Abstract
Impulse-control disorders are commonly observed during dopamine-replacement therapy in Parkinson’s disease, but the majority of patients seems “immune” to this side effect. Epidemiological evidence suggests that a major risk factor may be a specific difference in the layout of the dopaminergic-reinforcement system, of which the ventral striatum is a central player. A series of imaging studies of the dopaminergic system point toward a presynaptic reduction of dopamine-reuptake transporter density and dopamine synthesis capacity. Here, we review current evidence for a vulnerability-stress model in which a relative reduction of dopaminergic projections to the ventral striatum and concomitant sensitization of postsynaptic neurons represent a predisposing (hypodopaminergic) vulnerability. Stress (hyperdopaminergic) is delivered when dopamine replacement therapy leads to a relative overdosing of the already-sensitized ventral striatum. These alterations are consistent with consecutive changes in reinforcement mechanisms, which stimulate learning from reward and impede learning from punishment, thereby fostering the development of impulse-control disorders. This vulnerability-stress model might also provide important insights into the development of addictions in the non-Parkinsonian population.
Subject terms: Psychiatric disorders, Parkinson's disease, Human behaviour
Introduction
Addiction is a global social, economic, and health problem. So far, there is no effective treatment and pathophysiology is insufficiently understood1. A key feature of addiction is the reduced ability to control behavior (e.g., drug intake, gambling), despite of their obviously harmful effects2. There are substance-related and non-substance-related addictions, such as pathological gambling, which are also called behavioral addictions or impulse-control disorders (ICDs). It is conceivable that for the development of ICDs, both predisposing traits and triggering mechanisms play a role. These traits and triggers may have identifiable biological substrates. The main pathophysiological mechanism in these disorders has been linked to disrupted dopamine homeostasis2. While longitudinal data covering the entire development of ICDs are almost impossible to come by in the general population, a unique opportunity is provided in Parkinson’s disease (PD). In a large multicenter study, Weintraub et al. demonstrated that ICDs occur as a side effect of dopamine replacement therapy (DRT) in 14% of PD patients3–5. Newer research points out that the incidence of ICDs in PD could be much higher (up to 46%)6,7. Therefore, the development of ICDs in PD could serve as a model for all addictions8 because seemingly mentally healthy PD patients develop ICDs in a very short period of time.
The most popular pathophysiological concept for the development of ICDs in PD is the so-called overdose theory: among the basal ganglia loops, the motor loop is mainly affected by neurodegeneration in PD. Hence, when DRT is administered, the dopaminergic tone in the motor loop is balanced, but the relatively intact limbic loop is overdosed, which leads to a hyperdopaminergic state in the ventral striatum9,10. The dopamine signal of the limbic basal ganglia loop modulates conditional learning and has motivational impact on a person’s behavior: via dopaminergic signaling, we learn implicitly to approach stimuli with positive outcomes (Go-Learning) and to avoid the opposite (NoGo-Learning)11. Accordingly, patients with ICDs show altered dopamine-modulated behavior in the form of impulsivity, risk proneness, and overengagement in rewarding behavior as well as deficits in inhibitory control12–14. While this concept is attractive, it does not explain why only a fraction of patients develop the ICD phenotype. Furthermore, findings of several imaging and rodent studies leave doubts about the hyperdopaminergic concept for ICDs in PD and hint at hypodopaminergic changes in these patients, which may represent a premorbid biological vulnerability.
The aim of this review is to consolidate hypodopaminergic findings with the hyperdopaminergic overdose theory in the form of a vulnerability-stress model for the development of ICDs in PD. In general, this model states that persons have an intrinsic vulnerability (e.g., genetic), leading in combination with an extrinsic stressor (e.g., life crisis, drug abuse) to the development of mental illness15,16.
Additionally, we want to shed light on the relationship between apathy and ICDs since both conditions might underlie comparable changes within the dopaminergic reinforcement system.
Evidence for a premorbid vulnerability to ICDs
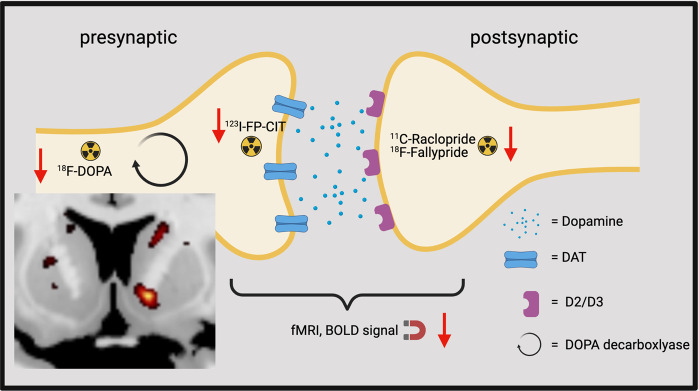
Human imaging studies found several hypodopaminergic changes in the ventral striatum of PD patients with ICDs: a reduced dopamine transporter (DAT) density in 123I-FP-CIT-SPECT17–20, a reduced dopamine synthesis capacity in 18F-DOPA-PET at rest21, a reduced BOLD activation at rest22, and a reduced D2/D3 receptor availability at rest23–25 (Fig. 1). These hypodopaminergic changes could be inherited or acquired (e.g., by neurodegeneration). When considering general PD populations, Fazio et al. found that 36% of early PD patients had a reduced DAT density in the ventral striatum26 and early PD patients showed reduced dopamine synthesis capacity as compared with healthy controls in the ventral striatum27. Further evidence for the possibility of a premorbid vulnerability, that may be unrelated to dopaminergic neurodegeneration, comes from various studies reporting ICD development in non-PD populations (e.g., patients with fibromyalgia or prolactinoma) with DRT28,29. These hypodopaminergic changes were also found in non-PD populations with behavioral or substance addictions: pathological gamblers30, alcoholics31, tobacco, and cannabis addicts32 showed a reduced DAT signal in the ventral striatum. Young people with internet addiction had a lower DAT binding33 and a reduced D2 receptor availability34 in the ventral striatum. Interestingly, a reduced DAT density was also found in healthy individuals with higher trait impulsivity35. Furthermore, a reduced dopamine synthesis capacity was also found in cocaine addicts36, cannabis users37, and binge eaters38.
