Abstract
Humpback whales (Megaptera novaeangliae) are a cosmopolitan species and perform long annual migrations between low-latitude breeding areas and high-latitude feeding areas. Their breeding populations appear to be spatially and genetically segregated due to long-term, maternally inherited fidelity to natal breeding areas. In the Southern Hemisphere, some humpback whale breeding populations mix in Southern Ocean waters in summer, but very little movement between Pacific and Atlantic waters has been identified to date, suggesting these waters constituted an oceanic boundary between genetically distinct populations. Here, we present new evidence of summer co-occurrence in the West Antarctic Peninsula feeding area of two recovering humpback whale breeding populations from the Atlantic (Brazil) and Pacific (Central and South America). As humpback whale populations recover, observations like this point to the need to revise our perceptions of boundaries between stocks, particularly on high latitude feeding grounds. We suggest that this “Southern Ocean Exchange” may become more frequent as populations recover from commercial whaling and climate change modifies environmental dynamics and humpback whale prey availability.
Subject terms: Animal migration, Behavioural ecology, Biogeography, Biooceanography, Climate-change ecology, Conservation biology, Ecological epidemiology, Evolutionary ecology, Population dynamics
Introduction
Humpback whale migration patterns and breeding stocks
Humpback whales (Megaptera novaeangliae) are a cosmopolitan species1 which can migrate up to 8500 km between seasonal breeding and feeding areas2,3, except for the non-migratory Arabian Sea breeding population4. In the Pacific and Atlantic Oceans, the southern distribution of some populations from the Northern Hemisphere overlaps in equatorial regions with the northern distribution of some populations from the Southern Hemisphere2,3,5. Despite these overlaps in distribution, these Northern Hemisphere and Southern Hemisphere populations are unlikely to co-occur in their breeding areas, because the seasons and directions of their northward and southward migrations segregate populations spatiotemporally6–8. High genetic differentiation of populations between the hemispheres suggest divergence possibly to the level of distinct subspecies9.
Humpback whale populations in the same hemisphere are roughly synchronized in the timing of their migration to and from feeding areas. In the ocean basins of the Southern Hemisphere, humpback breeding stocks show significant genetic population structure despite an absence of geographic barriers to dispersal10–13. This is promoted by maternally-directed site-fidelity where calves accompany mothers for approximately a year from natal breeding areas to the feeding areas and back14, and probably also by social facilitation, given growing evidence that behavior is culturally acquired in humpback whales15,16. Seven low-latitude breeding stocks (A to G) and six high-latitude feeding areas off the Antarctic continent (Areas I to VI) are recognized based on site fidelity and spatial segregation of catches by the International Whaling Commission (IWC) in the Southern Hemisphere17. Here we use the term breeding stock and breeding population interchangeably, defining them as a condition where all the individuals in an area are part of the same reproductive process, forming a self-contained unit, with emigration/immigration rates far lower than the intrinsic rate of population growth18.
The breeding area of humpback whales from the breeding stock ‘G’ (BSG) is situated in the tropical and subtropical waters along the Pacific coast of Central and South America from where they seasonally migrate to the Western Antarctic Peninsula (WAP) to feed 2,3,19,20. Some individuals from this population do not reach the Antarctic and instead feed around the Fueguian Archipelago of Southern Chile21. On the Atlantic coast of South America, breeding stock ‘A’ (BSA) breeds off the coast of Brazil, from the north to the southeast, especially on the Abrolhos Bank22,23. The longitudinal boundary between BSG and BSA feeding areas is believed to be at or around 40° W24 (Fig. 1c).
Figure 1.
Maps of the breeding areas of breeding stock ‘G’ (BSG in a) and breeding stock ‘A’ (BSA in b), and feeding areas off the Antarctic continent (c). Putative feeding grounds for BSA are shown on the bottom map (c) in a shade of green25–29 and for BSG in a shade of orange24. Colored symbols (circle for whales from BSA and triangles for whales from BSG) indicate where individuals were photo-identified. The green line within the BSG putative feeding area delimits where BSA whales are feeding in the WAP (this study) and the orange line indicates previous information of a BSG whale feeding on the putative BSA feeding area42. Maps were created using QGIS version 3.16 (2021, Open Source Geospatial Foundation Project, http://qgis.osgeo.org) and Inkscape version 0.92.5 (2020, Inkscape Project, https://inkscape.org).
Evidence provided by genetics, satellite-tracking, and photo-identification shows that BSA whales mainly feed near South Georgia and the South Sandwich Islands25–29 (Antarctic Area II, Fig. 1c). Satellite tracking and gravitational coordinates (local gravity accelerations associated with latitude and bed rock density) recently showed that BSA whales appear to have a persistent migratory fidelity to a corridor between Brazil and this specific sector of the South Atlantic and that those apparently “fixed” migratory trajectories do not seem to vary with changing oceanographic and geomagnetic conditions30. The feeding area for BSA extends west, at least to Shag Rocks (42º W; Fig. 1c) 240 km west of South Georgia31, and has been suggested to extend as far east as 10–20° W28, where stocks from West Africa (BSB) and BSA are believed to co-occur in space and time during summer feeding32.
Connectivity among breeding stocks in the Southern Hemisphere
Analyses of mitochondrial DNA (mtDNA) and microsatellite loci from whales feeding in nearshore waters of the WAP determined that the natal areas of most whales are situated off Colombia (BSG) with > 94% of samples assigned to this breeding area, a few are associated with breeding grounds off French Polynesia and Samoa (breeding stock ‘F’), yet no samples were genetically assigned to Brazil (BSA)20. Analysis of population connectivity between Colombia, Brazil, and the WAP using microsatellite loci also showed strong restrictions to gene flow between Brazil (BSA) and Colombia (BSG), with only ~ 1–2 animals estimated to migrate between the two breeding stocks each year33. In a broader comparison of all Southern Hemisphere breeding stocks, the level of maternally inherited gene flow was also lowest between breeding stocks A and G, reflecting strong and significant mtDNA differentiation between the two sites12 and hinting that there may be long-term, natural barriers to oceanic mixing between the Atlantic and Pacific breeding stocks.
Photo-identification efforts in the WAP feeding area (between 1997 and 2003, 375 individuals photo-identified) resulted in no matches to whales in the Brazilian breeding area (between 1989 and 2000, 983 individuals photo-identified)34. The photo-identification catalogue built with images from the WAP is large and goes back to 1986. Satellite tracking of humpback whales in the WAP has shown some movement into the South Atlantic, but no further east than 50° W to date35. Nonetheless, an Ecuadorian whale has been photo-identified and re-sighted in the South Orkney Islands (45–46° W), consistent with the hypothesis that the BSG feeding range boundary may be in the South Orkney islands region24. Humpback whale sightings and historical catches also suggest a hiatus in distribution between the South Orkneys region and the South Atlantic feeding areas in the northern and eastern Scotia Arc36. Altogether, these catches, sightings, individual movements, and molecular evidence suggest that some level of segregation occurs not only between the breeding areas but also between the feeding areas associated with BSA and BSG33,37.
Despite the genetic and photo-identification patterns suggesting low population connectivity between oceans, photo-identified sightings of individual animals show that humpbacks can move between breeding grounds and migrate into different oceans from their natal breeding areas. Some examples of such breeding area interchange among populations in different ocean basins include: (1) a male identified by skin biopsy samples who transited from the Indian Ocean to the South Atlantic Ocean38, (2) a photo-identified female who moved from Brazil (BSA) to Madagascar (BSC)39, (3) a female photographed off Ecuador (BSG) who was later photo-identified off Brazil40 and most recently (4) a whale photographed off Ecuador (BSG) who was later photo-identified off Brazil41. Previous photo-identifications also show that whales from BSG can feed in the same areas as whales from BSA; a single whale from BSG has been photographed both in Ecuador (2131 individuals photo-identified) and the South Sandwich Islands (36° W, 23 individuals photo-identified)42. These cases involve movements by individuals either between different Southern Hemisphere breeding areas, or into adjacent feeding areas that are believed to be used by a different population.
The most recent case noted above was identified using a user-friendly platform that allows citizens to upload whale photographs and have their records compared to others from a large database—Happywhale43. Happywhale identified an opportunity to quickly expand data coverage globally by citizen science. Democratizing science through public participation and engagement brings benefits that include low cost data acquisition and increased collaboration network44,45 while promoting universal and equitable access to scientific data and information46. Here we further explore the Happywhale database to investigate where individuals co-occur at high latitudes in the Southern Hemisphere between the genetically distinct breeding stocks, mainly if individuals from Brazil (BSA) are occuring in the assumed feeding area of whales from the Pacific Central and South America (BSG).
Results
Here we present evidence from fourteen individuals from Brazil (BSA, n = 12) and Pacific Central and South America (BSG, n = 2) matched to putative feeding areas (Table 1, Figs. 1, 2) of BSG (Fig. 1c, showed in a shade of orange) and BSA (Fig. 1c showed in a shade of green). Happywhale’s current dataset includes 4302 identified humpback whales from the WAP. Of these, 558 match to the west coasts of Central and South America (BSG), 12 match to Oceania (BSF) (T. Cheeseman, unpublished data), and six to Brazil (BSA; this study, Fig. 1 and Table 1). Two individuals from Brazil were matched to Shag Rocks, two to South Georgia, and two to the South Sandwich Islands, which are all known feeding areas for Brazilian whales, confirming the putative feeding area for the Brazilian population. But six other matches from Brazil were made with the WAP, which is the feeding area attributed to whales breeding in Pacific Central and South America (Figs. 1, 2). Additionally, two whales from Pacific Central and South America were matched at the eastern edge of the putative feeding area for BSG (South Orkney Islands). One of these two individuals (PAN-1055) was first sighted in 2007 in Central America (Panama, BSG), five years later was sighted in Colombia (Gorgona Island, BSG), matched to the WAP (Hovgaard Island) feeding area during the austral winter of 2019 and then, less than a year later, was found again in Antarctica off Signy Island, South Orkney Islands. This individual moved across the known range of BSG feeding areas within six months and may not have migrated during this time to low latitude waters (identified in Antarctica in July 2019 and in January 2020).
Table 1.
Photographic records of individual humpback whales from breeding stock ‘A’ (BSA in green) and breeding stock ‘G’ (BSG in orange).
| Whale ID | Records on breeding areas | Records on feeding areas | Δt | Additional information | Happywhale link |
|---|---|---|---|---|---|
| IBJ-4283 |

