Abstract
Chronic urticaria (CU) is a debilitating skin disease that lasts for more than 6 weeks with wheals and/or angioedema, including chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CIndU). In China, the prevalence of this disease is high, more than 1%, and on the rise. CU has a major impact on the quality of life (QoL) of patients who frequently experience sleep disturbance, depression, and anxiety. Nearly one-third of patients with CSU, in China, are resistant to second-generation H1-antihistamines (sgAHs), even at a fourfold dose (second line; off-label). Omalizumab is approved for the treatment of CSU treatment in Europe and shows remarkable efficacy and safety. In China, regulatory approval for the use of omalizumab is pending, and its use in clinical practice varies widely. Consensus on omalizumab CU treatment in China is urgently needed. The aim of this article is to propose a practical omalizumab treatment algorithm for the management of antihistamine-resistant CSU and CIndU in adults and special population including children and adolescents, and pregnant or breast feeding women, to guide daily clinical practice in China. In the development of this consensus, an expert group including mainly dermatologists, allergists, but also pulmonologists, ENTs, immunologists, and pediatricians in Allergic Disease Prevention and Control Committee, Chinese Preventive Medicine Association, reviewed the existing evidence and developed consensus on the use of omalizumab in CU patients from China. The goal of this consensus is to assist clinicians in making rational decisions in the management of refractory CU with omalizumab. The key clinical questions covered by the treatment algorithm are: 1) Omalizumab treatment routine strategy in both CSU and CIndU patients; 2) Recommended dose and treatment duration for different age stratification; 3) Treatment duration for CU patients with other allergic comorbidities; 4) Recommendation on omalizumab stopping strategy.
Keywords: Chronic spontaneous urticaria, Chronic inducible urticaria, Treatment algorithm, Omalizumab, China
Introduction
Chronic urticaria (CU) is a debilitating skin disease that lasts for more than 6 weeks with wheals and/or angioedema.1 CU can be divided into chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CIndU). CSU, the most frequent type, is usually not linked to external triggers, and autoimmunity rather than type I allergy is held to play a key role. CIndU can be further divided into symptomatic dermographism (SD), cold urticaria (ColdU), cholinergic urticaria (CholU), heat urticaria, solar urticaria, and other subtypes according to the relevant and specific triggers. CU is a global problem and affects people of all ages, with a worldwide prevalence estimated at 0.7%.2 In China, the prevalence of this disease is high, above 1%, and it is increasing.3 A recent study showed that the prevalence of CU among college freshmen in China was 4.2%.4 Most studies have shown that CU mainly affects young adults, with the average age of onset between 25 and 50 years old.5 However, a recent study in South Korea showed that children aged 0–9 and people aged 70–79 are also likely to suffer from CU.6 In addition, CU has a major impact on the quality of life (QoL) of patients, who frequently experience sleep disturbance, sexual dysfunction, depression, and anxiety.7,8 In short, CU comes with a heavy global burden for both patient and society.9,10
The etiology of CU is complex, and increasing evidence shows that IgE-dependent skin mast cell degranulation plays a key role. There are 2 types of autoimmune mechanisms in CSU.11 Type I autoimmune CSU comes with IgE to autoantigens such as thyroid peroxidase and interleukin 24. Patients sensitized to these autoantigens (IgE-mediated autoimmunity/autoallergy) are held to experience whealing and angioedema due to the activation of skin mast cells via IgE. Type IIb autoimmune CSU comes with mast cell-targeting and activating autoantibodies directed against IgE or its high affinity receptor (IgG-autoantibodies to IgE or FcεRI). The pathogenesis of CIndU remains unclear, however, IgE appears to be involved in many patients, and some studies suggest that the characterization of the role and relevance of IgE and mast cell has high priority.12 The role and relevance of IgE and its receptor in the pathogenesis of CU provide a theoretical basis for immune-targeted therapy with anti-IgE.
The EAACI/GA2LEN/EDF/WAO guideline and Chinese Guideline for diagnosis and treatment of urticaria recommend that second-generation H1-antihistamines (sgAHs) are the first line treatment in CU and can be increased up to a fourfold dose (second line; off-label).1,13 However, nearly one-third of CSU patients in China are refractory to sgAHs even at higher doses.14 The use of omalizumab, a recombinant humanized anti-IgE antibody, has been recommended for these antihistamine-resistant patients. Omalizumab provides a remarkable advancement in the management of CU and is not linked to the wide array of side effects of immunosuppressive treatment including ciclosporin A.15,16 The mechanisms of action of omalizumab in CU include its effects on IgE, lowering free IgE levels, and on the high affinity IgE receptor, FcϵRI, lowering its expression on mast cells and basophils. The reduction of FcϵRI levels is held to be the result of lowering IgE levels, as IgE bound to the receptor stabilizes its expression.17,18
Most studies on omalizumab in CU originate from Europe, Canada, South Korea, and the United States, where omalizumab is licensed for CSU in patients 12 years and older and that its use in CIndU and/or in patients younger than 12 years is off label.19 In China, omalizumab has only recently been approved for the treatment of moderate-to-severe asthma in 2017. Omalizumab is still an off-label drug for CU in China. Although several studies showed that Chinese patients with CU generally respond well to omalizumab,16,20,21 most physicians in China rely on their own clinical experience and use the drug in different ways. They are unsure about how to start, adapt, and stop omalizumab therapy with respect to treatment dosing and intervals. Some physicians are reluctant to use omalizumab for lack of guidance. There is, therefore, an urgent need for a national consensus on the use of omalizumab for the treatment of CU. The establishment of such a consensus may also benefit countries that are similar to China in terms of the availability, approval, and reimbursement of CU treatments including omalizumab.
Here, we put forward a practical omalizumab treatment algorithm for the management of antihistamine-resistant CSU and CIndU in adults and special populations including children and adolescents, and pregnant and breast-feeding patients, to guide routine clinical practice in China. The algorithm has been structured so that primary care physicians (who see most CU patients in China) as well as specialists including dermatologists, pediatricians, and allergists can use it. It is intended to support evidence-based treatment guidelines available at both the international and national level.
Methods
We reviewed the literature on omalizumab and CU published in the last 10 years, combined with the results of recent research done inside and outside of China, and formulated the expert consensus described below after a collective discussion among the members of the expert group. The expert group includes mainly dermatologists, allergists, but also pulmonologists, ENTs, immunologists, and pediatricians in Allergic Disease Prevention and Control Committee, Chinese Preventive Medicine Association. Professor Zhao and Professor Maurer developed the initial draft of the practical algorithm for the use of omalizumab in CU patients from China. This was reviewed and modified with the other authors according to relevant expertise, local knowledge, guidelines, and literature.
Clinical evidence for the treatment of antihistamine-resistant chronic urticaria
Assessment of disease activity/severity, impact, and control in patients with chronic urticaria
The use of patient reported outcomes (PROs) is crucial when evaluating and monitoring CU disease activity/severity, QoL impairment, and disease control. All need to be evaluated at the first and every follow- up visit.
We recommend the use of urticaria-specific PRO measures to do so. The Urticaria Activity Score (UAS),22 Urticaria Control Test (UCT),23,24 and Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL)25 should be used in patients who predominantly suffer from whealing. The Angioedema Activity Score (AAS),26,27 Angioedema Quality of Life Questionnaire (AE-QoL),28 and Angioedema Control Test (AECT)29,30 should be used in patients who predominantly suffer from angioedema. The generic, skin-specific QoL questionnaire Dermatology Life Quality Index (DLQI)31 may be used to complement the results of these PRO measures. The Chinese version of UCT, CU-Q2oL and DLQI have also been validated.24,25,31 Other assessment tools are available in English (https://moxie-gmbh.de).