Fig. 1. Imaging findings in ICDs in PD in the ventral striatum at the synaptic level.
The red arrows symbolize reduced tracer uptake and reduced BOLD signal. The coronar brain slice shows the reduced dopamine synthesis capacity in the right ventral striatum21. (Created with BioRender.com).
An important study in drug-naive PD patients has shed further light on the question of whether these changes pre-existed before medication is started. In total, 31 de novo, drug-naive PD patients underwent DAT single-photon emission computed tomography (SPECT) and were screened for ICDs. After an average follow-up of 32 months, 11 had developed ICD symptoms without having any at baseline. These patients showed significantly lower baseline DAT binding ratios in the right ventral striatum, right anterior dorsal striatum, and right posterior putamen. Additionally, the severity of ICD symptoms at follow-up correlated negatively with baseline DAT availability20.
A reduced signal in DAT-SPECT in the ventral striatum could be due to reduced DAT density per dopaminergic terminal or due to a reduction of dopaminergic projections from the midbrain or a combination thereof. For further clarification, a recently published paper by our group used 18F-DOPA-PET to detect changes in dopamine synthesis capacity21. We found a negative correlation between the dopamine synthesis capacity and ICD severity in the ventral striatum at rest. Consequently, a predominant reduction of dopaminergic projections per se seems a more likely scenario. Interestingly, a postmortem study found no differences in tyrosine hydroxylase staining and α-synuclein load in the ventral striatum between PD patients with and without ICDs, indicating that the results from imaging studies could rather present functional changes than pure cell loss due to neurodegeneration39.
All in all, the results from the above-mentioned studies in PD and non-PD populations point to a weaker dopaminergic input to the ventral striatum as a premorbid vulnerability to develop ICDs.
Beyond the dopaminergic reinforcement system
Although in this review we focus on dopamine, other neurotransmitter systems may play an important role for the development of ICDs. Serotonergic neurons project from the raphe nucleus to the ventral striatum and to the prefrontal cortex. Low serotonergic levels are associated with depression40 and trait impulsivity41,42, which in turn are associated with ICDs. Indeed, a PET study in de novo PD patients with apathy and depression did demonstrate a relative serotonergic denervation40. While these patients may be seen as “at-risk” to develop ICDs when medicated, there currently is no molecular serotoninergic imaging study in ICDs in PD. However, in non-PD binge eaters Majuri et al. found a reduced serotonin -transporter density in the ventral striatum43. Serotonin depletion in humans44 and rodents45 can lead to impulsive behavior, and polymorphisms in the serotonin-transporter protein are associated with addiction46. Moreover, perfusate serotonin increases dopamine release in the nucleus accumbens47. In sum, evidence is still lacking in PD, but a reduced serotoninergic input to the ventral striatum would be a plausible hypothesis. In a PET-study, a reduced μ-opioid receptor density38 was found in binge eaters in the ventral striatum. Furthermore, μ-opioid receptor stimulation in the nucleus accumbens amplifies hedonic wanting48. Interestingly, polymorphisms in the κ-opioid receptor were negatively associated with ICDs49. An animal study with microdialysis revealed that stimulation of these opioid receptors has an effect on striatal dopamine release50. Furthermore, an increase in glutaminergic projections from the prefrontal cortex to the ventral striatum leads to drug seeking51. Engeli et al. found a reduction of glutamate in the nucleus accumbens in cocaine addicts at rest and an increase in glutamate levels during cue-induced craving compared with healthy controls in a magnetic resonance spectroscopy paradigm52. All in all, changes in other neurotransmitter systems seem to influence the dopamine metabolism in the ventral striatum and might be associated with a hypodopaminergic state in the ventral striatum as described above.
Beyond the ventral striatum
Most of the imaging studies concerning ICDs in PD reported alterations in the ventral striatum. This mesolimbic reward circuit, including the ventral tegmental area and the nucleus accumbens, is crucial for mediating reward and the calculation of a reward prediction error11,53 and seems to be the key player for the development of addictions. An important feature of addiction and compulsion is that an action becomes habitual. The key player for habit formation is commonly seen in the dorsolateral, not the ventral striatum. Belin and Everitt could show that the ventral striatum is important for the initiation of drug seeking, while the dorsal striatum is more involved in sustaining it53–55. Interestingly, two studies found that a reduction in DAT density in the putamen and the anterior dorsal striatum20 as well as a reduced D2/D3 density in this area are associated with ICDs in PD24. In a recently published paper by our group, we found a negative correlation between dopamine synthesis capacity in the caudate and the severity of ICDs21. Other studies could show a reduced connectivity between the anterior cingulate cortex and the left putamen56,57. All in all, ICDs seem to be associated with alterations in dorsal striatum. A region that is more affected from neurodegeneration in PD than the ventral striatum. To sum up, we reason that the dopaminergic loss in the ventral striatum may be critical for the initiation of ICDs and that the dopaminergic loss in the dorsal striatum might play an important role for the long-term persistence of this behavior.