|

|
4 years 4 months |
Unknown sex | https://happywhale.com/individual/25898 |
| IBJ-4463 |

|

|
3 years 4 months |
Unknown sex | https://happywhale.com/individual/35050 |
| IBJ-2003 |

|

|
14 years 3 months |
Unknown sex | https://happywhale.com/individual/34743 |
| IBJ-3918 |

|

|
7 years 3 months |
Unknown sex | https://happywhale.com/individual/33763 |
| IBJ-0261 |

|

|
29 years 5 months |
Unknown sex | https://happywhale.com/individual/32898 |
| RSL-131 |

|

|
1 year 6 months |
Unknown sex | https://happywhale.com/individual/44788 |
| Raulzito Oleg |

|

|
3 years 3 months |
Male | https://happywhale.com/individual/45068 |
| IBJ-1309 |

|
|
8 years 4 months |
Male | https://happywhale.com/individual/19672 |
| Nina |

|

|
11 years 8 months |
Female, recorded as a calf in 2004 and pregnant in 201647 | https://happywhale.com/individual/26347 |
| IBJ-2907 |

|

|
8 years 1 months |
Unknown sex | https://happywhale.com/individual/2945 |
| IBJ-4636 |

|

|
3 years 6 months |
Unknown sex | https://happywhale.com/individual/37484 |
| PMC0003 |

|

|
2 years 6 months |
Unknown sex | https://happywhale.com/individual/25687 |
| PAN-1834 |

|

|
6 months | Unknown sex | https://happywhale.com/individual/36029 |
| PAN-1055 |

