Abstract
Background:
The COVID-19 pandemic has had a major impact on access to health care resources. Our objective was to estimate the impact of the COVID-19 pandemic on the incidence of childhood cancer in Canada. We also aimed to compare the proportion of patients who enrolled in clinical trials at diagnosis, presented with metastatic disease or had an early death during the first 9 months of the COVID-19 pandemic compared with previous years.
Methods:
We conducted an observational study that included children younger than 15 years with a new diagnosis of cancer between March 2016 and November 2020 at 1 of 17 Canadian pediatric oncology centres. Our primary outcome was the monthly age-standardized incidence rates (ASIRs) of cancers. We evaluated level and trend changes using interventional autoregressive integrated moving average models. Secondary outcomes were the proportion of patients who were enrolled in a clinical trial, who had metastatic or advanced disease and who died within 30 days. We compared the baseline and pandemic periods using rate ratios (RRs) and 95% confidence intervals (CIs).
Results:
Age-standardized incidence rates during COVID-19 quarters were 157.7, 164.6, and 148.0 per million, respectively, whereas quarterly baseline ASIRs ranged between 150.3 and 175.1 per million (incidence RR 0.93 [95% CI 0.78 to 1.12] to incidence RR 1.04 [95% CI 0.87 to 1.24]). We found no statistically significant level or slope changes between the projected and observed ASIRs for all new cancers (parameter estimate [β], level 4.98, 95% CI −15.1 to 25.04, p = 0.25), or when stratified by cancer type or by geographic area. Clinical trial enrolment rate was stable or increased during the pandemic compared with baseline (RR 1.22 [95% CI 0.70 to 2.13] to RR 1.71 [95% CI 1.01 to 2.89]). There was no difference in the proportion of patients with metastatic disease (RR 0.84 [95% CI 0.55 to 1.29] to RR 1.22 [0.84 to 1.79]), or who died within 30 days (RR 0.16 [95% CI 0.01 to 3.04] to RR 1.73 [95% CI 0.38 to 15.2]).
Interpretation:
We did not observe a statistically significant change in the incidence of childhood cancer, or in the proportion of children enrolling in a clinical trial, presenting with metastatic disease or who died early during the first 9 months of the COVID-19 pandemic, which suggests that access to health care in pediatric oncology was not reduced substantially in Canada.
Concerns have been raised that the COVID-19 pandemic disrupted health care–seeking behaviours and access to health care, affecting the diagnosis and management of other conditions such as cancer. Studies conducted in the Netherlands and United Kingdom using administrative data have shown as much as a 50% reduction in cancer incidence in adults after March 2020.1,2 Other studies in adult populations thus far have shown a decrease in the number of new cancer diagnoses, and cancer-related medical visits, therapies and surgeries, 1,3–5 raising concerns about potential excess cancer mortality in the upcoming years.6 This may be explained partly by the suspension or reduction of cancer-screening procedures, such as mammography, colonoscopy and cervical cytology by up to 90%,3,5,7 because these screening initiatives play a critical role in the detection of cancers in adults. A 2020 retrospective single-centre cohort study in Japan that involved 123 patients with colorectal cancer reported that significantly more of these patients presented with complete intestinal obstruction, which suggests that detection delays might have contributed to diagnosis at later stages of the disease.8 It is unclear whether these findings apply to childhood cancer because cancer screening is not part of routine pediatric care, and early detection may not be as important in childhood cancer than in its adult counterpart.9
In children, case series and single-centre retrospective cohort studies, notably from Italy and the United States, suggested a marked reduction in incident cancers, along with high acuity of care at presentation.10–13 Similar concerns of delayed clinical presentation were raised in other pediatric patient populations, with reports of children presenting at late stages of sepsis or diabetic ketoacidosis, which suggests a delay in seeking care.14,15
It is possible that fear of COVID-19 dissuaded families with children from seeking care for nonspecific symptoms such as pain, headache or fatigue, which are typical triggers leading to a pediatric cancer diagnosis. Understanding the indirect effects of health policies during the COVID-19 pandemic is important to guide policy-making and mitigate barriers to essential health care in future public health crises.
Our objective was to measure the impact of the COVID-19 pandemic and associated restrictions on the incidence of childhood cancer in Canada. We also aimed to compare the proportion of patients who enrolled in clinical trials at diagnosis, presented with metastatic disease or died during the first 9 months of the COVID-19 pandemic compared with previous years.
Methods
We conducted a retrospective interrupted time series analysis using a population-based active cancer registry (Cancer in Young People in Canada [CYP-C]).
Study setting
In Canada, pediatric oncology care is regionalized in 17 pediatric hematology–oncology centres, distributed across the country, which form the C17 Council (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210659/tab-related-content).
Data sources
The CYP-C is a collaboration between the Public Health Agency of Canada, the C17 Council and the Canadian Partnership Against Cancer. Trained clinical research assistants or data managers abstract data prospectively from medical records at each site. Multiple approaches are used to ensure high-quality data.16–18 Data from the 5 Ontario sites are collected into the Pediatric Oncology Group of Ontario Networked Information System (POGONIS) and are transferred to CYP-C.
For our study, all centres contributed to accelerated data collection to obtain contemporary data and entered all new cancer diagnoses up to Nov. 30, 2020, into CYP-C as of June 22, 2021. The CYP-C Management Committee and the Pediatric Oncology Group of Ontario granted approval to use data in CYP-C for the purpose of this study.
