of COPD might be associated with the prognosis of patients with advanced NSCLC.
Treatment for COPD has advanced in the past two decades due to modifications of older compounds, resulting in more potent, longer-acting drugs that can be delivered via improved devices20. Currently, long-acting bronchodilators (LABDs) are the standard treatment for COPD, and these LABDs have been reported to be associated with improvements in lung function, exercise capacity, quality of life, the rate of exacerbations, and prognosis21–25.
We hypothesize that treatment intervention for COPD might improve the prognosis of advanced NSCLC patients with COPD. We retrospectively examined the impact of COPD and pharmacological treatment for COPD on the survival outcomes of patients with locally advanced NSCLC in the present study.
Methods
Patients
The study flow chart is shown in Supplemental Fig. S1, Additional File 1. We enrolled patients with lung cancer who underwent chemotherapy for recurrence after curative treatment and locally advanced or metastatic NSCLC at Kyoto University Hospital from April 2007 to March 2014.
The entry criteria were as follows: pathological diagnosis of NSCLC, age > 40 years, and performance status (PS) better than 4. Curative treatment was defined as treatment with radiation therapy, radiochemotherapy, or surgery in the present study. The exclusion criteria were as follows: no spirometry, a combination of other respiratory diseases, such as asthma, the use of immunotherapy, including immune checkpoint inhibitors (ICIs), and no smoking history data.
Spirometry was conducted using a Chestac-8800 (Chest M.I., Inc., Tokyo, Japan), and the results obtained met the requirements of the Japanese Respiratory Society guidelines26. COPD was diagnosed based on smoking status as previously described27–30, and the functional definition, i.e., a forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) ratio less than 70%, in accordance with the documents of the Global Initiative for Chronic Obstructive Lung Disease (GOLD)31.
Data collection
The primary endpoint of this study was overall survival (OS), measured from the date of initiation of 1st-line chemotherapy to the date of death (event) or last known date of survival (censored). Data were collected from lung cancer patient records of Kyoto University Graduate School of Medicine. All patients’ vital statuses were confirmed in September 2015.
Pharmacological treatment of COPD
The use of COPD treatment was also collected from the medical records of our hospital. We defined the use of LABDs and inhaled corticosteroids with/without short-acting bronchodilators as pharmacological treatment of COPD according to GOLD documents31. We classified the COPD group into two groups, with COPD treatment and without COPD treatment, depending on the presence or absence of treatment.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine (http://www.ec.med.kyoto-u.ac.jp) (approval No. R0702-1) and performed in accordance with the Declaration of Helsinki, and informed consent from the patients was waived because this is a retrospective study. We have disclosed information about this study on the official site of our laboratory (http://kukonai.com/).
Statistical analysis
For comparisons of background features between the non-COPD and COPD groups or between the patients with COPD treatment and without COPD treatment, continuous variables are reported as the mean ± standard deviation (SD) or range. Student's t-test was used to compare the means of continuous variables that had a normal distribution, and the Wilcoxon signed-rank test was used to compare the means of continuous variables that did not have a normal distribution. The chi-squared test or Fisher’s exact test was used to compare the proportions of categorical variables (e.g., sex) between groups. The alpha level was set to 0.05. The significance level was set to p < 0.05 for two-sided tests.
Survival curves were estimated using the Kaplan–Meier method, and life expectancy between the two groups was assessed using the log-rank test. The multivariate Cox proportional hazards model was used to estimate the adjusted hazard ratio (HR) with the 95% confidence interval. Multivariate regression analysis was performed using age, sex, smoking history, histology, presence of concomitant platinum use, presence of TKI use, recurrence, performance status, and COPD in the COPD-non-COPD group comparison. Multivariate regression analysis of COPD patients was performed using age, sex, platinum use (yes or no), TKI use (yes or no), performance status, COPD severity, and COPD treatment (yes or no).
All statistical analyses were performed using the statistical software JMP Pro 14.0 for Windows (SAS Institute Inc, Tokyo, Japan, www.jmp.com). Kaplan–Mayer curves were visualized by GraphPad Prism 9.2 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com).
Results
Patient characteristics with advanced NSCLC
Detailed demographic clinical information is shown in Table 1. Of the 550 patients who underwent chemotherapy for recurrence after curative treatment and locally advanced or metastatic NSCLC, 347 patients (63.1%) who underwent pulmonary function tests were analyzed. Of them, 103 patients (18.9%) had COPD (COPD group), 219 (39.8%) did not (non-COPD group), and 25 were excluded due to diagnosis of asthma and radiation pneumonitis. As we expected, sex, smoking status, tyrosine kinase inhibitor (TKI) treatment, histology and PS were significantly different between the COPD group and the non-COPD group.
Table 1.
