Abstract
Aims
To investigate the clinical features and mitochondrial mutations for maternally inherited diabetes and deafness.
Methods
PubMed, Embase, Medline, Web of Science, the China National Knowledge Infrastructure, and Wanfang were searched with the following search terms: “Maternally inherited diabetes and deafness” OR “MIDD” OR “Mitochondrial diabetes”. The mutations and clinical features were analyzed. Correlation between the heteroplasmy levels of the m.3243A>G mutation in the peripheral blood and age at the onset of diabetes was conducted by Spearman test. The significance level was set as p < 0.05. Statistical analysis was performed using the Statistical Package for the Social Sciences version 26 for Windows.
Results
Totally 161 patients with 21 different mitochondrial mutations were enrolled. The most common mutation was the m.3243A>G mutation in 136 cases. Of 142 patients, 120 (84.51%) had family histories of diabetes or hearing loss. Hearing loss presented in 85.71% of the patients with mitochondrial mutations. Central nervous system diseases were found in 29.19%, myopathy in 22.98%, oculopathy in 23.60%, cardiac disease in 23.60%, and nephropathy in 13.66% of the patients. Forty-two of 101 (41.58%) patients were underweight. A significant negative correlation was found between the heteroplasmy levels of the m.3243A>G mutation in the peripheral blood and age at the onset of diabetes.
Conclusions
The young onset of diabetes with low or normal BMI, maternal inheritance, and presence of impairments of multiple systems should prompt a genetic testing in order to differentiate MIDD from other types of diabetes earlier.
Keywords: treatment, diagnosis, mitochondrial gene mutations, heteroplasmy, maternally inherited diabetes and deafness
Introduction
Diabetes mellitus is one of the most important chronic non-communicable disease, which has been a significant global public health problem. The incidence of monogenic diabetes mellitus has increased in recent years due to greater awareness and wider availability of genetic testing. Monogenic diabetes mellitus comprises a heterogeneous group of diabetes which are caused by a single gene defect (1). A subtype of monogenic diabetes associated with mutations in the mitochondrial DNA is referred to as maternally inherited diabetes and deafness (MIDD), which was first described in 1992 by Van den Ouveland et al. (2). The abnormality of glucose metabolism in MIDD is associated with a gradual decrease in insulin secretion due to reduced ATP production in pancreatic β-cells with abnormal mitochondria (3). The diagnosis and differentiation of MIDD from type 1 and type 2 diabetes are important in view of the implications for treatment and prognosis as well as for identification of family members at risk of diabetes (2). However, the clinical features of MIDD are variable, and MIDD is frequently misdiagnosed as other types of diabetes. Thus, it is extremely essential to recognize MIDD among the diabetic patients. It is a pity that there is no systematic summary about the disease up to now. Our study summarized the clinical features and mutations in reported MIDD to help the doctors better diagnose and manage these patients.
Subjects and Methods
PubMed, Embase, Medline, Web of Science, the China National Knowledge Infrastructure (CNKI), and Wanfang were searched from the date of their inception to February 10, 2021, without language restrictions. The key words used were “Maternally inherited diabetes and deafness” OR “MIDD” OR “Maternally inherited diabetes” OR “Mitochondrial diabetes.” Eligible studies met the following criteria: (1) Mitochondrial mutations were confirmed by genetic testing, and detection of the mutation was based on polymerase chain reaction (PCR) amplification. (2) The mutation sites were described. (3) The main clinical data of patients were described. The flow chart ( Supplementary Data Figure S1 ) showed the reasons for identification of eligible studies.
From the eligible studies, the following data were extracted: (1) country, (2) gender, (3) age at onset of diabetes and hearing loss, (4) family history, (5) therapies, (6) BMI, (7) fasting C-peptide, (8) mutation sites, (9) antibodies, (10) the heteroplasmy levels in tissues.
Statistical Analyses
Correlation between the heteroplasmy levels of the m.3243A>G mutation in the peripheral blood and age at the onset of diabetes was conducted by Spearman test. The significance level was set as p < 0.05. Statistical analysis was performed using the Statistical Package for the Social Sciences version 26 for Windows (SPSS).
Results
Epidemiological Characteristics and Gene Mutations
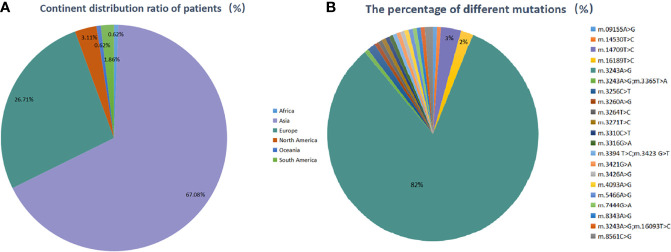
One hundred and sixteen studies including 161 patients were eligible with the aforementioned search terms. Among them, Asian cases accounted for the largest part (108 cases, 67.08%), followed by European (43 cases, 26.71%), North American (5 cases, 3.11%), South American (3 cases, 1.86%), African and Oceania (1 case, 0.62%, respectively). Among the Asian, cases from China, Japan, India, Iran, and Korea accounted for 39.75, 25.47, 0.62, 0.62, and 0.62%, respectively. Totally 21 different mtDNA mutations were identified. Most of the patients (158/161, 98.14%) had one mutation, and three (3/161, 1.86%) had two mutations. The most common mutation was the m.3243A>G mutation, which accounted for 84.47% of the cases, while other mutations were quite rare. The detailed information of enrolled countries and individuals are described in Table S1 ( Supplementary Materials ) and Figure 1 .
Figure 1.
(A) Geographical country distribution ratio among the patients (%), (B) the percentage of different mutation sites (%).