Study population
We included all patients entered into CYP-C who were younger than 15 years of age at cancer diagnosis (aligned with CYP-C eligibility criteria), diagnosed with a neoplasm included in the International Classification of Childhood Cancer (ICCC-3), third edition, 19 and diagnosed and received treatment at 1 of the 17 pediatric oncology centres during the study periods. More than 95% of patients aged younger than 15 years at diagnosis in Canada receive treatment in 1 of these hospitals.17,20 We defined the COVID-19 pandemic period as March to November 2020 to correspond with the first reports of local transmission within Canada.21 The pre-pandemic, baseline period was December 2017 to February 2020.
Outcomes
Our primary outcome was the age-standardized incidence rate (ASIR) of newly diagnosed cancers per million population. We used the date of the most definitive diagnostic procedure, typically biopsy, as the date of diagnosis. Secondary outcomes included the proportion of patients who were enrolled in a clinical trial at diagnosis, presented with metastatic disease or died within 30 days of cancer diagnosis (early death). We considered enrolment in a clinical trial an important outcome because improvements in childhood cancer outcomes are thought to be largely attributable to high participation rates in cooperative clinical trials,22 whereas metastatic disease and early deaths were considered as proxies of delayed presentation or management. For solid cancers, any distant extension was considered metastatic disease. We categorized acute lymphoblastic leukemia as no central nervous system (CNS) involvement (CNS 1) or CNS positive (CNS 2 or 3). Precursor B-cell acute lymphoblastic leukemia was categorized as standard or high risk using the National Cancer Institute (NCI) risk scale.23 The presence of metastatic disease at presentation, CNS status and enrolment in a clinical trial are explicitly collected by CYP-C and POGONIS. We only considered enrolment in a therapeutic clinical trial at the initial diagnosis (excluding trials for supportive care interventions and for relapsed or refractory disease). Early death included all causes of death within 30 days of cancer diagnosis.
Statistical analysis
To compare quarterly outcomes, we compared pediatric cancers diagnosed from December 2017 to February 2020 (baseline period) to pediatric cancers diagnosed between March and November 2020 (COVID-19 pandemic). Then, to evaluate the association between the pandemic and changes in the monthly ASIR, we used cancer diagnoses between March 2016 and November 2020.
We calculated ASIRs using the direct method to standardize the age-specific rates to the 2011 Canadian population. Monthly and quarterly population estimates were based on the quarterly population estimates by age and sex between 2016 and 2020, for all Canadian provinces and territories.24,25
We defined each pandemic quarter incidence rate ratio (RR) as the quarterly ASIR divided by the median of ASIRs across the quarters of the baseline period. We estimated the 95% confidence interval (CI) of the incidence RR using the method for the ASIR.26
To evaluate changes in the monthly observed ASIRs during the pandemic period compared with the projected ASIRs, we performed interrupted time series analysis using the autoregressive integrated moving average model.27 The COVID-19 impact was evaluated by the changes in the level (a step change) and trend (slope) of the ASIRs.27–30 The methodology is detailed in Appendix 1.27,31
We performed stratified analysis by sex, cancer type and geographic area. We categorized cancer types as leukemia and lymphoma, CNS tumours or extracranial solid tumours19 because the diagnosis of some cancer types might require additional diagnostic and imaging procedures and thus may be more affected by the pandemic. Extracranial solid tumours included all non-CNS solid tumours, such as hepatic, renal and bone tumours.19
We divided Canada into 5 geographic regions (British Columbia, Prairies [Manitoba, Saskatchewan and Alberta], Ontario, Quebec and Atlantic provinces [Nova Scotia, Prince Edward Island, New Brunswick, Newfoundland and Labrador]) to reflect their differential burden of COVID-19. As of Aug. 1, 2020, the number of cumulative COVID-19 cases per 100 000 population for British Columbia, the Prairies, Ontario, Quebec and Atlantic provinces was 71.8, 182.0, 282.5, 700.8 and 63.5, respectively.32 We used RRs and 95% CIs to compare differences in proportions for all secondary outcomes between pandemic and baseline periods.
We defined the RR as the ratio of the proportion for the pandemic quarter to the median of proportions in the baseline period. Whereas the asymptotic confidence limits for the RR were estimated for a large sample, we used exact unconditional confidence limits for RR based on the relative risk score statistic when the count was less than 5. When the count was zero, we computed an adjusted RR, a logit estimate of the RR and CI, using a 0.5 correction factor in each cell of 0.
All analyses were conducted using SAS (version 9.4). We considered p values of less than 0.05 to be significant, without adjustment for multiplicity. We used complete case analysis.
Ethics approval
The Pediatric Oncology Group of Ontario is a prescribed entity pursuant to Section 45 of the Ontario Personal Health Information Protection Act and, therefore, research ethics board approval was not required for the 5 Ontario sites. Collection of data in CYP-C was approved by the Research Ethics Boards of all participating sites outside Ontario with a waiver of the requirement for informed consent. The CYP-C Management Committee and the Pediatric Oncology Group of Ontario granted approval to use data in CYP-C for the purpose of this study. As we used aggregate data, approval was deemed unnecessary after evaluation by the CHU de Québec’s Research Ethics Board.
Results
During the COVID-19 pandemic quarters (March–May, June–August and September–November 2020), the ASIRs of new cancer diagnoses registered in CYP-C were 157.7, 164.6 and 148.0 per million population (Table 1). These estimates were comparable to ASIRs from December 2017 to February 2020 (median 158.4, interquartile range [IQR] 155.6 to 170.6), with incidence RRs of 1.00 (95% CI 0.83 to 1.19), 1.04 (95% CI 0.87 to 1.24) and 0.93 (95% CI 0.78 to 1.12) for each of the COVID-19 pandemic quarters, respectively.
