Summary
Although the concepts underpinning green chemistry have evolved over the past 30 years, the practice of green chemistry must move beyond the environmental and human health-related roots of green chemistry towards a more systems-based, life cycle-informed, and interdisciplinary practice of chemistry. To make a transition from green to sustainable chemistry, one must learn to think at a systems level; otherwise green chemistry-inspired solutions are unlikely to be sustainable. This perspective provides a brief description of why the current situation needs to change and is followed by how life cycle thinking helps chemists avoid significant systems-level impacts. The transition from batch to continuous flow processing and novel approaches to isolation and purification provide a case for interdisciplinary collaboration. Finally, an example of end-of-useful-life considerations makes the case that systems and life cycle thinking from an interdisciplinary perspective needs to inform the design of new chemical entities and their associated processes.
Subject areas: chemistry, organic chemistry, green chemistry, green engineering
Graphical abstract
Highlights
-
•
Green and sustainable chemistry must include a systems and life cycle perspective
-
•
Green and sustainable chemistry requires extensive interdisciplinary collaboration
-
•
Catalysis, purification and isolation, and batch to flow processing are discussed
Chemistry; Organic chemistry; Green chemistry; Green engineering
Introduction
For much of the past 30 years, green chemistry has been largely identified with two central ideas: the reduction or elimination of toxic substances and pollution prevention (U.S. E.P.A, 2021). Much of what has been written and spoken about green chemistry is rooted in environmentalism, environmental policies, and governmental regulations promulgated since the 1980's. For the period between 1995 and about 2010, proponents of green chemistry struggled with being seen as a legitimate part of chemistry within the traditional chemistry community for many reasons, but three stand out. The first is that green chemistry was seen as being environmentally-related, applied, and not innovative. The second is that because of the association with the environment, it was seen as more of an environmental movement and not science (Breyman and Woodhouse, 2005). The third is that many in industry felt that they had been doing the pollution prevention aspects incorporated in green chemistry for many years, but especially through the 1970's and 1980's (Murphy, 2018, 2020).
When considering chemistry research from a green chemistry perspective, another challenge to chemistry researchers in traditional chemistry disciplines is the necessity of drawing from multiple scientific and engineering disciplines to not only understand the underlying chemical and physical phenomena, but to better understand why the current approaches to chemistry need to change (Constable, 2017; Whitesides, 2015; Matlin, et al., 2016). Similar to non-traditional chemistry fields like biochemistry and nanochemistry, to be successful in green chemistry-related research, one must draw from many different disciplines.
I would also say that this need for an interdisciplinary approach is amplified as one moves from a singular focus on green chemistry to one that incorporates a consideration of two related ideas, sustainability and sustainable development. For the purposes of this article and the current argument, sustainability will be confined to thinking about environmental sustainability; i.e., actions and behaviors one must take to ensure that the chemistry being practiced is not creating current or generational environmental impacts. It should be understood that the use of the term sustainability typically envisions a “triple bottom line” approach that includes a concurrent consideration of environmental, societal and economic impacts (Elkington, 2018), but such considerations are generally not embraced by the chemistry community which fails to see the point or necessity of connecting molecular-scale chemical phenomena to macro-scale impacts. The most frequent definition of sustainable development is from “Our Common Future,” also known as the Brundtland Report (World Commission on Environment and Development, 1987), and is “Sustainable Development is development that meets the needs of the present without compromising the needs of future generations to meet their own needs.” The practice of chemistry is inherently rooted in the present and by design, on a time scale of less than a second to perhaps hours. In addition, for many academic research chemists and the institutions that fund them, “real” chemistry is decoupled from any notion of application or development; it is science to advance the science of chemistry, not to fulfill the needs of human society.
Therefore, to make a transition from green chemistry to sustainable chemistry, one must learn to think at a systems level, otherwise green chemistry-inspired solutions are unlikely to be sustainable. Although systems thinking is routinely taught in a variety of scientific and engineering disciplines, it has only recently been introduced to chemistry educators as something that needs to be included in chemistry education (Mahaffy, et al., 2018, 2019). There are a variety of definitions for systems thinking in science, engineering, social, and organizational contexts, and an agreed, or authoritative, or standard definition of a system or systems thinking for the chemistry context has yet to be established (York and Orgill, 2020). In essence, it is best to understand a system as being a logical construct or model of real-world phenomena that contains a collection of components or parts. These components are coherently organized and interconnected in patterns or in a structured and usually hierarchical manner to produce a characteristic set of behaviors, often classified as the system's “function” or “purpose,” or to answer a question related to systems outputs and outcomes. The study of changes in the state of a system over time and space are included in what is known as systems dynamics. Although it is beyond the scope of this article to discuss the details of systems thinking, one should appreciate that systems are everywhere, of different scales, and usually a part of a system of systems. This connectedness of system components with and between other systems is generally not explicitly seen as being a part of chemistry and that is one reason why systems thinking is critical to understanding how to practice green and sustainable chemistry.