|

|
6 years 7 months |
Unknown sex Note Recorded in WAP only 6 months before it was recorded in South Orkney Island |
https://happywhale.com/individual/34550 |
Credit is given for images used, with permission, when not owned by the authors and their institutions.
‘Δt’ is the shortest time interval (in years and months) between migratory destinations.
Figure 2.
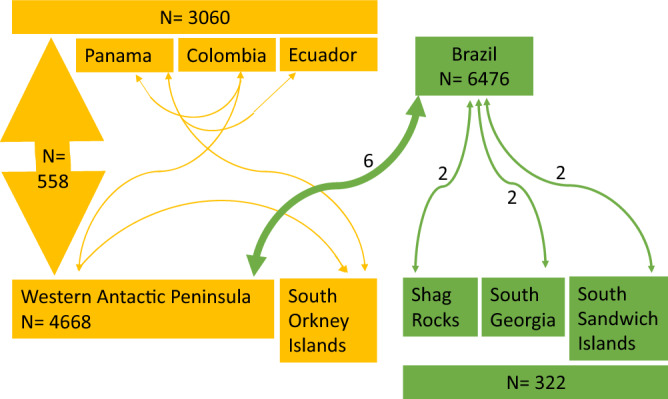
Summary of the matching results for individuals from breeding stock ‘A’ (BSA, in green) and breeding stock ‘G’ (BSG, in orange). N indicates the number of individual whales in the Happywhale catalogue from each region (N for Brazil is likely overestimated because all catalogues have not yet been cross-validated). The thickness of the arrows indicate the number of matched individuals (excepting the arrow linking the west coasts of Central and South America to the Western Antarctic Peninsula, not to scale because it is much greater, N = 558). Two particular BSG humpback whales are shown moving between the WAP all the way to the edge of the putative feeding area for the Central and South American Pacific breeding population. The number of matched individuals is noted if greater than one by each arrow connecting breeding and feeding areas.
Photoidentification and humpback whale sexual maturity age
These photo-identification records also revealed additional information about age of sexual maturity. For instance, the female named Nina was first photo-identified in her birth year in Abrolhos, Brazil, and then photo-identified pregnant 11 years later along the WAP (pregnancy determined by hormone analysis of a skin biopsy sample47). This time gap coincides with the mean age at first calving of humpback whales in Southeastern Alaska (11.8 years)48. However in this case we have no data to determine whether parturition was successful at the breeding area, nor whether this was the first pregnancy for this animal.
Discussion
We found new evidence of summer co-occurrence of two recovering humpback whale breeding populations from the Atlantic (Brazil, BSA) and Pacific (Central and South America, BSG) in the West Antarctic Peninsula feeding area. The temporal pattern of resightings of BSA individuals (older sightings in South Georgia and South Sandwich Islands and more recent sightings off the WAP) suggests BSA whales may be making more extended feeding area movements in recent years. Photo-identifications collected from South Georgia and South Sandwich Islands (Table 1) add to the previous evidence that this area is a known feeding area for whales belonging to BSA. For example, the oldest record of an individual photo-identified at Abrolhos Bank, Brazil in 1989 and matched to its feeding area was for whale IBJ-0261 (Table 1). This individual was photo-identified almost 30 years later in the South Sandwich Islands in 2019, the feeding area for BSA. Photo-identifications available in Happywhale from the South Georgia and South Sandwich Islands mostly come from recent efforts and presently include only 322 identified individuals, 7% of the WAP sample size, thus this effort offers a limited sighting history for photo-identification matching, perhaps explaining the lower number of matches compared to the matches seen between the WAP and wintering grounds in Pacific Central and South America.
Available photo-identification data from the South Orkney Islands is also limited in the Happywhale dataset, but of 17 individually identified humpback whales, two individuals (PAN-1055 and PAN-1834) have been resighted on BSG breeding areas off Ecuador, Colombia, and Panama (Table 1, Figs. 1, 2). Notably, individual PAN-1055 may have migrated north late (or not at all) during the 2019 winter providing information on recent unusual movements around southern polar waters. The breeding destination of the other 15 identified whales in the South Orkney Islands remains to be determined.
Despite differences in photo-identification effort among breeding areas, this study reveals the first evidence of whales moving from Brazil into BSG feeding areas, reinforcing the proposed “Southern Ocean Exchange”. As such, the WAP feeding area is used by two different breeding stocks, BSG and BSA, which were previously strongly differentiated, and now co-occur in polar waters. Increasing photo-identification efforts in this region should provide further evidence of this assessment. The number of individuals that migrate between these two breeding stocks has been estimated to be 1–1.5 whales per season33. Two BSA individuals (named Nina and Raulzito Oleg) were photo-identified off the WAP, crossing the boundary between feeding areas attributed to BSA and BSG in the same period (January 2016, austral summer) and relatively close together (Fig. 1c). This is strong evidence that at least in recent years the use of BSG feeding areas by BSA whales is not a rare event.
While humpback whales are nearly global in their distribution, there is strong evidence that the equator represents a barrier to movement of this species9,49. Mitochondrial and nuclear genetic differentiation is stronger among humpback whales from different hemispheres (North Atlantic, North Pacific, and Southern Hemisphere) than among humpback whales from three Southern Hemisphere ocean basins (Indian, South Pacific, and South Atlantic), suggesting that gene flow is greater across the Southern Hemisphere oceans than across the equator9,50. This has led to a proposal to separate humpback whales into North Atlantic, North Pacific, and Southern Hemisphere subspecies9,50. Levels of genetic differentiation are weaker among Southern Hemisphere breeding stocks12,13 but sufficiently strong that they have been considered demographically independent populations, with population assessments of recovery from exploitation carried out at the level of each breeding stock51. Within the Southern Hemisphere, inter-population genetic differentiation may be anticipated to decrease, as populations recover from whaling and migratory movements between populations increase. If the crossing of individuals from BSA into BSG feeding areas is a recent event, likely motivated by population recovery, the observations presented in this study support this hypothesis.