Table 1:
Quarterly age-standardized incidence rates per million among Canadian children younger than 15 years of age who had a new diagnosis of cancer between December 2017 and November 2020
| Parameter | Baseline period ASIR | COVID-19 pandemic ASIR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| December 2017–February 2018 | March–May 2018 | June–August 2018 | September–November 2018 | December 2018–February 2019 | March–May 2019 | June–August 2019 | September–November 2019 | December 2019–February 2020 | March–May 2020 | June–August 2020 | September–November 2020 | |
| All cancers combined | 166.9 | 158.4 | 155.6 | 173.5 | 156.4 | 175.1 | 170.6 | 150.3 | 153.0 | 157.7 | 164.6 | 148.0 |
|
| ||||||||||||
| Sex | ||||||||||||
|
| ||||||||||||
| Male | 179.7 | 166.8 | 173.7 | 189.5 | 172.9 | 183.7 | 188.2 | 159.9 | 163.4 | 167.7 | 194.1 | 151.6 |
|
| ||||||||||||
| Female | 153.5 | 149.5 | 136.6 | 156.8 | 139.2 | 166.2 | 152.5 | 140.2 | 142.1 | 147.2 | 133.5 | 144.1 |
|
| ||||||||||||
| Age, yr | ||||||||||||
|
| ||||||||||||
| < 1 | 262.8 | 265.0 | 297.2 | 325.8 | 230.8 | 338.1 | 203.5 | 221.7 | 321.5 | 312.9 | 309.0 | 253.9 |
|
| ||||||||||||
| 1–4 | 225.2 | 220.1 | 184.1 | 217.4 | 222.7 | 233.1 | 258.9 | 210.2 | 199.7 | 228.1 | 242.7 | 217.6 |
|
| ||||||||||||
| 5–9 | 150.0 | 102.5 | 118.1 | 121.8 | 112.0 | 135.5 | 141.1 | 109.6 | 121.3 | 97.8 | 95.9 | 109.7 |
|
| ||||||||||||
| 10–14 | 117.7 | 141.3 | 140.5 | 157.7 | 131.1 | 134.5 | 122.0 | 127.1 | 112.9 | 127.9 | 139.0 | 108.1 |
|
| ||||||||||||
| Region | ||||||||||||
|
| ||||||||||||
| Atlantic* | 156.1 | 191.1 | 152.1 | 171.0 | 142.4 | 158.8 | 152.1 | 113.2 | 60.4 | 177.6 | 123.1 | 201.4 |
|
| ||||||||||||
| Quebec | 166.4 | 183.3 | 166.1 | 217.2 | 168.1 | 215.6 | 144.0 | 128.0 | 137.5 | 151.0 | 167.8 | 131.0 |
|
| ||||||||||||
| Ontario | 177.9 | 161.3 | 167.8 | 183.6 | 167.8 | 177.3 | 188.6 | 165.5 | 168.5 | 162.7 | 169.8 | 153.1 |
|
| ||||||||||||
| Prairies† | 136.1 | 130.4 | 151.5 | 144.6 | 170.5 | 138.5 | 181.4 | 138.0 | 178.9 | 173.6 | 184.1 | 163.5 |
|
| ||||||||||||
| British Columbia | 199.0 | 142.9 | 113.7 | 120.0 | 79.4 | 176.4 | 158.8 | 191.4 | 130.1 | 108.4 | 133.2 | 113.6 |
|
| ||||||||||||
| Cancer type | ||||||||||||
|
| ||||||||||||
| Leukemia | 48.5 | 45.6 | 50.1 | 52.5 | 46.0 | 53.0 | 58.7 | 47.8 | 56.3 | 49.0 | 59.5 | 49.1 |
|
| ||||||||||||
| Lymphoma | 23.0 | 12.8 | 14.9 | 25.0 | 18.8 | 18.8 | 15.1 | 25.9 | 15.7 | 22.0 | 28.9 | 17.9 |
|
| ||||||||||||
| CNS | 39.5 | 41.4 | 37.3 | 36.8 | 46.6 | 47.1 | 45.5 | 30.2 | 28.9 | 35.4 | 30.2 | 35.3 |
|
| ||||||||||||
| Neuroblastoma | 13.8 | 11.7 | 12.6 | 15.9 | 9.8 | 14.0 | 12.5 | 8.2 | 10.3 | 13.8 | 11.3 | 11.0 |
|
| ||||||||||||
| Retinoblastoma | 2.8 | 1.4 | 1.4 | 2.1 | 1.4 | 6.2 | 1.4 | 4.2 | 3.5 | 2.7 | 4.9 | 3.5 |
|
| ||||||||||||
| Renal tumour | 8.1 | 10.9 | 3.4 | 5.3 | 5.5 | 8.1 | 4.8 | 7.3 | 8.2 | 9.5 | 7.5 | 8.3 |
|
| ||||||||||||
| Hepatic tumour | 4.1 | 4.2 | 4.2 | 5.5 | 4.2 | 4.2 | 2.8 | 2.0 | 6.2 | 2.8 | 4.8 | 3.5 |
|
| ||||||||||||
| Bone tumour | 5.4 | 6.7 | 6.1 | 6.7 | 6.0 | 7.4 | 6.6 | 5.2 | 5.2 | 4.7 | 3.3 | 4.5 |
|
| ||||||||||||
| Soft tissue sarcoma | 8.7 | 9.5 | 14.1 | 14.8 | 8.1 | 4.7 | 7.4 | 10.0 | 9.3 | 5.9 | 11.0 | 8.8 |
|
| ||||||||||||
| Germ cell tumour | 8.2 | 7.6 | 4.8 | 2.1 | 6.1 | 7.5 | 8.6 | 3.5 | 4.7 | 7.4 | 2.6 | 2.7 |
|
| ||||||||||||
| Carcinomas‡ | 3.4 | 6.1 | 5.5 | 6.0 | 4.0 | 2.7 | 7.3 | 6.0 | 4.6 | 4.0 | 0.7 | 2.7 |
|
| ||||||||||||
| Other | 1.3 | 0.7 | 1.4 | 0.7 | 0.0 | 1.4 | 0.0 | 0.0 | 0.0 | 0.7 | 0.0 | 0.7 |
Note: ASIR = age-standardized incidence rate, CNS = central nervous system.