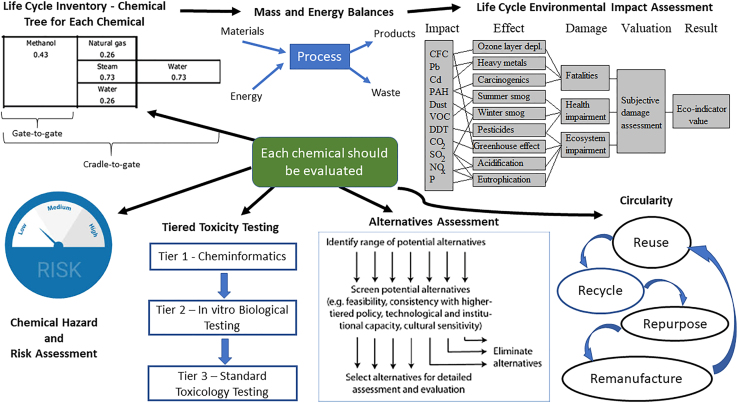
Systems thinking also helps one to manage the complexity that is inherent to sustainability and the implementation of green and sustainable chemistry (Constable, et al., 2019). Figure 1 shows a systems-level view of chemical evaluation. An important point to be made about thinking in systems within the chemistry context is that this should be accompanied by life cycle thinking, i.e., a consideration of environmental safety and health hazards and risks associated with the constituents of a material or product. Chemical trees may be used to visualize the gate-to-gate manufacturing processes and an inventory made of the associated inputs, outputs, and emissions for each step leading to the constituent parts of a material or product, from raw material extraction to a factory gate. Once the product is made, life cycle thinking considers a similar input/output inventory for the distribution, use and end-of-life phases of the product. In recent years, the end-of-life phase is increasingly incorporating a consideration of recycling/reuse and impacts related to waste management to advance the circular economy (Kirchherr et al., 2017). In a full life cycle inventory/assessment, there is a detailed, quantitative accounting for all the impacts and these impacts are combined into discrete categories (e.g., greenhouse gas equivalents, etc.) where they may be assessed for their cumulative impacts for the material or product life cycle. As is hopefully evident, life cycle thinking requires one to think of the material, process, or product in terms of a system of systems where the output of the life cycle is limited to the cumulative environmental impacts associated with the material or product. As important as life cycle thinking is, it should be understood that systems thinking is a broader, more comprehensive and holistic approach to considering material, process, or product benefits and impacts.
Figure 1.
Systems-level view of chemical evaluation
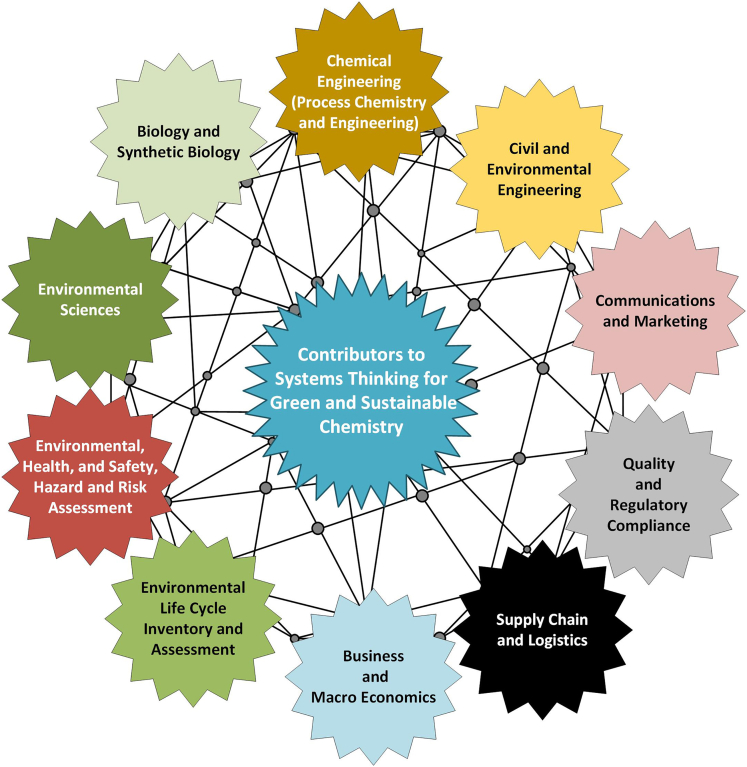
Life cycle and systems thinking should be practiced as complementary and synergistic lines of thinking. Merely formalizing a benchtop chemical reaction, or industrial chemical process, as a system, without consideration of a molecule's system and life cycle impacts defeats the purpose of systems thinking for green chemistry. Systems thinking also requires an interdisciplinary approach if one is to understand sustainability drivers and to correctly define the system, draw meaningful boundaries, recognize causal and feedback loops, and see the inter-system interactions that are common to considerations of sustainability. Chemistry impacts, and is impacted by, human/social systems, economic systems, and environmental systems. Figure 2 contains some, but by no means all, of the professions and skills that might contribute to systems thinking in chemistry and the system-of-systems supporting chemistry. Sustainable chemistry, to be successful, requires one to develop disciplinary skills outside of chemistry, and partner routinely with other scientists, engineers, businesses, and many other non-science-based professions. If a chemist does not do this, they will never arrive at a sustainable solution to chemical problems.