Clinical characteristics of patients with advanced NSCLC.
| Characteristic | Non-COPD n = 219 |
COPD n = 103 |
P value |
|---|---|---|---|
| Age (years) | 67.0 ± 10.2a | 68.6 ± 7.77a | 0.16b |
| Sex, male (%) | 118 (53.9) | 94 (91.3) | < 0.001c |
| Smoking status | < 0.001c | ||
| Nonsmoker | 112 (51.1) | 0 | |
| Former smoker | 63 (28.8) | 44 (42.7) | |
| Current smoker | 44 (20.1) | 59 (57.2) | |
| Chemotherapy | |||
| Platinum doublet | 138 (63.0) | 61 (59.2) | 0.54 |
| TKI | 131 (59.8) | 36 (35.0) | < 0.001 |
| Histology | 0.0001c | ||
| Squamous | 26 (11.9) | 29 (28.2) | |
| Adeno | 177 (80.8) | 59 (57.3) | |
| NSCLC | 11 (5.0) | 12 (11.7) | |
| Other | 5 (2.3) | 3 (2.9) | |
| NSCLC stage | 0.90 | ||
| 4 | 151 (69.0) | 70 (68.0) | |
| Recurrence | 68 (31.1) | 33 (32.0) | |
| Surgery/radiotherapy/chemoradiotherapy | 40/6/26 | 14/5/15 | |
| Performance status | 0.0136c | ||
| 0 | 119 (54.3) | 44 (42.7) | |
| 1 | 86 (39.3) | 47 (45.6) | |
| 2 | 9 (4.1) | 12 (11.7) | |
| 3 | 5 (2.3) | 0 (0) | |
Continuous variables are presented as the mean, and categorical variables are presented as the number (%). Comparisons were made by means of chi-squared tests unless otherwise indicated.
Significant values are in [italics].
TKI Tyrosine kinase inhibitors, NSCLC Non-small-cell lung cancer.
aMean ± SD.
bStudent’s t-test.
cFisher’s exact test.
Among the COPD group, 37 patients in the COPD group had received pharmacological treatment for COPD, mainly using LABDs. Thirty-three (89.1%) patients were treated with tiotropium, a long-acting muscarinic antagonist (LAMA). Other patients were treated with a long-acting beta agonist (LABA) alone or in combination with an inhaled corticosteroid (ICS), and 13 (36.1%) patients were treated with all three compounds (Table 2)32.
Table 2.
Univariate and multivariate analyses of the association between various clinical characteristics and OS.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HRadj (95% CI) | P value | |
| Age, < 75 years | 0.73 (0.56–0.96) | 0.021 | 0.89 (0.64–1.24) | 0.48 |
| Sex, male | 1.76 (1.36–2.30) | < 0.001 | 1.36 (0.89–2.06) | 0.15 |
| Smoking status, ≥ 10 pack-years | 1.97 (1.52–2.58) | < 0.001 | 1.17 (0.74–1.86) | 0.51 |
| Histology, squamous | 1.88 (1.39–2.56) | < 0.001 | 1.19 (0.84–1.68) | 0.32 |
| Chemotherapy | ||||
| Platinum doublet | 0.79 (0.62–1.01) | 0.060 | 0.83 (0.61–1.14) | 0.26 |
| TKI | 0.52 (0.41–0.67) | < 0.001 | 0.66 (0.49–0.88) | 0.0047 |
| Recurrence | 0.62 (0.47–0.80) | 0.0004 | 0.62 (0.47–0.83) | 0.0011 |
| Performance status, ≥ 2 | 3.92 (2.53–5.84) | < 0.001 | 3.36 (2.13–5.31) | < 0.001 |
| COPD | 1.62 (1.25–2.09) | 0.0002 | 1.06 (0.78–1.43) | 0.72 |
Comparisons were made by means of chi-squared tests unless otherwise indicated.
Significant values are in [italics].
TKI Tyrosine kinase inhibitors.