Clinical Characteristics
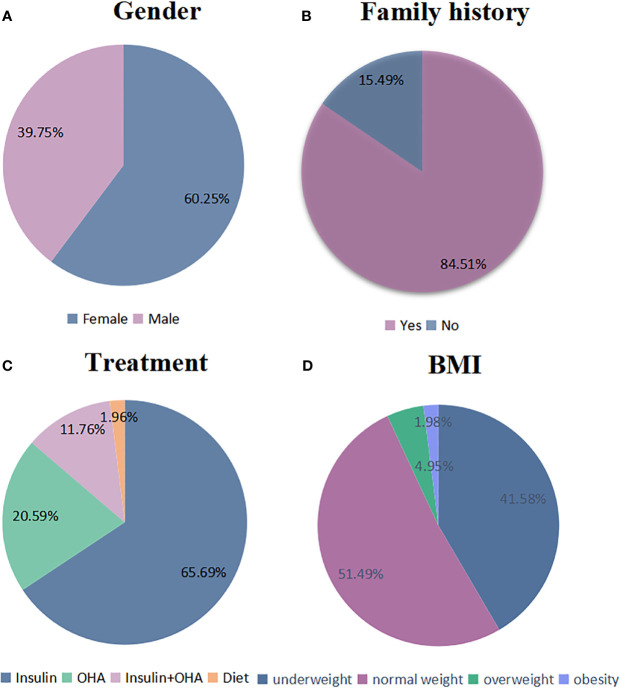
The clinical data at diagnosis of the patients are shown in Table S2 ( Supplementary Materials ) and Figures 2 and 3 . Among the 161 patients, 60.25% (97/161) were female and 39.75% (64/161) were male. Family histories were mentioned in 142 patients, and among them, 59.86% (85/142) had family histories of both diabetes and hearing loss; 21.83% (31/142) had family history of diabetes; 2.82% (4/142) had family history of hearing loss. Mitochondrial mutations were detected in 3.52% (5/142) of patients’ relatives who had no hyperglycemia or hearing loss. The body-mass-index (BMI) at diagnosis were available in 101 patients. Their BMI ranged from 12.5 to 36.89 kg/m² (mean 19.41 kg/m²). The group of underweight (<18.5 kg/m2), normal weight, overweight (25~29.9 kg/m2), or obese (≥30 kg/m2) accounted for 41.58, 51.49, and 6.93%, respectively, according to WHO standard.
Figure 2.
Clinical characteristics of patients with mitochondrial mutations. (A–D) The proportion of several clinical characteristics in enrolled patients: (A) gender (N: 161), (B) family histories (N: 142), (C) treatment (N: 102), and (D) BMI (N: 101).
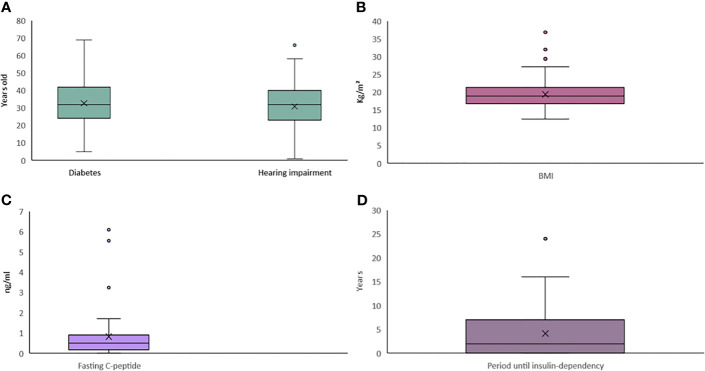
Figure 3.
Whisker plot for continuous clinical data of patients with mitochondrial mutations. (A–D) Continuous data for the variables of (A) Age at onset, (B) BMI, (C) Fasting C peptide, and (D) Period until insulin dependency.
Fasting C-peptide data were available in 42 patients, with a mean of 0.83 ng/ml, lower than the normal range (1.1~4.4 ng/ml). Diabetes antibodies were reported in 50 patients, and among them, only five (10.00%) patients had positive results (one case had GADA, two cases had IAA, and two cases had ICA).
The clinical manifestations of MIDD patients are shown in Table S3 ( Supplementary Materials ). Hearing impairment presented in 138 (85.71%) patients. Central nervous system diseases were found in 47 (29.19%) patients, including encephalatrophy (11.18%), cerebellar ataxia (6.21%), basal ganglia calcification (2.48%), migraine (4.97%), and cerebral infarction (5.59%). Peripheral neuropathy was found in 28 (17.39%) patients. Myopathy was presented in 37 (22.98%) patients manifesting muscle weakness (14.91%), myophagism (4.97%), ptosis (2.48%). Ragged red fibers on muscle biopsy were seen in 26 (16.15%) patients. Oculopathy was observed in 38 (23.60%) patients, and the prevalence of macular degeneration (9.32%) was higher than proliferative retinopathy (5.59%). Cardiac disease was present in 38 (23.60%) patients, and 19 (11.80%) patients had a manifestation of ventricular hypertrophy and 15 (9.32%) patients had a manifestation of arrhythmia. Nephropathy, defined by the presence of albuminuria and/or impairment in renal function, was found in 22 (13.66%) patients. Gastrointestinal symptoms were observed in 9 (5.95%) patients. In addition to diabetes, other endocrine disorders involving hypogonadism (1.86%) and osteoporosis (2.48%) were observed.