Downstream consequences of reduced DAT and dopamine synthesis capacity
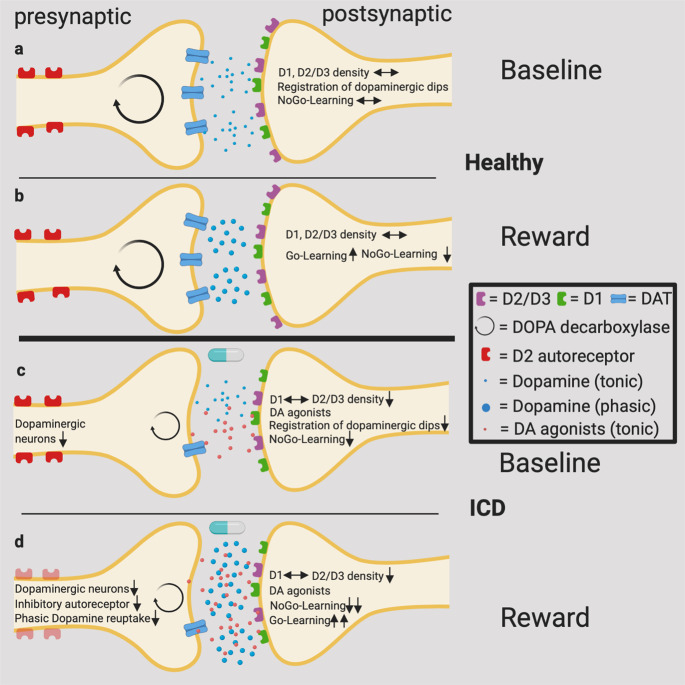
Dopamine release in the striatum can conceptually be divided into two relatively independent forms, tonic and phasic dopamine release, which relate to tonic and phasic activities of dopaminergic neurons, and have distinguishable roles in learning from outcomes. On the one hand, an unexpected reward leads to a phasic dopamine release from the ventral tegmental area to the ventral striatum which is then followed by D1 receptor activation (Go-Learning). On the other hand, punishment or the omission of an expected reward leads to a dopaminergic dip and NoGo-Learning via D2/D3 receptors is fostered11. D1 receptors are activated after phasic dopamine release, whereas the activation of D2/D3 receptors (having a higher affinity to dopamine than D1 receptors) is dominated by tonic dopaminergic levels58–60. D3 receptors have the highest affinity to dopamine and are mainly located in limbic areas such as the ventral striatum61. Therefore, there have been speculations that D3 receptors are primarily involved in the development of ICDs. Interestingly, the influence of phasic dopamine release seems strongly affected by DAT activity, whereas the tonic dopamine release is mainly affected by the overall activity of a dopaminergic neuron population per se62,63. See Fig. 2A and B.
Fig. 2. A vulnerability-stress model for the development of ICDs.
a Normal tonic dopamine release and balanced density of D1 and D2/D3 receptors. Dopaminergic dips via D2/D3 can be registered, NoGo-Learning is possible. b A reward leads to a phasic dopaminergic burst in the striatum, which is followed by D1 stimulation and Go-Learning. The phasic dopamine release is stopped by inhibitory autoreceptors and dopamine reuptake. c The tonic dopamine release and the postsynaptic D2/D3 density are reduced. In combination with dopamine agonists, dopaminergic dips cannot be registered. NoGo-Learning is attenuated. d The ending of phasic dopamine release is disturbed because of a reduction of DAT density and a reduced activation of inhibitory autoreceptors. Go-Learning is emphasized, whereas NoGo-Learning is attenuated. (Created with BioRender.com).
A possible downstream effect of reduced dopamine synthesis capacity could be a reduced tonic stimulation (i.e., occupation) of postsynaptic D2/D3 receptors. However, imaging studies found a reduced postsynaptic D2/D3 receptor availability in the ventral striatum at rest in PD patients with ICDs23,24. Then again, a reduced receptor availability measured by PET can have three different explanations: a reduction of receptor density, higher dopamine levels in the synaptic cleft (competing with the PET ligand), or a combination of both. In light of the reduced dopamine synthesis capacity at rest21, we may interpret a reduced D2/D3 receptor availability as primarily reflecting a reduction in receptor density. This fits well with a post mortem study, showing lower levels of D3 receptors in the ventral striatum of PD patients with ICDs. This study could not find changes regarding D2 receptors39. A reduced density of D2/D3 receptors in combination with an additional administration of dopamine agonists would hamper learning from negative feedback and lead as a consequence to ICDs (Fig. 2C)11. A concurrently reduced activation of presynaptic D2 autoreceptors, on the other hand, would lead to an increase in phasic dopamine, associated with a heightened propensity to reward-driven behavior (see also below).
Genetic studies point out that a reduction of DAT expression (polymorphism in DAT1 gene) is associated with addiction, PD, and ADHD5,64,65. Guo et al. found that individuals with the 10-repeat allele of the DAT1 had significantly more sexual partners66; and individuals with the 10-repeat allele had lower binding in DAT-SPECT as compared with patients with the 9-repeat allele67. Moreover, Volkow et al. found a reincrease in DAT density in abstinent cocaine addicts68. In animal studies DAT blocker enhances reactions to reward predicting cues69 and DAT knock-out mice show a greater locomotor sensitization to drugs, i.e., a greater progressive and persistent enhancement of the motor-stimulant effects of cocaine and ethanol70. Having in mind that the phasic dopamine release is mainly affected by the reuptake capacity of DAT in combination with the reduction of DAT density, as described above, we would measure more dopamine in the synaptic cleft as a consequence of a phasic dopamine release under reward conditions. In line with this hypothesis, imaging studies found a reduced D2 availability under reward conditions in PD patients with ICDs as compared with normal PD patients23,71. This would involve an increased dopamine release as well as a reduction in D2 receptors.
Furthermore, a chronic underexpression of DAT leads to a reduced function of midbrain D2 autoreceptors which may evoke higher extracellular dopamine levels72,73. According to a recently published review74, presynaptic D2 autoreceptors have three different possibilities to modulate dopamine metabolism: (1) reduction of the exocytotic dopamine release after a prior release, (2) regulating the dopamine uptake via an increase of DAT expression, and (3) downregulation of tyrosine hydroxylase (reduced filling of dopamine vesicles). Ray et al. found a reduced activation of these autoreceptors in PD patients with pathological gambling75, which could also explain increased phasic dopamine release. See Fig. 2D. Buckholtz et al. could show that healthy individuals with lower levels of D2 autoreceptor had a higher amphetamine-induced dopamine release76.
The role of the prefrontal cortex—a loss of inhibitory top-down control
Besides from changes in striatal regions, the prefrontal cortex plays an important role for development of ICDs. The anterior cingulate cortex is crucial for error monitoring77 and behavioral adaptions after negative feedback78. The lateral orbitofrontal cortex is responsible for punishment-based decision-making79 and is important for suppression of previously rewarded behavior80. Voon et al. found a reduced BOLD activation of the anterior cingulate cortex in ICD in PD during risk-taking14. Another study reported a reduced activation of the anterior cingulate cortex and lateral orbitofrontal cortex in PD gamblers as compared with PD controls in H2O-PET81. Several other studies found a reduced connectivity between the anterior cingulate cortex and the ventral striatum21,78. Another PET study found a higher availability of D2 and D3 receptors in this area, which could indicate low levels of synaptic dopamine in PD patients with75. All in all, imaging studies point out that there is a diminished top-down control of inhibitory cortical areas in ICDs5.