Figure 2.
Contributors to systems thinking for green and sustainable chemistry
The remainder of this article highlights a few key considerations and the interdisciplinary needs associated with selected aspects of making molecules from a mostly bioactive molecule perspective e.g., pharmaceuticals or crop protection agents. Green chemistry in the minds of many has been popularized as a limited number of easily accomplished practices a chemist needs to do to make a molecule green, greener, or more sustainable. If that was truly the case, there would be less of a reason to still be discussing the rationale for practicing and implementing green, greener, and now, more sustainable chemistry. What follows are several illustrations to show chemists need to employ systems thinking. The first is a high-level overview of some sustainability pitfalls of modern catalysis and what is needed to make it more sustainable. The second illustration is the underlying need to move as many batch chemical operations currently done in batch to a flow regime as warranted. The third illustration concerns the isolation, work-up and purification of biologically active molecules and the general need to reduce the impacts associated with this common operation. Finally, the case is made for chemists to develop a greater understanding of the life cycle impacts associated with the choices chemists make at the bench, pilot and process level, and work collaboratively with other scientists and engineers to make chemistry greener and more sustainable while addressing the world's grand challenges of sustainability (National Research Council, 2006).
Catalysis - metal and organometallic catalysts are generally not sustainable
It is probably best to use a short example to illustrate how systems thinking that includes an interdisciplinary mindset might be applied. One that immediately comes to mind is catalysis, which most will recognize as one of the 12 Anastas and Warner principles: “Catalytic reagents (as selective as possible) are superior to stoichiometric reagents (Anastas and Warner, 1998).” Catalysis is enormously important to the petrochemical and chemical processing industries, with many products relying on one or more catalytic steps in their synthesis routes. The use of catalysis is almost always considered to be green. From the earliest journal articles pertaining to green chemistry and in green chemistry-related programming at ACS National Meetings and Green Chemistry and Engineering conferences, catalysis has represented a significant part of the scientific discourse around green chemistry. Much of this discourse has been related to discoveries in catalysis where, for example, metal salts, zeolites, or complex organometallic compounds are used.
With respect to metals like Zn, Sn, Co, Ni, etc., or the transition metals centered around platinum on the periodic table that are used in organometallic catalysts, environmental and occupational toxicologists help the chemist understand environmental and human health hazards and risks. Interestingly, the greenness of the organometallic catalyst synthesis itself, where large or complex ligands are made using hazardous reagents, and difficult, inefficient, multi-step syntheses are common, is not featured in most catalysis discussions. Or, the catalytic reaction requires the use of reagents like triphenylphosphine, organoboranes, or similarly activating reagents that are not only mass inefficient, but hazardous. Moreover, many of these catalysts are only active as homogeneous catalysts; their binding to an inert substrate renders them less active or inactive. And while some in academic research think about catalyst recovery, homogeneous catalyst recovery and reuse remains technically difficult and largely an afterthought.
From a sustainability, systems, and life cycle perspective, the type and chemical nature of the catalyst matters. Where and how the metals, the reagents, chemicals, and solvents are sourced, the reaction conditions required for the catalysis to proceed, the disposition of these components in use and in final disposal, should all be carefully considered. At this point, a sustainability minded green and sustainable chemist should in the first instance be thinking about delivering catalytic function in some other way than organometallic catalysts, perhaps considering how the chemical transformation could be carried out enzymatically or with organocatalysts. Instead of creating an organometallic enzyme mimic, evolve an enzyme. Secondarily, if you have to use a precious metal catalyst, develop protocols that reduce the quantity of metal required to the ppm level as has been done with, e.g., performing reactions in micellar aqueous solutions using nanoparticulate, earth-abundant iron (Fe) particles containing low concentrations of the precious metals (Lipshutz, 2017; Lipshutz et al., 2018; Romney, et al., 2018).