Mixing of Southern Hemisphere humpback whales on high latitude feeding areas (the Southern Ocean Exchange), sometimes leading to movements between breeding stocks, might be influenced by geographic features such as landmasses, and oceanographic barriers such as major ocean currents that restrict movements. Additionally, El Niño and La Niña climatic events influence the distribution of prey and therefore may potentially influence the migratory destination and timing of migration41. The observed long-range movements between Southern Hemisphere oceans may also result from increasing population size52 and expanding distribution range, as whales colonize new areas and reoccupy historical breeding and feeding areas5,53,54.
Unique features that determine oceanographic and ecological characteristics of the Southern Ocean, such as climate and sea-ice extent variability that affect krill productivity and distribution dynamics, can also influence individual decision-making about migratory paths to these feeding destinations55. Observed shifts in migration timing of humpback whales from Antarctic feeding areas to earlier arrival at breeding areas in the Eastern Tropical Pacific off Colombia are also believed to be associated with population growth (e.g., high pregnancy rates detected in WAP56) and reductions in sea ice coverage and pack mass during the austral autumn, which, in turn, should influence krill availability in Antarctica57. When the autumn ice sheet has a larger mass, more krill can find food in winter58,59 and during the following summer, there are more prey available for whales. In years with larger autumn ice sheets, humpback whales arrive later to Colombian waters the following winter. Prey availability is probably used by whales as a cue to time their migration to wintering areas57.
Krill availability along the WAP correlates with reproductive rates in BSG whales, suggesting the importance of krill to the dynamics of this population60. Although krill abundance in the southwest Atlantic sector is one of the largest in the Southern Ocean, krill density in South Georgia is declining61,62 and show interannual fluctuation linked to climate variability63, with population-level impacts on other krill predators using South Georgia waters64. High krill density values (> 30 g m−2) are interspersed with years with low density (< 30 g m−2) with fluctuations every 4–5 years65. The relationship between BSA whale population growth (12% per year66) and krill availability is yet to be established but is assumed to be similar to that found for BSG. Increasing fluctuations in krill availability in the northern Scotia Arc may also be forcing humpback whales to venture further from their traditional feeding areas in order to feed. Melting sea ice around the WAP may open up new habitats and modulate krill densities and distributions across the Peninsula and into the Scotia Arc67 which, in turn, may have a dampening effect on whale population growth while increasing the likelihood that humpback whales from different populations will venture into areas that were not traditionally utilized for feeding in that part of the ocean.
Behavioural traits such as humpback whale song and its evolution and transmission are also facilitated by the Southern Ocean Exchange. In humpback whales, songs are produced by males68,69. Molecular data also suggests most movements between breeding grounds, and associated gene flow, may be male-driven33. Male carriers of a particular song may therefore introduce it to another population. Cultural transmission of song patterns by males has been shown to occur between west and east coasts of Australia70 and across the South Pacific Ocean15. The lower site fidelity of males therefore facilitates song sharing among breeding grounds in the Southern Hemisphere16,71. The co-occurrence of whales from different populations could also contribute to the spread of infectious diseases. In seabird populations, extensive winter mixing has been suggested as an important factor in disease transmission72. For example, morbillivirus was recently identified in the blow of humpback whales in Brazil73 and it could be introduced to other breeding areas through whales that migrate away from their natal breeding and feeding grounds.
Here we have considered how population growth, climate change and oceanic productivity may be acting synergistically to increase the porosity of boundaries among feeding areas of different humpback whale breeding stocks. Underscoring the Southern Ocean Exchange hypothesis of porous Southern Ocean boundaries for humpbacks are the international collaborations among academics and citizens which have made these observations possible. Traditionally, fluke comparisons between regions were very time-consuming as they were carried out manually by researchers visually inspecting all images74. New algorithms and tools for automated fluke matching, such as the one developed and implemented by Happywhale43 have enormously improved matching rates and also facilitate the detection of low-frequency movements. Photo-identification sample sizes used in humpback whale stock comparisons jumped 50-fold when citizens started to participate in biodiversity monitoring, facilitated by public campaigns, tourism, and the development of user-friendly platforms to engage and receive feedback on their contributions. In ours and other cases, these contributions add great complementary value to dedicated scientific research75,76. Global citizen engagement informs, at a much faster pace, stakeholders’ actions towards conservation of marine life. The relative cost of citizen science compared to more academic approaches in wildlife monitoring may change how conservation science is made and perceived by society in the near future.