Treatment
The treatments of 102 patients were provided in the original articles. Seventy-nine patients (79/102, 77.45%) injected insulin, and 23 patients (23/102, 22.55%) did not. Among them, 67 patients (67/102, 65.69%) received insulin without oral hypoglycemic agent. Twenty-two patients (21/102, 20.59%) received oral hypoglycemic agents (OHA) without insulin, and 12 (12/102, 11.76%) received both insulin and OHA. Two patients (2/102, 1.96%) underwent diet therapy only. Among the patients who received insulin, 37.18% (29/78) initiated once diabetes was diagnosed. The mean time from the diagnosis of diabetes to insulin therapy was 4.15 years. Onset of the diabetes usually occurred at an early age with a mean age of 32.79 years, and the mean age of onset of hearing impairment was 30.84 years.
Heteroplasmy Levels
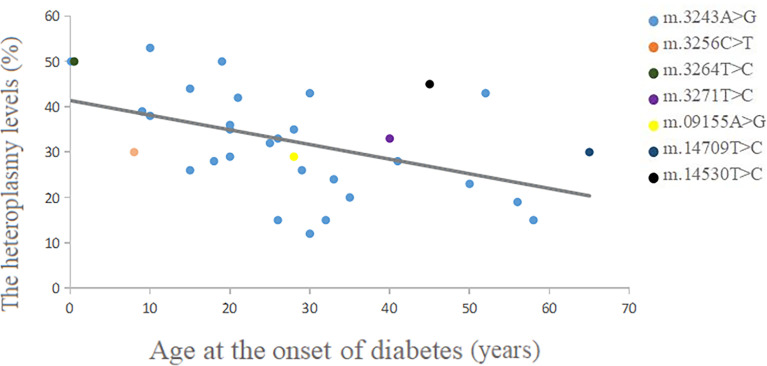
The heteroplasmy levels of the m.3243A>G mutation in different tissues are shown in Table 1 . The blood heteroplasmy levels in 27 patients ranged from 0.102 to 58%, with a mean value of 26.97%. The mean heteroplasmy levels in urothelium, muscle, hair follicle, buccal mucosa, nail, and myocardium were 58.13, 49.53, 41.35, 48.25, 32.00, and 67.50% respectively. We used different colors representing different mutations in the scattergram, which showed a negative correlation between blood heteroplasmy levels and age at the onset of diabetes ( Figure 4 ). A significant negative correlation was found between the blood mutation levels of the m.3243A>G mutation and age at the onset of diabetes (P<0.01) ( Table 2 ).
Table 1.
The heteroplasmy levels of the m.3243A>G mutation in different tissues.
| Tissue | N | Heteroplasmy levels (%) | ||
|---|---|---|---|---|
| Min | Max | Mean | ||
| Peripheral blood leukocyte | 27 | 0.102 | 58 | 26.97 |
| Urothelium | 3 | 31.4 | 76 | 58.13 |
| Muscle | 7 | 0.024 | 90 | 49.53 |
| Buccal mucosa | 4 | 40 | 58 | 48.25 |
| Hair follicle | 2 | 12.7 | 70 | 41.35 |
| Nail | 1 | 32 | 32 | 32.00 |
| Myocardium | 2 | 60 | 75 | 67.50 |
N, number of patients.
Figure 4.
Correlation between blood heteroplasmy levels and age at the onset of diabetes.
Table 2.
Correlation between blood heteroplasmy levels of the m.3243A>G mutation and age at the onset of diabetes.
| Age at onset | The heteroplasmy levels in peripheral blood | blood |
|---|---|---|
| r | p | |
| Diabetes | −0.612 | 0.001** |
**p < 0.01
Discussion
Since MIDD was first described, there have been some reports about this disease. However, most studies about MIDD involved only a small series of patients. Unlike previous studies, we summarized the clinical features and mutations of 161 patients with mitochondrial mutations. Our study demonstrated the MIDD patients with most mutations had the following clinical characteristics: (1) high incidence of progressive neurosensory deafness (85.71%); (2) early onset of diabetes and deafness; (3) high incidence of maternal inheritance (84.51%); (4) thin and short stature (41.58%); (5) absence of diabetes antibodies (90.00%); (6) progressive insulin secretory defect.
But not all patients with mitochondrial mutations met the above characteristics. The m.3426 A>G and m.16189T>C mutation were considered as susceptibility factors for insulin resistance and T2DM, which were also maternal inherited (4–6). The patients with m.3426 A>G mutation did not have deafness and had late onset age of diabetes (mean 42.14 years) with the BMI above the normal range (mean 27.14 kg/m²) and usually had no complications (4). In addition, the laboratory characteristics of patients with m.3426A>G mutation was that they had normal levels of fasting C-peptide and usually took oral hypoglycemic agents for treatment (4, 6). The patients with m.16189T>C mutation were also usually overweight (mean BMI 28.12 kg/m²) and had high HOMA-IR (5). The reason for the above differences is not clear yet. Previous studies have demonstrated the m.3243A>G mutation is associated with both insulin deficiency and reduced insulin sensitivity in patients with MIDD (7). Sequential follow-up of patients with the 3243 mutation documented decreased sensitivity to insulin prior to the development of insulin deficiency (7). A presumable supposition is that reduced sensitivity to insulin could be a consequence of minor mitochondrial dysfunction, and deficiency only occurred with a severe defect. The mutations 3426A>G and 16189T>C have less impact on mitochondrial function than 3243A>G.
MtDNA is passed from the mother to offspring; therefore, it is generally accepted mitochondrial diabetes is also maternally inherited (8, 9). However, in our study, 22 patients were sporadic cases without family histories of diabetes and hearing loss. Among the 22 patients, mitochondrial mutations were detected in five patients’ relatives who had no hyperglycemia or hearing loss. One possible reason for this seems to be that mitochondrial inheritance is characterized by heteroplasmy and threshold (10). The percentage of affected mitochondria is variable among different tissues and among cells within a given tissue, which is called heteroplasmy (11). Typically, the carrier exhibits clinical phenotypes when the mutant mtDNA reaches more than 60%, which is the threshold (12). The other reason is that the patient was the propositus and had the first mutation in his family (13).