Includes Nova Scotia, Prince Edward Island, Newfoundland and Labrador, and New Brunswick.
Includes Manitoba, Alberta and Saskatchewan.
Includes adrenocortical carcinoma, thyroid carcinoma, nasopharyngeal carcinoma, malignant melanoma, skin carcinoma, and other and unspecified carcinomas.
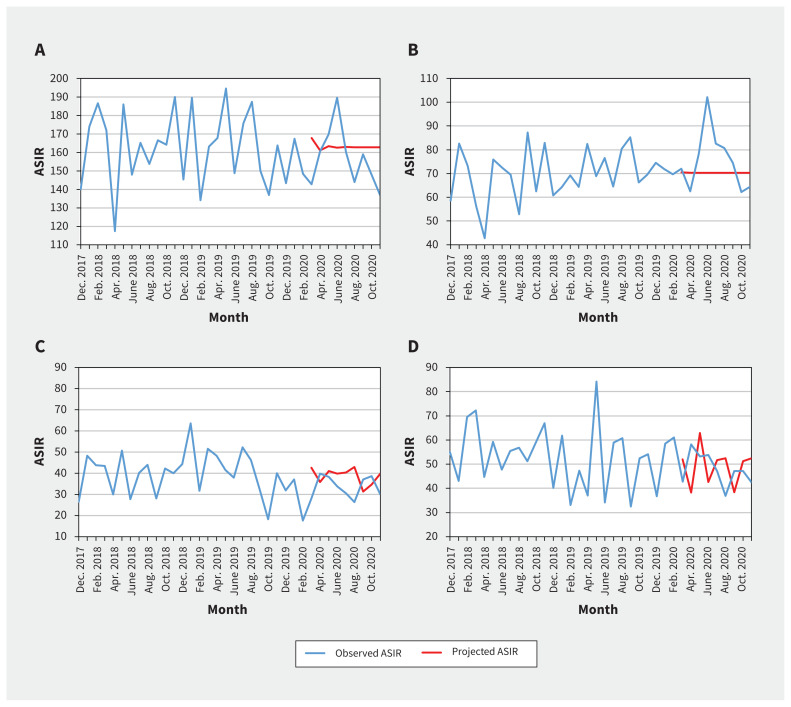
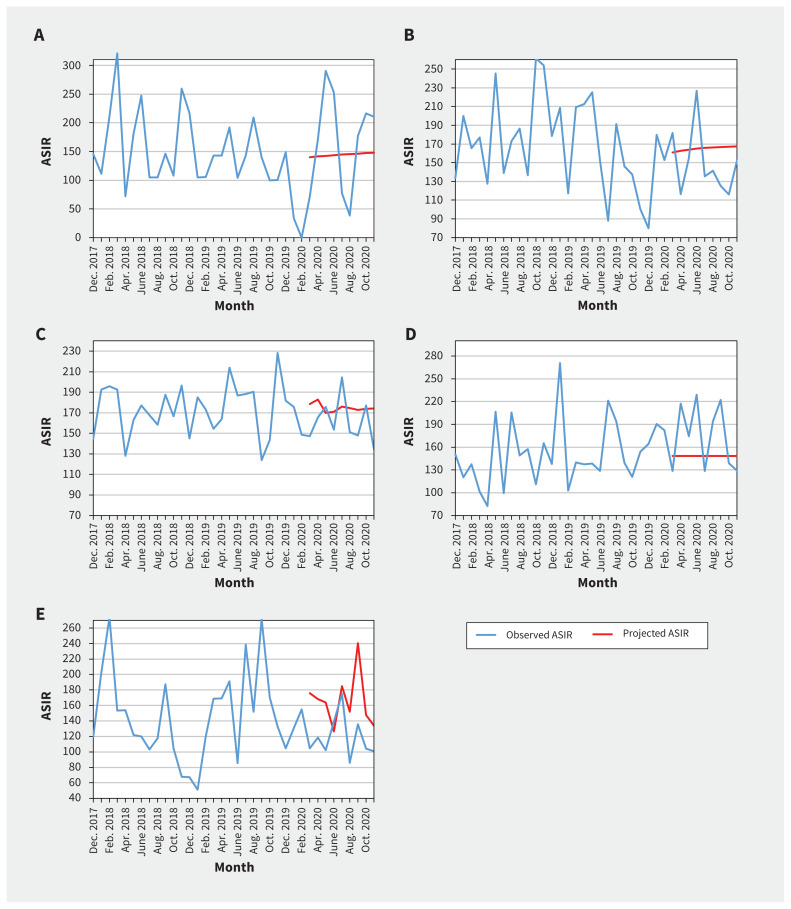
We found that the quarterly ASIRs of new cancer diagnoses were comparable to baseline values for both males and females (Table 1 and Appendix 1, Supplementary Table S1). We did not find statistically significant change in level and trend between projected and observed monthly ASIRs of new cancer diagnoses overall, when stratified by sex, cancer type (Table 2, Figure 1) or geographical area (Table 2 and Figure 2).