Methods
Digital images from research collaborator and citizen science contributors of the ventral surfaces of humpback whale tails (flukes) photographed in Central and South American breeding areas (~ 6476 photo-identified individuals in BSA and 3060 photo-identified in BSG) and feeding areas near South Georgia and the South Sandwich Islands (associated with BSA, n = 322 photo-identified individuals) and the WAP (associated with BSG, n = 4668) were uploaded to the web platform Happywhale (Table 1)43. The number of Brazilian individuals identified may be overestimated since the catalogues from Brazil are not fully reconciled (i.e., there may be repeat sightings of individuals in this dataset) and are pending cross-validation on Happywhale. Uploaded images were matched via automated image recognition43 to a dataset of 47,122 known individual humpback whales worldwide, of which 4990 (10.6%) have been photo-identified in the feeding areas of BSG or BSA. Individual fluke matches are presented in Table 1.
Acknowledgements
We thank Alexandre D. Paro, Rodrigo S. Amaral, Lucas G. Collares, Leiv Poncet, Ihor Dykyy, Daniela Abras, Kátia Groch and Xavier Stump for field efforts. We thank the Captain of the Kotic sailboat, Oleg Belly, “in memoriam” of his contribution to Antarctic exploration, as well as Brazilian Captains: Ubirajara “Bira” dos Santos, Carlo L. D’Angelo and Bernardo Cerqueira and the other members of the crews of the boats Tomara, Coronado and Piloto. We thank Projeto Baleia Jubarte sponsored by Petróleo Brasileiro S.A. (PETROBRAS) for logistic support. We thank the International Association of Antarctic Tour Operators (IAATO), Government of South Georgia and the South Sandwich Islands (GSGSSI), British Antarctic Survey (BAS) particularly Mick Baines and Maren Reichlt and the DY098 expedition, South Georgia Heritage Trust (SGHT) and all other Antarctic expedition tour operators, guides and guests who have contributed to building the Happywhale Antarctic humpback whale photo-identification dataset. We thank PETROBRAS for kindly authorizing the use of data collected during the Projeto de Monitoramento de Cetáceos na Bacia de Santos (PMC-BS). The PMC-BS is one of the monitoring programs required by Brazil’s federal environmental agency, IBAMA, for the environmental licensing process of the oil production and transport by PETROBRAS at the Santos Basin pre-salt province (process no. 02001.114279/2017-80, ACCTMB no. 657/2015). In Colombia, we thank Alberto Parra (Pacífico Extremo) and Diana Grajales for contributing humpback whale photos and the support of the Colombian Ministry of Science, Technology and Innovation (Call number 848, 2019). State Institution National Antarctic Scientific Center, Ministry of Education and Science of Ukraine funded research in the framework of the State Special-Purpose Research Program in Antarctica for 2011–2020 (Permit Series AP No 075-19/2). Biopsy samples were collected under NMFS Permits 14809 and 23095, ACA 2015-011, and UCSC IACUC Friea2004. Panacetacea thanks the Moore Charitable Foundation and the Islas Secas Foundation for their support. We thank Vanessa Pirotta and one anonymous reviewer for their suggestions and words of appreciation about our work.
Author contributions
R.S.S.L. secured financial support for research in Brazil, carried out data collection in the field, photoidentified individuals, organized the UFRN fluke catalogue and uploaded it to Happywhale, drafted and edited the manuscript. R.C.F. and M.F.A. carried out data collection in the field, identified individuals and contributed to the manuscript. T.C. carried out data collection in the field, photoidentified individuals, built and managed the Happywhale web platform and image recognition algorithm and edited the manuscript. M.O. carried out data collection in the field, photoidentified individuals, managed and matched data in Happywhale. V.B. carried out data collection in the field. M.C.C.M. carried out data collection in the field and contributed to the manuscript. J.D.F.S. carried out data collection in the field, organized IBJ fluke catalogue and photoidentified individuals. L.L.W. and F.G.D.J. collected and managed data from Santos Basin (Brazil) and revised the manuscript. A.F. and L.P. carried out data collection in the field, organized and contributed the Biotelemetry and Behavioral Ecology fluke catalogue from the WAP, and edited the manuscript. I.C.A. carried out data collection in the field, photoidentified individuals and contributed to the manuscript. O.S. carried out data collection in the field, organized and contributed to the fluke catalogue of the National Antarctic Scientific Center of Ukraine, and commented on the manuscript. D.M.P. participated in fieldwork in Panama and edited the manuscript. A.S.K. is the creator and curator of the South Georgia humpback whale catalog. K.R. carried out data collection in Panama, photodientified individuals and commented on the manuscript. J.A.J. coordinated part of the field research in Antartica, contributed images and edited the manuscript. M.B. and F.G.D.J. made the maps. M.B. also contributed to the first draft and edited the manuscript. All other authors contributed data and edited the manuscript.
Funding