In addition to diabetes mellitus and hearing loss, other clinical phenotypes may present differently since there are variabilities in heteroplasmy in different tissues (14, 15). Mitochondria are presented in every cell of the human body except red blood cells, so the organs depending on energy metabolism are subjected to be affected in patients with MIDD, such as central nervous system, retina, skeletal muscle, heart, kidney, and endocrine. We found some complications involving other systems are characteristic, which might help to identify MIDD from other types of diabetes.
The prevalence of macular dystrophy (9.32%) was higher than proliferative retinopathy (5.59%), which was consistent with a previous study (16). Macular pattern dystrophy might protect against the development of diabetic retinopathy through a reduction in retinal metabolism and a decrease in oxygen consumption in the retina (17). Macular dystrophy is thought be used as a marker to select the MIDD patients (17). This typical retinal dystrophy should raise suspicion as to the diagnosis for MIDD. Therefore, it is of clinical importance for diabetes to perform specific examinations by a trained ophthalmologist.
Our results also suggested a high frequency of myopathy, especially muscle weakness and myophagism. Skeletal muscle requires a great deal of ATP to function, and when patients increase the demand on the muscles, the dysfunctional mitochondria are unable to meet the demand and develop muscular symptoms (18). Further, ragged red fibers on muscle biopsy are typically seen in MIDD, which indicate damaged muscles and are thought to be pathognomonic of mitochondrial disease (19). Muscle biopsy may show ragged-red fibers that are characteristic of mitochondrial disorders and contribute to the diagnosis of MIDD (20).
In the UK MRC Mitochondrial Disease Patient Cohort Study involving 129 patients with the m.3243A>G mutation, the patients were associated with other clinical syndromes involving the nervous and muscular systems including mitochondrial encephalopathy lactic acidosis and stroke-like episodes (MELAS) and chronic progressive external ophthalmoplegia (CPEO). Thirty percent had MIDD, 6% MELAS/MIDD, and 5% MIDD/CPEO overlap syndromes (21). Our study also showed high prevalence of central nervous system involvement (29.19%) and myopathy (22.98%) in MIDD patients. Brain and skeletal muscle are susceptible to be affected due to a great demand for ATP (3). Basal ganglia high signal lesions (2.48%) or encephalatrophy (11.18%) on the brain computerized tomography (CT) are specific manifestations, which might have implications for the diagnosis.
Cardiac abnormalities in MIDD mainly manifested as cardiomyopathy or arrhythmias. The decrease in ATP leads to a decrease in contractility and subsequently results in decreased stroke volume. The increase in end diastolic volume and pressure in the left ventricle causes further left ventricular remodeling, most often, left ventricular hypertrophy (22). Cardiac conductance disorders might occur by a rearrangement of the cardiac conductance system induced by the mitochondrial dysfunction. Common cardiac conduction abnormalities seen in MIDD include Wolff Parkinson White syndrome, frequent ventricular extrasystoles, and atrial fibrillation (20, 22). Cardiac conductance disorders might contribute to sudden deaths; therefore, patients diagnosed with MIDD should undergo a comprehensive cardiac examination to avoid adverse outcomes (15).
Although the prevalence of nephropathy (13.66%) and gastrointestinal symptoms (5.59%) was relatively lower, it was not insignificant. Renal biopsy of MIDD often shows focal glomerulosclerosis (FSGS), not typical diabetic nephropathy, and is the most prevalent finding (23). The main gastrointestinal symptoms were usually mild and presented as constipation, diarrhea, or succession of both. Intestinal pseudo-obstruction is a rare but serious complication of mitochondrial disease of alimentary localization with a mortality rate of approximately 50% (24). In addition, there have also been rare reports of pancreatitis, which may be related to MIDD (25, 26). Apart from diabetes, the most common endocrine manifestation of MIDD was short stature. Hypothyroidism and hypogonadism were also observed in MIDD (27–29). For MIDD patients, in addition to glycemic control, other endocrine function should be cared.
In clinical practice, testing for mutations in mitochondrial DNA is routinely done in DNA isolated from blood; however, the level of mutation may be low and even undetectable in blood. Our study indicated that mutational loads of m.3243A>G varied widely among different tissues. The mean mutation levels of m.3243A>G in urothelium, muscle, hair follicle, buccal mucosa, nail, and myocardium were 58.13, 49.53, 41.35, 48.25, 32.00, and 67.50%. DNA from urothelium, myocardium, and buccal mucosa had the higher while blood had the lowest proportion of mutant genomes. These results are consistent with a previous research (20). Each time a somatic cell undergoes mitosis, mtDNA will be randomly distributed to the progeny cells along with mitochondria. Therefore, the mutation load of mtDNA in the tissue will change with the division of the tissue cells (30, 31). The above results indicate that buccal mucosa and urinary sediment are tissues of choice for the diagnosis of mtDNA mutations, as they are easy to obtain and their mutation loads are greater than blood.