In CIndU, the threshold of the eliciting factor(s) should be determined to assess disease activity and response to treatment. Examples include cold and heat urticaria, where a Peltier element-based provocation device (TempTest®)32 is available, symptomatic dermographism for which dermographometers (eg, FricTest®) have been developed, and delayed pressure urticaria. The assessment routine should follow the consensus recommendations on the definition, diagnostic testing and management of CIndUs.33
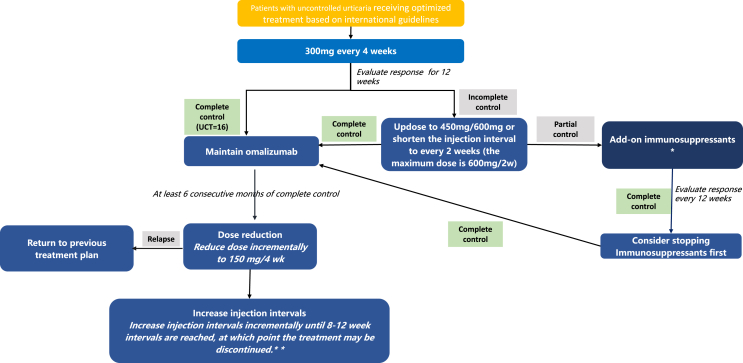
UCT, which works in all types of CU, is the simplest tool with 4 items to assess the control of the disease under the treatment (poor-controlled: UCT <12, well-controlled: UCT≥ 12, complete controlled: UCT = 16) (Fig. 1). In this consensus, we recommend to evaluate the on-treatment response with the UCT and to adjust dosing according to its outcome.
Fig. 1.
Omalizumab Treatment Paradigm and Recommendation on Omalizumab Stopping Strategy. ∗Options include cyclosporine A, methotrexate and cyclophosphamide. A short course (less than 1 week) of glucocorticosteroids may be considered in case of severe exacerbation. CSU: chronic spontaneous urticaria; UCT: urticaria control test. ∗∗ If control is lost (UCT<16) after adjustment of dose or interval, return patient to effective omalizumab dosing and treatment intervals and reevaluate after 3 months of complete control. CSU: chronic spontaneous urticaria; UCT: urticaria control test
Omalizumab in chronic spontaneous urticaria
When to start omalizumab treatment in patients with chronic spontaneous urticaria
Most physicians use sgAHs as the first line treatment in CSU, as recommended. However, many patients continue to experience CSU signs and symptoms despite taking sgAHs, with as many as 60% of patients with CSU not achieving symptom control at approved doses. According to the current EAACI/GA2LEN/EDF/WAO guidelines for CU,1 omalizumab is recommended as the only third-line treatment option in CSU patients refractory to sgAHs. We also recommend to start omalizumab in patients with CSU who failed sgAH treatment (combination 1st- and 2nd-generation H1-antihistamines as well as up to fourfold). Omalizumab is approved as add-on therapy for patients with inadequate response to H1 antihistamine treatment; but real world data show that most patients with complete response to omalizumab do not need to continue their antihistamine treatment. In patients who start omalizumab treatment, we recommend continuing antihistamine treatment until complete response is achieved and to consider stopping the antihistamine treatment once complete response has been reached for more than one month.
The clinical efficacy and safety of omalizumab in CSU has been shown in numerous clinical trials and real-life studies including several from China.16,20,33, 34, 35, 36, 37, 38 A real-world study from the Beijing Urticaria Center of Reference and Excellence (UCARE)39 showed that 75.3% of CU patients achieved complete response with omalizumab therapy, and an additional 14.6% had partial response. Patients experienced substantial improvements of symptoms as early as after 1 month of omalizumab treatment and continued to benefit throughout the entire treatment period.16 The therapeutic effect and speed of onset of effect for omalizumab were comparable between patients with CSU, CIndU, and both.20
How to start omalizumab treatment in patients with chronic spontaneous urticaria
The recommended starting dose of omalizumab in patients with CSU is 300 mg/4 weeks in adult and adolescent patients.1 Responder rates in patients with CSU started on 150 mg omalizumab reportedly range from 15% to 35%, lower than the responder rates of patients started on 300 mg omalizumab.40,41 In addition, dosing at 150 mg/4 weeks was inferior in controlling angioedema episodes and improving QoL.35,42 We recommend to start omalizumab treatment in adult and adolescent patients with CSU at 300 mg every 4 weeks (Table 1).
Table 1.
Recommended dose and treatment duration for different age stratification
| Age, in year | Recommended dose∗(mg) | Treatment duration∗, in month |
|---|---|---|
| 0–3 | 75–150 | 6 |
| 3–6 | 75–150 | 6–12 |
| 6–12 | 150–300 | 6–12 |
| 12–18 | 150–300 | 6–12 |
| 18–65 | 300–600 | 6–12 |
| >65 | 300–600 | 6–12 |
∗It is suggested to adjust it according to clinical conditions.
Recommendation for special age groups.
Pediatric and adolescent population: In clinical studies enrolled children and adolescents, omalizumab is safe and has a similar incidence of adverse events as placebo. Whereas omalizumab is indicated for CSU in population 12 years of age and older both China16,20 and worldwide.36 Omalizumab should be administered with caution in children under 12 years of age, especially in children under 3 years of age.
Elderly: There are limited data available on the use of Omalizumab in patients older than 65 years but there is no evidence that elderly patients require a different dose from younger adult patients
CSU patients with low IgE levels or a positive basophil activation test (BAT) tend to respond slowly and poorly to omalizumab.16,20 Type IIb autoimmune mechanisms with mast cell-targeting and activating autoantibodies directed against IgE or its high affinity receptor may be relevant. Assessing patients for markers of type IIb autoimmune CSU such as low IgE and a positive basophil test can help to predict the outcome of omalizumab treatment.43 If the patient is diagnosed with type IIb autoimmune CSU and CSU is poorly controlled (UCT<12) after 12 weeks of treatment, we recommend updosing omalizumab to or shortening the injection interval to every 2 weeks instead of turning to another therapy immediately.
How to optimize omalizumab treatment in patients with chronic spontaneous urticaria
We recommend to evaluate patients started on omalizumab for their disease activity and control with the aim of optimizing treatment. The aim of treatment with omalizumab is complete control. After 12 weeks of omalizumab treatment a UCT should be performed. If CSU is poorly controlled (UCT<12), we recommend updosing omalizumab to 450 or 600 mg every 4 weeks or shortening the injection interval to every 2 weeks, as higher dosage and shorter injection intervals appear to result in better therapeutic effect in a subgroup of patients.44 The maximum recommended dose is 600 mg every 2 weeks. The decision of increasing the dose or shortening injection interval will depend on provider discretion. In case of partial control (12 < UCT<16), we suggest to evaluate disease control at every visit to guide treatment optimization as needed (Fig. 1). After 24 weeks of treatment, if the patient has still not achieved complete control (UCT<16), add-on immunosuppressants should be considered. In CSU patients treated with omalizumab, the adjustment of dosage should be based on disease activity and disease control status.
