Summary
Background
Sepsis bundles, promulgated by Surviving Sepsis Campaign have not been widely adopted because of variability in sepsis identification strategies, implementation challenges, concerns about excess antimicrobial use, and limited evidence of benefit.
Methods
A 1-hour septic shock and a 3-hour sepsis bundle were implemented using a Breakthrough Series Collaborative in 14 public hospitals in Queensland, Australia. A before (baseline) and after (post-intervention) study evaluated its impact on outcomes and antimicrobial prescription in patients with confirmed bacteremia and sepsis.
Findings
Between 01 July 2017 to 31 March 2020, of 6976 adults presenting to the Emergency Departments and had a blood culture taken, 1802 patients (732 baseline, 1070 post-intervention) met inclusion criteria. Time to antibiotics in 1-hour 73.7% vs 85.1% (OR 1.9 [95%CI 1.1-3.6]) and the 3-hour bundle compliance (48.2% to 63.3%, OR 1.7, [95%CI 1.4 to 2.1]) improved post-intervention, accompanied by a significant reduction in Intensive Care Unit (ICU) admission rates (26.5% vs 17.5% (OR 0.5, [95%CI 0.4 to 0.7]). There were no significant differences in-hospital and 30-day post discharge mortality between the two phases. In a post-hoc analysis of the post-intervention phase, sepsis pathway compliance was associated with lower in-hospital mortality (9.7% vs 14.9%, OR 0.6, 95%CI 0.4 to 0.8). The proportions of appropriate antimicrobial prescription at baseline and post-intervention respectively were 55.4% vs 64.1%, (OR 1.4 [95%CI 0.9 to 2.1]).
Interpretation
Implementing 1-hour and 3-hour sepsis bundles for patients presenting with bacteremia resulted in improved bundle compliance and a reduced need for ICU admission without adversely influencing antimicrobial prescription.
Research in context.
Evidence before this study
We searched Pubmed (date of search June 1, 2021) for English language articles using the terms “Sepsis” and “bundles”. Sepsis affects 55 million patients worldwide and causes 11 million deaths annually. The Surviving Sepsis Campaign (SSC) guidelines advocate time-based sepsis bundles to facilitate early recognition, timely antimicrobials, source control and supportive treatments. The original SSC guidelines in 2012 recommended a 3-hour and 6-hour bundle. Although several publications report significant risk adjusted improvement in mortality with bundle compliance, there are few studies comparing bundle compliance and outcomes pre and post bundle implementation. A 2018 update of the SSC guidelines combined the 3-hour and 6-hour guidelines into a 1-hour bundle with the explicit intention of beginning resuscitation and management immediately. However, these bundles have not been widely adopted owing to the lack of strong evidence supporting certain elements of the bundle, challenges of implementation, variability in sepsis identification strategies, concerns about unnecessary testing, placing demand on ICU resources, and excess antimicrobial use.
Added value of this study
We evaluated the efficacy of 1-hour and 3-hour sepsis bundle implementation for septic shock and sepsis respectively in a pre and post-study by screening 6976 patients across 14 public hospitals in the State of Queensland in Australia as a quality improvement initiative over a 3 year period. We used a Breakthrough Series Collaborative approach for the education and training of staff at each site. To improve the specificity of the diagnosis of sepsis, we specifically studied patients who had confirmed bacteraemia. We also assessed the appropriateness of anti-microbial prescription as part of bundle implementation. Compliance with the 3-hour bundle for sepsis improved from 48% to 63% and antibiotic administration in 1-hour for septic shock improved from 74% to 85%. We observed a 10% reduction in the need for ICU admission post bundle implementation. The proportions of appropriate antimicrobial prescription remained similar across both phases without adversely influencing antimicrobial prescription.
Implications of all available evidence
These results provide evidence for 1-hour septic shock and 3-hour sepsis bundles in specific contexts. We provide a key piece of evidence highlighting that sepsis bundle implementation reduces the need for ICU admission without adversely influencing antimicrobial prescription. As sepsis is a global health priority, these findings are important for understanding sepsis bundle implementation and for strategic planning worldwide to improve patient outcomes.
Alt-text: Unlabelled box
Introduction
Sepsis affects 55 million patients worldwide and causes 11 million deaths annually.1 The Surviving Sepsis Campaign guidelines advocate time-based sepsis bundles to facilitate early recognition, timely antimicrobials, source control and supportive treatments.2 Several publications report significant risk adjusted improvement in mortality with bundle compliance,3,4 although few report comparisons pre and post-bundle implementation. A 2018 update of the SSC guidelines combined the 3-hour and 6-hour guidelines into a 1-hour bundle with the explicit intention of beginning resuscitation and management immediately.5 However this 1-hour bundle has not been well received owing to the lack of strong evidence supporting certain elements of the bundle and challenges of implementation in the emergency department.6 Concerns voiced by clinicians about the bundles include the variability in sepsis identification strategies7, conflation of sepsis and septic shock, and the potential for excess antimicrobial use,8 although this has not been studied in detail. Moreover, not all centres using these bundles report a consistent improvement in patient centred outcomes.9,10
Ensuring uniform implementation of existing guidelines at individual hospital level, and educating a high turnover workforce in emergency departments, where the bulk of the patients with sepsis present,11 has been a challenge. Consequently, the bundles have not been widely adopted in clinical practice.12
We used a before-and-after study to test the hypothesis that implementing an evidence-based sepsis bundle -1 hour bundle for patients with septic shock and a 3-hour bundle for patients with sepsis - no shock, using a Breakthrough Series Collaborative approach developed by the Institute for Healthcare Improvement (IHI),13 would result in improved process of care and patient outcomes. To improve the specificity of the diagnosis of sepsis, we only studied patients who had confirmed bacteremia. In a nested cohort, we also assessed the appropriateness of antimicrobial prescription.