COPD and OS in patients with advanced NSCLC
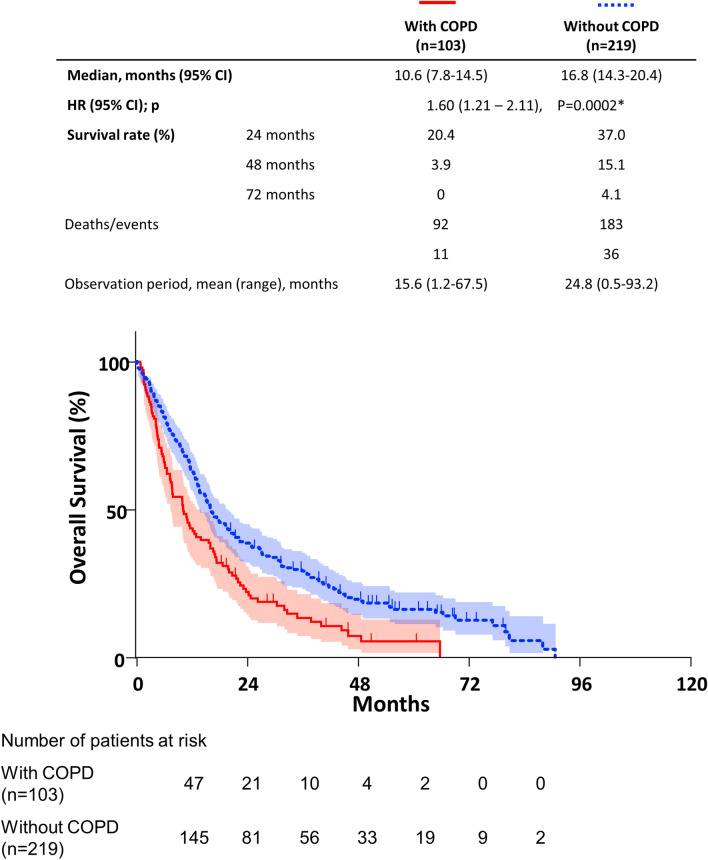
Kaplan–Meier curves and log-rank tests showed that COPD was associated with significantly shorter OS in advanced NSCLC (Fig. 1). The median OS in the COPD group (10.6 months) was lower than that in the non-COPD group (16.8 months).
Figure 1.
Kaplan–Meier curve of OS stratified by COPD. Coexisting COPD was associated with a significantly shorter OS in advanced NSCLC patients (P = 0.0002*, log-rank test). CI confidence interval, HR hazard ratio.
In the univariate Cox proportional hazard model, after adjusting for clinicopathologic variables, sex (male), smoking status (≥ 10 pack-years), histology (squamous carcinoma), non-TKI treatment and better PS were significantly associated with worse OS in patients with advanced NSCLC (Table 2).
In the multivariate Cox proportional hazard model, after adjusting for clinicopathologic variables, TKI treatment, recurrence after curative treatment and better PS were significantly associated with better OS in patients with advanced NSCLC (Table 2). However, the adjusted hazard ratio (HRadj) for COPD patients compared with non-COPD patients was 1.06 (95% confidence interval (CI) 0.78–1.43).
Patient characteristics in the COPD group
Detailed demographic clinical information of the COPD group is shown in Table 3. Classification of airflow limitation severity in COPD (GOLD) and recurrence were significantly different between the patients who received COPD treatment and those who did not. The proportion of patients with severe COPD was 37.8% in the COPD treatment group and 6.1% in the group without COPD treatment. The proportion of patients with recurrence in the patients with and without COPD treatment was 46% and 24.2%, respectively.
Table 3.
Clinical characteristics in COPD group.
| Characteristic | With COPD treatment n = 37 |
Without COPD treatment n = 66 |
P value |
|---|---|---|---|
| Age (years) | 68.0 ± 7.8a | 69.0 ± 7.8a | 0.84b |
| Sex, male | 33 (89.2) | 61 (92.4) | 0.72c |
| Smoking status | 0.94 | ||
| Nonsmoker | 0 | 0 | |
| Former smoker | 16 (43.2) | 28 (42.4) | |
| Current smoker | 21 (56.8) | 38 (57.6) | |
| GOLD | 0.0002c | ||
| 1 | 4 (10.8) | 20 (30.3) | |
| 2 | 19 (51.4) | 42 (63.6) | |
| 3 | 11 (29.7) | 4 (6.1) | |
| 4 | 3 (8.1) | 0 (0) | |
| COPD treatment | |||
| LAMA | 32 (86.5) | – | |
| ICS/LABA | 20 (54.1) | – | |
| Triple therapy | 13 (35.1) | – | |
| Comorbidities | |||
| Interstitial pneumonitis | 3 (8.1) | 1 (1.5) | 0.13c |
| History of cardiovascular events | 4 (10.8) | 11(16.7) | 0.56c |
| Chemotherapy | |||
| Platinum doublet | 21 (56.8) | 40 (60.6) | 0.70 |
| TKI | 9 (24.3) | 27 (40.9) | 0.13c |
| Number of regimens (range) | 3.16 (1–13) | 2.39 (1–11) | 0.45b |
| Histology | 0.63c | ||
| Squamous | 10 (27.0) | 19 (28.8) | |
| Adeno | 22 (59.5) | 37 (56.1) | |
| NSCLC | 3 (8.1) | 9 (13.6) | |
| Other | 2 (5.4) | 1 (1.5) | |
| NSCLC stage | 0.0248 | ||
| 4 | 20 (54.1) | 50 (75.8) | |
| Recurrence | 17 (46.0) | 16 (24.2) | |
| Surgery/radiotherapy/chemoradiotherapy | 6/3/9 | 8/2/6 | |
| Performance status | 0.84c | ||
| 0 | 16 (43.2) | 28 (42.4) | |
| 1 | 16 (43.2) | 31 (47.0) | |
| 2 | 5 (13.5) | 7 (10.6) | |
| 3 | 0 (0) | 0 (0) | |
| 4 | 0 (0) | 0 (0) | |
Continuous variables are presented as the mean, and categorical variables are presented as the number (%). Comparisons were made by means of chi-squared tests unless otherwise indicated.