Table 2:
Results for the interventional autoregressive integrated moving average model summarizing the association of the COVID-19 pandemic and monthly age-standardized incidence rates for new pediatric cancer diagnoses per million population
| Parameter | ARIMA (p d q)(P D Q)12* | Level shift intervention† | Trend change intervention‡ | ||
|---|---|---|---|---|---|
|
|
|
||||
| Estimate of β (95% CI) | p value | Estimate of β (95% CI) | p value | ||
| Cancer type | |||||
|
| |||||
| All cancers combined | (1 0 0)(0 0 0)¶** | 4.98 (−15.1 to 25.04) | 0.6 | −2.09 (−5.63 to 1.44) | 0.25 |
|
| |||||
| Leukemia and lymphoma | (1 0 0)(0 0 0) | 10.27 (−0.72 to 23.25) | 0.1 | −1.00 (−3.27 to 1.27) | 0.4 |
|
| |||||
| CNS tumour | (0 0 0)(0 1 1)¶** | −5.11 (−11.7 to 1.45) | 0.1 | NA‡ | – |
|
| |||||
| Extracranial solid tumour | (0 0 0)(0 1 1)¶** | −1.33 (−11.4 to 8.73) | 0.8 | NA | – |
|
| |||||
| Geographic region | |||||
|
| |||||
| Atlantic§ | (1 0 1)(0 0 0) | 11.98 (0.38.3 to 62.22) | 0.6 | NA | – |
|
| |||||
| Quebec | (1 0 1)(0 0 0) | −16.0 (−51.1 to 19.12) | 0.4 | NA | – |
|
| |||||
| Ontario | (2 0 0)(0 0 0) | −7.09 (−32.6 to 18.42) | 0.6 | NA | 0.7 |
|
| |||||
| Prairies¶ | (0 1 1)(0 1 1) | −38.7 (−126.0 to 48.41) | 0.4 | NA | – |
|
| |||||
| British Columbia | (0 0 0)(0 0 0) | 25.22 (−3.01 to 53.45) | 0.09 | NA | – |
Note: ARIMA = autoregressive integrated moving average, ASIR = age-standardized incidence rate, CI = confidence interval, CNS = central nervous system, NA = not applicable.
For methodological considerations regarding ARIMA models, see Appendix 1, Supplement 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210659/tab-related-content.
The level change measures an immediate change after the onset of the pandemic.
The trend (slope) change measures a progressive increase or decrease in ASIRs in the pandemic period compared with the hypothetical continuations of the trends from the baseline period of March 2016 to February 2020.
Includes Nova Scotia, Prince Edward Island, Newfoundland and Labrador, and New Brunswick.
Includes Manitoba, Alberta and Saskatchewan.
Indicates that the trend change variable was not selected in the final model.
Figure 1:
Observed and projected monthly age-standardized incidence rates (ASIRs) of new pediatric cancer diagnoses per million, overall and stratified by cancer type: A) all cancers combined, B) leukemia and lymphoma, C) central nervous system tumour and D) extracranial solid tumour. We compared the monthly observed ASIRs in the pandemic period of March to November 2020 with the projected hypothetical continuation of the trends in the prepandemic period of March 2016 to February 2020.
Figure 2:
Observed and projected monthly age-standardized incidence rates (ASIRs) for all pediatric cancers combined per million, by geographic area: A) Atlantic provinces (Nova Scotia, Prince Edward Island, New Brunswick and Newfoundland and Labrador), B) Quebec, C) Ontario, D) Prairie provinces (Manitoba, Saskatchewan and Alberta) and E) British Columbia. We compared the monthly observed ASIRs in the pandemic period of March to November 2020 with the projected hypothetical continuation of the trends in the prepandemic period of March 2016 to February 2020.
Table 3 shows that the percentage of patients enrolled in clinical trials was 10.7% between March and May 2020, 14.0% between June and August 2020, and 15.0% between September and November 2020. The median enrolment rate was 8.8% (IQR 7.0% to 9.5%) during the baseline period. There was a significant increase in enrolment in clinical trials during the third quarter of the pandemic (RR 1.71, 95% CI 1.01 to 2.89) compared to baseline, which was largely attributable to an increase in enrolment rate for leukemia and lymphoma during that period (Table 2).