Five patients were positive for diabetes antibodies. The patient with GADA positive was diagnosed as MIDD combined with latent autoimmune diabetes in adults (LADA) (32). The two patients with ICA positive were diagnosed as MIDD. The original literatures considered that metabolic damage to beta cells could possibly trigger an autoimmune response and ICA might be a secondary phenomenon in mitochondrial diseases (33, 34). As for two patients with IAA positive, one was diagnosed as MIDD (32). The other patient repeated antibody tests, and the results were negative for all antibodies in the first test and positive for IAA in the second test. Since the patient had high HOMA-IR, the patient was diagnosed as T2DM combined with mitochondrial gene mutation (35). Actually, the three diabetes antibodies all have false-positive rates, which can be positive in some patients without diabetes, and some drugs such as methimazole could result in positive antibodies (36, 37).
We investigated the correlation between the heteroplasmy levels in the peripheral blood and age at the onset of diabetes. The scattergram showed an overall trend that the age at onset of diabetes was negatively correlated with the heteroplasmies in the peripheral blood. Especially the age at onset of m.3243A>G was significantly negatively correlated with the heteroplasmies in the peripheral blood, which was consistent with previous studies (38). The heteroplasmy load of the m.3243A>G mutation declines with age in blood leukocytes at a mean rate of 1.4% per year (39). Moreover, as the patient’s age increased, all tissue showed a declining proportion of mutant mtDNA (40), which might indicate that early genetic testing is easier to detect mutations, and the positive rate of detection decreases with age. And one previous study suggested age-adjusted blood m.3243A>G mutation load might be an indicator of disease burden (41).
For MIDD patients, the clinical management is also a complicated issue to be discussed. Our study showed that most of the MIDD patients (79/102,77.45%) received insulin therapy, 37.18% (29/78) initiated insulin once diabetes was diagnosed, and less than one-fourth (24/102,23.53%) did not use insulin. The mean time from the diagnosis of diabetes to insulin therapy was 4.15 years. Our results might indicate that only minority of MIDD patients did not need insulin injection as they were diagnosed. As islet function declines, insulin is usually required. There is a research suggesting a cocktail therapy combining CoQ10, lipoic acid, L-carnitine, and thiamine (42). CoQ10 is an antioxidant and mitochondrial cofactor that plays an important role in the mitochondrial respiratory chain, which may enhance insulin secretion, slow hearing loss, improve symptoms of myopathy and congestive heart failure in the setting of mitochondrial disease (43–45). Some drugs should be avoided, such as metformin as it might increase the risk of lactic acidosis (46). Additionally, some antibiotics such as tetracycline (47) and chloramphenicol (48), antiepileptic drugs such as sodium valproate phenytoin and phenobarbital (49), antiretroviral drugs (50), and statins (51) should be taken carefully due to their mitochondrial toxicity, which may exacerbate mitochondrial damage. For hearing impairment, cochlear implantation could improve listening in patients of all ages, provided there are intact neural components to function (52).
Our study has several limitations. First, to analyze the characteristics of patients with MIDD, studies with insufficient information were excluded, so that we were unable to analyze the characteristics of some rare mutations. Second, this study might have selective bias, because typical or more severe patients tend to be reported. Additionally, the mechanism of different mutations leading to various clinical features still remains unclear. Further studies are needed for the purpose of explaining the more precise molecular mechanism of MIDD.
Conclusions
In summary, our study shows that MIDD is a heterogeneous disease which is various in clinical features and treatment. The age of onset of diabetes is negatively correlated with the heteroplasmies in the peripheral blood. These patients who present with low body mass index, young onset of diabetes but without positive antibodies, multiple systems involved especially hearing loss should prompt an investigation for MIDD and genetic counseling as soon as possible.
Author Contributions
MY contributed to the conception and design of the study. CX, YC, and LX performed the literature search and study selection. LX performed the data extraction. MY and SJ performed the statistical analyses. MY, LL, SJ and JD drafted the manuscript and revised it critically. SJ polished the draft. All authors gave final approval for submitted manuscript content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was funded by the National Natural Science Foundation of China (81770822).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.728043/full#supplementary-material
Abbreviations
MIDD, maternally inherited diabetes and deafness; mtDNA, mitochondrial DNA; tRNA, transfer RNA; PCR, polymerase chain reaction. GADA, glutamic acid decarboxylase antibody; IAA, insulin autoantibody; ICA, islet cell antibody; OHA, oral hypoglycemic agents; MELAS, mitochondrial encephalopathy lactic acidosis and stroke like episodes; CPEO, chronic progressive external ophthalmoplegia; FSGS, focal glomerulosclerosis.