Significant values are in [italics].
GOLD The Global Initiative for Chronic Obstructive Lung Disease, LAMA Long-acting muscarinic antagonist, ICS Inhaled corticosteroid, LABA Long-acting beta agonist, TKI Tyrosine kinase inhibitors, NSCLC Non-small-cell lung cancer.
aMean ± SD.
bWilcoxon signed-rank test.
cFisher’s exact test.
COPD treatment and OS
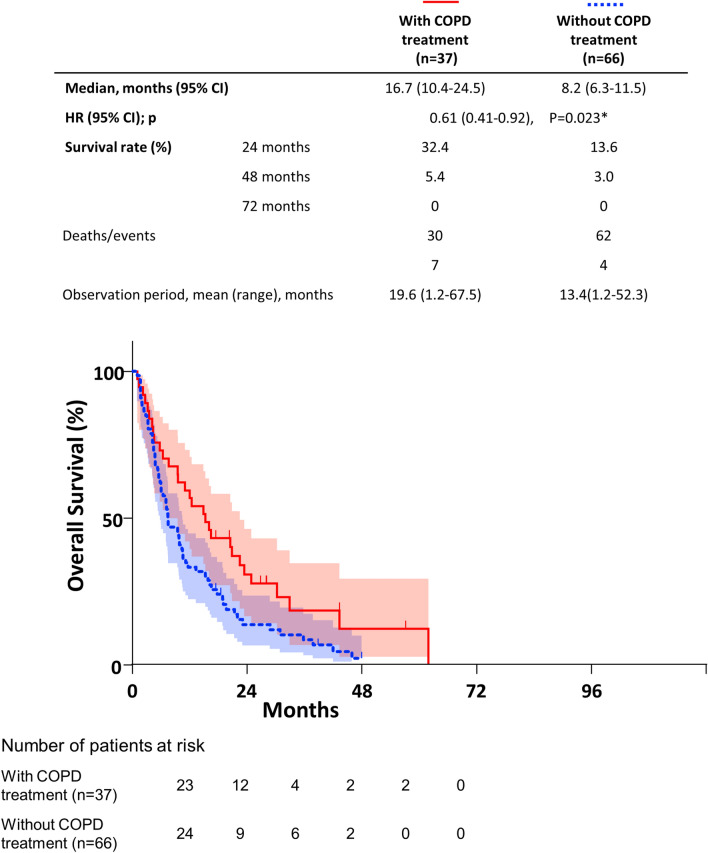
Kaplan–Meier curves and log-rank tests showed that COPD treatment was associated with significantly longer OS in advanced NSCLC (Fig. 2). The median OS in patients without COPD treatment (8.2 months) was lower than that in patients with COPD treatment (16.8 months). In the univariate Cox proportional hazard model, recurrence, better PS and COPD treatment were significantly associated with better OS in the COPD group (Table 4). In the multivariate Cox proportional hazard model, recurrence, mild COPD stage, better PS, and COPD treatment were significantly associated with better OS in patients with advanced NSCLC, and the HRadj for COPD treatment was 0.52 (95% CI 0.31–0.87).
Figure 2.
Kaplan–Meier curve of OS stratified by COPD treatment. COPD treatment was associated with a significantly longer OS in advanced NSCLC patients (P = 0.023*, log-rank test). CI confidence interval, HR hazard ratio.
Table 4.
Univariate and multivariate analyses of the association between various clinical characteristics and OS in the COPD group.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HRadj (95% CI) | P value | |
| Age, < 75 years | 0.89 (0.56–1.45) | 0.63 | 1.16 (0.68–1.97) | 0.59 |
| Sex, male | 0.94 (0.48–2.11) | 0.86 | 1.14 (0.52–2.50) | 0.75 |
| Recurrence | 0.49 (0.30–0.78) | 0.0037 | 0.45 (0.27–0.76) | 0.0017 |
| Chemotherapy | ||||
| Platinum doublet | 0.79 (0.52–1.20) | 0.26 | 0.76 (0.48–1.23) | 0.27 |
| TKI | 0.94 (0.60–1.45) | 0.79 | 0.76 (0.47–1.23) | 0.25 |
| %FEV1 < 80% | 1.48 (0.92–2.47) | 0.12 | 2.63 (1.53–4.70) | 0.0007 |
| Performance status, ≥ 2 | 2.96 (1.51–5.31) | 0.0006 | 2.79 (1.40–5.57) | 0.0037 |
| COPD treatment, yes | 0.60 (0.38–0.93) | 0.0243 | 0.52 (0.31–0.87) | 0.0134 |
Comparisons were made by means of chi-squared tests unless otherwise indicated.