Funding is provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) finance code 001 and scholarship number AUXPE 541/2016 to R. S. Sousa-Lima, Conselho Nacional de Desenvolvimento Científico e Tecnológico Fellowships to M. Bassoi (DTI 443305/2019-6) and R. S. Sousa-Lima (PPq 312763/2019-0), Petróleo Brasileiro S. A. (PETROBRAS), Cheesemans’ Ecology Safaris, EU BEST medium grant number 1594 awarded to J. A. Jackson, DARWIN award number DPLUS057 awarded to J. A. Jackson, WWF UK, South Georgia Heritage Trust, Friends of South Georgia Island, British Antarctic Survey Polar Science for Planet Earth Programme, National Science Foundation Office of Polar Programs grants 1643877 and 1440435 awarded to A.S. Friedlaender and L. Pallin, Colombian Ministry of Science, Technology and Innovation Call 848 of 2019 awarded to I. C. Avila, Society for Marine Mammalogy Small-Grants-in-Aid of Research Grant awarded to R. S. Sousa-Lima, The Canon National Parks Science Scholars Program 2003 Technology Innovation grant awarded to R. S. Sousa-Lima, Canon U.S.A, Animal Behavior Society Cetacean Conservation grant awarded to R. S. Sousa-Lima, Fundação O Boticário de Proteção à Natureza, and MacArthur Foundation.
Data availability
The matched images are publicly available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License (http://www.happywhale.com).
Code availability
An open-source repository for the original algorithm, as posted at the completion of the Kaggle competition though which it was developed, is available via https://www.kaggle.com/c/humpback-whale-identification/discussion. Access to the implemented algorithm and supporting information architecture is available for use, at no cost, via the web platform www.happywhale.com.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clapham, P. J. & Mead, J. G. Sharing the space: Review of humpback whale occurrence in the Amazonian equatorial coast. In: Mammalian Species: Megaptera novaeangliae. American Society of Mammalogists Issue, vol 604, 5 (1999). 10.1016/j.gecco.2019.e00854.
- 2.Rasmussen K, et al. Southern Hemisphere humpback whales wintering off Central America: Insights from water temperature into the longest mammalian migration. Biol. Lett. 2007;3:302–305. doi: 10.1098/rsbl.2007.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Weerdt J, Ramos EA, Cheeseman T. Northernmost records of Southern Hemisphere humpback whales (Megaptera novaeangliae) migrating from the Antarctic Peninsula to the Pacific coast of Nicaragua. Mar. Mamm. Sci. 2020;36:1015–1021. doi: 10.1111/mms.12677. [DOI] [Google Scholar]
- 4.Mikhalev YA. Humpback whales Megaptera novaeangliae in the Arabian Sea. Mar. Ecol. Prog. Ser. 1997;149:13–21. doi: 10.3354/meps149013. [DOI] [Google Scholar]
- 5.Ristau NG, et al. Sharing the space: Review of humpback whale occurrence in the Amazonian Equatorial Coast. Glob. Ecol. Conserv. 2020;22:e00854. doi: 10.1016/j.gecco.2019.e00854. [DOI] [Google Scholar]
- 6.Kellogg, R. What is known of the migration of some of the whalebone whales U.S.G.P.O. In Publication Smithsonian Institution,2997 Rex Nan Kivell Collection,NK5765, 467e494, 2997 (2) leaves of plates (Smithsonian Publication, 1929).
- 7.Clapham, P. J. Humpback whale. In Megaptera novaeangliae. Encyclopedia of Marine Mammals, 3rd edn, 489–492. (Academic Press, 2018). 10.1016/B978-0-12-804327-1.00154-0.
- 8.Chereskin E, et al. Song structure and singing activity of two separate humpback whales populations wintering off the coast of Caño Island in Costa Rica. J. Acoust. Soc. Am. 2020;146:EL509–EL515. doi: 10.1121/1.5139205. [DOI] [PubMed] [Google Scholar]
- 9.Jackson J, et al. Global diversity and oceanic divergence of humpback whales (Megaptera novaeangliae) Proc. R. Soc. B. 2014;281:20133222. doi: 10.1098/rspb.2013.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker CS, et al. Abundant mitochondrial DNA variation and world-wide population structure in humpback whales. Proc. Natl. Acad. Sci. 1993;90:8239–8243. doi: 10.1073/pnas.90.17.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palsbøll PJ, et al. Distribution of mtDNA haplotypes in North Atlantic humpback whales: The influence of behaviour on population structure. Mar. Ecol. Progr. Ser. 1995;116:1–10. doi: 10.3354/meps116001. [DOI] [Google Scholar]
- 12.Rosenbaum HC, et al. First circumglobal assessment of Southern Hemisphere humpback whale mitochondrial genetic variation and implications for management. Endang. Species Res. 2017;32:551–567. doi: 10.3354/esr00822. [DOI] [Google Scholar]
- 13.Kershaw F, et al. Multiple processes drive genetic structure of humpback whale (Megaptera novaeangliae) populations across spatial scales. Mol. Ecol. 2017;26:977–994. doi: 10.1111/mec.13943. [DOI] [PubMed] [Google Scholar]
- 14.Baker CS, et al. Strong maternal fidelity and natal philopatry shape genetic structure in North Pacific humpback whales. Mar. Ecol. Progr. Ser. 2013;494:291–306. doi: 10.3354/meps10508. [DOI] [Google Scholar]
- 15.Garland EC, et al. Dynamic horizontal cultural transmission of humpback whale song at the ocean basin scale. Curr. Biol. 2011;21:687–691. doi: 10.1016/j.cub.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Garland EC, et al. Humpback whale song on the Southern Ocean feeding grounds: Implications for cultural transmission. PLoS ONE. 2013;8:11. doi: 10.1371/journal.pone.0079422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan, G. A. Review of IWC stock boundaries. In Report of the International Whaling Commission (Special Issue), vol. 13, 39–68 (1991).
- 18.IWC. JCRM (Supplement), vol. 15, 287–288 (2014).
- 19.Félix F, Guzmán HM. Satellite tracking and sighting data analyses of Southeast Pacific humpback whales (Megaptera novaeangliae): Is the migratory route coastal or oceanic? Aquat. Mamm. 2014;40:329–340. doi: 10.1578/AM.40.4.2014.329. [DOI] [Google Scholar]
- 20.Albertson GR, et al. Temporal stability and mixed-stock analyses of humpback whales (Megaptera novaeangliae) in the nearshore waters of the Western Antarctic Peninsula. Polar Biol. 2018;41:323–340. doi: 10.1007/s00300-017-2193-1. [DOI] [Google Scholar]
- 21.Acevedo J, et al. First evidence of interchange of humpback whales (Megaptera novaeangliae) between the Magellan Strait and Antarctic Peninsula feeding grounds. Polar Biol. 2021;44:613–619. doi: 10.1007/s00300-021-02827-2. [DOI] [Google Scholar]
- 22.Andriolo A, Kinas PG, Engel MH, Martins CCA, Rufino AM. Humpback whales within the Brazilian breeding ground: Distribution and population size estimate. Endanger. Species Res. 2010;11:233–243. doi: 10.3354/esr00282. [DOI] [Google Scholar]