References
- 1. Henzen C. Monogenic Diabetes Mellitus Due to Defects in Insulin Secretion. Swiss Med Wkly (2012) 142:w13690. doi: 10.4414/smw.2012.13690 [DOI] [PubMed] [Google Scholar]
- 2. van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, Struyvenberg PA, et al. Mutation in Mitochondrial Trna(Leu)(UUR) Gene in a Large Pedigree With Maternally Transmitted Type II Diabetes Mellitus and Deafness. Nat Genet (1992) 1(5):368–71. doi: 10.1038/ng0892-368 [DOI] [PubMed] [Google Scholar]
- 3. Murphy R, Turnbull DM, Walker M, Hattersley AT. Clinical Features, Diagnosis and Management of Maternally Inherited Diabetes and Deafness (MIDD) Associated With the 3243A>G Mitochondrial Point Mutation. Diabetes Med (2008) 25(4):383–99. doi: 10.1111/j.1464-5491.2008.02359.x [DOI] [PubMed] [Google Scholar]
- 4. Yu P, Yu D. Study on a New Point Mutation of Nt3426 a→G of Mitochondrial DNA in a Diabetes Mellitus Family. Chin J Med Genet (2003) 03):63–5. Chinese. [PubMed] [Google Scholar]
- 5. Poulton J, Brown MS, Cooper A, Marchington DR, Phillips DI. A Common Mitochondrial DNA Variant Is Associated With Insulin Resistance in Adult Life. Diabetologia (1998) 41(1):54–8. doi: 10.1007/s001250050866 [DOI] [PubMed] [Google Scholar]
- 6. Shin CS, Kim SK, Park KS, Kim WB, Kim SY, Cho BY, et al. A New Point Mutation (3426, a to G) in Mitochondrial NADH Dehydrogenase Gene in Korean Diabetic Patients Which Mimics 3243 Mutation by Restriction Fragment Length Polymorphism Pattern. Endocr J (1998) 45(1):105–10. doi: 10.1507/endocrj.45.105 [DOI] [PubMed] [Google Scholar]
- 7. Gebhart SS, Shoffner JM, Koontz D, Kaufman A, Wallace D. Insulin Resistance Associated With Maternally Inherited Diabetes and Deafness. Metabolism (1996) 45(4):526–31. doi: 10.1016/s0026-0495(96)90231-0 [DOI] [PubMed] [Google Scholar]
- 8. Greenfield A, Braude P, Flinter F, Lovell-Badge R, Ogilvie C, Perry ACF. Corrigendum: Assisted Reproductive Technologies to Prevent Human Mitochondrial Disease Transmission. Nat Biotechnol (2018) 36(2):196. doi: 10.1038/nbt0218-196b [DOI] [PubMed] [Google Scholar]
- 9. van den Ameele J, Li AYZ, Ma H, Chinnery PF. Mitochondrial Heteroplasmy Beyond the Oocyte Bottleneck. Semin Cell Dev Biol (2020) 97:156–66. doi: 10.1016/j.semcdb.2019.10.001 [DOI] [PubMed] [Google Scholar]
- 10. Dimauro S, Davidzon G. Mitochondrial DNA and Disease. Ann Med (2005) 37(3):222–32. doi: 10.1080/07853890510007368 [DOI] [PubMed] [Google Scholar]
- 11. Laloi-Michelin M, Meas T, Ambonville C, Bellanné-Chantelot C, Beaufils S, Massin P, et al. The Clinical Variability of Maternally Inherited Diabetes and Deafness Is Associated With the Degree of Heteroplasmy in Blood Leukocytes. J Clin Endocrinol Metab (2009) 94(8):3025–30. doi: 10.1210/jc.2008-2680 [DOI] [PubMed] [Google Scholar]
- 12. Bacman SR, Kauppila JHK, Pereira CV, Nissanka N, Miranda M, Pinto M, et al. Mitotalen Reduces Mutant MtDNA Load and Restores tRNA(Ala) Levels in a Mouse Model of Heteroplasmic Mtdna Mutation. Nat Med (2018) 24(11):1696–700. doi: 10.1038/s41591-018-0166-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maassen JA, Biberoglu S, t Hart LM, Bakker E, de Knijff P. A Case of a De Novo A3243G Mutation in Mitochondrial DNA in a Patient With Diabetes and Deafness. Arch Physiol Biochem (2002) 110(3):186–8. doi: 10.1076/apab.110.3.186.8294 [DOI] [PubMed] [Google Scholar]
- 14. Li HZ, Li RY, Li M. A Review of Maternally Inherited Diabetes and Deafness. Front Biosci (Landmark Ed) (2014) 19:777–82. doi: 10.2741/4244 [DOI] [PubMed] [Google Scholar]
- 15. Robinson KN, Terrazas S, Giordano-Mooga S, Xavier NA. The Role of Heteroplasmy in the Diagnosis and Management of Maternally Inherited Diabetes and Deafness. Endocr Pract (2020) 26(2):241–6. doi: 10.4158/ep-2019-0270 [DOI] [PubMed] [Google Scholar]
- 16. Massin P, Virally-Monod M, Vialettes B, Paques M, Gin H, Porokhov B, et al. Prevalence of Macular Pattern Dystrophy in Maternally Inherited Diabetes and Deafness. GEDIAM Group. Ophthalmology (1999) 106(9):1821–7. doi: 10.1016/s0161-6420(99)90356-1 [DOI] [PubMed] [Google Scholar]
- 17. Guillausseau PJ, Massin P, Dubois-LaForgue D, Timsit J, Virally M, Gin H, et al. Maternally Inherited Diabetes and Deafness: A Multicenter Study. Ann Intern Med (2001) 134(9 Pt 1):721–8. doi: 10.7326/0003-4819-134-9_part_1-200105010-00008 [DOI] [PubMed] [Google Scholar]
- 18. Tanaka K, Takada Y, Matsunaka T, Yuyama S, Fujino S, Maguchi M, et al. Diabetes Mellitus, Deafness, Muscle Weakness and Hypocalcemia in a Patient With an A3243G Mutation of the Mitochondrial DNA. Intern Med (2000) 39(3):249–52. doi: 10.2169/internalmedicine.39.249 [DOI] [PubMed] [Google Scholar]