Dopamine replacement therapy—when it comes to stress
In case of L-DOPA, 7.2% of PD patients develop ICDs, 14% in case of dopamine agonists, and 17.7% in case of both4.
According to our proposed theory, due to hypodopaminergic changes and the associated mechanisms, the system becomes vulnerable to relatively small alterations in dopaminergic levels. The system is adjusted to low levels of tonic dopamine and reduced D2 receptors. Then, as a consequence of DRT administration, the system becomes easily overdosed. Thereby, phasic effects are boosted and dips in dopamine release are drowned by the tonic D2/D3 overstimulation82.
Interestingly, patients taking dopamine agonists have twice the risk for ICD than patients taking L-DOPA alone. A reason could be the altered function of D2 autoreceptors in the midbrain75, which downregulate phasic striatal dopamine release. Chronic treatment with dopamine agonists may lead to a desensitization of D2 autoreceptors in the midbrain with consecutive dysregulation of phasic dopamine release83. Furthermore, dopamine agonists reduce the activity of inhibitory control areas in PD patients with ICDs, whereas they increase the activity in these areas in PD controls81. Likewise, dopamine agonszts diminish reward processing in the lateral orbitofrontal cortex during negative errors of reward prediction82.
In the same vein, a combination therapy with agonszts and L-DOPA will lead to the highest prevalence of ICDs because increased D1 effects (higher dopamine release because of L-DOPA and low D2 autoreceptor function) and D2/D3 overstimulation (dopamine agonists) are combined.
Astonishingly, time onset of ICD diagnosis after the initiation of DRT is highly variable (from 3 months up to 10 years)84. A reason could be the association between the cumulative dopamine agonist dose and the development of ICDs6. Likewise, Perez et al. could find a correlation between agonist dose and ICDs85 as opposed to Weintraub et al. using a different pharmacological model4. So, in every prone PD patient, there might be an individual (cumulative) dose threshold. After discontinuation of dopamine agonists, ICDs resolve in about 50% of the patients6. Two longitudinal studies could show an improvement of ICDs after reduction of agonists or a switch to L-DOPA86,87, whereas personality traits associated with ICDs persisted. So far, variable rates of relapse or remission are not fully understood and further research is needed.
Is apathy the counterpart of ICDs?
Arguably, one could arrange the motivational spectrum of behavior in such a way that ICDs would be at the positive end and apathy at the negative end of the spectrum. Apathy generally is even more prevalent in PD patients than ICDs, including early stages of the disease88. While there potentially are multiple mechanisms leading to apathy, it is interesting that some forms of apathy are clearly temporally correlated with a reduction of dopaminergic stimulation. Apathy occurs following deep-brain stimulation, especially when DRT is reduced to a large degree89. Apathy can also be found as a part of dopamine-agonist withdrawal syndrome (DAWS). Intriguingly, dopamine agonist withdrawal in PD patients with DAWS almost always was preceded by ICDs, and in patients without ICDs, dopamine agonist withdrawal did not lead to apathy90. Additionally, when comparing PD patients with these two PD subgroups, overlaps in behavior were found91,92. Scott et al. showed in a recently published cohort study that more than a third of PD patients with apathy also suffer from ICDs. Interestingly, these were the patients with the longest disease duration93.
In addition, rodent studies94,95 as well as human imaging studies96 hint at a hypodopaminergic state in the striatum predisposing for apathy. Therefore, apathy and ICD might share the same pathophysiological principle, i.e., hypodopaminergic changes in the striatum, which then leads to either ICDs or apathy, depending on DRT. Sierra et al. use the term “Ying and Yang” of dopamine-dependent behavior97. Figure 3 describes hypothetical differences in dose–response relationships, implicating that vulnerable PD patients switch between the extremes in response to only small changes in dopaminergic medication. Additionally, it seems worth mentioning that not only might apathy share pathomechanisms with ICD but also dyskinesia (for detailed review see Voon et al.)98.
Fig. 3. Between the extremes.
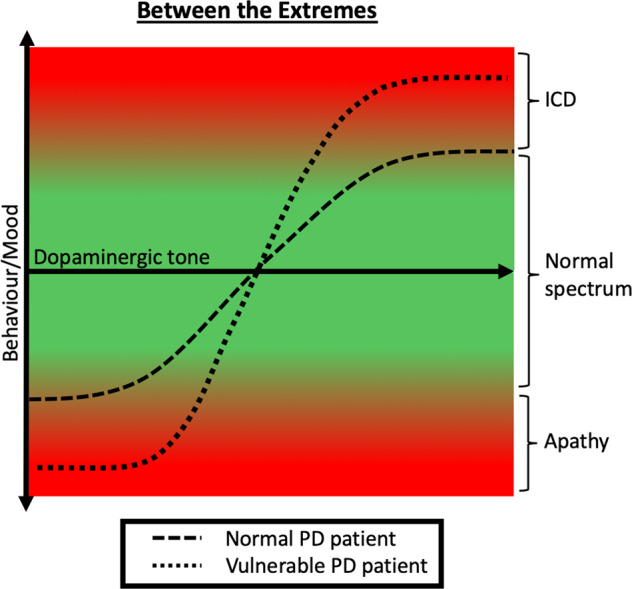
Relation between dopaminergic tone and behavior illustrated as sigmoid curves in PD patients, with and without vulnerability. In vulnerable PD patients, the relationship between behavior and dopaminergic tone resembles a “flip-flop” switch scenario.
Rodent studies—a possibility for validation of our vulnerability-stress model?