- 23.Martins CCA, Andriolo A, Engel MH, Kinas PG, Saito CH. Identifying priority areas for humpback whale conservation at Eastern Brazilian Coast. Ocean Coast. Manag. 2013;75:63–71. doi: 10.1016/j.ocecoaman.2013.02.006. [DOI] [Google Scholar]
- 24.Dalla Rosa L, et al. Feeding ground of the eastern South Pacific humpback whale population include the south Orkney island. Polar Res. 2012;31:17324. doi: 10.3402/polar.v31i0.17324. [DOI] [Google Scholar]
- 25.Zerbini AN, et al. Satellite-monitored movements of humpback whales Megaptera novaeangliae in the southwest Atlantic Ocean. Mar. Ecol. Prog. Ser. 2006;313:295e304. doi: 10.3354/meps313295. [DOI] [Google Scholar]
- 26.Zerbini A, et al. Migration and summer destinations of humpback whales (Megaptera novaeangliae) in the western South Atlantic Ocean. J. Cetacean Res. Manag. 2011;3:113–118. doi: 10.47536/jcrm.vi.315. [DOI] [Google Scholar]
- 27.Engel MH, et al. Mitochondrial DNA diversity of the Southwestern Atlantic humpback whale (Megaptera novaeangliae) breeding area off Brazil, and the potential connections to Antarctic feeding areas. Conserv. Genet. 2008;9:1253e1262. doi: 10.1007/s10592-007-9453-5. [DOI] [Google Scholar]
- 28.Engel MH, Martin AR. Feeding grounds of the western South Atlantic humpback whale population. Mar. Mamm. Sci. 2009;25:964e969. doi: 10.1111/j.1748-7692.2009.00301.x. [DOI] [Google Scholar]
- 29.IWC. Report of the workshop on the comprehensive assessment of Southern hemisphere humpback whales. J. Cetacean Res. Manag. 1, 1–50 (2011).
- 30.Horton T, Zerbini A, Andriolo A, Danilewicz D, Sucunza F. Multi-decadal humpback whale migratory route fidelity despite oceanographic and geomagnetic change. Front. Mar. Sci. 2020;7:414. doi: 10.3389/fmars.2020.00414. [DOI] [Google Scholar]
- 31.Stevick PT, et al. Population spatial structuring on the feeding grounds in North Atlantic humpback whales (Megaptera novaeangliae) J. Zool. 2006;270:244e255. doi: 10.1111/j.1469-7998.2006.00128.x. [DOI] [Google Scholar]
- 32.IWC. Report of the scientific committee. Rep. Int. Whal. Commun. 48, 53–118 (1998).
- 33.Cypriano-Souza AL, et al. Genetic differentiation between humpback whales (Megaptera novaeangliae) from Atlantic and Pacific breeding grounds of South America. Mar. Mamm. Sci. 2017;33:457–479. doi: 10.1111/mms.12378. [DOI] [Google Scholar]
- 34.IWC. J. Cetacean Res. Manag. (Supplement) 7, 235–246 (2005).
- 35.Dalla Rosa L, Secchi ER, Maia YG, Zerbini AN, Heide-Jørgensen MP. Movements of satellite-monitored humpback whales on their feeding ground along the Antarctic Peninsula. Polar Biol. 2008;31:771–781. doi: 10.1007/s00300-008-0415-2. [DOI] [Google Scholar]
- 36.Bombosch A, et al. Predictive habitat modelling of humpback (Megaptera novaeangliae) and Antarctic minke (Balaenoptera bonaerensis) whales in the Southern Ocean as a planning tool for seismic surveys. Deep Sea Res. (I Oceanogr. Res. Pap.) 2014;91:101–114. doi: 10.1016/j.dsr.2014.05.017. [DOI] [Google Scholar]
- 37.Stevick P, et al. Migrations of individually identified humpback whales between the Antarctic Peninsula and South America. J. Cetacean Res. Manag. 2004;6:109–113. [Google Scholar]
- 38.Pomilla C, Rosenbaum HC. Against the current: An inter-oceanic whale migration event. Biol. Lett. 2005;1:476–479. doi: 10.1098/rsbl.2005.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevick PT, et al. A quarter of a world away: Female humpback whale moves 10 000 km between breeding areas. Biol. Lett. 2011;7:299–302. doi: 10.1098/rsbl.2010.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevick PT, et al. Inter-oceanic movement of an adult female humpback whale between Pacific and Atlantic breeding grounds off South America. J. Cetacean Res. Manag. 2013;13:159–162. [Google Scholar]
- 41.Félix F, et al. A new case of interoceanic movement of a humpback whale in the Southern hemisphere: The El Niño link. Aquat. Mamm. 2020;46:578–583. doi: 10.1578/AM.46.6.2020.578. [DOI] [Google Scholar]
- 42.Castro, C. Engel, M., Martin, A. & Kaufman, G. Comparison of humpback whale catalogues between Ecuador, and South Georgia and Sandwich Island: Evidence of increased feeding area I boundary or overlap between feeding areas I and II? Report of the scientific committee. Rep. Int. Whal. Comm. SC/66/SH (2015).
- 43.Cheeseman, T. et al. Advanced image recognition: A fully automated, high-accuracy photo-identification matching system for humpback whales. Mamm. Biol. 10.1007/s42991-021-00180-9 (in press).
- 44.Gura T. Citizen science: Amateur experts. Nature. 2013;496:259–261. doi: 10.1038/nj7444-259a. [DOI] [PubMed] [Google Scholar]
- 45.Chandler M, et al. Contribution of citizen science towards international biodiversity monitoring. Biol. Conserv. 2017;213:280–294. doi: 10.1016/j.biocon.2016.09.004. [DOI] [Google Scholar]
- 46.de Sherbinin A, et al. The critical importance of citizen science data. Front. Clim. 2021;3:650760. doi: 10.3389/fclim.2021.650760. [DOI] [Google Scholar]
- 47.Pallin LJ, Robbins J, Kellar N, Bérubé M, Friedlaender A. Validation of a blubber-based endocrine pregnancy test for humpback whales. Conserv. Physiol. 2018 doi: 10.1093/conphys/coy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gabriele, C. M., Straley, J. M. & Neilson, J. L. Age at first calving of female humpback whales in Southeastern Alaska. In Proceedings of the Fourth Glacier Bay Science Symposium, October 26–28, 2004:U.S. Geological Survey Scientific Investigations Report (eds. Piatt, J. F. & Gende, S. M.) vol. 2007–5047, 159–162 (2007).
- 49.Baker CS, Medrano-González L. Worldwide distribution and diversity of humpback whale mitochondrial DNA lineages. In: Pfeiffer CJ, editor. Molecular and Cell Biology of Marine Mammals. Krieger Publishing Company; 2002. pp. 84–99. [Google Scholar]
- 50.Bettridge, S. et al.Status Review of the Humpback Whale (Megaptera novaeangliae) under the Endangered Species Act. NOAA-TM-NMFS-SWFSC-540, ID#4883, 241. https://repository.library.noaa.gov/view/noaa/4883 (2015).