Transitioning from batch to flow
Outside of the laboratory and in a manufacturing environment, there are two main types of chemical processing technologies that are used in the chemical and allied industries. These are batch chemical operations carried out in multi-purpose chemical plants to produce low tonnages of chemicals, and chemical processing in flow, such as are found in large, high-volume petrochemical operations. The operation of chemistry in flow within the large petrochemical operations is dominated by chemical engineering expertise whereas batch chemical operations are dominated by process chemists and in many respects, are scaled-up laboratory operations. High-volume, petrochemical flow operations are also characterized by comparatively chemically simpler reactions taking place and with higher efficiency in terms of low mass and energy intensity, but it would be wrong to conclude that the control of these reaction spaces is unsophisticated. In fact, there is a high degree of automation and sophistication in the design of the reaction system and its control technology to ensure heat and mass transfer and reaction kinetics are optimized to achieve required high process mass and energy efficiencies for low-margin commodity chemicals. Higher process mass and energy efficiency is a key measure of greenness although it is only one part of it.
As there is a push towards converting more batch chemical operations to flow (Ding, 2018) to take advantage of the unrealized potential in chemical reaction technologies such as photochemistry (Noel, 2017; Halperin, et al., 2015), electrochemistry (Noël et al., 2019; Tanbouza et al., 2020) and others (Glasnov and Kappe, 2011; Dallinger and Kappe, 2017), there will be a need for greater interdisciplinary collaboration, especially between chemists and chemical engineers, to achieve the required level of quality or purity, cost, sophistication in design, and reaction control for molecules that are inherently more complex. Because the volumetric demand for chemicals is relatively smaller in batch operations compared to petrochemical or commodity chemical flow operations, the process trains will be much smaller, modular, highly automated, and in many respects, more technologically complex. It should be understood that flow is not a batch chemical panacea for making processes greener or more sustainable, especially if a chemist uses highly hazardous reactants and reagents as suggested by some (Gutmann et al., 2015), or there is a reduction in overall process efficiency. One challenge in the transition from batch to flow is the frequent need for larger volumes of solvent to ensure the appropriate flow regime. This is a decidedly unwelcome trade-off in making a process greener or more sustainable especially if in-process and out-of-process recycle and reuse options (Chea et al., 2020) are limited.
Isolation and purification
Isolation and purification, especially in batch chemical operations for small molecules (MW < 750), accounts for a significant portion of the total process mass intensity (Jimenez-Gonzalez et al., 2011). Process mass intensity is a very good proxy for energy and the associated life cycle environmental impacts associated with batch processes because at least 80% of the mass is organic solvents and water. Isolation and purification in most cases entails solvent and water removal via distillation in multiple steps of the synthesis process and distillation is the main driver of energy intensity in batch process operations. Given this fact, there are currently few strategies that one can employ to overcome the mass intensity associated with isolation and purification. The first obvious one is to do as few isolations and purifications as possible. The second would be to employ one-pot, multi-step syntheses, but this is very difficult to do in practice at scale for a variety of competing reasons beyond the scope of this article.
To optimize a multi-step process requires a close collaboration between process chemists and engineers to select solvents, use as few solvent classes as possible across multiple steps, avoid azeotropes and emulsions, optimize reflux or near-reflux conditions for extended reactions at high temperatures, and finally, optimize distillation in the process and solvent recovery. As with the preceding examples, there needs to be a close collaboration between process chemists and chemical engineers who are generally better trained in distillation, azeotrope formation, and process optimization.
As bioactive molecules common to the pharmaceutical industry, e.g., oligonucleotides, polypeptides, antibodies, drug-antibody conjugates, etc., become much larger and more prevalent, isolation and purification across compound synthesis and manufacture becomes an even bigger driver of process mass intensity (Isidro-Llobet et al., 2019; Andrews et al., 2021). Solvent use is greater and the separation technology changes to solid phase linking and large-scale chromatographic processing in the place of distillation. Opportunities to green this kind of process are more limited (Bryan et al., 2018; Jiménez-González et al., 2000) and will require the development of novel synthetic approaches and more efficient chromatographic processing, but once again, close collaboration between chemists and chemical engineers will be highly beneficial.
Towards avoiding life cycle impacts
Most chemists I encounter have little idea about how the basic chemicals and framework molecules they use are made at an industrial scale and they do not they possess an intuitive sense of the life cycle environmental impacts associated with chemicals or the molecules they synthesize. In my opinion, every chemist should employ life cycle thinking as noted in the introduction, but the ability to perform a modular cradle-to-gate life cycle inventory/assessment (Jiménez-González et al., 2000, 2001; Jiménez-González and Overcash, 2000) is a skill a chemist is well advised to develop, or they should partner with someone (usually a chemical engineer) who can perform one. I say modular cradle-to-gate methodologies purposefully since these approaches are the only ones that visualize how chemicals are built through the chemical processing steps that lead to the desired molecule, chemical, or product. Every element in the periodic table, and every chemical, has a history with origins that are spread across the world. Although I think chemists understand the elements that appear on a periodic table don't originate at a local chemical supplier, they don't typically know which minerals or ores are mined and the chemical purification processes that are used to obtain the pure elements or salts they use in their experiments. Modular cradle-to-gate life cycle inventory/assessment methodologies enable one to see inputs and outputs each step of the journey from raw material extraction through each processing step.