Rodent studies of the dopaminergic reward system show interesting insights into the development of ICDs. Lesions with 6-hydroxydopamine (6-OHDA) can imitate our theory of a premorbid vulnerability: Cardinal et al. produced a dopaminergic lesion in the nucleus accumbens with 6-OHDA, leading to impulsiveness in a delay-discounting task99. In other designs, dopaminergic lesions were set in the posterior VTA. After the submission of DRT, the animals showed impulsive behavior in a place-preference task100,101. Holtz et al. also used 6-OHDA to produce a dopaminergic lesion in the striatum. In a delay-discounting task, rats showed risk-taking behavior when pramipexole was administered. Interestingly, mirtazapine leads to a reduction of risk-taking102. In another design, 6-OHDA was administered at the substantia nigra. After surgery, animals took less from a rewarding sucrose solution. As a more general claim, rats became apathetic, which was fully reversed after the intake of pramipexole94, which supports our theory-comparable changes within the dopaminergic reinforcement system in apathy and ICD described above.
Interesting insights into the effect of dopaminergic medication, stress in our model, can be derived from chemogenetics. “Designer receptor exclusively activated by designer drugs” (DREADDs) can be used to activate or inactivate certain types of dopaminergic receptors. Boender et al. injected a D1-activating DREADD in the nucleus accumbens of rats, leading to an increased intake of sucrose pellets, which was annulated by the administration of a D1 antagonist103. Zhu and colleges injected D2 receptor activating and inhibiting DREADDs in the nucleus accumbens of rats. D2 activation reduced locomotion and running, whereas D2 inhibition had the opposite effect104.
In all, rodent studies—despite their limitations in comparability—corroborate and validate biological concepts of the vulnerability-stress model of ICD development.
Conclusions
We discuss a hypothetical model of hypodopaminergic changes in the ventral striatum that would act as a biological vulnerability toward addictive behavior. These alterations predispose the dopaminergic system (vulnerability), which, in combination with DRT (stress), leads to ICDs. As the most likely scenario, a reduction of dopaminergic projections in combination with a reduced DAT density and autoreceptor function results in adjustment processes at the postsynaptic membrane. Furthermore, it comes to a diminished top-down control of inhibitory cortical areas. As a consequence, DRT overwhelms the prone system. So, a combination of a premorbid vulnerability and overdosing could lead to ICDs in PD and can be seen as a vulnerability-stress model. It is tempting to speculate that similar biological processes may underlie other drug or non-substance addictions in the non-PD population.
Apathy is associated with a reduced DAT density in the dorsal striatum, whereas patients with ICDs have also a reduction of DAT in the ventral striatum. We assume that apathy and ICDs go along with a hypodopaminergic state in striatal regions and therefore with an increased sensibility to DRT. There is an overlap in patients suffering from both ICDs and apathy.
Limitations
The model of hypodopaminergic changes in the ventral striatum, leading to a vulnerability for DRT and thereby to ICDs, is only hypothetic and, of course, a simplification of the complex development of ICDs. So, there are some limitations to consider. In this review, we mainly shed light on the so-called dopaminergic reinforcement system, but also other neurotransmitters, as mentioned above, play an important role.
Additionally, we do not have de novo data concerning the postsynaptic membrane. We do not know whether the reduced D2 availability might predate the presynaptic changes. Hence, theoretically, the model described above could be vice versa. Changes in D2 density could lead to a hypodopaminergic state in the striatum.
Furthermore, imaging data do not always point in the same direction: One study found an increase in dopamine synthesis capacity in impulsive PD patients105. In addition, Boileau et al. could not find differences in D2/D3 availability between pathological gamblers and healthy controls106. Similarly, the results of genetic studies in PD with ICDs are not consistent107. Altogether, despite many converging results around a premorbid biological vulnerability, more rigorous studies with larger samples are needed to consolidate the genetic and molecular features of this vulnerability.
Future directions
It would be interesting to measure D2 receptor availability and DAT density in de novo PD patients and after the development of ICDs under DRT. With this approach, it would be possible to check if postsynaptic changes also predate the development of ICDs. In addition, it would be interesting to measure dopamine synthesis capacity under reward conditions since all existing 18F-DOPA-PET studies measure baseline dopamine synthesis capacity. This would help to classify the reduced D2 receptor availability under reward conditions. Furthermore, a validation of our vulnerability-stress model in a rodent model combined with PET imaging would be of great interest for the understanding of ICDs in PD in particular but also for the development of addiction in general.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Author contributions
H.T. contributed in the conception, design, writing of the first draft, idea and creation of the figures, C.P. contributed in conception, design, and writing to the first draft, P.O.F. contributed in design, consulting, review, and critique of the paper, and T.v.E. contributed in conception, design, consulting, review, and critique of the paper.
Funding
This work was partly funded by the German Research Foundation (DFG, CRC-1451, project C03, project-ID 431549029). Open Access funding enabled and organized by Projekt DEAL.
Data availability
No datasets were generated or analyzed during the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-021-00253-z.
References
- 1.World Health Organization (WHO). Drugs (2021) https://www.who.int/westernpacific/health-topics/drugs-psychoactive.