- 19. Lien LM, Lee HC, Wang KL, Chiu JC, Chiu HC, Wei YH. Involvement of Nervous System in Maternally Inherited Diabetes and Deafness (MIDD) With the A3243G Mutation of Mitochondrial DNA. Acta Neurol Scand (2001) 103(3):159–65. doi: 10.1034/j.1600-0404.2001.103003159.x [DOI] [PubMed] [Google Scholar]
- 20. Fukao T, Kondo M, Yamamoto T, Orii KE, Kondo N. Comparison of Mitochondrial A3243G Mutation Loads in Easily Accessible Samples From a Family With Maternally Inherited Diabetes and Deafness. Mol Med Rep (2009) 2(1):69–72. doi: 10.3892/mmr_00000063 [DOI] [PubMed] [Google Scholar]
- 21. Nesbitt V, Pitceathly RD, Turnbull DM, Taylor RW, Sweeney MG, Mudanohwo EE, et al. The UK MRC Mitochondrial Disease Patient Cohort Study: Clinical Phenotypes Associated With the M.3243A>G Mutation–Implications for Diagnosis and Management. J Neurol Neurosurg Psychiatry (2013) 84(8):936–8. doi: 10.1136/jnnp-2012-303528 [DOI] [PubMed] [Google Scholar]
- 22. Hoptasz M, Szczuciński A, Losy J. Heterogeneous Phenotypic Manifestations of Maternally Inherited Deafness Associated With the Mitochondrial A3243G Mutation. Case Report. Neurol Neurochir Pol (2014) 48(2):150–3. doi: 10.1016/j.pjnns.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 23. Cao XY, Wei RB, Wang YD, Zhang XG, Tang L, Chen XM. Focal Segmental Glomerulosclerosis Associated With Maternally Inherited Diabetes and Deafness: Clinical Pathological Analysis. Indian J Pathol Microbiol (2013) 56(3):272–5. doi: 10.4103/0377-4929.120392 [DOI] [PubMed] [Google Scholar]
- 24. Narbonne H, Paquis-Fluckinger V, Valero R, Heyries L, Pellissier JF, Vialettes B. Gastrointestinal Tract Symptoms in Maternally Inherited Diabetes and Deafness (MIDD). Diabetes Metab (2004) 30(1):61–6. doi: 10.1016/s1262-3636(07)70090-3 [DOI] [PubMed] [Google Scholar]
- 25. Schleiffer T, t Hart LM, Schürfeld C, Kraatz K, Riemann JF. Maternally Inherited Diabetes and Deafness (MIDD): Unusual Occult Exocrine Pancreatic Manifestation in an Affected German Family. Exp Clin Endocrinol Diabetes (2000) 108(2):81–5. doi: 10.1055/s-2000-5800 [DOI] [PubMed] [Google Scholar]
- 26. Verny C, Amati-Bonneau P, Letournel F, Person B, Dib N, Malinge MC, et al. Mitochondrial DNA A3243G Mutation Involved in Familial Diabetes, Chronic Intestinal Pseudo-Obstruction and Recurrent Pancreatitis. Diabetes Metab (2008) 34(6 Pt 1):620–6. doi: 10.1016/j.diabet.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 27. Finsterer J, Frank M. The Tip of the Iceberg in Maternally Inherited Diabetes and Deafness. Oman Med J (2018) 33(5):437–40. doi: 10.5001/omj.2018.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kytövuori L, Lipponen J, Rusanen H, Komulainen T, Martikainen MH, Majamaa K. A Novel Mutation M.8561C>G in MT-ATP6/8 Causing a Mitochondrial Syndrome With Ataxia, Peripheral Neuropathy, Diabetes Mellitus, and Hypergonadotropic Hypogonadism. J Neurol (2016) 263(11):2188–95. doi: 10.1007/s00415-016-8249-2 [DOI] [PubMed] [Google Scholar]
- 29. Moraes CT, Ciacci F, Bonilla E, Jansen C, Hirano M, Rao N, et al. Two Novel Pathogenic Mitochondrial DNA Mutations Affecting Organelle Number and Protein Synthesis. Is the Trna(Leu(UUR)) Gene an Etiologic Hot Spot? J Clin Invest (1993) 92(6):2906–15. doi: 10.1172/jci116913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao L, Shitara H, Horii T, Nagao Y, Imai H, Abe K, et al. The Mitochondrial Bottleneck Occurs Without Reduction of Mtdna Content in Female Mouse Germ Cells. Nat Genet (2007) 39(3):386–90. doi: 10.1038/ng1970 [DOI] [PubMed] [Google Scholar]
- 31. Chinnery PF, Howell N, Lightowlers RN, Turnbull DM. MELAS and MERRF. The Relationship Between Maternal Mutation Load and the Frequency of Clinically Affected Offspring. Brain (1998) 121(Pt 10):1889–94. doi: 10.1093/brain/121.10.1889 [DOI] [PubMed] [Google Scholar]
- 32. Rong Y. Mitochondrial Diabetes Mellitus With 3 Cases. Hebei Medical University; (2011). MA thesis. Hebei, Chinese. [Google Scholar]
- 33. Xiu L. Clinical Characterizations of Familial Diabete Mellitus Associated With Mitochondrial Gene Mutation. Natl Med J China (1997) 06:111–3. doi:CNKI : SUN:ZHYX.0.1997-06-007. Chinese. doi: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2002.0036 [DOI] [PubMed] [Google Scholar]
- 34. Oexle K, Oberle J, Finckh B, Kohlschütter A, Nagy M, Seibel P, et al. Islet Cell Antibodies in Diabetes Mellitus Associated With a Mitochondrial Trna(Leu(UUR)) Gene Mutation. Exp Clin Endocrinol Diabetes (1996) 104(3):212–7. doi: 10.1055/s-0029-1211445 [DOI] [PubMed] [Google Scholar]
- 35. Yu Z, Li J. Islet Cell Antibodies in Diabetes Mellitus Associated With a Mitochondrial Trnal(U) Gene Mutation. Int J Endocrinol Metab (2020) 40(04):279–83. Chinese. [Google Scholar]