Different functional groups on molecules provide a desired property or function in a chemical process or product, but their presence may also have adverse effects on living organisms, the facilities we work in, and the environment. Chemists need to be trained to recognize functional groups and structural motifs that lead to hazardous properties (environmental, safety, and human health) and make a conscious effort to avoid them. This is an important point that should be repeated in a different way. Chemists currently accept the inherent hazard of many molecules as just the way chemistry is done; i.e., the chemistry system is inherently hazardous and there is no other way to do chemistry but through the use of highly hazardous substances. Chemists should recognize and pursue ways to change the chemistry system to one that has minimal adverse impacts and promotes a more sustainable planet.
To understand and avoid negative life cycle impacts associated with chemical process inputs and outputs, a chemist must rely on other disciplines like toxicology, where chemical interactions with, and effects on, living organisms are studied (DeVito, 1996; Anastas, 2016; Maertens et al., 2014). Regardless of the science or engineering discipline or sub-discipline, the common theme is that the chemistry, chemical mechanisms, and chemical technology govern a system's processes and outcomes. Understanding environmental chemistry, the molecular drivers of eco- or human toxicology, environmental fate (where chemicals distribute; i.e., air, water, and land), etc., are all dependent on a good understanding of fundamental chemistry, chemical properties, and chemical phenomena. What other science and engineering disciplines like toxicology, molecular genetics, safety engineering, chemical engineering, public health experts, etc. should provide to the research and development chemist is continuing insight and molecular design guidance that would enable the chemist to avoid problematic molecular structural motifs, functional groups, and other chemical and physico-chemical properties that lead to environmental, safety, and health impacts. A chemist needs to know how to understand, interpret, and apply these environmental, safety, and health data and most importantly, to avoid the use of materials that are hazardous. This is an important point worth repeating in a different way; the status quo of the chemistry system means that chemists routinely, knowingly, and purposefully use hazardous materials (environmental safety and health) to make new molecules and this simply is not sustainable.
End of useful life
Humanity is using an ever-increasing quantity of materials and energy to drive its pursuit of affluence. To put this into perspective, in 1900, global material use was estimated to be 7.1 Gt per year, which increased to 70 Gt by 2010 (Krausmann et al., 2017). Sadly, most materials moving through a modern economy become waste in a short period of time. Obviously, this is not all chemical waste, but a significant proportion of it is related to energy, and chemical production does require a significant amount of energy. Among OECD countries, it is estimated that 1.75 million metric tons of solid waste are produced every day, whereas on a global scale, solid waste is projected to grow from 3.5 million metric tons/day in 2010 to 6 million metric tons/day in 2025 (Hoornweg et al., 2013). This is a somewhat sobering realization and there are at least two main ideas worth pondering. The first is that managing this waste and moving from a linear to a circular economy where waste is reused is only now becoming a topic of serious consideration and will require multiple disciplines to overcome the profound challenges involved. Green chemistry has always been associated with the reduction or elimination of waste through pollution prevention by source reduction, but the scope of that has typically been limited to manufacturing, not the rest of the product life cycle. Moreover, manufacturing waste is something that is disposed of and it becomes someone else's problem, not something that is repurposed or reused, so there's a psychological barrier to overcome.
An additional burden for waste reuse in the pharmaceutical realm is the institutional imperative surrounding the purity of the drug substance. This is completely understandable in that no one wants an impurity appearing in the final formulation containing the active pharmaceutical ingredient that has not been previously observed, characterized, and has passed through all clinical testing proving it does not have any adverse impacts on the patient. Purity is clearly a design constraint for the system that produces a pharmaceutical product. An unintended consequence of this, however, is that solvent recycling can be perceived as a potential source for the introduction of impurities in the final drug substance, and solvent recycling is generally not considered to be a design constraint for the pharmaceutical product system. Solvent recycling does of course occur in the pharmaceutical industry (Tiwari, 2019), but the industry also incinerates a large amount of solvent and water to avoid potential issues like pharmaceuticals in the environment or human health impacts. Analogous concerns are associated with food production and personal care products like cosmetics.
The second big idea is that apart from food, most products are made to be durable since product durability is greatly valued by society. Simply put, there is a desire for the products on the market to retain their original as-manufactured appearance and functionality for as long as possible. Another way to say this is that the system that produces a product is constrained by the perceived value of durability. The implications of this for chemists is that the chemistry subsystem that is part of the pharmaceutical product system sets molecular design constraints to synthesize new molecules that are difficult to degrade chemically, through physical processes, or through biological processes that are a part of human and environmental systems. Designing chemicals, and the products we make from chemicals, to deliver a desired function at a robust performance level for only as long as we need them, and then have a low-energy, low-mass process to return them to raw materials, should be a design constraint for every product system, and it is a problem that chemistry alone will not solve. The chemistry subsystem of the product systems needs to be informed by biology to know, e.g., common microbiological degradation mechanisms; by environmental science to understand the fate and effects of chemicals and predict environmental risk; by engineering to assist with treatment and closing the loop; by business to help make the business case; by waste management practices to seek opportunities for design for circularity, etc.