Studies show that relapsed patients who underwent retreatment regain symptom control with a second course of treatment in the vast majority of cases indicating that retreatment with omalizumab is an effective therapy option for patients who discontinue omalizumab and previously benefited from this drug.45,46
When and how to stop omalizumab treatment in patients with chronic spontaneous urticaria
Omalizumab is not a curative or disease-modifying treatment in CSU. Therefore, instead of treating patients for a fixed length of time, omalizumab should be used until the disease is gone, until spontaneous remission. To this end, we recommend that CSU patients who achieve complete control are maintained on omalizumab treatment for 6 to 12 months (Table 1) before treatment discontinuation is considered. In patients with completely controlled CSU (UCT = 16) for 6 to 12 months of treatment, we recommend following a stopping strategy, where first the dose is reduced to 150 mg every 4 weeks and then, if complete control is maintained, the dosing interval is incrementally increased to every 8–12 weeks. Patients who experience, during this tapering of omalizumab, loss of complete control of their CSU, should return to effective omalizumab dosing and treatment intervals and be reevaluated after 3 months of complete control (Fig. 1.)
Patients with higher baseline total IgE levels and longer disease duration are more likely to experience relapse after discontinuation of omalizumab treatment.20 In a recent study from China, patients with disease relapse after omalizumab discontinuation had higher pre-treatment total IgE levels and longer disease duration than patients without disease relapse. High pre-treatment IgE levels are also linked to faster relapse in patients who experience relapse upon omalizumab discontinuation.47 Assessing patients for pre-treatment IgE levels may help to guide decisions on when and how omalizumab should be stopped in complete responders.
Most relapse patients who are retreated with omalizumab regain symptom control. We recommend to reinitiate treatment with omalizumab in CSU patients who previously benefited from this drug and show relapse after stopping omalizumab.
Omalizumab in chronic inducible urticaria
When to start omalizumab treatment in patients with chronic inducible urticaria
Nearly half of CIndU patients are resistant to sgAHs up to fourfold doses, and this percentage is even higherwhen angioedema is present.48,49 Omalizumab has been reported as an effective therapy in antihistamine-resistant CIndU, including SD, ColdU, CholU, solar urticaria, heat urticaria, as well as delayed pressure urticaria.50 We recommend to start omalizumab in patients with CIndU who failed sgAHs. In CIndU, the omalizumab treatment plan should be individually designed for each patient based on CIndU type, activity, and control.
How to start omalizumab treatment in patients with chronic inducible urticaria
The recommended starting dose of omalizumab in adult and adolescent patients with CIndU is 300 mg/4 weeks, the same as in CSU. There have been several randomized controlled trials comparing omalizumab and placebo. A randomized controlled trial in ColdU showed that both 150 mg and 300 mg of omalizumab significantly improved the symptoms after 3 subcutaneous injections at intervals of 4 weeks.51,47 Similar efficacy was found in patients with SD and CholU.52,53 Gastaminza et al observed that a progressive improvement in the rate of negative exercise challenge tests from 7.7% at 16th week to 31.3% with extending treatment duration for CholU at 300mg/4 weeks.53 In the real-life study, the response of patients with CholU was significantly better than that of patients with SD at 300mg/4weeks.54 We recommend to start omalizumab treatment in all types of CIndU in adult and adolescent patients at 300 mg every 4 weeks (Table 2).
Table 2.
Recommended dose and treatment duration for adult and adolescent patients with different type of CIndU
| Type of CIndU∗ | Recommended dose | Recommended treatment duration |
|---|---|---|
| Symptomatic dermographism | 300 mg/4 week | At least 12 months, then the dose and treatment duration should be adjusted according to clinical needs |
| Cold urticaria | ||
| Cholinergic urticaria |
∗In clinical studies enrolled patients with CIndU, omalizumab is safe and has a similar incidence of adverse events as placebo. Omalizumab is indicated for symptomatic dermographism, cold urticaria, cholinergic urticaria in China and worldwide.50, 51, 52 Due to limited clinical evidence for delayed pressure urticaria, vibratory angioedema, heat urticaria, contact urticaria or aquagenic urticaria, it is recommended to consider according to the clinical situation. Recommended dose and treatment duration for patients younger than 12 years old with different type of CIndU refer to Table 1. CIndU: chronic inducible urticaria
As of yet, there are no reliable predictors of response to omalizumab treatment in CIndU. A history of atopy and higher baseline total serum IgE were linked to a faster response to omalizumab.54 However, larger multi-centered studies in CIndU patients are needed to explore the factors that affect response to omalizumab.
How to optimize omalizumab treatment in patients with chronic inducible urticaria
We recommend to evaluate patients with CIndU when they begin to use omalizumab for their disease control and on-treatment response with the aim of optimizing treatment. The aim of treatment with omalizumab is complete control. The initial 300mg/4 weeks of omalizumab should be used for 12 weeks.50 After that if CIndU is well-controlled or completely controlled (12 < UCT≤16), we recommend to maintain the previous treatment dose and interval. If CIndU is poorly controlled (UCT<12), We also recommend to adopt same adjustment strategy as CSU to increase the dose of omalizumab or reduce the dosing interval (Fig. 1). There is no preference toward increasing dose or increasing frequency first, and that the decision will depend on provider discretion. As for increasing the dose, we recommend increasing the dose to 450 mg/4w first, if it does not work, continue to increase the dose to 600 mg/4w, and the maximum dose is 600 mg/2w. If the urticaria has still not been complete controlled (UCT<16), add-on immunosuppressants should be considered.
Real-life studies in solar urticaria, ColdU, SD, CholU, delayed pressure urticaria, aquagenic urticaria, and heat urticaria found that rates of patients with well-controlled disease increased when the dose was increased to 450 mg or 600mg/4weeks in non-responders.54, 55, 56 According to the evidence and our clinical experience in CIndU, we recommend that the dose of omalizumab for the 3 most common CIndU, ColdU, CholU, and SD, is 150–600 mg/4 weeks.56 For solar urticaria, we recommend 150mg/4 weeks or higher dose according to clinical efficacy.16,20 Due to limited clinical evidence for delayed pressure urticaria, vibratory angioedema, heat urticaria, contact urticaria, and aquagenic urticaria, we recommend omalizumab dosing and treatment duration be guided by the clinical situation (Table 2).
When and how to stop omalizumab treatment in patients with chronic inducible urticaria
Omalizumab, in CIndU, should be used until the disease is gone, until spontaneous remission. We recommend that the treatment duration of CIndU is at least 12 months. Complete control should be achieved before reducing the dose or prolonging the interval. If patients with completely controlled CIndU (UCT = 16) after 12 months of treatment, we recommend the same stopping strategy as CSU where first the dose is reduced to 150 mg every 4 weeks and then, if complete control is maintained, the dosing interval is incrementally increased to every 8–12 weeks. If patients who experience relapse and cannot achieve complete control of their urticaria during the period of reducing the dose or prolonging the interval, should return to effective omalizumab dosing and treatment intervals and be reevaluated after 3 months of complete control (Fig. 1).