Methods
The Collaborative “Could this be Sepsis?” program was a before-and-after study launched by Queensland Health's Clinical Excellence Queensland (CEQ) involving emergency departments (ED) in 16 major public hospitals (Table S1 and Figure S1 and S2– Supplementary Appendix) to reduce mortality, intensive care admission and hospital length of stay by improving early recognition and treatment of sepsis. The program incorporated a sepsis screening tool, and a treatment bundle. Prior to the Collaborative, CEQ facilitated a pilot project at Gold Coast University Hospital ED to develop and test the delivery of the screening tool, treatment bundle, data collection tools, and testing and measuring changes aimed at improved screening and treatment.
CEQ provided operational support, including a data capture system and provision of Improvement Advisors trained by the IHI (Boston, MA, USA). Trained coordinators collected data at each site and entered the information into a web-based database.. Statistical analysis was performed by an independent team of statisticians from The University of Queensland. Data analysis was performed on data collected for patients who presented to EDs both before (baseline) and after (post-intervention) implementation of the bundle. The authors vouch for the accuracy of the data and statistical analyses.
Site selection and training
All Queensland Health hospitals with EDs assessed at Level 4-6 according to the Clinical Services Capability Framework (CSCF) were eligible for inclusion. Clinical services are categorised into up to six capability levels with Level 1 managing the least complex patients and Level 6 managing the highest level of patient complexity (Table S2- Supplementary Appendix). The site selection and individual training are described in Tables S3 and S4- Supplementary Appendix). All sites had access to sepsis online learning and instructions on how to use the screening tool and pathway (Figure S3, Supplementary Appendix).
Study population
Inclusion criteria: The criteria for inclusion were
-
a)
Adult Patients (≥18 years old) and
-
b)
presenting to the ED with symptoms and signs suggestive of an infection or fever or hypothermia or signs of clinical deterioration and
-
c)
who had blood cultures collected in the ED which were subsequently reported to be positive and
-
d)
Required admission to the hospital and
-
e)
an ICD 10 coded diagnosis of sepsis at admission into the hospital. (Table S5 – Supplementary Appendix).
Patients were excluded if they met any of the following criteria
-
a)
<18 years of age
-
b)
pregnant or post-delivery,
-
c)
retrieved from other hospitals with sepsis bypassing the emergency department and directly admitted to ICU at participating sites;
-
d)
If a pathogen in a positive blood culture was deemed likely to be a contaminant by the microbiology laboratory
Patients meeting the above criteria for inclusion in the study were deemed to have septic shock during their stay in the ED if they had a systolic blood pressure of < 90 mmHg AND a plasma lactate concentration ≥ 2 mmol/L AND despite a fluid bolus14 OR where data on fluid bolus response was not available, a plasma lactate ≥4 mmol/L was required for inclusion.
Interventions
The interventions consisted of a sepsis screening tool and a treatment bundle. These included collection of two sets of blood cultures with at least one prior to administration of antimicrobials, measurement of the serum lactate concentration, and antibiogram adjusted antibiotic guidelines15 for the administration of broad-spectrum antimicrobials within three hours for patients with sepsis and one hour for those with septic shock and a fluid bolus of 20ml/kg for hypotension defined as a systolic blood pressure of less than 90 mm Hg.
In the post-intervention phase, it was required that where clinical care of patients utilised the sepsis pathway a copy of the pathway was affixed in the chart as evidence of use of the pathway.
Data collection and reporting
Baseline data (pre-intervention) was collected retrospectively (Table S6-Supplementary Appendix). Post-intervention data was collected prospectively following implementation of the sepsis pathway. Each site was provided with a weekly list of all patients from the Queensland Health Pathology System, who had presented to ED who had blood cultures collected that were positive.
Patient level data included data on demographic characteristics, co-morbidities, and characteristics of sepsis or septic shock. Date and time stamps for protocol initiation and the elements of 3-hour bundled care were required for patients in whom a sepsis protocol was initiated. Time zero for sepsis was defined as the time of triage in the ED. Time zero for septic shock was defined as the time that the first systolic blood pressure of < 90 mmHg was recorded. In both phases, compliance with the sepsis treatment bundle was recorded. In addition, in the post-intervention phase. evidence of use of the pathway was documented.
In a nested cohort sub-study from 3 participating sites, which had electronic medical records, the appropriateness of empirical first antibiotic prescriptions16 were scrutinised by antimicrobial pharmacists and infectious disease physicians based on blood culture and other laboratory results (Table S7-Supplementary Appendix).
Data were collected from patient medical records and laboratory systems and entered into a secure online database, Research Electronic Data Capture (REDCap, Vanderbilt University, USA).17 Data was sourced both from the REDCap Sepsis Collaborative audit database, and Queensland Health Admitted Patient Data Collection (QHAPDC) and linked prior to analysis.
Data monitoring and source data verification were conducted over the course of the Collaborative (Table S8 Supplementary Appendix). The study oversight was provided by the Queensland Sepsis Steering Committee and designed by the project working groups and clinical experts.and the Governance Structure is described in Figure S4, Supplementary Appendix). This study was approved with a waiver of informed consent by the Metro North Human Resource Ethics Committee LNR/2019/QPCH/5089.
Outcomes
The process measures included proportion of eligible patients screened, had evaluation of plasma lactate, collection of two sets of blood cultures, had broad-spectrum antimicrobials administered within 3-hours for patients with sepsis and 1-hour for those with septic shock, patients with fluid bolus administered less than 1 hour of shock, and proportion of patients in whom there was compliance with 1 hour and 3-hour bundles. These are defined in Table S9 – Supplementary appendix
The clinical outcome measures were in-hospital mortality, combined in-hospital and 30-day post hospital discharge mortality, proportion of patients requiring ICU admission, length of stay in ICU and non-ICU length of stay. ICU admission was defined as patient admitted to the ICU at any time during the hospital admission
Appropriateness of antimicrobial prescription was assessed using the National Antimicrobial Prescribing Survey tool criteria16 (Figure S5, Supplementary Appendix).