Significant values are in [italics].
TKI Tyrosine kinase inhibitors, FEV1 forced expiratory volume in 1 s.
Discussion
Here, we found that coexisting COPD might be associated with a worse survival outcome in patients with advanced NSCLC in our observational retrospective cohort study. Interestingly, among patients with coexisting COPD, more than half of the patients did not receive pharmacological treatment for COPD, and these patients had a substantially worse prognosis than patients with treatment for COPD. Our results indicated that even in patients with advanced NSCLC, coexisting COPD must be treated accordingly. To the best of our knowledge, this is the first study to identify the survival impact of pharmacotherapy for coexisting COPD in patients with advanced NSCLC.
COPD is often a comorbid condition in patients with lung cancer, but its effect on survival is not well understood. In the present study, almost half of the patients who had a smoking history and spirometry had COPD (coexisting COPD). These patients tended to be male, infrequently treated without TKIs, infrequently diagnosed without squamous carcinoma and have a poorer PS than patients without COPD. These factors may be associated with a poor prognosis. Although univariate Cox regression analysis revealed a negative impact of coexisting COPD for patients with advanced lung cancer, multivariate regression analysis showed that coexisting COPD did not have a significant impact on OS. These results are somehow inconsistent with those of previous studies. A meta-analysis including 21 studies (60,764 participants and 11,270 cases) revealed that COPD had a significant impact on OS in lung cancer patients33. However, several reports show that COPD does not have a significant deleterious impact on OS34,35. A previous meta-analysis also showed that the impact of coexisting COPD may be smaller in patients with advanced cancer than in patients with early-stage cancer33.
Notably, the median OS of patients with treatment for coexisting COPD was significantly prolonged relative to that of the patients without treatment (16.7 vs. 8.2 months, Fig. 1) and was equivalent to that in the non-COPD groups (16.8 months, Supplemental Fig. S1, Additional File 1). Since all of the patients were treated according to guidelines for COPD in a previous study34, our results are quite consistent with those of previous studies.
On the other hand, a lack of treatment for coexisting COPD is an important issue. In the present study, 64% of the patients with coexisting COPD did not receive treatment for COPD, including LABDs. Furthermore, the patients without COPD treatment had a lower proportion of severe to very severe COPD than those with COPD treatment. The proportions of patients with mild to moderate COPD and severe to very severe COPD in the patients with treatment were 62.2% and 37.8%, whereas in the patients without treatment, they were 93.9% and 6.1%, respectively. However, mild COPD patients may not complain much compared to severe COPD patients and may not request treatment for their symptoms. As a result, the patients without treatment showed a worse survival outcome despite the milder severity of COPD. These results suggest that we must consider pharmacological treatment for coexisting COPD to improve OS in patients with advanced NSCLC even in the mild stage of COPD.
Regarding pharmacological treatment for COPD, three major classes are currently available, including LAMAs, LABAs, and ICSs32. Treatment with these drugs (mono- or combination therapy) was associated with improvements in lung function, exercise capacity, quality of life, the rate of exacerbations, and prognosis21–25. In this study, 89.1% of patients with COPD treatment were treated with tiotropium (LAMA). During our enrolment period of April 2007 to March 2014, tiotropium and combined fluticasone/salmeterol were the most popular medications. Due to the small sample size and because 13 patients used combined medications (such as “triple therapy”), performing an additional analysis to compare the effects of two medications in the present study was difficult; however, pharmacological treatment for COPD might contribute to preventing a PS decline in advanced NSCLC patients with COPD.
The mechanisms underlying the positive impact on the prognosis of advanced NSCLC patients with coexisting COPD are still unclear, and the biological mechanisms related to the anti-oncogenesis or anti-inflammatory effects are uncertain36,37, although a possible mechanism may be prevention of a functional status decline due to breathlessness, resulting in an increased number of chemotherapy regimens. Unfortunately, no significant difference was found in the number of chemotherapy regimens between the two groups, whereas according to the effect of palliative care, appropriate treatment and care for breathlessness may have a positive impact on OS38. Patients with lung cancer and coexisting COPD who received care from a pulmonologist were significantly more likely to undergo surgery and experience improved survival39. Pulmonologists might improve symptom management and decrease respiratory complications by preventing functional status declines.