- 36. Hu L. The Clinical Significance of Serum Autoantibodies to Pancreatic Islet in One Thousand and Four Hundreds Patients With Diabetes. J Modern Lab Med (2006) 02):15–7. doi:CNKI : SUN:SXYN.0.2006-02-007Chinese. doi: 10.3969/j.issn.1671-7414.2006.02.005 [DOI] [Google Scholar]
- 37. Di Mario U, Perfetti R, Anastasi E, Contreas G, Crisà L, Tiberti C, et al. Autoantibodies to Insulin do Appear in Non-Diabetic Patients With Autoimmune Disorders: Comparison With Anti-Immunoglobulin Antibodies and Other Autoimmune Phenomena. Acta Endocrinol (Copenh) (1990) 122(3):303–8. doi: 10.1530/acta.0.1220303 [DOI] [PubMed] [Google Scholar]
- 38. Suzuki S, Oka Y, Kadowaki T, Kanatsuka A, Kuzuya T, Kobayashi M, et al. Clinical Features of Diabetes Mellitus With the Mitochondrial DNA 3243 (a-G) Mutation in Japanese: Maternal Inheritance and Mitochondria-Related Complications. Diabetes Res Clin Pract (2003) 59(3):207–17. doi: 10.1016/s0168-8227(02)00246-2 [DOI] [PubMed] [Google Scholar]
- 39. van Essen EH, Roep BO, ′t Hart LM, Jansen JJ, Van den Ouweland JM, Lemkes HH, et al. HLA-DQ Polymorphism and Degree of Heteroplasmy of the A3243G Mitochondrial DNA Mutation in Maternally Inherited Diabetes and Deafness. Diabetes Med (2000) 17(12):841–7. doi: 10.1046/j.1464-5491.2000.00379.x [DOI] [PubMed] [Google Scholar]
- 40. Liou CW, Huang CC, Wei YH. Molecular Analysis of Diabetes Mellitus-Associated A3243G Mitochondrial DNA Mutation in Taiwanese Cases. Diabetes Res Clin Pract (2001) 54 Suppl 2:S39–43. doi: 10.1016/s0168-8227(01)00334-5 [DOI] [PubMed] [Google Scholar]
- 41. Grady JP, Pickett SJ, Ng YS, Alston CL, Blakely EL, Hardy SA, et al. MtDNA Heteroplasmy Level and Copy Number Indicate Disease Burden in M.3243A>G Mitochondrial Disease. EMBO Mol Med (2018) 10(6):e8262. doi: 10.15252/emmm.201708262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial Effects of Creatine, Coq10, and Lipoic Acid in Mitochondrial Disorders. Muscle Nerve (2007) 35(2):235–42. doi: 10.1002/mus.20688 [DOI] [PubMed] [Google Scholar]
- 43. Fragaki K, Chaussenot A, Benoist JF, Ait-El-Mkadem S, Bannwarth S, Rouzier C, et al. Coenzyme Q10 Defects may be Associated With a Deficiency of Q10-Independent Mitochondrial Respiratory Chain Complexes. Biol Res (2016) 49:4. doi: 10.1186/s40659-015-0065-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salles JE, Moisés VA, Almeida DR, Chacra AR, Moisés RS. Myocardial Dysfunction in Mitochondrial Diabetes Treated With Coenzyme Q10. Diabetes Res Clin Pract (2006) 72(1):100–3. doi: 10.1016/j.diabres.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 45. Suzuki S, Hinokio Y, Ohtomo M, Hirai M, Hirai A, Chiba M, et al. The Effects of Coenzyme Q10 Treatment on Maternally Inherited Diabetes Mellitus and Deafness, and Mitochondrial DNA 3243 (a to G) Mutation. Diabetologia (1998) 41(5):584–8. doi: 10.1007/s001250050950 [DOI] [PubMed] [Google Scholar]
- 46. Kim NH, Siddiqui M, Vogel J. MELAS Syndrome and MIDD Unmasked by Metformin Use: A Case Report. Ann Intern Med (2021) 174(1):124–5. doi: 10.7326/l20-0292 [DOI] [PubMed] [Google Scholar]
- 47. Chatzispyrou IA, Held NM, Mouchiroud L, Auwerx J, Houtkooper RH. Tetracycline Antibiotics Impair Mitochondrial Function and its Experimental Use Confounds Research. Cancer Res (2015) 75(21):4446–9. doi: 10.1158/0008-5472.Can-15-1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leiter LM, Thatte HS, Okafor C, Marks PW, Golan DE, Bridges KR. Chloramphenicol-Induced Mitochondrial Dysfunction Is Associated With Decreased Transferrin Receptor Expression and Ferritin Synthesis in K562 Cells and Is Unrelated to IRE-IRP Interactions. J Cell Physiol (1999) 180(3):334–44. doi: [DOI] [PubMed] [Google Scholar]
- 49. Lam CW, Lau CH, Williams JC, Chan YW, Wong LJ. Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis and Stroke-Like Episodes (MELAS) Triggered by Valproate Therapy. Eur J Pediatr (1997) 156(7):562–4. doi: 10.1007/s004310050663 [DOI] [PubMed] [Google Scholar]
- 50. Moyle G. Mitochondrial Toxicity: Myths and Facts. J HIV Ther (2004) 9(2):45–7. [PubMed] [Google Scholar]
- 51. Okuyama H, Langsjoen PH, Hamazaki T, Ogushi Y, Hama R, Kobayashi T, et al. Statins Stimulate Atherosclerosis and Heart Failure: Pharmacological Mechanisms. Expert Rev Clin Pharmacol (2015) 8(2):189–99. doi: 10.1586/17512433.2015.1011125 [DOI] [PubMed] [Google Scholar]
- 52. Sinnathuray AR, Raut V, Awa A, Magee A, Toner JG. A Review of Cochlear Implantation in Mitochondrial Sensorineural Hearing Loss. Otol Neurotol (2003) 24(3):418–26. doi: 10.1097/00129492-200305000-00012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.