- 2.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. AJP. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 3.Probst CC, van Eimeren T. The functional anatomy of impulse control disorders. Curr. Neurol. Neurosci. Rep. 2013;13:386. doi: 10.1007/s11910-013-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weintraub D, et al. Impulse control disorders in parkinson disease: a cross-sectional study of 3090 patients. Arch. Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 5.Cilia R, van Eimeren T. Impulse control disorders in Parkinson’s disease: seeking a roadmap toward a better understanding. Brain Struct. Funct. 2011;216:289–299. doi: 10.1007/s00429-011-0314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corvol J-C, et al. Longitudinal analysis of impulse control disorders in Parkinson disease. Neurology. 2018;91:e189–e201. doi: 10.1212/WNL.0000000000005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonini A, et al. ICARUS study: prevalence and clinical features of impulse control disorders in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2017;88:317–324. doi: 10.1136/jnnp-2016-315277. [DOI] [PubMed] [Google Scholar]
- 8.Potenza MN. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotham AM, Brown RG, Marsden CD. ‘Frontal’ cognitive function in patients with Parkinson’s disease ‘on’ and ‘off’ levodopa. Brain. 1988;111:299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- 10.Cools R, Robbins TW. Chemistry of the adaptive mind. Philos. Trans. A Math. Phys. Eng. Sci. 2004;362:2871–2888. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- 11.Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in Parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 12.Grant JE, Potenza MN, Weinstein A, Gorelick DA. Introduction to behavioral addictions. Am. J. Drug Alcohol Abus. 2010;36:233–241. doi: 10.3109/00952990.2010.491884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voon V, Dalley JW. Parkinson disease: impulsive choice-Parkinson disease and dopaminergic therapy. Nat. Rev. Neurol. 2011;7:541–542. doi: 10.1038/nrneurol.2011.139. [DOI] [PubMed] [Google Scholar]
- 14.Voon V, et al. Dopamine agonists and risk: impulse control disorders in Parkinson’s; disease. Brain. 2011;134:1438–1446. doi: 10.1093/brain/awr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zubin J, Spring B. Vulnerability-a new view of schizophrenia. J. Abnorm. Psychol. 1977;86:103–126. doi: 10.1037//0021-843x.86.2.103. [DOI] [PubMed] [Google Scholar]
- 16.Goh C, Agius M. The stress-vulnerability model how does stress impact on mental illness at the level of the brain and what are the consequences? Psychiatr. Danub. 2010;22:198–202. [PubMed] [Google Scholar]
- 17.Cilia R, et al. Reduced dopamine transporter density in the ventral striatum of patients with Parkinson’s disease and pathological gambling. Neurobiol. Dis. 2010;39:98–104. doi: 10.1016/j.nbd.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Smith KM, Xie SX, Weintraub D. Incident impulse control disorder symptoms and dopamine transporter imaging in Parkinson disease. J. Neurol. Neurosurg. Psychiatry. 2016;87:864–870. doi: 10.1136/jnnp-2015-311827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voon V, et al. Impulse control disorders in Parkinson’s disease: decreased striatal dopamine transporter levels. J. Neurol. Neurosurg. Psychiatry. 2014;85:148–152. doi: 10.1136/jnnp-2013-305395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vriend C, et al. Reduced dopamine transporter binding predates impulse control disorders in Parkinson’s disease: reduced DaT BR Predates ICD in PD. Mov. Disord. 2014;29:904–911. doi: 10.1002/mds.25886. [DOI] [PubMed] [Google Scholar]
- 21.Hammes J, et al. Dopamine metabolism of the nucleus accumbens and fronto-striatal connectivity modulate impulse control. Brain. 2019;142:733–743. doi: 10.1093/brain/awz007. [DOI] [PubMed] [Google Scholar]
- 22.Rao H, et al. Decreased ventral striatal activity with impulse control disorders in Parkinson’s disease. Mov. Disord. 2010;25:1660–1669. doi: 10.1002/mds.23147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steeves TDL, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark, A. J. et al. Nigrostriatal and mesolimbic D2/3receptor expression in Parkinson’s disease patients with compulsive reward-driven behaviors. J. Neurosci. 10.1523/JNEUROSCI.3082-17.2018 (2018). [DOI] [PMC free article] [PubMed]
- 25.Pagano G, et al. Impulse control disorders are associated with lower ventral striatum dopamine D3 receptor availability in Parkinson’s disease: a [11C]-PHNO PET study. Parkinsonism Relat. Disord. 2021;90:52–56. doi: 10.1016/j.parkreldis.2021.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Fazio P, et al. Nigrostriatal dopamine transporter availability in early Parkinson’s disease. Mov. Disord. 2018;33:592–599. doi: 10.1002/mds.27316. [DOI] [PubMed] [Google Scholar]
- 27.Jokinen P, et al. Simple ratio analysis of 18F-fluorodopa uptake in striatal subregions separates patients with early Parkinson disease from healthy controls. J. Nucl. Med. 2009;50:893–899. doi: 10.2967/jnumed.108.061572. [DOI] [PubMed] [Google Scholar]
- 28.Bancos I, et al. Impulse control disorders in patients with dopamine agonist-treated prolactinomas and nonfunctioning pituitary adenomas: a case-control study. Clin. Endocrinol. 2014;80:863–868. doi: 10.1111/cen.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holman AJ. Impulse control disorder behaviors associated with pramipexole used to treat fibromyalgia. J. Gambl. Stud. 2009;25:425–431. doi: 10.1007/s10899-009-9123-2. [DOI] [PubMed] [Google Scholar]
- 30.Pettorruso M, et al. Striatal presynaptic dopaminergic dysfunction in gambling disorder: A 123 I-FP-CIT SPECT study. Addict. Biol. 2019;24:1077–1086. doi: 10.1111/adb.12677. [DOI] [PubMed] [Google Scholar]
- 31.Yen C-H, et al. Reduced dopamine transporter availability and neurocognitive deficits in male patients with alcohol dependence. PLoS ONE. 2015;10:e0131017. doi: 10.1371/journal.pone.0131017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leroy C, et al. Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: a high-resolution PET study. Addict. Biol. 2012;17:981–990. doi: 10.1111/j.1369-1600.2011.00356.x. [DOI] [PubMed] [Google Scholar]
- 33.Hou, H. et al. Reduced striatal dopamine transporters in people with internet addiction disorder. BioMed Res. Int.10.1155/2012/854524 (2012) https://www.hindawi.com/journals/bmri/2012/854524/. [DOI] [PMC free article] [PubMed]
- 34.Kim SH, et al. Reduced striatal dopamine D2 receptors in people with Internet addiction. Neuroreport. 2011;22:407–411. doi: 10.1097/WNR.0b013e328346e16e. [DOI] [PubMed] [Google Scholar]