In addition, some evidence has shown that retreatment with omalizumab can also provide a rapid and significant improvement in CSU and CIndU patients who previously benefited and experienced relapse after discontinuation of treatment.45
Omalizumab in special populations
Omalizumab in children younger than 12 years old with chronic urticaria
Few data are available on the epidemiology of CU in children. In Europe, the prevalence of CU and CSU in children was reported to be 1.4% and 0.8%, respectively.57 In pediatric populations, remission of CSU at 5 years from onset occurs in 38%–72% of patients.58 CU in childhood is disabling and has a great impact on children's physical and psychological state, impairs their QoL, and affects performance at school.1
According to the EAACI/GA2LEN/EDF/WAO guidelines for CU,1 a second-generation H1-antihistamine at approved dose is the first-line treatment for CU, and updosing to up to 4 times the approved dose is the second-line treatment. However, many pediatric CU patients are resistant to antihistaminic treatment.59 In a recent study, only 25 of 66 (38%) children with CU treated with a standard-dosed sgAH responded.60 Omalizumab is licensed for children with asthma 6 years or older at higher doses than in CSU, which also suggests that omalizumab is generally safe for the use for children. Based on the available evidence, although small, and our clinical experience, we suggest that omalizumab may be an effective and safe choice for children with antihistamine-refractory CU.21,61, 62, 63
We conducted a retrospective, observational study in Chinese patients under 16 years old with CU to investigate the efficacy and safety of omalizumab. Patients (n = 12) were treated with 150 or 300 mg of omalizumab every 4 weeks, and all of them including a 1-year-old child achieved well or completely controlled urticaria with the first four months of treatment.21 In addition, a literature review of reports on omalizumab treatment in CU patients younger than 12 years old with 16 cases demonstrated that a 150- to 300-mg monthly dose of omalizumab achieved a complete response in 81% and a partial response in 19%.61 Passanisi and coworkers reported on 6 pediatric CU patients treated with omalizumab, 2 of them younger than 12 years when started on omalizumab, had a clinically meaningful response, and added 4 cases with complete response to omalizumab on the basis of the former review.62 A multi-center retrospective case series including 19 patients with CSU aged between 6 and 16.9 years, 9 of them <12 years old, also showed a response rate of 84% with omalizumab treatment.63
Up to now, although off-label, no adverse events have been reported in children with CSU treated with omalizumab. In summary, for the children younger than 3 years old, we recommend to treat with 75–150 mg/4 weeks for 6 months. The patients with age between 3 and 6 years old, we recommend to treat with 75–150 mg/4 weeks for 6 to 12 months. For patients younger than 12 years old but older that 6 years old, we recommend that to treat with 150–300 mg/4 weeks for 6 to 12 months (Table 1).
Omalizumab use in pregnant and lactating women
Based on previous evidence64, 65, 66, 67, 68 and our clinical experience with using omalizumab in China, patients with CU who want to become pregnant, are pregnant, and are breastfeeding can be treated with omalizumab, weighing the benefits against possible risks.68
In addition to a large body of data on omalizumab treatment in pregnant and breast-feeding asthma patients, there are some reports on the use of omalizumab in pregnant or breastfeeding CU patients. In total, 11 CU patients receiving omalizumab during 13 pregnancies have been reported.64, 65, 66, 67, 68 Updosing of omalizumab during pregnancy was only reported in one previous CU patient,69 who was the first CU patient ever reported to receive omalizumab during pregnancy, disease activity decreased markedly with omalizumab 150 mg/4 weeks, and she achieved complete remission when intervals were shortened to every 2 weeks. All omalizumab treated CU patients gave birth to healthy children and no adverse effects have been reported. There were also no abnormalities observed in CU patients during lactation in the 6 breastfed children.64, 65, 66, 67, 68 We reported two cases of pregnant women with CSU, both achieved complete controlled urticaria and did not have any adverse events.
Of note, omalizumab has been assigned pregnancy category-B risk status by the Food and Drug Administration (FDA)70 and China Food and Drug Administration (CFDA).71 Omalizumab is expected to cross the placental barrier and be present in human milk in small amounts.72 In animal reproductive studies, no evidence of maternal toxicity, embryotoxicity, or teratogenicity was observed in Cynomolgus monkeys with subcutaneous doses of omalizumab up to approximately 10 times the maximum recommended human dose (MRHD) throughout the period of organogenesis. A large body of data on safety of omalizumab treatment in pregnant and breast-feeding asthma patients have been reported. In the EXPECT registry, a prospective cohort of pregnancy with asthma, this study compared 250 pregnant patients with an age-adjusted frequencies in a disease (asthma) matched external cohort of 1153 pregnant women without exposure to omalizumab, showed no increase in the rate of major birth defects or miscarriage.73 In the cohort pregnancy exposure registry study of patients with asthma, compared with infants who were not breastfed, or infants who were breastfed without exposure to omalizumab, adverse events such as “infections and infestations” were not significantly increased in breastfed infants who were exposed to omalizumab.72 An increased rate of low birth weight was observed among registry infants, but difficult to determine whether it was due to omalizumab exposure or the disease severity.
In summary, if clinically needed, we recommend that the use of omalizumab may be considered during pregnancy and lactation (Table 1).
Omalizumab in patients with multiple comorbid allergic diseases
Allergic diseases are often considered as a series of systemic disease which contain atopic dermatitis, asthma, allergic rhinitis, food allergy, allergic conjunctivitis, and so on. It is gradually accepted that these allergic diseases have common characteristics in the pathogenesis and may be different manifestations of atopic patients at different stages.74,75 Similar to CSU and CIndU, patients with multiple comorbid allergic diseases may benefit from omalizumab. Omalizumab is a recombinant humanized monoclonal antibody which inhibits the binding of IgE to the high-affinity IgE receptor (FcεRI). This treatment approach also applies to other IgE-mediated diseases.
So far, omalizumab already received approval for treatment of chronic rhinosinusitis with nasal polyps (CRSwNP) by European Medicines Agency (EMA) and US Food & Drug Administration (FDA),76 as well as severe seasonal allergic rhinitis in Japan.77 For CSU patients with CRSwNP, it is recommended to follow the asthma medication table at a dose of 300 mg or higher for at least 6 months, and then adjust the dose and treatment duration according to clinical need.78 For patients with CSU and allergic rhinitis, a dose of 300 mg or higher is recommended according to the asthma dosing table. The on-treatment response should be evaluated after 16 weeks. If the patient shows a good response, the treatment can continue for a year, after which re-evaluation should be considered.
In 2018, FDA granted Breakthrough Therapy Designation for omalizumab for the prevention of severe allergic reaction following accidental exposure to one or more foods in people with allergies.76 An on-going phase III study is exploring omalizumab alone or in combination with multi-allergen oral immunotherapy helping people with multiple food allergies to consume foods without dose-limiting symptoms.77 For patients with CSU and food allergies, we recommend considering a dose of 300 mg or higher according to the asthma dose table. Efficacy should be evaluated after 16 to 20 weeks. If patients respond well to the treatment, omalizumab can be continued for up to 24 weeks (Table 3).
Table 3.
Recommended dose and treatment duration for patients with other allergic comorbidities
| CSU with other allergic comorbidities | Recommended dose | Recommended treatment duration |
|---|---|---|
| CSU with CRSwNP76,78 | 300 mg or higher dose according to asthma dosing table | At least 6 months, then the dose and treatment duration should be adjusted according to clinical needs |
| CSU with food allergy77∗ | 300 mg or higher dose according to asthma dosing table | For food allergy: evaluate response after 16 or 20 weeks treatment, continue to 24 weeks if good response |
| CSU with ABPA79,80∗ | 375 mg/2 weeks | At least 6 months, then the dose and treatment duration should be adjusted according to clinical needs |
| CSU with allergic asthma81 | 300 mg or higher dose according to asthma dosing table | Evaluate response after 16 weeks, At least 12 months, then the dose and treatment duration should be adjusted according to clinical needs |
| CSU with allergic rhinitis81 | 300 mg or higher dose according to asthma dosing table | Evaluate response after 16 weeks, re-evaluate after 1-year treatment if good response |
| CSU with AD82∗ | 150–450mg/2 week | Refer to Fig. 1 for stopping strategy |
∗Limited clinical evidence, it is recommended to base treatment decision on the clinical situation. In cases where both the dose strength and frequency differ, result in the higher monthly dose is recommended.