Table 3:
Proportion of children enrolled in a clinical trial, with metastatic disease at cancer diagnosis and who died within 30 days
| Patient characteristic | Baseline period | Pandemic | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| December 2017–February 2020 | March–May 2020 | June–August 2020 | September–November 2020 | ||||
|
|
|
|
|
||||
| Median % (range) | No.* (%) n = 235 |
RR (95% CI) | No.(%) n = 240 |
RR (95% CI) | No. (%) n = 220 |
RR (95% CI) | |
| Patients enrolled in a clinical trial at cancer diagnosis | |||||||
|
| |||||||
| All cancers combined | 8.8 (4.7–15.6) | 25 (10.7) | 1.22 (0.70–2.13) | 35 (14.0) | 1.60 (0.95–2.69) | 35 (15.0) | 1.71 (1.01–2.89) |
|
| |||||||
| Leukemia and lymphoma | 12.3 (4.6–30.8) | 20 (19.1) | 1.55 (0.83–2.91) | 30 (21.2) | 1.73 (0.96–3.12) | 35 (31.0) | 2.52 (1.43–4.47) |
|
| |||||||
| CNS tumour | 2.5 (1.2–12.1) | 5 (3.8) | 1.13 (0.08–16.80) | < 5 (4.4) | 1.33 (0.09–19.76) | 0 (0) | 0.23§ (0.01–4.60) |
|
| |||||||
| Extracranial solid tumour | 8.5 (4.7–15.6) | 5 (4.0) | 0.61 (0.07–2.73) | 5 (6.1) | 0.93 (0.21–3.79) | < 5 (3.0) | 0.46 (0.05–2.26) |
|
| |||||||
| Patients with metastatic disease at cancer diagnosis | |||||||
|
| |||||||
| All cancers combined, except leukemia | 22.7 (16.9–28.0) | 30 (19.1) | 0.84 (0.55–1.29) | 45 (27.7) | 1.22 (0.84–1.79) | 30 (22.5) | 0.99 (0.65–1.49) |
|
| |||||||
| Lymphoma | 34.8 (21.1–43.2) | 5 (18.2) | 0.51 0.21–1.22) | 15 (34.1) | 0.95 (0.50–1.82) | 15 (44.4) | 1.24 (0.65–2.39) |
|
| |||||||
| CNS tumour | 4.7 (3.3–13.0) | < 5 (1.9) | 0.42 (0.02–4.08) | 10 (15.6) | 3.47 (0.96–22.07) | 5 (7.6) | 1.69 (0.37–12.46) |
|
| |||||||
| Extracranial solid tumour | 30.4 (22.4–37.2) | 25 (31.6) | 1.02 (0.64–1.63) | 20 (31.8) | 1.03 (0.64–1.67) | 15 (25.4) | 0.82 (0.49–1.39) |
|
| |||||||
| Patients with acute lymphoblastic leukemia with advanced disease | |||||||
|
| |||||||
| NCI high risk† | 28.3 (22.2–43.1) | 15 (33.3) | 1.18 (0.66–2.10) | 15 (27.9) | 0.99 (0.56–1.75) | 15 (27.8) | 0.98 (0.53–1.80) |
|
| |||||||
| CNS involvement‡ | 16.1 (8.7–26.9) | 15 (25.0) | 1.56 (0.73–3.30) | 20 (28.6) | 1.78 (0.89–3.56) | 15 (23.3) | 1.45 (0.68–3.09) |
|
| |||||||
| Patients who died within 30 days | |||||||
|
| |||||||
| All cancers combined | 1.2 (0.8–1.3) | 5 (2.1) | 1.73 (0.38–15.19) | < 5 (0.8) | 0.67 (0.08–4.14) | 0 (0) | 0.16§ (0.01–3.04) |
Note: CI = confidence interval, CNS = central nervous system, NCI = National Cancer Institute, RR = rate ratio.
The number of new cases were randomly rounded up or down to a multiple of 5 to reduce risk of disclosure, in line with Canadian Cancer Registry requirements, although percentages were based upon the true number of cases.
NCI high risk defined as age < 1 year or ≥ 10 years, or initial white blood cell count > 50 × 109/L.23
Central nervous system involvement was defined as those with CNS2 or CNS3 status.
We added a correction factor of 0.5 in every cell containing a zero.
For each pandemic quarter, 19.1%, 27.7% and 22.5%, respectively, of patients presented with metastatic disease, which was comparable to baseline overall (median 22.7%, IQR 20.5% to 24.9%) and stratified by cancer type (Table 3). Similarly, the proportion of patients with leukemia who presented with CNS involvement or NCI high-risk disease was comparable to baseline (Table 3).
The percentage of patients who died within 30 days of presentation was 2.1% between March and May 2020, 0.8% between June and August 2020 and 0.0% between September and November 2020, which was comparable to the baseline rate (median 1.2%, IQR 0.8% to 1.3%). Analyses using a weighted model (time-dependent variance) rather than an unweighted model (using constant variance) yielded similar results (data not shown).
Interpretation
We did not find a statistically significant difference in the incidence of new cancer diagnoses in Canadian children aged younger than 15 years during the first 9 months of the COVID-19 pandemic compared with previous years. In addition, we observed no significant differences in the proportion of patients enrolled in a clinical trial, presenting with metastatic disease or who died within 30 days of presentation.
Our findings suggest that among children in Canada, cancer diagnosis was not delayed during the pandemic, unlike findings described in previous reports.10–13 Although access to emergency departments markedly dropped during the pandemic, there may have been less reluctance by families and health care professionals to access health care for serious symptomatology.33,34 Furthermore, most newly diagnosed patients in Canada have access to publicly funded health care, lessening the impact of financial hardship. Alternatively, it may also suggest that delays were sufficiently short that they did not affect cancer incidence rates when calculated monthly. Of note, we did not observe a reduction in new cases of extracranial solid tumours or CNS tumours; diagnosis of these cancers is dependent on adequate access to diagnostic imaging and surgical procedures. We studied the first 9 months of the pandemic; it is possible that changes in cancer detection and outcomes will be appreciable only in the longer term.