Statistical analysis
The primary unit of analysis was the patient. Three key subgroups were defined to best represent the populations required to assess each of the measures:
-
•
All Sepsis - sepsis (shock and non-shock) defined as patients with positive blood culture and an ICD sepsis code
-
•
Septic shock only
-
•
Sepsis only (not shock)
The primary comparison of interest was baseline versus post-intervention; secondary comparisons were: evidence of sepsis pathway utilisation versus no evidence of pathway utilisation (post-intervention phase only), bundle compliance achieved versus not achieved, and time to antibiotics achieved versus not achieved. Bivariate analyses (logistic regression for binary outcomes; Cox regression for length of stay) were performed for these comparisons with each of the outcomes to determine the unadjusted relationship, within each subgroup. Multivariable models were then created to quantify the relationship between the exposure variable (comparison group) controlling for demographics, admission features and comorbidities. The following covariates were included in the adjusted models based on clinical and statistical significance: season, admission time (7am–3pm, 3pm–11pm, 11pm-7am), age, Indigenous status, triage category, regionality and comorbidity (Charlson Comorbidity Index). Due to low prevalence rates, triage categories four and five were combined, as were Remote, Very Remote and Outer Regional categories. In addition, a random intercept for hospital, as well as other hospital level variables, was explored in the adjusted models, however, these effects were not related to the outcome and resulted in poor model convergence, and were subsequently not included.
The unadjusted and adjusted odds ratios (aORs) (hazard ratio for length of stay outcomes) and 95% confidence intervals (CIs) are reported for each of the main comparison groups, within each subgroup.
The times to death in the baseline and post-intervention phases and with and without pathway use were described with the use of Kaplan–Meier plots with results censored at 30 days after triage.
StataSE version 14.1 (StataCorp Pty Ltd, College Station, Texas) and Python were used to undertake the analyses. As this was a quality improvement initiative, an a priori sample size calculation was not undertaken; therefore, all statistical comparisons are interpreted with caution. Corrections for multiple comparisons were not undertaken, however multiplicity was taken into account when interpreting the results. There was no missing data points for any of the reported outcomes and less than 10% for covariates of interest; therefore, complete-case analysis was undertaken.
Role of the funding source
The funding source did not have a role in data analysis, interpretation or in the writing of the manuscript or the decision to submit to publication.
Results
The Collaborative commenced in August 2018. The pathway implementation date varied for each site with the earliest implementation date being 03 December 2018. Of 21 eligible sites, sixteen hospitals (14 adult, 2 paediatric) opted to participate. (Data from paediatric sites not reported). Only one of the participating sites had an alternative sepsis pathway in the baseline phase. Baseline data was collected retrospectively at 13 hospitals between 01 July 2017 to 30 June 2018. The post-intervention phase commenced in July 2018, was implemented in the various hospitals between July 2018 and June 2019 and data collected prospectively till March 2020. Post intervention data from participating hospitals was only included from the date the pathway was introduced. The COVID-19 pandemic led to the premature conclusion of the project.
During the study period, a total of 6976 patients who presented to the participating hospitals and had a blood culture collected in the ED were screened. Of these there were 1802 patients (732 in the baseline phase and 1070 in the post-intervention phase) who met the study criteria for sepsis or septic shock (Figure S6-Supplementary appendix). The status of infection or the diagnosis of sepsis were unclear in the remaining 5174 patients (Table S10 – Supplementary Appendix). Of these 600 patients had a negative blood culture and a positive sepsis ICD code on admission, 931 patients had a positive blood culture and a negative sepsis ICD code and the remainder had both a negative blood culture and a negative ICD code.
The characteristics of the patients were similar in the baseline and the post-intervention phases overall (Table 1) and in the subgroups of sepsis and septic shock (Table S12 – Supplementary Appendix). The median (IQR) age of the patients was 72.0 years (62.0-81.0) and 72.0 years (60.0-82.0), the percentages of male patients 57.5% and 54.3%, and the percentages of Indigenous patients 6.1% and 6.7% in the baseline and post-intervention groups respectively. The triage category, the time of triage, distribution of co-morbidities the time from triage to ICU admission or the proportion of patients deemed palliative in the two phases (Table 1) and primary sites of infection (Table S13 – Supplementary Appendix) were similar in the two groups.
Table 1.
Demographic characteristics.