In the case of pharmaceuticals that are small molecules (MW < 750), because most are designed to survive the digestive system, to make it to the desired organ and produce the intended effect, have reasonable shelf lives, i.e., be resistant to photochemical degradation and/or humidity, etc., they are very durable and retain their bioactivity, which has led to increased concerns about the presence of pharmaceuticals in the environment (Kümmerer, 2010; aus der Beek et al., 2016). Ideally, pharmaceuticals would produce the desired physiological effect only for as long as they are in our body and then they and their metabolites would degrade into benign products. For many of these molecules, the design for stability system constraint during the use phase of their life cycle means that the molecule does not readily degrade in the environment during its end-of-useful-life phase of their life cycle, although in most cases it will be inherently biodegradable over time. By contrast, large pharmaceutical molecules like oligonucleotides, peptides, monoclonal antibodies, and other macromolecular molecules have very selective physiological effects and they are readily biodegradable during the end-of-useful-life phase of their life cycle although they have a much larger process mass intensity in the manufacturing phase of their life cycle than a small molecule. As can be seen, a change in the pharmaceutical product system to these large molecules involves a few environmental trade-offs, but the larger process mass intensity associated with these larger molecules is likely to be reduced over time through changes in synthesis technology, adoption of semi-synthetic approaches and better water management practices.
Conclusions
For chemistry to thrive in the future, it needs to be increasingly focused on meeting the needs of the world as envisioned through the U.N. Sustainable Development Goals (United Nations, 2015). Chemistry and chemical engineering are absolutely essential to society's ability to achieve these goals given the considerable amount of work to be accomplished. As essential as these two science and engineering disciplines are, it is also essential for chemists to think in terms of the systems that are impacted by the chemicals and materials they make, and they must draw on a wide range of disciplines to ensure that the most sustainable chemistry and chemical engineering are accomplished; they cannot act in isolation or only for the advance of the discipline. To reiterate, in order to make a transition from green to sustainable chemistry, chemists must learn to think at a systems level; otherwise green chemistry-inspired solutions are unlikely to be sustainable. Moreover, there is no doubt that the world will need to innovate in chemistry and related disciplines at an unprecedented rate to avoid major reductions in quality of life. As a recently published patent analysis revealed, the rate of green and sustainable chemistry-related U.S. patents has held steady at about 1.2% per year for the past 30 years (Constable, 2020) and this suggests change in the status quo is essential. The flip side of the waste dilemma noted previously is the ever-increasing and unprecedented rate of material extraction to meet societal demands that are directly correlated with increasing affluence in many nations throughout the world. Without a change in the material and energy intensity of our collective affluence, the future looks anything but sustainable. Chemists and chemical engineers working in collaboration and partnership with their science and engineering peers have an enormous role to play in making the world more sustainable.
Acknowledgments
Declaration of interests
The author declares no competing interests.
Inclusion and diversity
While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
References
- Anastas N.D. Connecting toxicology and chemistry to ensure safer chemical design. Green. Chem. 2016;18:4325–4331. doi: 10.1039/C6GC00758A. [DOI] [Google Scholar]
- Anastas P.T., Warner J.C. Oxford Univ. Press; 1998. Green Chemistry: Theory and Practice. [Google Scholar]