Our findings regarding stable enrolment in clinical trials also diverge from the experiences of other researchers.35–39 For example, the Innovative Therapies for Children with Cancer consortium in Europe reported a profound disruption in pediatric early clinical research owing to a reduction in personnel, cancellation of monitoring visits and difficulty with sample transportation,36 which led to a 75% reduction in enrolment.37 Our results mostly reflect enrolment in phase III clinical trials led by academic consortia and are probably not generalizable to enrolment in early phase or translational trials that may require travel to quaternary hospitals or may not have been prioritized by institutions because of resource constraints. The ongoing recruitment in clinical trials that we observed might be explained by enrolment in therapeutic trials being considered an integral component of high-quality care in pediatric cancer22 and thus being prioritized by institutions. It is also possible that the impact of the COVID-19 pandemic was minimized by the proactive and extensive changes established by some research consortia and funding agencies, such as flexibility for patients’ enrolment and follow-up schedules, and implementation of remote monitoring visits,35 which could benefit children and their families as the pandemic wanes.
Limitations
Interpretation of our findings requires consideration of some limitations. The CYP-C collects data only from pediatric oncology centres. It is possible that some children are not yet properly referred to tertiary centres. However, this would be more concerning if new diagnoses had decreased. External factors unrelated to the COVID-19 pandemic might affect enrolment in clinical trials, given that the availability of clinical trials varies over time. For example, accrual in the Children’s Oncology Group front-line trials for acute B-cell lymphoblastic leukemia started in 2020 for several Canadian hospitals and may partly explain the increase in clinical trial enrolment for patients with leukemia. Moreover, estimates of enrolment rate may be unstable given the relatively small number of patients and clinical trials. Some positive findings could be attributed to type I errors caused by the large number of comparisons without adjustment for multiplicity. Finally, the study follow-up period does not allow the full range of the possible negative impacts of the COVID-19 pandemic to be understood. Our data likely did not capture adjustment of treatments such as delays in chemotherapy, radiotherapy or hematopoietic stem cell transplantation. As subsequent waves of COVID-19 cases unfold in Canada and abroad, continued surveillance is necessary to understand the ramifications of the extensive changes made in response to the COVID-19 pandemic, such as reorganization of care and more generalized use of telehealth.
Conclusion
We did not observe a statistically significant change in the ASIRs of childhood cancer during the first 9 months of the COVID-19 pandemic in Canada compared with the period before the pandemic. Moreover, enrolment in clinical trials remained stable, and we did not observe an increase in the proportion of patients with metastatic disease or early mortality. Although these results are reassuring, continued surveillance is necessary to ascertain potential long-term negative effects of the COVID-19 pandemic among children with cancer.
Supplementary Material
Acknowledgements
The authors are grateful for the contributions of study participants, participating pediatric oncology centres, members of the Cancer in Young People in Canada (CYP-C) Management and Advisory Committees and the Pediatric Oncology Group of Ontario. The authors also thank all data managers and principal investigators at the 17 CYP-C sites for their collaboration and dedicated work.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All authors contributed to research design and data interpretation. Lin Xie, Marie-Claude Pelland-Marcotte, Sulaf Elkhalifa, Mylene Frechette, Jaskiran Kaur and Jay Onysko were involved in data analysis. Marie-Claude Pelland-Marcotte prepared the first version of this manuscript. All of the authors reviewed the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: Cancer in Young People in Canada is funded by the Public Health Agency of Canada. No additional funding was provided for this study. Lillian Sung is supported by the Canada Research Chair in Pediatric Oncology Supportive Care.
Data sharing: As per Cancer in Young People in Canada policies, individual participant data will not be shared. Application for utilization of data can be submitted through the C17 Council website (available at http://www.c17.ca/index.php?cID=70 [login required]).
Disclaimer: Data used in this publication are from the Cancer in Young People in Canada Surveillance Program and are used with the permission of the Public Health Agency of Canada.
References
- 1.IJzerman M, Emery J. Is a delayed cancer diagnosis a consequence of COVID-19? Victoria (AU): The University of Melbourne. Available: https://pursuit.unimelb.edu.au/articles/is-a-delayed-cancer-diagnosis-a-consequence-of-covid-19 (accessed 2021 Mar. 31). [Google Scholar]
- 2.Lai AG, Pasea L, Banerjee A, et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open 2020;10:e043828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform 2020;4:1059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman HW, Chen Z, Niles J, et al. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open 2020;3:e2017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.London JW, Fazio-Eynullayeva E, Palchuk MB, et al. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform 2020;4:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 2020;21:1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller MJ, Xu L, Qin J, et al. Impact of COVID-19 on cervical cancer screening rates among women aged 21–65 years in a large integrated health care system – Southern California, January 1–September 30, 2019, and January 1–September 30, 2020. MMWR Morb Mortal Wkly Rep 2021;70:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuno R, Ganeko R, Takeuchi G, et al. The number of obstructive colorectal cancers in Japan has increased during the COVID-19 pandemic: a retrospective single-center cohort study. Ann Med Surg (Lond) 2020;60:675–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasme JF, Morfouace M, Grill J, et al. Delays in diagnosis of paediatric cancers: a systematic review and comparison with expert testimony in lawsuits. Lancet Oncol 2012;13:e445–59. [DOI] [PubMed] [Google Scholar]