| Characteristic | All Patients (N = 1802) |
|
|---|---|---|
| Baseline | Post | |
| (N = 732) | (N = 1070) | |
| Age [years], Median (IQR) | 72.0 (62.0, 81.0) | 72.0 (60.0, 82.0) |
| Gender [M/F], N (%) | 421/311 (57.5,42.5) | 581/489 (54.3,45.7) |
| Indigenous Status [Y/N], N (%) | 45/683 (6.1,93.3) | 72/989 (6.7,92.4) |
| Admission Characteristics | ||
| Triage Category | ||
| Category 1 | 46 (6.3%) | 47 (4.4%) |
| Category 2 | 330 (45.1%) | 623 (58.2%) |
| Category 3 | 326 (44.5%) | 371 (34.7%) |
| Category 4 | 30 (4.1%) | 28 (2.6%) |
| Triage Time | ||
| 12am-6am | 84 (11.5%) | 141 (13.2%) |
| 6am-12pm | 207 (28.3%) | 264 (24.7%) |
| 12pm-6pm | 242 (33.1%) | 378 (35.3%) |
| 6pm-12am | 199 (27.2%) | 287 (26.8%) |
| Time from triage to ICU admission | ||
| Time [hours] | 9:01 (5:57, 19:13) | 9:23 (6:36, 19:41) |
| New Onset Organ Dysfunction | ||
| CNS | 187 (25.5%) | 274 (25.6%) |
| CVS | 269 (36.7%) | 364 (34.0%) |
| Renal | 154 (21.0%) | 146 (13.6%) |
| Haematology | 59 (8.1%) | 93 (8.7%) |
| Respiratory | 112 (15.3%) | 202 (18.9%) |
| Hepatobiliary | 32 (4.4%) | 31 (2.9%) |
| >2 Organs affected | 226 (30.9%) | 304 (28.4%) |
| Co-morbidities | ||
| Charlson Comorbidity Index | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) |
| Infectious disease | 329 (44.9%) | 508 (47.5%) |
| Neoplasms | 81 (11.1%) | 110 (10.3%) |
| Endocrine, metabolic or immune diseases | 372 (50.8%) | 591 (55.2%) |
| Diabetes | 231 (31.6%) | 348 (32.5%) |
| Blood Disorders | 130 (17.8%) | 163 (15.2%) |
| Mental Disorders | 71 (9.7%) | 83 (7.8%) |
| Drug or alcohol use | 89 (12.2%) | 114 (10.7%) |
| Diseases of the nervous system or sense organs | 43 (5.9%) | 50 (4.7%) |
| Eye disease | 24 (3.3%) | 33 (3.1%) |
| Diseases of the circulatory system | 244 (33.3%) | 326 (30.5%) |
| Diseases of the respiratory system | 148 (20.2%) | 224 (20.9%) |
| Diseases of the digestive system | 139 (19.0%) | 218 (20.4%) |
| Chronic renal disease | 145 (19.8%) | 179 (16.7%) |
| Diseases of the genitourinary system | 316 (43.2%) | 433 (40.5%) |
| Chronic skin ulcer | 2 (0.3%) | 5 (0.5%) |
| Diseases of the musculoskeletal system and connective tissue | 47 (6.4%) | 84 (7.9%) |
| Other | 146 (19.9%) | 201 (18.8%) |
| Patients deemed palliative | ||
| Beginning of hospitalisation | 3 (0.4%) | 7 (0.7%) |
| End of hospitalisation | 32 (4.4%) | 79 (7.4%) |
IQR: Interquartile range.
CNS: Central Nervous system.
CVS: Cardiovascular.
Process measures
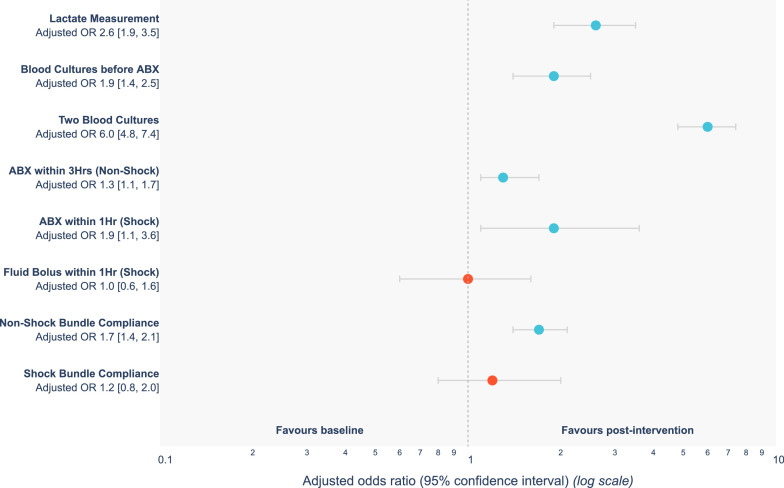
There was a significant improvement in the use of sepsis bundles in the post-intervention phase with respect to measurement of plasma lactate OR 2.6 [95%CI 1.9-3.5], collection of blood cultures prior to antibiotic administration OR 1.9 [95%CI 1.4-2.5], collection of two sets of blood cultures OR 6.0 [95%CI 4.8-7.4], antibiotic administration within three hours of the diagnosis of sepsis OR 1.3 [95%CI 1.1-1.7] and one hour of septic shock OR 1.9 [95%CI 1.1-3.6] and the three-hour bundle compliance in patients with sepsis OR 1.7 [95%CI 1.4-2.1] (Figure 1 and Table 2). “The median times from triage to antibiotics in the baseline and post-intervention phases for sepsis were 106 and 109 min, and for septic shock were 15 and 13 min.”
Figure 1.
A Forest plot demonstrating a comparison of process measures between baseline and post-intervention phases.
Table 2.
Process measures.
| PROCESS MEASURES | ||||||
|---|---|---|---|---|---|---|
| Measure | Baseline a | Post-Intervention |
Adjusted effect size & (95% CI): Baseline vs Post-intervention | |||
| All b | No Pathway c | With Pathway d | Adjusted effect size # (95% CI): No pathway vs with pathway | |||
| Lactate Measurement | 607 (82.9) | 997 (93.2) | 357 (87.1) | 640 (97.0) | 4.5 (2.7, 7.8) ** | 2.6 (1.9, 3.5) ** |
| Blood cultures before antibiotics | 596 (81.4) | 956 (89.3) | 342 (83.4) | 614 (93.0) | 2.6 (1.7, 3.8) ** | 1.9 (1.4, 2.5) ** |
| Two sets of blood cultures | 232 (31.7) | 782 (73.1) | 237 (57.8) | 545 (82.6) | 3.6 (2.7, 4.8) ** | 6.0 (4.8, 7.4) ** |
| Antibiotics within 3 hours of triage (sepsis alone) | 380 (61.5) | 630 (69.8) | 195 (55.6) | 435 (78.9) | 3.0 (2.2, 4.1) ** | 1.3 (1.1, 1.7) ** |
| Antibiotics within 1 hour of shock (septic shock) | 84 (73.7) | 143 (85.1) | 50 (84.7) | 93 (85.3) | 1.0 (0.4, 2.6) | 1.9 (1.1, 3.6) ** |
| IV fluid bolus within 1 hour (septic shock) | 50 (43.9) | 71 (42.3) | 25 (42.4) | 46 (42.2) | 1.0 (0.5, 2.0) | 1.0 (0.6, 1.6) |
| Compliance with 3-hour sepsis bundle (sepsis alone) | 298 (48.2) | 571 (63.3) | 157 (44.7) | 414 (75.1) | 3.8 (2.8, 5.1) ** | 1.7 (1.4, 2.1) ** |
| Compliance with 1-hour septic shock bundle (septic shock) | 48 (42.1) | 79 (47.0) | 24 (40.7) | 55 (50.5) | 1.7 (0.9, 3.4) | 1.2 (0.8, 2.0) |
CI confidence interval; IV intravenous; ICU intensive care unit.
n (%), effect estimate = odds ratio;
# post-intervention no pathway vs with pathway.
& baseline vs post-intervention;
a N (Baseline) All Sepsis = 732; Septic Shock = 114; Sepsis Alone = 618.
b N (Post-Intervention) All Sepsis = 1070; Septic Shock = 168; Sepsis Alone = 902.
c N (No Pathway) All Sepsis = 410; Septic Shock = 59; Sepsis Alone = 351.
d N (With Pathway) All Sepsis = 660; Septic Shock = 109; Sepsis Alone = 551.