- Andrews B.I., Antia F.D., Brueggemeier S.B., Diorazio L.J., Koenig S.G., Kopach M.E., Lee H., Olbrich M., Watson A.L. Sustainability challenges and opportunities in oligonucleotide manufacturing. J. Org. Chem. 2021;86:49–61. doi: 10.1021/acs.joc.0c02291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- aus der Beek T., Weber F.A., Bergmann A., Hickmann S., Ebert I., Hein A., Küster A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Tox. Chem. 2016;35:823–835. doi: 10.1002/etc.3339. [DOI] [PubMed] [Google Scholar]
- Breyman S., Woodhouse E.J. Green chemistry as social movement? Sci. Technol. Hum. Values. 2005;30:199–222. doi: 10.1177/0162243904271726. [DOI] [Google Scholar]
- Bryan M.C., Dunn P.J., Entwistle D., Gallou F., Koenig S.G., Hayler J.D., Hickey M.R., Hughes S., Kopach M.E., Moine G., et al. Key green chemistry research areas from a pharmaceutical manufacturers’ perspective revisited. Green. Chem. 2018;20:5082–5103. doi: 10.1039/C8GC01276H. [DOI] [Google Scholar]
- Chea J.D., Lehr A.L., Stengel J.P., Savelski M.J., Slater C.S., Yenkie K.M. Evaluation of solvent recovery options for economic feasibility through a superstructure-based optimization framework. Ind. Eng. Chem. Res. 2020;59:5931–5944. doi: 10.1021/acs.iecr.9b06725. [DOI] [Google Scholar]
- Constable D.J.C. The practice of chemistry still needs to change. Cur. Op. Green. Sustainable Chem. 2017;7:60–62. doi: 10.1016/j.cogsc.2017.08.002. [DOI] [Google Scholar]
- Constable D.J.C. What do patents tell us about the implementation of green and sustainable chemistry? ACS Sustainable Chem. Eng. 2020;8:14657–14667. doi: 10.1021/acssuschemeng.0c05496. [DOI] [Google Scholar]
- Constable D.J.C., Jimenez-Gonzalez C., Matlin S.A. Navigating complexity using systems thinking in chemistry, with implications for chemistry education. J. Chem. Educ. 2019;96:2689–2699. doi: 10.1021/acs.jchemed.9b00368. [DOI] [Google Scholar]
- Dallinger D., Kappe C.O. Why flow means green – Evaluating the merits of continuous processing in the context of sustainability. Cur. Op. Gr. Sust. Chem. 2017;7:6–12. doi: 10.1016/j.cogsc.2017.06.003. [DOI] [Google Scholar]
- DeVito S.C. In: Designing Safer Chemicals. DeVito S.C., Garrett R.L., editors. Vol 640. American Chemical Society; 1996. pp. 16–59. (ACS Symposium Series). [Google Scholar]
- Ding B. Pharma Industry 4.0: Literature review and research opportunities in sustainable pharmaceutical supply chains. Proc. Saf. Env. Prot. 2018;119:115–130. doi: 10.1016/j.psep.2018.06.031. [DOI] [Google Scholar]
- Elkington J. 25 years ago I coined the phrase “triple bottom line.” Here’s why it’s time to rethink it. Harvard Business Review. 2018. https://hbr.org/2018/06/25-years-ago-i-coined-the-phrase-triple-bottom-line-heres-why-im-giving-up-on-it
- Glasnov T.N., Kappe C.O. The microwave-to-flow paradigm: Translating high-temperature batch microwave chemistry to scalable continuous-flow processes. Chem. Eur. J. 2011;17:11956–11968. doi: 10.1002/chem.201102065. [DOI] [PubMed] [Google Scholar]
- Gutmann B., Cantillo D., Kappe C.O. Continuous-flow technology—A tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. 2015;54:6688–6728. doi: 10.1002/anie.201409318. [DOI] [PubMed] [Google Scholar]
- Halperin S.D., Kwon D., Holmes M., Regalado E.L., Campeau L.C., DiRocco D.A., Britton R. Development of a direct photocatalytic C–H fluorination for the preparative synthesis of odanacatib. Org. Lett. 2015;17:5200–5203. doi: 10.1021/acs.orglett.5b02532. [DOI] [PubMed] [Google Scholar]
- Hoornweg D., Bhada-Tata P., Kennedy C. Waste production must peak this century. Nature. 2013;502:615–617. doi: 10.1038/502615a. [DOI] [PubMed] [Google Scholar]
- Isidro-Llobet A., Kenworthy M.N., Mukherjee S., Kopach M.E., Wegner K., Gallou F., Smith A.G., Roschangar F. Sustainability challenges in peptide synthesis and purification: From R&D to production. J. Org. Chem. 2019;84:4615–4628. doi: 10.1021/acs.joc.8b03001. [DOI] [PubMed] [Google Scholar]
- Jiménez-González C., Kim S., Overcash M.R. Methodology of developing gate-to-gate life cycle analysis information. Int. JLCA. 2000;5:153–159. doi: 10.1007/BF02978615. [DOI] [Google Scholar]
- Jiménez-González C., Overcash M.R. Energy sub-modules applied in life cycle inventory of processes. J. Clean. Prod. Process. 2000;2:57–66. doi: 10.1007/s100980050051. [DOI] [Google Scholar]
- Jimenez-Gonzalez C., Ponder C.S., Broxterman Q.B., Manley J.B. Using the right green yardstick: Why process mass intensity is used in the pharmaceutical industry to drive more sustainable processes. Org. Process. Res. Dev. 2011;15:912–917. doi: 10.1021/op200097d. [DOI] [Google Scholar]