A limitation of this study is the small sample size from a single center. Therefore, unexpected confounding biases and the influence of data deficiency cannot be excluded. Since this study was retrospective cohort, the choice of whether or not to perform spirometry was not random, and confounding bias could not be excluded. As shown in Table S2, patients who were excluded from the study due to no spirometry had mostly stage 4 disease and a relatively poor PS (Table S2, Additional File 1). In fact, these patients may not have benefited from COPD treatment if they had COPD. However, if they had undergone spirometry before the worsening of their condition, COPD treatments may have helped to improve their condition to some extent. We think that physicians should be encouraged to perform spirometry to detect COPD in patients with lung cancer, and if they have COPD, COPD treatments should be considered. Moreover, there was the possibility that patients might be treated for COPD due to the physician's expectations regarding the long-term prognosis or expectation of good organ function. In most cases of patients who had COPD in their medical records but did not receive treatment, there was no reason noted in the medical record, even though some of the patients clearly had COPD (Table S3, Additional File 1). However, due to the supportive results in the univariate and multivariate analyses, we believe that potential biases were adequately considered and may not have had a considerable impact on the present analysis. To overcome this limitation, we are currently undergoing a multi-institutional joint study to confirm the efficacy of COPD treatment in NSCLC patients with COPD. Another limitation is that patients who received immunotherapy were excluded from the present analysis. Because ICIs have an extensive impact on survival in advanced NSCLC patients and have already changed the basic chemotherapy strategies for NSCLC, we had to eliminate these effects in the present study. Moreover, regarding this issue, we performed an additional validation analysis on patients with advanced NSCLC who were treated with ICIs after 2016 (“Supplemental information”, Additional File 1), and we were able to confirm similar positive impacts of treatment for coexisting COPD (by log-rank test, P = 0.036, Supplemental Table S1, Figs. S3, S4, Additional File 1). Since strategies involving ICIs may significantly prolong the expected survival of advanced NSCLC patients, treatment for coexisting chronic diseases, such as COPD should be appropriately considered.
The current guidelines for lung cancer treatment do not describe how to treat lung cancer with COPD and are mostly dedicated to describing recent advances in genomic-targeted therapy for advanced NSCLC40. With regard to ICIs, the anti-PD-1/PD-L1 antibody had more curative properties in current or former smokers41. Therefore, considering management of coexisting COPD in patients with advanced NSCLC should become more important. Symptoms of COPD, such as dyspnea on exertion, sputum, and cough, may be masked by symptoms of advanced lung cancer. Our findings have clinical implications for advanced NSCLC, especially in an era when “living with cancer and comorbidities” is required.
Conclusion
We found that untreated coexisting COPD may have a considerable impact on the prognosis of patients with advanced NSCLC. Pharmacological treatment for coexisting COPD, might have the potential to improve the prognosis of patients with advanced NSCLC with or without treatment with ICIs.
Supplementary Information
Acknowledgements
The authors also thank Atsuyasu Sato and Naoya Tanabe at Kyoto University Hospital for help with acquiring preliminary data.
Abbreviations
- NSCLC
Non-small-cell lung cancer
- BASCs
Bronchoalveolar stem cells
- COPD
Chronic obstructive pulmonary disease
- OS
Overall survival
- PS
Performance status
- HRadj
Adjusted hazard ratio
- CI
Confidence interval
- BMI
Body mass index
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- LABDs
Long-acting bronchodilators
- LAMA
Long-acting muscarinic antagonist
- ICS
Inhaled corticosteroid
- LABA
Long-acting beta agonist
- TKI
Tyrosine kinase inhibitors
- ICIs
Immune checkpoint inhibitors
Author contributions
H.A., H.O.: study concept and design, statistical analysis, interpretation of the data, and drafting of the manuscript; S.S.: study concept and design, interpretation of the data, critical revision of the manuscript, and funding acquisition for the study; T.F.: acquisition and interpretation of the data; Y.S.: acquisition and interpretation of the data; K.K.: acquisition of the data; T.N., T.O., K.H., M.Y., T.T., H.Y., R.I., Y.H.K.: acquisition and interpretation of the data; K.U.: supervision of statistical analysis; S.M., T.H.: interpretation of the data and finalization of the manuscript.
Funding
This study was supported by a Grant from Nippon Boehringer Ingelheim.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
This study was supported by a grant from Nippon Boehringer Ingelheim (BI). SS received this grant from BI. BI had no role in the design, analysis or interpretation of the study or in the writing of the manuscript. BI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BI substances, as well as intellectual property considerations. All other authors have no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained errors in content. Modifications have been made to the Results and Discussion sections, Table 2 and Table 4. In addition, the Supplementary Information file published with this Article contained an error in Figure S4. Full information regarding the corrections made can be found in the correction for this Article.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/6/2023
A Correction to this paper has been published: 10.1038/s41598-023-29102-0
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-03139-5.