** - 95% Confidence limits indicate a significant change.
In the subgroup of patients with septic shock, there were no significant differences with regards to the use of one-hour bundle compliance or administration of fluid bolus for hypotension. There was a significant trend towards a greater compliance with process measures when there was evidence of pathway utilisation (Table 2).
Outcomes
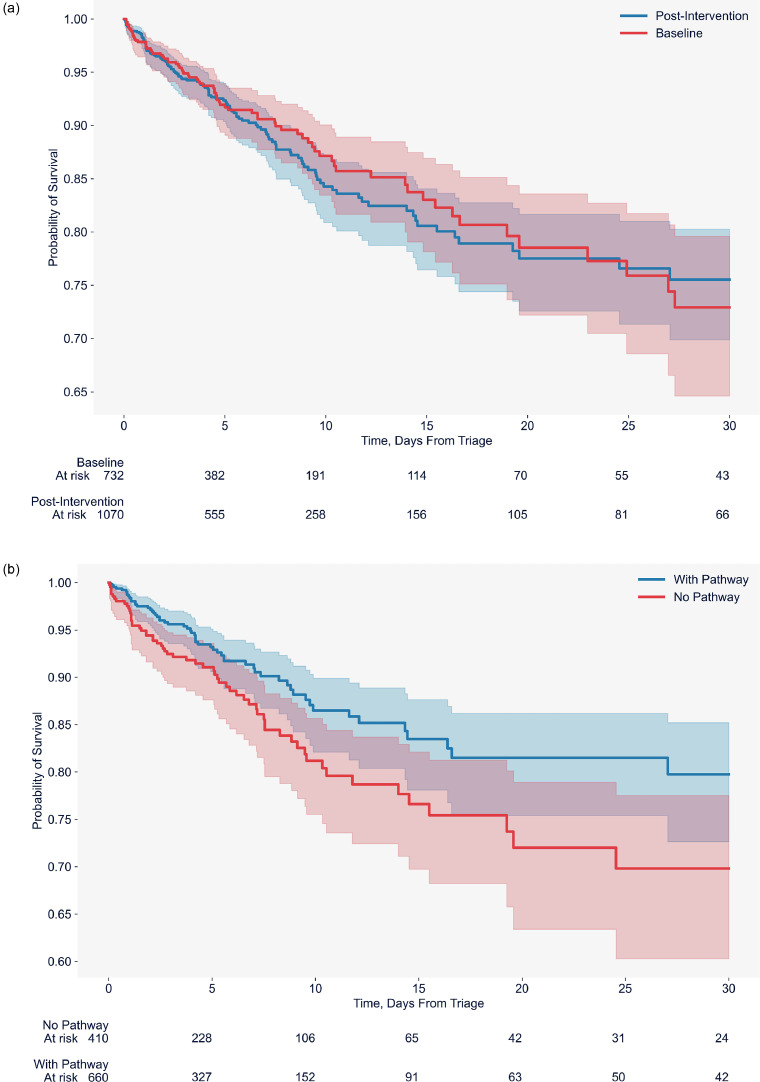
There were no significant differences in the in-hospital [Adjusted OR 1.1 [95%CI 0.8-1.5]) and 30-day post discharge mortality rates [Adjusted OR 1.0 [95%CI 0.8-1.4]) between the baseline and the post-intervention phases overall or in the subgroups of sepsis and septic shock (Table 3, Figure 2a).
Table 3.