- Jiménez-González C., Overcash M.R., Curzons A.D. Treatment modules – A partial life cycle inventory. J. Chem. Technol. Biotechnol. 2001;76:707–716. doi: 10.1002/jctb.426. [DOI] [Google Scholar]
- Kirchherr J., Reike D., Hekkert M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conservation Recycling. 2017;127:221–232. doi: 10.1016/j.resconrec.2017.09.005. [DOI] [Google Scholar]
- Krausmann F., Schandl H., Eisenmenger N., Giljum S., Jackson T. Material flow accounting: Measuring global material use for sustainable development. Annu. Rev. Environ. Res. 2017;42:647–675. doi: 10.1146/annurev-environ-102016-060726. [DOI] [Google Scholar]
- Kümmerer K. Pharmaceuticals in the environment. Ann. Rev. Environ. Res. 2010;35:57–75. doi: 10.1146/annurev-environ-052809-161223. [DOI] [Google Scholar]
- Lipshutz B.H. When does organic chemistry follow nature’s lead and “make the switch”. J. Org. Chem. 2017;82:2806–2816. doi: 10.1021/acs.joc.7b00010. [DOI] [PubMed] [Google Scholar]
- Lipshutz B.H., Ghorai S., Cortes-Clerget M. The hydrophobic effect applied to organic synthesis: Recent synthetic chemistry “in water”. Chem. Eur. J. 2018;24:6672–6695. doi: 10.1002/chem.201705499. [DOI] [PubMed] [Google Scholar]
- Matlin S.A., Mehta G., Hopf H., Krief A. One-world chemistry and systems thinking. Nat. Chem. 2016;8:393–398. doi: 10.1038/nchem.2498. [DOI] [PubMed] [Google Scholar]
- Maertens A., Anastas N., Spencer P.J., Stephens M., Goldberg A., Hartung T. Green toxicology. ALTEX - Alternatives Anim. Experiment. 2014;31:243–249. doi: 10.14573/altex.1406181. [DOI] [PubMed] [Google Scholar]
- Mahaffy P.G., Brush E.J., Haack J.A., Ho F.M. Special issue on reimagining chemistry education: systems thinking, and green and sustainable chemistry. J. Chem. Ed. 2018;95:1689–1691. doi: 10.1021/acs.jchemed.8b00764. [DOI] [Google Scholar]
- Mahaffy P., Matlin S.A., Holme T.A., MacKellar J. Systems thinking for education about the molecular basis of sustainability. Nat. Sust. 2019;2:362–370. doi: 10.1038/s41893-019-0285-3. [DOI] [Google Scholar]
- Murphy M. Early industrial roots of green chemistry and the history of the BHC Ibuprofen process invention and its Quality connection. Found. Chem. 2018;20:121–165. doi: 10.1007/s10698-017-9300-9. [DOI] [Google Scholar]
- Murphy M.A. Early industrial roots of green chemistry - II. International “pollution prevention”efforts during the 1970’s and 1980’s. Substantia. 2020;4:15–57. doi: 10.13128/Substantia-894. [DOI] [Google Scholar]
- National Research Council . The National Academies Press; 2006. Sustainability in the Chemical Industry: Grand Challenges and Research Needs. [DOI] [Google Scholar]
- Noel T., editor. Photochemical Processes in Continuous Flow Reactors: From Engineering Principles to Chemical Applications. World Scientific Publishing; 2017. [Google Scholar]
- Noël T., Cao Y., Laudadio G. The fundamentals behind the use of flow reactors in electrochemistry. Acc. Chem. Res. 2019;52:2858–2869. doi: 10.1021/acs.accounts.9b00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romney D.K., Arnold F.H., Lipshutz B.H., Li C.J. Chemistry takes a bath: Reactions in aqueous media. J. Org. Chem. 2018;83:7319–7322. doi: 10.1021/acs.joc.8b01412. [DOI] [PubMed] [Google Scholar]
- Tanbouza N., Ollevier T., Lam K. Bridging lab and industry with flow electrochemistry. IScience. 2020;23:101720. doi: 10.1016/j.isci.2020.101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A. Study of various solvent recycling and treatment methods in a pharmaceutical manufacturing plant. Intl. J. Pharm. Pract. Pharma. Sci. 2019;1:55–61. [Google Scholar]
- York S., Orgill M. ChEMIST table: a tool for designing or modifying instruction for a systems thinking approach in chemistry education. J. Chem. Edu. 2020;97:2114–2129. doi: 10.1021/acs.jchemed.0c00382. [DOI] [Google Scholar]
- United Nations. 2015. https://sdgs.un.org/goals
- U.S. E.P.A. 2021. https://www.epa.gov/greenchemistry/basics-green-chemistry
- Whitesides G.M. Reinventing chemistry. Angew. Chem. Int. Ed. 2015;54:3196–3209. doi: 10.1002/anie.201410884. [DOI] [PubMed] [Google Scholar]
- World Commission on Environment and Development . Oxford University Press; 1987. Our Common Future; p. 27. ISBN 019282080X. [Google Scholar]