- 35.Smith CT, et al. Ventral striatal dopamine transporter availability is associated with lower trait motor impulsivity in healthy adults. Transl. Psychiatry. 2018;8:269. doi: 10.1038/s41398-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu JC, et al. Decreasing striatal 6-FDOPA uptake with increasing duration of cocaine withdrawal. Neuropsychopharmacology. 1997;17:402–409. doi: 10.1016/S0893-133X(97)00089-4. [DOI] [PubMed] [Google Scholar]
- 37.Bloomfield MAP, et al. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol. Psychiatry. 2014;75:470–478. doi: 10.1016/j.biopsych.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 38.Majuri J, et al. Dopamine and opioid neurotransmission in behavioral addictions: a comparative PET study in pathological gambling and binge eating. Neuropsychopharmacology. 2017;42:1169–1177. doi: 10.1038/npp.2016.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbosa P, et al. Lower nucleus accumbens α-synuclein load and D3 receptor levels in Parkinson’s disease with impulsive compulsive behaviours. Brain. 2019;142:3580–3591. doi: 10.1093/brain/awz298. [DOI] [PubMed] [Google Scholar]
- 40.Maillet A, et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain. 2016;139:2486–2502. doi: 10.1093/brain/aww162. [DOI] [PubMed] [Google Scholar]
- 41.Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 42.Potenza MN. The neurobiology of pathological gambling. Semin Clin. Neuropsychiatry. 2001;6:217–226. [PubMed] [Google Scholar]
- 43.Majuri J, et al. Serotonin transporter density in binge eating disorder and pathological gambling: A PET study with [11C]MADAM. Eur. Neuropsychopharmacol. 2017;27:1281–1288. doi: 10.1016/j.euroneuro.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Worbe Y, Savulich G, Voon V, Fernandez-Egea E, Robbins TW. Serotonin depletion induces ‘waiting impulsivity’ on the human four-choice serial reaction time task: cross-species translational significance. Neuropsychopharmacology. 2014;39:1519–1526. doi: 10.1038/npp.2013.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology. 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- 46.Thompson MD, Kenna GA. Variation in the serotonin transporter gene and alcoholism: risk and response to pharmacotherapy. Alcohol Alcohol. 2016;51:164–171. doi: 10.1093/alcalc/agv090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parsons LH, Justice JB. Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. Brain Res. 1993;606:195–199. doi: 10.1016/0006-8993(93)90984-u. [DOI] [PubMed] [Google Scholar]
- 48.Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J. Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erga AH, et al. Dopaminergic and opioid pathways associated with impulse control disorders in Parkinson’s disease. Front Neurol. 2018;9:109. doi: 10.3389/fneur.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl Acad. Sci. USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engeli, E. J. E. et al. Impaired glutamate homeostasis in the nucleus accumbens in human cocaine addiction. Mol. Psychiatry10.1038/s41380-020-0828-z (2020). [DOI] [PubMed]
- 53.Lipton DM, Gonzales BJ, Citri A. Dorsal striatal circuits for habits, compulsions and addictions. Front. Syst. Neurosci. 2019;13:28. doi: 10.3389/fnsys.2019.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 55.Everitt BJ, et al. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carriere N, Lopes R, Defebvre L, Delmaire C, Dujardin K. Impaired corticostriatal connectivity in impulse control disorders in Parkinson disease. Neurology. 2015;84:2116–2123. doi: 10.1212/WNL.0000000000001619. [DOI] [PubMed] [Google Scholar]
- 57.Premi E, et al. Impulse control disorder in PD: a lateralized monoaminergic frontostriatal disconnection syndrome? Parkinsonism Relat. Disord. 2016;30:62–66. doi: 10.1016/j.parkreldis.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 58.Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- 59.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat. Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 60.Grieder TE, et al. Phasic D1 and tonic D2 dopamine receptor signaling double dissociate the motivational effects of acute nicotine and chronic nicotine withdrawal. Proc. Natl Acad. Sci. USA. 2012;109:3101–3106. doi: 10.1073/pnas.1114422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Payer D, Balasubramaniam G, Boileau I. What is the role of the D3 receptor in addiction? A mini review of PET studies with [11C]-(+)-PHNO. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;52:4–8. doi: 10.1016/j.pnpbp.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 62.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 63.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Bannon MJ, Michelhaugh SK, Wang J, Sacchetti P. The human dopamine transporter gene: gene organization, transcriptional regulation, and potential involvement in neuropsychiatric disorders. Eur. Neuropsychopharmacol. 2001;11:449–455. doi: 10.1016/s0924-977x(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 65.Forbes EE, et al. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol. Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo G, Tong Y, Xie C-W, Lange LA. Dopamine transporter, gender, and number of sexual partners among young adults. Eur. J. Hum. Genet. 2007;15:279–287. doi: 10.1038/sj.ejhg.5201763. [DOI] [PubMed] [Google Scholar]
- 67.Jacobsen LK, et al. Prediction of dopamine transporter binding availability by genotype: a preliminary report. AJP. 2000;157:1700–1703. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- 68.Volkow ND. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J. Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135:1025–1033. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 70.Morice E, Denis C, Giros B, Nosten-Bertrand M. Evidence of long-term expression of behavioral sensitization to both cocaine and ethanol in dopamine transporter knockout mice. Psychopharmacology. 2010;208:57–66. doi: 10.1007/s00213-009-1707-0. [DOI] [PubMed] [Google Scholar]
- 71.O’Sullivan SS, et al. Cue-induced striatal dopamine release in Parkinson’s disease-associated impulsive-compulsive behaviours. Brain. 2011;134:969–978. doi: 10.1093/brain/awr003. [DOI] [PubMed] [Google Scholar]
- 72.Jones SR, et al. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat. Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- 73.Gainetdinov RR, Jones SR, Caron MG. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol. Psychiatry. 1999;46:303–311. doi: 10.1016/s0006-3223(99)00122-5. [DOI] [PubMed] [Google Scholar]