Outcome measures
| OUTCOME MEASURES | |||||||
|---|---|---|---|---|---|---|---|
| Measure | Baseline a | Post-Intervention |
Adjusted effect size & (95% CI): Baseline vs Post-intervention | ||||
| All b | No Pathway c | With pathway d | Adjusted effect size # (95% CI): No pathway vs with pathway | ||||
| In-hospital mortality^ | All | 81 | 125 | 61 | 64 | 0.6 | 1.1 |
| Sepsis | (11.1) | (11.7) | (14.9) | (9.7) | (0.4, 0.9) ** | (0.8, 1.5) | |
| Septic Shock | 29 | 50 | 26 | 24 | 0.3 | 1.3 | |
| (25.4) | (29.8) | (44.1) | (22.0) | (0.2, 0.7) ** | (0.7, 2.2) | ||
| Sepsis Alone | 52 | 75 | 35 | 40 | 0.6 | 1.0 | |
| (8.4) | (8.3) | (10.0) | (7.3) | (0.4, 1.1) | (0.7, 1.5) | ||
| Suspected Sepsis | - | 61 | 30 | 31 | 0.5 | - | |
| (10.2) | (12.7) | (8.5) | (0.3, 1.0) | ||||
| 30-day post discharge mortality^ | All Sepsis | 124 (16.9) | 184 (17.2) | 79 (19.3) | 105 (15.9) | 0.8 (0.5, 1.1) | 1.0 (0.8, 1.4) |
| Septic Shock | 35 | 63 | 29 | 34 | 0.4 | 1.4 | |
| (30.7) | (37.5) | (49.2) | (31.2) | (0.2, 0.9) ** | (0.8, 2.5) | ||
| Sepsis Alone | 89 | 121 | 50 | 71 | 0.8 | 0.9 | |
| (14.4) | (13.4) | (14.2) | (12.9) | (0.6, 1.3) | (0.7, 1.3) | ||
| Suspected Sepsis | - | 92 | 40 | 52 | 0.7 | - | |
| (15.3) | (16.9) | (14.3) | (0.4, 1.1) | ||||
| ICU admission^ | All | 194 (26.5) | 187 | 68 | 119 | 1.1 | 0.5 |
| Sepsis | (17.5) | (16.6) | (18.0) | (0.8, 1.6) | (0.4, 0.7) ** | ||
| Septic Shock | 50 | 67 | 19 | 48 | 1.7 | 0.8 | |
| (43.9) | (39.9) | (32.2) | (44.0) | (0.9, 3.5) | (0.5, 1.4) | ||
| Sepsis Alone | 144 | 120 | 49 | 71 | 0.9 | 0.5 | |
| (23.3) | (13.3) | (14.0) | (12.9) | (0.6, 1.4) | (0.3, 0.6) ** | ||
| Suspected Sepsis | - | 113 | 57 | 56 | 0.7 | - | |
| (18.8) | (24.2) | (15.4) | (0.4, 1.0) | ||||
| Length of ICU stay (days)* | All Sepsis | 2.3 (0.9, 4.2) | 2.3 (1.1, 4.9) | 2.5 (0.9, 5.4) | 2.3 (1.2, 4.5) | 1.1 (0.8, 1.5) | 0.9 (0.7, 1.0) |
| Septic Shock | 2.6 (1.1, 4.5) | 2.6 (1.5, 4.0) | 2.3 (1.6, 3.2) | 2.7 (1.5, 4.4) | 0.6 (0.3, 1.1) | 1.1 (0.8, 1.6) | |
| Sepsis Alone | 2.2 (0.8, 4.2) | 2.3 (1.0, 5.4) | 2.8 (0.8, 6.0) | 2.3 (1.1, 4.5) | 1.1 (0.8, 1.7) | 0.8 (0.6, 1.0) | |
| Suspected Sepsis | - | 2.9 (1.7, 5.1) | 3.3 (1.8, 5.1) | 2.8 (1.5, 5.0) | 1.1 (0.8, 1.7) | - | |
| Length of non-ICU hospital stay (days)* | All Sepsis | 5.4 (2.7, 10.3) | 5.2 (2.9, 9.8) | 5.8 (3.0, 10.4) | 4.9 (2.7, 9.3) | 1.1 (1.0, 1.2) | 1.0 (0.9, 1.1) |
| Septic Shock | 4.6 (1.7, 9.5) | 5.1 (1.8, 9.7) | 4.4 (1.9, 10.1) | 5.3 (1.5, 9.3) | 1.0 (0.8, 1.4) | 0.9 (0.7, 1.1) | |
| Sepsis Alone | 5.4 (2.8, 10.5) | 5.4 (3.0, 9.8) | 6.0 (3.2, 10.4) | 4.9 (2.8, 9.4) | 1.1 (1.0, 1.3) | 1.0 (0.9, 1.1) | |
| Suspected Sepsis | - | 4.1 (2.0, 7.8) | 5.0 (2.3, 9.8) | 3.7 (1.9, 6.7) | 1.3 (1.1, 1.5) ** | - | |
CI confidence interval; IV intravenous; ICU intensive care unit.
^ n (%), effect estimate = odds ratio; # post-intervention no pathway vs with pathway.
& baseline vs post-intervention; * median (IQR), effect estimate = hazard ratio.
a N (Baseline) All Sepsis = 732; Septic Shock = 114; Sepsis Alone = 618.
b N (Post-Intervention) All Sepsis = 1070; Septic Shock = 168; Sepsis Alone = 902.
c N (No Pathway) All Sepsis = 410; Septic Shock = 59; Sepsis Alone = 351.
d N (With Pathway) All Sepsis = 660; Septic Shock = 109; Sepsis Alone = 551.
** - 95% Confidence limits indicate a significant change.
Figure 2a.
A Kaplan-Meier plot of survival analysis between baseline and post-intervention phases Figure 2b A Kaplan-Meier plot of survival analysis in the post intervention phase comparing evidence of pathway use versus no evidence of pathway use.
In the post-intervention phase, the pathway utilisation rates varied across the 14 hospitals ranging from 32.5% to 86%. A subgroup analysis of the post-intervention phase revealed that in-hospital mortality rate (9.7% vs 14.9%, adjusted OR 0.6, [95%CI 0.4-0.8] was significantly lower in the overall cohort and in the subgroup of septic shock in whom there was evidence of compliance with sepsis pathway. (Table 3, Figure 2b).
The proportion of patients requiring ICU admission was significantly reduced in the post-intervention phase after adjustment for covariates from 26.5% vs 17.5% (aOR 0.5, 95%CI 0.4-0.7)]. (Table 3)
There were no differences in ICU (median [IQR] 2.3 [0.9-4.2] vs 2.3[1.1-4.9]) days or non-ICU length of hospital stay (median [IQR] 5.4 (2.7-10.3) vs 5.2[2.9-9.8]) in the overall cohort between the two phases. (Table 3).
In the subgroup of 600 patients with negative blood cultures and a positive ICD code, the use of the pathway was associated with trends in reductions in ICU and 30-day post discharge mortality and need for ICU admission, although these results were not statistically significant. (Table 3)
Across the study duration, timely administration of antibiotics and bundle compliance were associated with lower mortality and ICU admission rates in patients with sepsis (Table S13-Supplementary Appendix)
Although the proportion of appropriate antimicrobial prescription increased in the post-intervention phase (55.4% vs 64.1%, OR 1.4 [95%CI 0.9-2.1]) this was not statistically significant. (Table S14 - Supplementary appendix)
Discussion
The implementation of the Collaborative resulted in significantly improved process measures with all elements of the 3-hour sepsis bundle and antibiotic administration in the 1-hour septic shock bundle in the post-intervention phase. There was a significantly reduced need for ICU admission. Although there were no significant differences in mortality between baseline and post-intervention phases, evidence of pathway utilization in the post-intervention phase was associated with improved mortality. The use of a sepsis bundle did not adversely impact on the appropriateness of antimicrobial prescription.