- 10.Chiaravalli S, Ferrari A, Sironi G, et al. A collateral effect of the COVID-19 pandemic: delayed diagnosis in pediatric solid tumors. Pediatr Blood Cancer 2020;67:e28640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Offenbacher R, Knoll MA, Loeb DM. Delayed presentations of pediatric solid tumors at a tertiary care hospital in the Bronx due to COVID-19. Pediatr Blood Cancer 2021;68:e28615. [DOI] [PubMed] [Google Scholar]
- 12.Ding YY, Ramakrishna S, Long AH, et al. Delayed cancer diagnoses and high mortality in children during the COVID-19 pandemic. Pediatr Blood Cancer 2020;67:e28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carai A, Locatelli F, Mastronuzzi A. Delayed referral of pediatric brain tumors during COVID-19 pandemic. Neurooncol 2020;22:1884–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynn RM, Avis JL, Lenton S, et al. Delayed access to care and late presentations in children during the COVID-19 pandemic: a snapshot survey of 4075 paediatricians in the UK and Ireland. Arch Dis Child 2021;106:e8. [DOI] [PubMed] [Google Scholar]
- 15.Lazzerini M, Barbi E, Apicella A, et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health 2020;4:e10–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pole JD, Barber R, Bergeron R-É, et al. Most children with cancer are not enrolled on a clinical trial in Canada: a population-based study. BMC Cancer 2017;17:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitra D, Hutchings K, Shaw A, et al. Status report: the cancer in young people in Canada surveillance system. Health Promot Chronic Dis Prev Can 2015;35:73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer in young people in Canada: a report from the enhanced childhood cancer surveillance system. Ottawa: Public Health Agency of Canada; 2017, modifed 2019 Dec. 9:1–58. Available: https://www.canada.ca/en/health-canada/services/publications/science-research-data/cancer-young-people-canada-surveillance-2017.html (accessed 2021 July 9). [Google Scholar]
- 19.Steliarova-Foucher E, Stiller C, Lacour B, et al. International Classification of Childhood Cancer, third edition. Cancer 2005;103:1457–67. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg ML, Barr RD, DiMonte B, et al. Childhood cancer registries in Ontario, Canada: lessons learned from a comparison of two registries. Int J Cancer 2003;105:88–91. [DOI] [PubMed] [Google Scholar]
- 21.Zhao N, Liu Y, Smargiassi A, et al. Tracking the origin of early COVID-19 cases in Canada. Int J Infect Dis 2020;96:506–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bond MC, Pritchard S. Understanding clinical trials in childhood cancer. Paediatr Child Health 2006;11:148–50. [PMC free article] [PubMed] [Google Scholar]
- 23.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol 1996;14:18–24. [DOI] [PubMed] [Google Scholar]
- 24.Annual demographic estimates: Canada, provinces and territories, 2020. Cat no 91-215-X. Ottawa: Statistics Canada; 2020. Available: https://www150.statcan.gc.ca/n1/pub/91-215-x/91-215-x2020001-eng.htm (accessed 2021 Mar. 2). [Google Scholar]
- 25.Table 91-002-X: Quarterly demographic estimates. Ottawa: Statistics Canada. Available: https://www150.statcan.gc.ca/n1/en/catalogue/91-002-X (accessed 2021 Mar. 2). [Google Scholar]
- 26.Newman S. Biostatistical methods in epidemiology. New York: John Wiley and Sons; 2001. [Google Scholar]
- 27.Box GEP, Jenkins GM. Time series analysis: forecasting and control. San Francisco: Holden-Day; 1976. [Google Scholar]
- 28.Schaffer AL, Dobbins TA, Pearson S-A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: a guide for evaluating large-scale health interventions. BMC Med Res Methodol 2021;21:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campitelli MA, Bronskill SE, Maclagan LC, et al. Comparison of medication prescribing before and after the COVID-19 pandemic among nursing home residents in Ontario, Canada. JAMA Netw Open 2021;4:e2118441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Box GEP, Tiao GC. Intervention analysis with applications to economic and environmental problems. J Am Stat Assoc 1975;70:70–9. [Google Scholar]
- 31.Kim HJ, Fay MP, Yu B, et al. Comparability of segmented line regression models. Biometrics 2004;60:1005–14. [DOI] [PubMed] [Google Scholar]
- 32.Berry I, Soucy J-PR, Tuite A, et al. COVID-19 Canada Open Data Working Group. Open access epidemiologic data and an interactive dashboard to monitor the COVID-19 outbreak in Canada. CMAJ 2020;192:E420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu X, Zhang L, Du H, et al. Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 infection in children. N Engl J Med 2020;382:1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Nardo M, van Leeuwen G, Loreti A, et al. A literature review of 2019 novel coronavirus (SARS-CoV2) infection in neonates and children. Pediatr Res 2021;89:1101–8. [DOI] [PubMed] [Google Scholar]
- 35.Tolaney SM, Lydon CA, Li T, et al. The impact of COVID-19 on clinical trial execution at the Dana–Farber Cancer Institute. J Natl Cancer Inst 2020;djaa144. doi: 10.1093/jnci/djaa144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubio-San-Simón A, André N, Cefalo MG, et al. Impact of COVID-19 in paediatric early-phase cancer clinical trials in Europe: a report from the Innovative Therapies for Children with Cancer (ITCC) consortium. Eur J Cancer 2020;141:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubio-San-Simón A, Verdú-Amorós J, Hladun R, et al. Challenges in early phase clinical trials for childhood cancer during the COVID-19 pandemic: a report from the new agents group of the Spanish Society of Paediatric Haematology and Oncology (SEHOP). Clin Transl Oncol 2021;23:183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waterhouse DM, Harvey RD, Hurley P, et al. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: findings from an American Society of Clinical Oncology survey. JCO Oncol Pract 2020;16:417–21. [DOI] [PubMed] [Google Scholar]
- 39.Unger JM, Blanke CD, LeBlanc M, et al. Association of the coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw Open 2020;3:e2010651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.