CSU: chronic spontaneous urticaria; CRSwNP: chronic rhinosinusitis with nasal polyps; ABPA: allergic bronchopulmonary aspergillosis; AD: atopic dermatitis
Four patients with allergic bronchopulmonary aspergillosis (ABPA) were treated with 375mg/2 week omalizumab for 1 year, an improvement in asthma control test symptom scores for both daytime and nighttime were found.79 Different studies used various dosages of omalizumab ranging from 225 mg to 750 mg according to weight and serum IgE level, and dosing frequency ranged from once per week to once monthly while the most commonly used dose was 375 mg every 2 weeks.80 The maximum recommended dose of 600 mg every 2 weeks for patient with a higher total IgE beyond the higher limit of 1500 IU/mL. For patients with ABPA and CSU, we recommend that patients can be treated with 375 mg every 2 weeks for at least 6 months, then the dose and treatment duration should be adjusted according to clinical needs (Table 3).
Researches on omalizumab for the treatment of patients with atopic dermatitis are rare. A systematic review including 15 studies showed that 43% of the patients with atopic dermatitis (AD) could achieve remarkable clinical response after omalizumab treatment.81 The dosing regimens varied from 150 to 900 mg/month in the included studies. Serum IgE concentrations of less than 700 IU/mL may be more favorable clinical responses.81 The pathogenesis of the diseases is remain unclear. Omalizumab has a higher therapeutic effect for those IgE-mediated diseases, while the pathogenesis of atopic dermatitis is otherwise complex, and IgE does not necessarily play a decisive role in pathogenicity. According to previous evidence and our clinical experience, we recommend that patient with CSU and AD can be treated with 150–450mg/2 weeks (Table 3). The stopping strategy is similar to CSU (Fig. 1).
In conclusion, patients with multiple comorbid allergic diseases may benefit from omalizumab treatment according to limited clinical evidence. We recommend to consider the higher dosage according to the clinical situation for CU patients (both CSU and CIndU) with other comorbid allergic diseases (Table 3).
Other special populations
There have been no studies on the effect of impaired renal or hepatic function on the pharmacokinetics of omalizumab. Because omalizumab clearance at clinical doses is dominated by the reticuloendothelial system, it is unlikely to be altered by renal or hepatic impairment. While no dose adjustment is recommended for these patients, omalizumab should be administered with caution. No association between omalizumab and increase risk of malignancy has been demonstrated, Omalizumab can be used for the treatment of CSU in patients with comorbid malignancy.
Omalizumab treatment algorithm for antihistamine-resistant CU in China
The present consensus, as agreed by all authors, provides an omalizumab treatment algorithm for patients with antihistamine-resistant CU in China (Fig. 1). This algorithm follows a stepwise approach, brings on board other treatments as needed, and applies for adults and special populations of CSU patients including children, adolescents, and pregnant and breast-feeding women (Fig. 1, Table 1, Table 2, Table 3).
Conclusions
CU is a major health issue, globally and in China. The risk/benefit profile of omalizumab supports its use in CU patients who do not achieve complete control with antisthistamine treatment. Omalizumab is a well established, licensed and reimbursed treatment in many countries outside of China. Similar to other countries where omalizumab is available but off label and paid for by patients, Chinese physicians who treat patients with CU are in need for guidance on when, how, and for how long to use omalizumab in this setting. This consensus document aims to provide this guidance. We encourage all urticarialogists in China and in countries with similar settings to apply our recommendations in their routine clinical management of CU, report on the outcomes and help to improve them moving forward.
Abbreviations
CU: chronic urticaria; CSU: chronic spontaneous urticaria; CIndU: chronic inducible urticaria; QoL: quality of life; sgAHs:second-generation H1-antihistamines; SD:symptomatic dermographism; ColdU:cold urticaria; CholU: cholinergic urticaria; UCARE: Urticaria Center of Reference and Excellence; UAS: Urticaria Activity Score; UCT: Urticaria Control Test; CU-Q2oL: Chronic Urticaria Quality of Life Questionnaire; AAS: The Angioedema Activity Score; AE-QoL: Angioedema Quality of Life Questionnaire; AECT: Angioedema Control Test; DLQI: Dermatology Life Quality Index; CRSwNP: chronic rhinosinusitis with nasal polyps; EMA: European Medicines Agency; FDA: Food & Drug Administration; ABPA: allergic bronchopulmonary aspergillosis; AD: Atopic Dermatitis; CRSwNP: chronic rhinosinusitis with nasal polyps; ABPA: allergic bronchopulmonary aspergillosis.
Authors' contributions
Zuo-tao Zhao and Marcus Maurer have made substantial contributions to conception and study design; reviewed the article critically for important intellectual content. The other authors have drafted the manuscript. All authors were involved in the development of this consensus and final approval of the manuscript.
Funding
None.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics statement
The authors declare that this manuscript complies with the ethics in publishing guidelines. The ethics approval and consent to participate is not applicable here.
Submission declaration
We confirm that the manuscript is original, has not been published before, is not currently being considered for publication elsewhere. All authors agree to the publication.
Declaration of competing interest
ZT Zhao has participated as Principal Investigator in clinical trials sponsored by Novartis, Pfizer. He has received consultancy/speaker honoraria from Novartis, Pfizer, Astellas, Galderma, Janssen, GSK, BAYER, LEO, MEDA Pharma (a Mylan Company), and has acted as scientific Advisory Board member for Pfizer, Novartis, Astellas, LEO, MEDA Pharma (a Mylan Company).
XH Gao has received consultancy/speaker honoraria from Novartis, Pfizer, Astellas, Galderma, Janssen, Sinofi, GSK, LEO, Lilly, MEDA Pharma (a Mylan Company), and has acted as scientific Advisory Board member for Novartis, Lilly, Sinofi, MEDA Pharma (a Mylan Company).
YS Guo has received consultancy/speaker honoraria from Novartis, Janssen, BAYER, AstraZeneca, Sanofi/Regeneron, ALK-Abello, Allergopharma, Takeda Pharmaceutical, MSD and Thermo Fisher Scientific.
H Long has received consultancy or speaker honoraria from Novartis, AbbVie, Lilly, Janssen, Astellas, GSK, BAYER, LEO, Menarini, Sanofi, BrightFuture, and CMS.
MM is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Celldex, Centogene, CSL Behring, FAES, Genentech, GIInnovation, Gilead, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Roche, Sanofi/Regeneron, Third HarmonicBio, UCB, and Uriach.
The rest of the authors declare that they have no relevant conflicts of interest.
Acknowledgements
We thank Miao Yu and Xiaoting Song for expert help with formatting and revising the manuscript as well as its submission. We thank Jia Yu for her help in establishing communication between all authors.
Footnotes
Full list of author information is available at the end of the article. http://doi.org/10.1016/j.waojou.2021.100610
Contributor Information
Zuotao Zhao, Email: zhaozuotaotao@163.com.
Marcus Maurer, Email: marcus.maurer@charite.de.