Despite a significant reduction in the need for Intensive Care admission, we did not observe a significant difference in in-hospital or 30-day post discharge mortality overall in the post-intervention phase. An explanation for the lack of a beneficial effect on mortality may be inadequate statistical power. It is also possible that early intervention may influence hemodynamic stability and organ function to reduce the need for ICU admission, but may not influence the trajectory and progression of sepsis. It is noteworthy that in the post-intervention phase, pathway utilisation was associated with improved process measures and outcomes; however as this is a post hoc analysis, this finding must be considered exploratory. The results of our study accord with data from previous single centre studies 18, 19, 20, 21, 22, multicentre quality improvement programs23,24 and randomized trials of protocolised early goal directed therapy.25
However, it differs from previously published reports of sepsis bundles in several respects. The strengths of our study include the use of the IHI Collaborative Methodology, and incorporating a before and after study design and therefore able to assess compliance with the care bundle. Although the bundle elements were similar to the Surviving Sepsis Campaign Guidelines, we used different time windows for sepsis and septic shock, avoiding conflation of the two syndromes. A high proportion of patients received the bundle intervention and few patients were lost to follow up. We not only evaluated in-hospital but also 30-day post-discharge mortality as patient-centered outcomes and specifically targeted a population of patients with positive blood cultures to improve the diagnostic accuracy of sepsis in the emergency department. In contrast to the study by Seymour et al,4 we did not observe a relationship between increased time to antibiotic administration and achievement of bundle compliance and worsening mortality in septic shock. Our results are also at variance with those reported in a recent study by Barbash et al where they reported an increase in the need for ICU and no changes in mortality following SEP-1 sepsis bundle implementation.26 This may be due to differences in patient populations, the use of differing criteria for identifying patients with septic shock and different time bundles. We also incorporated an antimicrobial prescription assessment to assess the impact of the sepsis bundle on antimicrobial usage. All sites were provided with online relevant documents and training manuals and there was participation from 14 hospitals representing tertiary, metropolitan and provincial hospitals.
Our study had limitations. Our study was a before and after observational study and therefore there was potential for bias by residual confounding. The high turnover workforce in emergency departments may have impacted on the level of staff awareness of the bundle and pathway during the post-intervention phase. The pathway filed in the notes was used as evidence that a clinician providing care for the patient was aware of the sepsis bundle intervention. The premature termination of the Collaborative may have resulted in fewer enrollments in the post-intervention phase, potentially reducing the power of the study. We did not use Sepsis-3 criteria for the diagnosis of sepsis and the initiation of the bundle in the ED. Whilst the Sepsis-3 definition represents a better risk stratification tool it is not recommended as a trigger for initiation of treatment. We used a modified Sepsis-3 definition to identify patients with septic shock. Collection of detailed physiological data and additional propensity matched analysis adjusting for receipt of pathway would have been useful for rigorous risk adjustment but was not undertaken as this was a quality improvement project. Data on vasopressor use in the ED was not collected as patients are often transferred to intensive care for such therapies. Evaluation of antimicrobial usage was confined to a nested cohort predominantly amongst patients who had confirmed blood culture positivity thus limiting extrapolation to patients with blood culture negative sepsis. These data were collected in hospitals in one state in Australia and therefore limits the generalisability of our findings. The differing patient populations, bundle timings, choice of antibiotics have been highlighted in a recent position paper as another factor which limit the applicability of sepsis bundles.27
Our study provides evidence about the role of sepsis bundles in the management of patients presenting to the emergency department with bacteremia and sepsis. Important patient centred outcomes such as reduced need for ICU intervention were better in the post-intervention phase. These have important implications for ICU resources and triage. A detailed cost–benefit assessment of these results was not done, but such an analysis may inform clinicians about the overall cost-effectiveness of bundles in the management of patients with sepsis.
In conclusion, the use of a Breakthrough Collaborative for Sepsis management resulted in improved uptake of a 1-hour septic shock and a 3-hour sepsis bundle and a reduced need for intensive care admission in patients presenting to the emergency department with bacteremia and sepsis. There was no adverse impact on antimicrobial use.
Funding
Queensland Health
Authors’ contributions
BV: Conceptualisation; Data curation; formal analysis; investigation; methodology; interpretation of results; writing-original draft; writing- review and editing
LS: Data curation; interpretation of results investigation; writing- review and editing
DM: Data curation; interpretation of results; methodology; review and editing
KW: Data curation; interpretation of results writing- review and editing
RS: Data curation; interpretation of results; methodology; review and editing
PL (Lister): Data curation; investigation; writing- review and editing
AI: Interpretation of results; writing- review and editing
PL (Lane): Data curation; investigation; writing- review and editing
LR: Data curation; Methodology; investigation; review and editing
KG: Data curation; statistical analysis; review and editing
EE: Data curation; statistical analysis; review and editing
MR: Data curation; Methodology; investigation; review and editing
Data sharing statement
Deidentified patient data will be made available (participant data with identifiers, data dictionary), beginning 6 months after publication of the study and ending at 2 years. All requests for data sharing must be accompanied by a formal request, a study proposal with clear statement of aims and hypotheses, and a statistical analysis plan. All applications will be assessed by the Sepsis Collaborative Steering Committee. Applications from investigators with suitable academic capability to conduct the proposed work will be given consideration. Any proposal will require approval from the ethics committee which approved the conduct of this trial prior to sharing of any patient data. If a proposal is approved, a signed data transfer agreement will be required before data sharing.
Declaration of Competing Interest
None
Acknowledgement
We thank the patients and their families for their participation in this collaborative and the clinical and research staff at all participating sites.