- 74.Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ray NJ, et al. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson’s patients with medication-induced pathological gambling: A [11C] FLB-457 and PET study. Neurobiol. Dis. 2012;48:519–525. doi: 10.1016/j.nbd.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buckholtz JW, et al. Dopaminergic Network Differences in Human Impulsivity. Science. 2010;329:532–532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carter CS, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 78.Cilia R, et al. Pathological gambling in patients with Parkinson’s disease is associated with fronto-striatal disconnection: a path modeling analysis. Mov. Disord. 2011;26:225–233. doi: 10.1002/mds.23480. [DOI] [PubMed] [Google Scholar]
- 79.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 80.Rolls ET. The orbitofrontal cortex and reward. Cereb. Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 81.van Eimeren T, et al. Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology. 2010;75:1711–1716. doi: 10.1212/WNL.0b013e3181fc27fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Eimeren T, et al. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in Parkinson’s disease? Neuropsychopharmacology. 2009;34:2758–2766. doi: 10.1038/sj.npp.npp2009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chernoloz O, Mansari ME, Blier P. Sustained administration of pramipexole modifies the spontaneous firing of dopamine, norepinephrine, and serotonin neurons in the rat brain. Neuropsychopharmacology. 2009;34:651–661. doi: 10.1038/npp.2008.114. [DOI] [PubMed] [Google Scholar]
- 84.Bastiaens J, Dorfman BJ, Christos PJ, Nirenberg MJ. Prospective cohort study of impulse control disorders in Parkinson’s disease. Mov. Disord. 2013;28:327–333. doi: 10.1002/mds.25291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perez-Lloret S, et al. Prevalence and pharmacological factors associated with impulse-control disorder symptoms in patients with Parkinson disease. Clin. Neuropharmacol. 2012;35:261–265. doi: 10.1097/WNF.0b013e31826e6e6d. [DOI] [PubMed] [Google Scholar]
- 86.Mamikonyan E, et al. Long-term follow-up of impulse control disorders in Parkinson’s disease. Mov. Disord. 2008;23:75–80. doi: 10.1002/mds.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J-Y, et al. Behavioural and trait changes in parkinsonian patients with impulse control disorder after switching from dopamine agonist to levodopa therapy: results of REIN-PD trial. J. Neurol. Neurosurg. Psychiatry. 2019;90:30–37. doi: 10.1136/jnnp-2018-318942. [DOI] [PubMed] [Google Scholar]
- 88.Aarsland D, et al. Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 1999;67:492–496. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thobois S, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain. 2010;133:1111–1127. doi: 10.1093/brain/awq032. [DOI] [PubMed] [Google Scholar]
- 90.Rabinak CA, Nirenberg MJ. Dopamine agonist withdrawal syndrome in Parkinson disease. Arch. Neurol. 2010;67:58–63. doi: 10.1001/archneurol.2009.294. [DOI] [PubMed] [Google Scholar]
- 91.Ahearn DJ, McDonald K, Barraclough M, Leroi I. An exploration of apathy and impulsivity in parkinson disease. Curr. Gerontol. Geriatr. Res. 2012;2012:390701. doi: 10.1155/2012/390701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leroi I, et al. Apathy and impulse control disorders in Parkinson’s disease: a direct comparison. Parkinsonism Relat. Disord. 2012;18:198–203. doi: 10.1016/j.parkreldis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Scott BM, et al. Co-occurrence of apathy and impulse control disorders in Parkinson disease. Neurology. 2020;95:e2769–e2780. doi: 10.1212/WNL.0000000000010965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Favier M, et al. Pramipexole reverses Parkinson’s disease-related motivational deficits in rats. Mov. Disord. 2014;29:912–920. doi: 10.1002/mds.25837. [DOI] [PubMed] [Google Scholar]
- 95.Drui G, et al. Loss of dopaminergic nigrostriatal neurons accounts for the motivational and affective deficits in Parkinson’s disease. Mol. Psychiatry. 2014;19:358–367. doi: 10.1038/mp.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Santangelo G, et al. Apathy and striatal dopamine transporter levels in de-novo, untreated Parkinson’s disease patients. Parkinsonism Relat. Disord. 2015;21:489–493. doi: 10.1016/j.parkreldis.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 97.Sierra, M. et al. Apathy and impulse control disorders: Yin & Yang of dopamine dependent behaviors. J. Parkinson’s Dis.5, 10.3233/JPD-150535 (2015). [DOI] [PubMed]
- 98.Voon V, et al. Impulse control disorders and levodopa-induced dyskinesias in Parkinson’s disease: an update. Lancet Neurol. 2017;16:238–250. doi: 10.1016/S1474-4422(17)30004-2. [DOI] [PubMed] [Google Scholar]
- 99.Cardinal RN, et al. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 100.Ouachikh O, Dieb W, Durif F, Hafidi A. Differential behavioral reinforcement effects of dopamine receptor agonists in the rat with bilateral lesion of the posterior ventral tegmental area. Behavioural Brain Res. 2013;252:24–31. doi: 10.1016/j.bbr.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 101.Carvalho MM, et al. Effect of levodopa on reward and impulsivity in a rat model of Parkinson’s disease. Front. Behav. Neurosci. 2017;11:145. doi: 10.3389/fnbeh.2017.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holtz NA, Tedford SE, Persons AL, Grasso SA, Napier TC. Pharmacologically distinct pramipexole-mediated akinesia vs. risk-taking in a rat model of Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;70:77–84. doi: 10.1016/j.pnpbp.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boender AJ, et al. Combined use of the Canine Adenovirus-2 and DREADD-technology to activate specific neural pathways in vivo. PLoS ONE. 2014;9:e95392. doi: 10.1371/journal.pone.0095392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu X, Ottenheimer D, DiLeone RJ. Activity of D1/2 receptor expressing neurons in the nucleus accumbens regulates running, locomotion, and food intake. Front. Behav. Neurosci. 2016;10:66. doi: 10.3389/fnbeh.2016.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lawrence AD, Brooks DJ, Whone AL. Ventral Striatal Dopamine Synthesis Capacity Predicts Financial Extravagance in Parkinson’s Disease. Front. Psychol. 2013;4:90. doi: 10.3389/fpsyg.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boileau I, et al. The D2/3 dopamine receptor in pathological gambling: a positron emission tomography study with [11C]-(+)-propyl-hexahydro-naphtho-oxazin and [11C]raclopride. Addiction. 2013;108:953–963. doi: 10.1111/add.12066. [DOI] [PubMed] [Google Scholar]
- 107.Vallelunga A, et al. Role of genetic polymorphisms of the dopaminergic system in Parkinson’s disease patients with impulse control disorders. Parkinsonism Relat. Disord. 2012;18:397–399. doi: 10.1016/j.parkreldis.2011.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.