References
- 1.Zuberbier T., Aberer W., Asero R., et al. The eaaci/ga2len/edf/wao guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 2.Fricke J., Ávila G., Keller T., et al. Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta-analysis. Allergy. 2020;75(2):423–432. doi: 10.1111/all.14037. [DOI] [PubMed] [Google Scholar]
- 3.Chung M.C., Symons C., Gilliam J., et al. Stress, psychiatric co-morbidity and coping in patients with chronic idiopathic urticaria. Psychol Health. 2010;25(4):477–490. doi: 10.1080/08870440802530780. [DOI] [PubMed] [Google Scholar]
- 4.Xiao Y., Huang X., Jing D., et al. The prevalence of atopic dermatitis and chronic spontaneous urticaria are associated with parental socioeconomic status in adolescents in China. Acta Derm Venereol. 2019;99(3):321–326. doi: 10.2340/00015555-3104. [DOI] [PubMed] [Google Scholar]
- 5.Maurer M., Costa C., Gimenez Arnau A., et al. Antihistamine-resistant chronic spontaneous urticaria remains undertreated: 2-year data from the aware study. Clin Exp Allergy. 2020;50(10):1166–1175. doi: 10.1111/cea.13716. [DOI] [PubMed] [Google Scholar]
- 6.Kim B.R., Yang S., Choi J.W., et al. Epidemiology and comorbidities of patients with chronic urticaria in korea: a nationwide population-based study. J Dermatol. 2018;45(1):10–16. doi: 10.1111/1346-8138.14075. [DOI] [PubMed] [Google Scholar]
- 7.Zhong H., Song Z., Chen W., et al. Chronic urticaria in Chinese population: a hospital-based multicenter epidemiological study. Allergy. 2014;69(3):359–364. doi: 10.1111/all.12338. [DOI] [PubMed] [Google Scholar]
- 8.Gonçalo M., Gimenéz-Arnau A., Al-Ahmad M., et al. The global burden of chronic urticaria for the patient and society. Br J Dermatol. 2021;184(2):226–236. doi: 10.1111/bjd.19561. [DOI] [PubMed] [Google Scholar]
- 9.Ertaş R., Erol K., Hawro T., et al. Sexual functioning is frequently and markedly impaired in female patients with chronic spontaneous urticaria. J Allergy Clin Immunol Pract. 2020;8(3):1074–1082. doi: 10.1016/j.jaip.2019.10.046. [DOI] [PubMed] [Google Scholar]
- 10.Dong W., An J., Geng P., et al. Years lost due to disability from skin diseases in China 1990-2017: findings from the global burden of disease study 2017. Br J Dermatol. 2020;182(1):248–250. doi: 10.1111/bjd.18329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolkhir P., Church M.K., Weller K., et al. Autoimmune chronic spontaneous urticaria: what we know and what we do not know. J Allergy Clin Immunol. 2017;139(6):1772–1781. doi: 10.1016/j.jaci.2016.08.050. e1771. [DOI] [PubMed] [Google Scholar]
- 12.Gruber B.L., Baeza M.L., Marchese M.J., et al. Prevalence and functional role of anti-ige autoantibodies in urticarial syndromes. J Invest Dermatol. 1988;90(2):213–217. doi: 10.1111/1523-1747.ep12462239. [DOI] [PubMed] [Google Scholar]
- 13.Urticaria Research Center soDaV, Chinese Medical Association Guideline for diagnosis and treatment of urticaria in China (2018) Chin J Dermatol. 2019;52(1):1–5. doi: 10.3760/cma.j.issn.0412-4030.2019.01.001. [DOI] [Google Scholar]
- 14.Zhang L., Wu J., Qi Y., et al. Long-term combinations and updosing of second-generation h(1)-antihistamines show efficacy and safety in the treatment of chronic spontaneous urticaria: a multicenter real-life pilot study. J Allergy Clin Immunol Pract. 2020;8(5):1733–1736. doi: 10.1016/j.jaip.2019.12.006. e1711. [DOI] [PubMed] [Google Scholar]
- 15.Carrillo D.C., Borges M.S., García E., et al. Omalizumab vs. Placebo in the management of chronic idiopathic urticaria: a systematic review. World Allergy Organ J. 2014;7(1):72. doi: 10.1186/s40413-014-0050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y., Yu M., Huang X., et al. Omalizumab treatment and outcomes in Chinese patients with chronic spontaneous urticaria, chronic inducible urticaria, or both. World Allergy Organ J. 2021;14(1):100501. doi: 10.1016/j.waojou.2020.100501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Yd H.Y., Sui H.J., Geng P., Tu P., Zhao Z.T. The use of anti-ige monoclonal antibody in treatment of allergic diseases. Chin J Allergy Clin Immunol. 2018;12(3):43–48. [Google Scholar]
- 18.Chen Y.d. G.P., Zhao J.H., Tu P., Zhao Z.T. Chronic spontaneous urticaria: therapeutic mechanism of omalizumab and assessment of its clinical efficacy. Chin J Dermatol. 2019;52(9):652–655. [Google Scholar]
- 19.Bernstein J.A., Kavati A., Tharp M.D., et al. Effectiveness of omalizumab in adolescent and adult patients with chronic idiopathic/spontaneous urticaria: a systematic review of 'real-world' evidence. Expet Opin Biol Ther. 2018;18(4):425–448. doi: 10.1080/14712598.2018.1438406. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y.D., Maurer M., Yu M., et al. Addition of omalizumab to antihistamine treatment in chronic urticaria: a real-world study in China. Ann Allergy Asthma Immunol. 2020;125(2):217–219. doi: 10.1016/j.anai.2020.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Song X.T., Chen Y.D., Yu M., et al. Omalizumab in children and adolescents with chronic urticaria: a 16-week real-world study. Allergy. 2021;76(4):1271–1273. doi: 10.1111/all.14686. [DOI] [PubMed] [Google Scholar]
- 22.Młynek A., Zalewska-Janowska A., Martus P., et al. How to assess disease activity in patients with chronic urticaria? J Allergy. 2008;63(6):777–780. doi: 10.1111/j.1398-9995.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 23.Weller K., Groffik A., Church M.K., et al. Development and validation of the urticaria control test: a patient-reported outcome instrument for assessing urticaria control. J Allergy Clin Immunol. 2014;133(5):1365–1372. doi: 10.1016/j.jaci.2013.12.1076. 1372.e1361-1366. [DOI] [PubMed] [Google Scholar]
- 24.Yu M.C.Y., Liu B., Song X.T., Zhao Z.T. The Chinese version of the urticaria control test and validation of its reliability and validity. Chin J Dermatol. 2020;53(7):533–538. [Google Scholar]
- 25.Yu M.C.Y., Liu B., Song X.T., Zhao Z.T. The Chinese version of chronic urticaria quality of life questionnaire (cu-q2ol) : validation of reliability and validity. Chin J Dermatol. 2020;53(12):992–997. [Google Scholar]
- 26.Weller K., Groffik A., Magerl M., et al. Development, validation, and initial results of the angioedema activity score. Allergy. 2013;68(9):1185–1192. doi: 10.1111/all.12209. [DOI] [PubMed] [Google Scholar]
- 27.Kulthanan K., Chularojanamontri L., Rujitharanawong C., et al. Angioedema activity score (aas): a valid and reliable tool to use in asian patients. BioMed Res Int. 2019;2019:9157895. doi: 10.1155/2019/9157895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weller K., Magerl M., Peveling-Oberhag A., et al. The angioedema quality of life questionnaire (ae-qol) - assessment of sensitivity to change and minimal clinically important difference. Allergy. 2016;71(8):1203–1209. doi: 10.1111/all.12900. [DOI] [PubMed] [Google Scholar]