We thank the sepsis consumers Mary Steele and Matthew Ames for their enormous contribution to and advocacy for the program.
We acknowledge the contribution by Dr. Julian Williams in the early phases of the set up of the collaborative.
Footnotes
All authors in Australia unless stated.
for the Queensland Sepsis Collaborative
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2021.100305.
Appendix. Supplementary materials
References
- 1.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive care med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes A, Phillips G, Beale R. al.The Surviving Sepsis Campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study) Intensive Care Med. 2015;41:1620–1628. doi: 10.1007/s00134-015-3906-y. [DOI] [PubMed] [Google Scholar]
- 4.Seymour CW, Gesten F, Prescott HC, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Medicine. 2018;44:925–928. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert JA. Sepsis care bundles: a work in progress. Lancet Respir Med. 2018;6:821–823. doi: 10.1016/S2213-2600(18)30362-X. [DOI] [PubMed] [Google Scholar]
- 7.IDSA Sepsis Task Force Infectious Diseases Society of America (IDSA) POSITION STATEMENT: Why IDSA Did Not Endorse the Surviving Sepsis Campaign Guidelines. Clin Infect Dis. 2018;66:1631–1635. doi: 10.1093/cid/cix997. May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klompas M, Calandra T, Singer M. Antibiotics for Sepsis-Finding the Equilibrium. JAMA. 2018;320:1433–1434. doi: 10.1001/jama.2018.12179. [DOI] [PubMed] [Google Scholar]
- 9.Machado FR, Ferreira EM, Schippers P, et al. Implementation of sepsis bundles in public hospitals in Brazil: a prospective study with heterogeneous results. Crit Care. 2017;21(1):268. doi: 10.1186/s13054-017-1858-z. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrell AR, McLaws ML, Fullick M, Sullivan RB, Sindhusake D. SEPSIS KILLS: early intervention saves lives. Med J Aust. 2016;204:73. doi: 10.5694/mja15.00657. [DOI] [PubMed] [Google Scholar]
- 11.Wang HE, Jones AR, Donnelly JP. Revised National Estimates of Emergency Department Visits for Sepsis in the United States. Crit Care Med. 2017;45:1443–1449. doi: 10.1097/CCM.0000000000002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reade MC, Huang DT, Bell D, et al. Variability in management of early severe sepsis. Emerg Med J. 2010;27:110–115. doi: 10.1136/emj.2008.070912. [DOI] [PubMed] [Google Scholar]
- 13.Resar R, Griffin FA, Haraden C, Nolan TW. IHI Innovation Series white paper. Institute for Healthcare Improvement; Cambridge, Massachusetts: 2012. Using Care Bundles to Improve Health Care Quality. [Google Scholar]
- 14.Shankar-Hari M, Phillips GS, Levy ML, et al. 2016, 'Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)'. JAMA, 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randhawa V, Sarwar S, Walker S, Elligsen M, Palmay L, Daneman N. Weighted-incidence syndromic combination antibiograms to guide empiric treatment of critical care infections: a retrospective cohort study. Crit Care. 2014;18:R112. doi: 10.1186/cc13901. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James R., Upjohn L., Cotta M., et al. Measuring antimicrobial prescribing quality in Australian hospitals: development and evaluation of a national antimicrobial prescribing survey tool. J Antimicrob Chemother, 2015;70:1912–1918. doi: 10.1093/jac/dkv047. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trzeciak S, Dellinger RP, Abate NL, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest. 2006;129:225–232. doi: 10.1378/chest.129.2.225. [DOI] [PubMed] [Google Scholar]
- 19.Micek ST, Roubinian N, Heuring T, et al. Before after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2006;34:2707–2713. doi: 10.1097/01.CCM.0000241151.25426.D7. [DOI] [PubMed] [Google Scholar]
- 20.Kortgen A, Niederprum P, Bauer M. Implementation of an evidence-based “standard operating procedure” and outcome in septic shock. Crit Care Med. 2006;34:943–949. doi: 10.1097/01.CCM.0000206112.32673.D4. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35:1105–1112. doi: 10.1097/01.CCM.0000259463.33848.3D. [DOI] [PubMed] [Google Scholar]
- 22.Gao F, Melody T, Daniels DF, Giles S, Fox S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit Care. 2005;9:R764–R770. doi: 10.1186/cc3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrer R, Artigas A, Levy MM, Blanco J, González-Díaz G, Garnacho-Montero J, Ibáñez J, Palencia E, Quintana M, de la Torre-Prados MV. Edusepsis Study Group. Improvement in Process of Care and Outcome After a Multicenter Severe Sepsis Educational Program in Spain. JAMA. 2008;299:2294–2303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 24.Rhee C, Filbin MR, Massaro AF, et al. Compliance With the National SEP-1 Quality Measure and Association With Sepsis Outcomes: A Multicenter Retrospective Cohort Study. Crit Care Med. 2018;46:1585–1591. doi: 10.1097/CCM.0000000000003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PRISM Investigators. Rowan KM, Angus DC, Bailey M, et al. Early, Goal-Directed Therapy for Septic Shock - A Patient-Level Meta-Analysis. N Engl J Med. 2017;376:2223–2234. doi: 10.1056/NEJMoa1701380. [DOI] [PubMed] [Google Scholar]
- 26.Barbash IJ, Davis BS, Yabes JG, Seymour CW, Angus DC, Kahn JM. Treatment Patterns and Clinical Outcomes After the Introduction of the Medicare Sepsis Performance Measure (SEP-1) Ann Intern Med. 2021;174(7):927–935. doi: 10.7326/M20-5043. Jul10.7326/M20-5043.Epub 2021 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee C, et al. Infectious Diseases Society of America Position Paper: Recommended Revisions to the National Severe Sepsis and Septic Shock Early Management Bundle (SEP-1) Sepsis Quality Measure. Clin Infect Dis. 2021;72(4):541–552. doi: 10.1093/cid/ciaa059. 02 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.