- 51.IWC. Annex H: Report of the sub-committee on other Southern hemisphere whale stocks. J. Cetacean Res. Manag.(Supplement) 17, 250–282 (2016).
- 52.Zerbini A, et al. Assessing the recovery of an Antarctic predator from historical exploitation. R. Soc. Open Sci. 2019;6:190368. doi: 10.1098/rsos.190368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zerbini AN, Clapham PJ, Wade PR. Assessing plausible rates of population growth in humpback whales from life-history data. Mar. Biol. 2010;157:1432e1793. doi: 10.1007/s00227-010-1403-y. [DOI] [Google Scholar]
- 54.Gonçalves MIC, et al. Low latitude habitat use patterns of a recovering population of humpback whales. J. Mar. Biol. Assoc. U. K. 2018;98:1087–1096. doi: 10.1017/S0025315418000255. [DOI] [Google Scholar]
- 55.Riekkola L, et al. Longer migration not necessarily the costliest strategy for migrating humpback whales. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020;1:12. doi: 10.1002/aqc.3295. [DOI] [Google Scholar]
- 56.Pallin LJ, et al. High pregnancy rates in humpback whales (Megaptera novaeangliae) around the Western Antarctic Peninsula, evidence of a rapidly growing population. R. Soc. Open Sci. 2018;5:180017. doi: 10.1098/rsos.180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avila IC, et al. Whales extend their stay in a breeding ground in the Tropical Eastern Pacific. ICES J. Mar. Sci. 2020;77:109–118. doi: 10.1093/icesjms/fsz251. [DOI] [Google Scholar]
- 58.Fritsen CH, Memmott JC, Stewart FJ. Inter-annual sea-ice dynamics and micro-algal biomass in winter pack ice of Marguerite Bay, Antarctica. Deep Sea Res II Top. Stud. Oceanogr. 2008;55:2059–2067. doi: 10.1016/j.dsr2.2008.04.034. [DOI] [Google Scholar]
- 59.Meyer B. The overwintering of Antarctic krill, Euphausia superba, from an ecophysiological perspective. Polar Biol. 2012;35:15–37. doi: 10.1007/s00300-011-1120-0. [DOI] [Google Scholar]
- 60.Seyboth E, et al. Influence of krill (Euphausia superba) availability on humpback whale (Megaptera novaeangliae) reproductive rate. Mar. Mamm. Sci. 2021 doi: 10.1111/mms.12805. [DOI] [Google Scholar]
- 61.Atkinson AA, Siegel V, Pakhomov E, Rothery P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 2004;432:100–103. doi: 10.1038/nature02996. [DOI] [PubMed] [Google Scholar]
- 62.Atkinson A, et al. Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat. Clim. Change. 2019;9:142–147. doi: 10.1038/s41558-018-0370-z. [DOI] [Google Scholar]
- 63.Loeb VJ, Santora JA. Climate variability and spatiotemporal dynamics of five Southern Ocean krill species. Prog. Ocean. 2015;134:93–122. doi: 10.1016/j.pocean.2015.01.002. [DOI] [Google Scholar]
- 64.Forcada J, Trathan P, Murphy EJ. Life history buffering in Antarctic mammals and birds against changing patterns of climate and environmental variation. Glob. Change Biol. 2008;14:2473–2488. doi: 10.1111/j.1365-2486.2008.01678.x. [DOI] [Google Scholar]
- 65.Fielding S, et al. Interannual variability in Antarctic krill (Euphausia superba) density at South Georgia, Southern Ocean: 1997–2013. ICES J. Mar. Sci. 2014;71:2578–2588. doi: 10.1093/icesjms/fsu104. [DOI] [Google Scholar]
- 66.Wedekin LL, et al. Running fast in the slow lane: Rapid population growth of humpback whales after exploitation. Mar. Ecol. Prog. Ser. 2017;575:195–206. doi: 10.3354/meps12211. [DOI] [Google Scholar]
- 67.Rogers AD, et al. Antarctic futures: An assessment of climate-driven changes in ecosystem structure, function, and service provisioning in the Southern Ocean. Ann. Rev. Mar. Sci. 2020;12:87–120. doi: 10.1146/annurev-marine-010419-011028. [DOI] [PubMed] [Google Scholar]
- 68.Glockner, D. A. & Venus, S. Determining the sex of humpback whales (Megaptera novaeangliae) in their natural environment. In Behavior and Communication of Whales. (Westview Press, 1983).
- 69.Darling JD, Berubé M. Interactions of singing humpback whales with other males. Mar. Mamm. Sci. 2001;17:570–584. doi: 10.1111/j.1748-7692.2001.tb01005.x. [DOI] [Google Scholar]
- 70.Noad MJ, Cato DH, Bryden MM, Jenner MN, Jenner KCS. Cultural revolution in whale songs. Nature. 2000;408:537–537. doi: 10.1038/35046199. [DOI] [PubMed] [Google Scholar]
- 71.Darling DJ, Sousa-Lima RS. Songs indicate interaction between humpback whale (Megaptera novaeangliae) populations in western and eastern South Atlantic Ocean. Mar. Mamm. Sci. 2006;21:557–566. doi: 10.1111/j.1748-7692.2005.tb01249.x. [DOI] [Google Scholar]
- 72.McKnight A, Allyn AJ, Duffy DC, Irons DB. ‘Stepping stone’ pattern in Pacific Arctic tern migration reveals the importance of upwelling areas. Mar. Ecol. Prog. Ser. 2013;491:253–264. doi: 10.3354/meps10469. [DOI] [Google Scholar]
- 73.Groch KR, et al. Cetacean morbilivirus in humpback whale's exhaled breath. Transbound. Emerg. Dis. 2020 doi: 10.1111/tbed.13883. [DOI] [PubMed] [Google Scholar]
- 74.Ballance LT. Contributions of photographs to cetacean science. Aquat. Mamm. 2018;44:668–682. doi: 10.1578/AM.44.6.2018.668. [DOI] [Google Scholar]
- 75.Kosmala M, Wiggins A, Swanson A, Simmons B. Assessing data quality in citizen science. Front. Ecol. Environ. 2016;14:551–560. doi: 10.1002/fee.1436. [DOI] [Google Scholar]
- 76.Vieira EA, Souza LR, Longo GO. Diving into science and conservation: Recreational divers can monitor reef assemblages. Perspect. Ecol. Conserv. 2020;18:51–59. doi: 10.1016/j.pecon.2019.12.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The matched images are publicly available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License (http://www.happywhale.com).
An open-source repository for the original algorithm, as posted at the completion of the Kaggle competition though which it was developed, is available via https://www.kaggle.com/c/humpback-whale-identification/discussion. Access to the implemented algorithm and supporting information architecture is available for use, at no cost, via the web platform www.happywhale.com.