- 29.Weller K., Donoso T., Magerl M., et al. Development of the angioedema control test-a patient-reported outcome measure that assesses disease control in patients with recurrent angioedema. Allergy. 2020;75(5):1165–1177. doi: 10.1111/all.14144. [DOI] [PubMed] [Google Scholar]
- 30.Weller K., Donoso T., Magerl M., et al. Validation of the angioedema control test (aect)-a patient-reported outcome instrument for assessing angioedema control. J Allergy Clin Immunol Pract. 2020;8(6):2050–2057. doi: 10.1016/j.jaip.2020.02.038. e2054. [DOI] [PubMed] [Google Scholar]
- 31.Liu J.B., Yao M.Z., Si A.L., et al. Life quality of Chinese patients with chronic urticaria as assessed by the dermatology life quality index. J Eur Acad Dermatol Venereol. 2012;26(10):1252–1257. doi: 10.1111/j.1468-3083.2011.04277.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y.d. L.B., Song X.T., Zhao Z.T. Clinical application of critical temperature threshold measurement in cold contact urticaria. Chin J Dermatol. 2020;53(5):352–355. [Google Scholar]
- 33.Magerl M., Altrichter S., Borzova E., et al. The definition, diagnostic testing, and management of chronic inducible urticarias - the eaaci/ga(2) len/edf/unev consensus recommendations 2016 update and revision. Allergy. 2016;71(6):780–802. doi: 10.1111/all.12884. [DOI] [PubMed] [Google Scholar]
- 34.Saini S.S., Bindslev-Jensen C., Maurer M., et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on h1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135(3):925. doi: 10.1038/jid.2014.512. [DOI] [PubMed] [Google Scholar]
- 35.Maurer M., Rosén K., Hsieh H.J., et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368(10):924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Z.T., Ji C.M., Yu W.J., et al. Omalizumab for the treatment of chronic spontaneous urticaria: a meta-analysis of randomized clinical trials. J Allergy Clin Immunol. 2016;137(6):1742–1750. doi: 10.1016/j.jaci.2015.12.1342. e1744. [DOI] [PubMed] [Google Scholar]
- 37.Vadasz Z., Tal Y., Rotem M., et al. Omalizumab for severe chronic spontaneous urticaria: real-life experiences of 280 patients. J Allergy Clin Immunol Pract. 2017;5(6):1743–1745. doi: 10.1016/j.jaip.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 38.Cherrez-Ojeda I., Maurer M., Bernstein J.A., et al. Learnings from real-life experience of using omalizumab for chronic urticaria in Latin america. World Allergy Organ J. 2019;12(2):100011. doi: 10.1016/j.waojou.2019.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maurer M., Metz M., Bindslev-Jensen C., et al. Definition, aims, and implementation of ga(2) len urticaria centers of reference and excellence. Allergy. 2016;71(8):1210–1218. doi: 10.1111/all.12901. [DOI] [PubMed] [Google Scholar]
- 40.Larenas-Linnemann D.E.S., Parisi C.A.S., Ritchie C., et al. Update on omalizumab for urticaria: what's new in the literature from mechanisms to clinic. Curr Allergy Asthma Rep. 2018;18(5):33. doi: 10.1007/s11882-018-0787-5. [DOI] [PubMed] [Google Scholar]
- 41.Sussman G., Hébert J., Gulliver W., et al. Omalizumab re-treatment and step-up in patients with chronic spontaneous urticaria: optima trial. J Allergy Clin Immunol Pract. 2020;8(7):2372–2378. doi: 10.1016/j.jaip.2020.03.022. e2375. [DOI] [PubMed] [Google Scholar]
- 42.Casale T.B., Bernstein J.A., Maurer M., et al. Similar efficacy with omalizumab in chronic idiopathic/spontaneous urticaria despite different background therapy. J Allergy Clin Immunol Pract. 2015;3(5):743–750. doi: 10.1016/j.jaip.2015.04.015. e741. [DOI] [PubMed] [Google Scholar]
- 43.Schoepke N., Asero R., Ellrich A., et al. Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria: results of the purist study. Allergy. 2019;74(12):2427–2436. doi: 10.1111/all.13949. [DOI] [PubMed] [Google Scholar]
- 44.Salman A., Comert E. The real-life effectiveness and safety of omalizumab updosing in patients with chronic spontaneous urticaria. J Cutan Med Surg. 2019;23(5):496–500. doi: 10.1177/1203475419847956. [DOI] [PubMed] [Google Scholar]
- 45.Metz M., Ohanyan T., Church M.K., et al. Retreatment with omalizumab results in rapid remission in chronic spontaneous and inducible urticaria. JAMA Dermatol. 2014;150(3):288–290. doi: 10.1001/jamadermatol.2013.8705. [DOI] [PubMed] [Google Scholar]
- 46.Türk M., Yılmaz İ., Bahçecioğlu S.N. Treatment and retreatment with omalizumab in chronic spontaneous urticaria: real life experience with twenty-five patients. Allergol Int. 2018;67(1):85–89. doi: 10.1016/j.alit.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Ertas R., Ozyurt K., Ozlu E., et al. Increased ige levels are linked to faster relapse in patients with omalizumab-discontinued chronic spontaneous urticaria. J Allergy Clin Immunol. 2017;140(6):1749–1751. doi: 10.1016/j.jaci.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Mellerowicz E., Weller K., Zuberbier T., et al. Real-life treatment of patients with cholinergic urticaria in German-speaking countries. J Dtsch Dermatol Ges. 2019;17(11):1141–1147. doi: 10.1111/ddg.13979. [DOI] [PubMed] [Google Scholar]
- 49.Kocatürk E., Can P.K., Akbas P.E., et al. Management of chronic inducible urticaria according to the guidelines: a prospective controlled study. J Dermatol Sci. 2017;87(1):60–69. doi: 10.1016/j.jdermsci.2017.02.283. [DOI] [PubMed] [Google Scholar]
- 50.Maurer M., Metz M., Brehler R., et al. Omalizumab treatment in patients with chronic inducible urticaria: a systematic review of published evidence. J Allergy Clin Immunol. 2018;141(2):638–649. doi: 10.1016/j.jaci.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 51.Metz M., Schütz A., Weller K., et al. Omalizumab is effective in cold urticaria-results of a randomized placebo-controlled trial. J Allergy Clin Immunol. 2017;140(3):864–867. doi: 10.1016/j.jaci.2017.01.043. e865. [DOI] [PubMed] [Google Scholar]
- 52.Maurer M., Schütz A., Weller K., et al. Omalizumab is effective in symptomatic dermographism-results of a randomized placebo-controlled trial. J Allergy Clin Immunol. 2017;140(3):870–873. doi: 10.1016/j.jaci.2017.01.042. e875. [DOI] [PubMed] [Google Scholar]
- 53.Gastaminza G., Azofra J., Nunez-Cordoba J.M., et al. Efficacy and safety of omalizumab (xolair) for cholinergic urticaria in patients unresponsive to a double dose of antihistamines: a randomized mixed double-blind and open-label placebo-controlled clinical trial. J Allergy Clin Immunol Pract. 2019;7(5):1599–1609. doi: 10.1016/j.jaip.2018.12.025. e1591. [DOI] [PubMed] [Google Scholar]