References
- 1.Takiguchi Y, Sekine I, Iwasawa S, Kurimoto R, Tatsumi K. Chronic obstructive pulmonary disease as a risk factor for lung cancer. World J. Clin. Oncol. 2014;5:660–666. doi: 10.5306/wjco.v5.i4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World health organization fact sheets; Fact sheets on cancer from IARC Updated March 2019. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 10 July 2021.
- 4.Koshiol J, Rotunno M, Consonni D, Pesatori AC, De Matteis S, Goldstein AM, et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case–control study. PLoS ONE. 2009;4:e7380. doi: 10.1371/journal.pone.0007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am. J. Respir. Crit. Care Med. 2007;176:285–290. doi: 10.1164/rccm.200612-1792OC. [DOI] [PubMed] [Google Scholar]
- 6.Congleton J, Muers MF. The incidence of airflow obstruction in bronchial carcinoma, its relation to breathlessness, and response to bronchodilator therapy. Respir. Med. 1995;89:291–296. doi: 10.1016/0954-6111(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 7.Loganathan RS, Stover DE, Shi W, Venkatraman E. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest. 2006;129:1305–1312. doi: 10.1378/chest.129.5.1305. [DOI] [PubMed] [Google Scholar]
- 8.Media AS, Persson M, Tajhizi N, Weinreich UM. Chronic obstructive pulmonary disease and comorbidities' influence on mortality in non-small cell lung cancer patients. Acta Oncol. 2019;58:1102–1106. doi: 10.1080/0284186X.2019.1612942. [DOI] [PubMed] [Google Scholar]
- 9.Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J. 2009;34:380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 10.Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: A systematic review and meta-analysis. Thorax. 2005;60:570–575. doi: 10.1136/thx.2004.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balata H, Harvey J, Barber PV, Colligan D, Duerden R, Elton P, et al. Spirometry performed as part of the Manchester community-based lung cancer screening programme detects a high prevalence of airflow obstruction in individuals without a prior diagnosis of COPD. Thorax. 2020;75:655–660. doi: 10.1136/thoraxjnl-2019-213584. [DOI] [PubMed] [Google Scholar]
- 12.Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir. Med. 2013;1:73–83. doi: 10.1016/S2213-2600(12)70060-7. [DOI] [PubMed] [Google Scholar]
- 13.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 2001;34:50s–59s. doi: 10.1183/09031936.01.00229701. [DOI] [PubMed] [Google Scholar]
- 14.Zeskind JE, Lenburg ME, Spira A. Translating the COPD transcriptome: Insights into pathogenesis and tools for clinical management. Proc. Am. Thorac. Soc. 2008;5:834–841. doi: 10.1513/pats.200807-074TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee G, Walser TC, Dubinett SM. Chronic inflammation, chronic obstructive pulmonary disease, and lung cancer. Curr. Opin. Pulm. Med. 2009;15:303–307. doi: 10.1097/MCP.0b013e32832c975a. [DOI] [PubMed] [Google Scholar]
- 16.O'Callaghan DS, O'Donnell D, O'Connell F, O'Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J. Thorac. Oncol. 2010;5:2024–2036. doi: 10.1097/jto.0b013e3181f387e4. [DOI] [PubMed] [Google Scholar]
- 17.Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nat. Med. 2008;14:1023–1024. doi: 10.1038/nm1008-1023. [DOI] [PubMed] [Google Scholar]
- 18.Sekine Y, Yamada Y, Chiyo M, Iwata T, Nakajima T, Yasufuku K, et al. Association of chronic obstructive pulmonary disease and tumor recurrence in patients with stage IA lung cancer after complete resection. Ann. Thorac. Surg. 2007;84:946–950. doi: 10.1016/j.athoracsur.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 19.Zhai R, Yu X, Shafer A, Wain JC, Christiani DC. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest. 2014;145:346–353. doi: 10.1378/chest.13-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celli BR. Pharmacological therapy of COPD: Reasons for optimism. Chest. 2018;154:1404–1415. doi: 10.1016/j.chest.