- 54.Exposito-Serrano V., Curto-Barredo L., Aguilera Peiro P., et al. Omalizumab for the treatment of chronic inducible urticaria in 80 patients. Br J Dermatol. 2021;184(1):167–168. doi: 10.1111/bjd.19425. [DOI] [PubMed] [Google Scholar]
- 55.Proceedings of the canadian society of allergy and clinical immunology annual scientific meeting 2020. Allergy Asthma Clin Immunol. 2021;17(Suppl 1):33. doi: 10.1186/s13223-021-00519-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu M., Terhorst-Molawi D., Altrichter S., et al. Omalizumab in chronic inducible urticaria: a real-life study of efficacy, safety, predictors of treatment outcome and time to response. Clin Exp Allergy. 2021;51(5):730–734. doi: 10.1111/cea.13838. [DOI] [PubMed] [Google Scholar]
- 57.Balp M.M., Weller K., Carboni V., et al. Prevalence and clinical characteristics of chronic spontaneous urticaria in pediatric patients. Pediatr Allergy Immunol. 2018;29(6):630–636. doi: 10.1111/pai.12910. [DOI] [PubMed] [Google Scholar]
- 58.Caffarelli C., Paravati F., El Hachem M., et al. Management of chronic urticaria in children: a clinical guideline. Ital J Pediatr. 2019;45(1):101. doi: 10.1186/s13052-019-0695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doshi D.R., Weinberger M.M. Experience with cyclosporine in children with chronic idiopathic urticaria. Pediatr Dermatol. 2009;26(4):409–413. doi: 10.1111/j.1525-1470.2009.00869.x. [DOI] [PubMed] [Google Scholar]
- 60.Sarti L., Barni S., Giovannini M., et al. Efficacy and tolerability of the updosing of second-generation non-sedating h1 antihistamines in children with chronic spontaneous urticaria. Pediatr Allergy Immunol. 2021;32(1):153–160. doi: 10.1111/pai.13325. [DOI] [PubMed] [Google Scholar]
- 61.Al-Shaikhly T., Rosenthal J.A., Ayars A.G., et al. Omalizumab for chronic urticaria in children younger than 12 years. Ann Allergy Asthma Immunol. 2019;123(2):208–210. doi: 10.1016/j.anai.2019.05.003. e202. [DOI] [PubMed] [Google Scholar]
- 62.Passanisi S., Arasi S., Caminiti L., et al. Omalizumab in children and adolescents with chronic spontaneous urticaria: case series and review of the literature. Dermatol Ther. 2020;33(4) doi: 10.1111/dth.13489. e13489. [DOI] [PubMed] [Google Scholar]
- 63.Ari A., Levy Y., Segal N., et al. Efficacy of omalizumab treatment for pediatric chronic spontaneous urticaria: a multi-center retrospective case series. Pediatr Dermatol. 2020;37(6):1051–1054. doi: 10.1111/pde.14360. [DOI] [PubMed] [Google Scholar]
- 64.Ensina L.F., Cusato-Ensina A.P., Camelo-Nunes I.C., et al. Omalizumab as third-line therapy for urticaria during pregnancy. J Investig Allergol Clin Immunol. 2017;27(5):326–327. doi: 10.18176/jiaci.0179. [DOI] [PubMed] [Google Scholar]
- 65.Saito J., Yakuwa N., Sandaiji N., et al. Omalizumab concentrations in pregnancy and lactation: a case study. J Allergy Clin Immunol Pract. 2020;8(10):3603–3604. doi: 10.1016/j.jaip.2020.05.054. [DOI] [PubMed] [Google Scholar]
- 66.González-Medina M., Curto-Barredo L., Labrador-Horrillo M., et al. Omalizumab use during pregnancy for chronic spontaneous urticaria (csu): report of two cases. J Eur Acad Dermatol Venereol. 2017;31(5):e245–e246. doi: 10.1111/jdv.14034. [DOI] [PubMed] [Google Scholar]
- 67.Cuervo-Pardo L., Barcena-Blanch M., Radojicic C. Omalizumab use during pregnancy for ciu: a tertiary care experience. Eur Ann Allergy Clin Immunol. 2016;48(4):145–146. [PubMed] [Google Scholar]
- 68.Ghazanfar M.N., Thomsen S.F. Successful and safe treatment of chronic spontaneous urticaria with omalizumab in a woman during two consecutive pregnancies. Case Rep Med. 2015;2015:368053. doi: 10.1155/2015/368053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Türk M., Carneiro-Leão L., Kolkhir P., et al. How to treat patients with chronic spontaneous urticaria with omalizumab: questions and answers. J Allergy Clin Immunol Pract. 2020;8(1):113–124. doi: 10.1016/j.jaip.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 70.Us fda xolair (omalizumab): safety information.[J]. Accessed 05.01, 2019.doi:https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/103976s5234lbl.pdf.
- 71.Xolair label[J]. updated 07/06/2016.doi:http://101.110.118.69/www.accessdata.fda.gov/drugsatfda_docs/label/2016/103976s5225lbl.pdf.
- 72.Namazy J., Cabana M.D., Scheuerle A.E., et al. The xolair pregnancy registry (expect): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol. 2015;135(2):407–412. doi: 10.1016/j.jaci.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 73.Namazy J.A., Blais L., Andrews E.B., et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J Allergy Clin Immunol. 2020;145(2):528–536. doi: 10.1016/j.jaci.2019.05.019. e521. [DOI] [PubMed] [Google Scholar]
- 74.Torres T., Ferreira E.O., Gonçalo M., et al. Update on atopic dermatitis. Acta Med Port. 2019;32(9):606–613. doi: 10.20344/amp.11963. [DOI] [PubMed] [Google Scholar]
- 75.Bantz S.K., Zhu Z., Zheng T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5(2) doi: 10.4172/2155-9899.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dantzer J.A., Wood R., A. Update on omalizumab in allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2021 Dec;121(6):559–568. doi: 10.1097/ACI.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 77.Omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen Oit in Food Allergic Participants (outmatch). Accessed on 14 Jan 2021.doi:https://clinicaltrials.gov/ct2/show/NCT03881696#wrapper.
- 78.Sui Hj, Hu Y., Chen Y.D., et al. Three cases of refractory sinusitis with nasal polyps treated with anti ige monoclonal antibody. Chin J Otorhinolaryngol Head Neck Surg. 2021;56(2) doi: 10.3760/cma.j.cn115330-20201112-00864. [DOI] [PubMed] [Google Scholar]
- 79.Collins J., Devos G., Hudes G., et al. Allergic bronchopulmonary aspergillosis treated successfully for one year with omalizumab[J] J Asthma Allergy. 2012;5:65–70. doi: 10.2147/jaa.S34579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J.X., Fan L.C., Li M.H., et al. Beneficial effects of omalizumab therapy in allergic bronchopulmonary aspergillosis: a synthesis review of published literature. Respir Med. 2017;122:33–42. doi: 10.1016/j.rmed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 81.Hutyrová B., Bystroň J. The effect of omalizumab treatment on severe allergic asthma and allergic comorbidities: real-life experience from the Czech anti-ige registry. Postepy Dermatol Alergol. 2018;35(5):510–515. doi: 10.5114/ada.2018.77243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H.H., Li Y.C., Huang Y.C. Efficacy of omalizumab in patients with atopic dermatitis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2016;138(6):1719–1722. doi: 10.1016/j.jaci.2016.05.038. e1711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.