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Tashkin DP, Celli BR, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 22.Ichinose M, Taniguchi H, Takizawa A, Grönke L, Loaiza L, Voß F, et al. The efficacy and safety of combined tiotropium and olodaterol via the Respimat (®) inhaler in patients with COPD: results from the Japanese sub-population of the Tonado (®) studies. Int. J. Chron. Obstruct. Pulmon. Dis. 2016;11:2017–2027. doi: 10.2147/COPD.S110389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Donnell D, Casaburi R, Frith P, Kirsten A, De Sousa D, Hamilton A, et al. Effects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPD. Eur. Respir. J. 2017;49:1601348. doi: 10.1183/13993003.01348-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maltais F, Aumann JL, Kirsten AM, Nadreau É, Macesic H, Jin X, et al. Dual bronchodilation with tiotropium/olodaterol further reduces activity-related breathlessness versus tiotropium alone in COPD. Eur. Respir. J. 2019;53:1802049. doi: 10.1183/13993003.02049-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calverley PMA, Anzueto AR, Carter K, Grönke L, Hallmann C, Jenkins C, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): A double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir. Med. 2018;6:337–344. doi: 10.1016/S2213-2600(18)30102-4. [DOI] [PubMed] [Google Scholar]
- 26.Kubota M, Kobayashi H, Quanjer PH, Omori H, Tatsumi K, Kanazawa M. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir. Investig. 2014;52:242–250. doi: 10.1016/j.resinv.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Vermeersch K, Gabrovska M, Aumann J, Demedts IK, Corhay JL, Marchand E, et al. Azithromycin during acute chronic obstructive pulmonary disease exacerbations requiring hospitalization (BACE). A multicenter, randomized, double-blind, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2019;200(7):857–868. doi: 10.1164/rccm.201901-0094OC. [DOI] [PubMed] [Google Scholar]
- 28.Boschetto P, Fucili A, Stendardo M, Malagù M, Parrinello G, Casimirri E, et al. Occurrence and impact of chronic obstructive pulmonary disease in elderly patients with stable heart failure. Respirology. 2013;18(1):125–130. doi: 10.1111/j.1440-1843.2012.02264.x. [DOI] [PubMed] [Google Scholar]
- 29.Tanabe N, Sato S, Oguma T, Shima H, Kubo T, Kozawa S, et al. Influence of asthma onset on airway dimensions on ultra-high-resolution computed tomography in chronic obstructive pulmonary disease. J. Thorac. Imaging. 2021;36(4):224–230. doi: 10.1097/RTI.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe N, Kaji S, Sato S, Yokoyama T, Oguma T, Tanizawa K, et al. A homological approach to a mathematical definition of pulmonary fibrosis and emphysema on computed tomography. J. Appl. Physiol. 1985;131(2):601–612. doi: 10.1152/japplphysiol.00150.2021. [DOI] [PubMed] [Google Scholar]
- 31.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report GOLD executive summary. Am. J. Respir. Crit. Care Med. 2017;195:557–882. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 32.Singh D, Brooks J, Hagan G, Cahn A, O'Connor BJ. Superiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63:592–598. doi: 10.1136/thx.2007.087213. [DOI] [PubMed] [Google Scholar]
- 33.Gao YH, Guan WJ, Liu Q, Wang HQ, Zhu YN, Chen RC, et al. Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies. Respirology. 2016;21(2):269–279. doi: 10.1111/resp.12661. [DOI] [PubMed] [Google Scholar]
- 34.Izquierdo JL, Resano P, El Hachem A, Graziani D, Almonacid C, Sánchez IM. Impact of COPD in patients with lung cancer and advanced disease treated with chemotherapy and/or tyrosine kinase inhibitors. Int. J. Chron. Obstruct Pulmon. Dis. 2014 doi: 10.2147/COPD.S68766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omote N, Hashimoto N, Morise M, Sakamoto K, Miyazaki S, Ando A, et al. Impact of mild to moderate COPD on feasibility and prognosis in non-small cell lung cancer patients who received chemotherapy. Int. J. Chron. Obstruct. Pulmon. Dis. 2017;12:3541–3547. doi: 10.2147/COPD.S149456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau JK, Brown KC, Thornhill BA, Crabtree CM, Dom AM, Witte TR, et al. Inhibition of cholinergic signaling causes apoptosis in human bronchioalveolar carcinoma. Cancer Res. 2013;73:1328–1339. doi: 10.1158/0008-5472.CAN-12-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CH, Lee CH, Ho YS. Nicotinic acetylcholine receptor-based blockade: Applications of molecular targets for cancer therapy. Clin. Cancer Res. 2011;17:3533–3541. doi: 10.1158/1078-0432.CCR-10-2434. [DOI] [PubMed] [Google Scholar]
- 38.Higginson IJ, Bausewein C, Reilly CC, Gao W, Gysels M, Dzingina M, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: A randomised controlled trial. Lancet Respir. Med. 2014;2:979–987. doi: 10.1016/S2213-2600(14)70226-7. [DOI] [PubMed] [Google Scholar]
- 39.Deepak JA, Ng X, Feliciano J, Mao L, Davidoff AJ. Pulmonologist involvement, stage-specific treatment, and survival in adults with non-small cell lung cancer and chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2015;12:742–751. doi: 10.1513/AnnalsATS.201406-230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.