Abstract
Background and Aims:
For e-cigarettes to be a viable substitute for combustible cigarettes, it is likely that they must be rewarding enough for regular use, indicated by factors such as craving and dependence, important aspects of reinforcement. This study aimed to understand short-term changes in measures of nicotine dependence between groups differing by use trajectory in a switching trial, and within group changes of these measures.
Design:
Secondary data analysis of one arm of an e-cigarette randomized clinical trial.
Setting:
San Diego, California and Kansas City, Missouri, USA.
Participants:
114 African American (n=60) and Latinx (n=54) smokers (58.8% male) attempting to switch to nicotine salt pod system (NSPS) e-cigarettes in a 6-week trial.
Measurements:
At week 6, participants were classified by use trajectory: exclusive smokers (n=16), exclusive e-cigarette (n=32), or dual users (n=66). E-cigarette, cigarette, and total nicotine dependence (cigarette+e-cigarette), use patterns, cigarette craving and nicotine withdrawal, and cotinine were assessed at baseline and week 6 using standard measures.
Findings:
In between group comparisons, exclusive e-cigarette and dual users showed greater reductions in cigarette dependence (e-cigarette:−32.38, 95%CI=−37.7,−27.1; dual:−18.48, 95%CI=−22.2,−14.7), withdrawal (e-cigarette:−6.25, 95%CI=−8.52,−3.98; dual:−3.18, 95%CI=−5.02,−1.34), craving (e-cigarette:−11.44, 95%CI=−14.2,8.7; dual:−9.59, 95%CI=−11.6,−7.59), and cigarettes per day (CPD; e-cigarette:−11.19, 95%CI=−13.1,−9.27; dual:−9.39, 95%CI=−11.3,−7.52) compared with exclusive smokers. In within group analyses, e-cigarette and dual users showed reductions in craving and withdrawal from baseline to week 6. Exclusive e-cigarette and dual users, maintained cotinine levels (ps>.05) and showed reductions in CPD and cigarette dependence (ps<.01). Findings were inconclusive regarding changes in total nicotine dependence from baseline to week 6 among exclusive e-cigarette users (p=.123). Dual users showed increased total nicotine dependence (p<.001) and smokers showed decreased total dependence (p=.004).
Conclusions:
Smokers who switch to nicotine salt pod system (NSPS) e-cigarettes maintain their nicotine levels and transfer their dependence, suggesting that NSPS e-cigarettes have a similar reinforcement potential to cigarettes and facilitate switching.
Keywords: ENDS, nicotine dependence, cigarette smoking, dual use, JUUL, reinforcement value
Introduction
Despite substantial declines in use of combustible cigarettes over the past several decades, smoking remains the leading cause of preventable death worldwide and contributes to more than 400,000 deaths per year in the United States alone.1,2 Electronic cigarettes (e-cigarettes) provide an alternate means of nicotine delivery by heating e-liquid to produce an aerosol that is inhaled by the user. E-cigarettes have increased in popularity since they were first marketed in the U.S. in 2007, with 27.7% of adult smokers reporting e-cigarette use in the past 30 days.3 E-cigarettes are widely considered to be less harmful than combustible cigarettes.4 In particular, completely switching to e-cigarettes results in reduced exposure to a number of carcinogens and toxicants compared to combustible cigarettes.5,6 Due to the reduced harms associated with exclusive e-cigarette use, some smokers have turned to these devices to help them reduce or quit use of combustible cigarettes.7
Smokers who switch completely to e-cigarettes receive the maximum harm reduction benefits, but a product that provides nicotine delivery and is as reinforcing as cigarettes is likely necessary for nicotine dependent smokers to completely switch.8,9 JUUL, a highly nicotinized (3-5% nicotine), fourth generation e-cigarette that uses disposable, nicotine salt-based pods, exemplifies an e-cigarette that has demonstrated a similar pharmacokinetic profile to combustible cigarettes10,11 and appears to have struck a “sweet spot” in terms of appeal, reinforcement, and reduced toxicity.9 This may render JUUL and similar devices feasible and effective alternatives for smokers willing to switch from combustible cigarettes to e-cigarettes. One recent cross-sectional study found that JUUL users showed elevated e-cigarette dependence which differed as a function of their smoking history, such that dual users showed the greatest dependence followed by exclusive e-cigarette users (i.e., former smokers) and then never smokers.12 However, no studies have investigated this question prospectively. One way to examine whether fourth generation e-cigarettes have comparable reinforcement value is by evaluating cigarette and e-cigarette dependence scores, as individuals who successfully switch from combustible cigarettes to e-cigarettes would theoretically demonstrate decreased cigarette dependence and increased e-cigarette dependence. Examining dependence and experiences of craving and withdrawal over time represents a novel approach to understanding the relative reinforcement of cigarettes, e-cigarettes, and the role of these aspects of reinforcement in successful switching.
Uptake of e-cigarettes has been slower among racial/ethnic minorities compared to whites. While approximately 14% of current smokers transition to e-cigarette use each year, a disproportionate percent are white (76%) while only 9% are African American (AA) and 8% are Latinx.13 Because e-cigarettes serve as a potential harm reduction tool, low rates of uptake among minorities could widen tobacco-related health disparities.14 Therefore, examining e-cigarette substitution and reinforcement value among minority populations is imperative to avoid further increasing health inequities.
The purpose of the current study was to examine among AA and Latinx smokers randomized to e-cigarette use in a 6-week e-cigarette switching trial: 1) between group (i.e., exclusive cigarette smoking, dual use, and exclusive e-cigarette use; categorized based on week 6 use data) changes in cigarette dependence (primary), nicotine withdrawal, cigarette craving, cigarettes per day, cotinine (the primary metabolite of nicotine), and e-cigarette dependence; 2) within group changes in the same measures from baseline to week 6; and 3) in an exploratory approach, within group changes in total nicotine dependence (combustible and e-cigarette dependence combined) from baseline to week 6. We hypothesized that exclusive e-cigarette users would experience the greatest reduction in cigarette dependence and greatest increase in e-cigarette dependence at week 6, followed by dual users of e-cigarettes and cigarettes (i.e., lesser reduction in cigarette dependence than exclusive e-cigarette users and greater increase in e-cigarette dependence than exclusive smokers), followed by exclusive smokers (i.e., the least reduction in cigarette dependence and increase in e-cigarette dependence).
Methods
Participants
Participants were recruited as part of a randomized clinical trial15 in which AA and Latinx smokers were randomized (2:1) to JUUL e-cigarettes and encouraged to switch completely or an assessment-only control (AOC) which continued smoking as usual. Participants were recruited from 2018 to 2019 in Kansas City (AA sample) and San Diego (Latinx sample) using flyers, radio, newspaper, and television advertisements, and word of mouth. Eligibility criteria are listed in Table 1. Participants were compensated $20 at baseline, $40 at week 2, and $60 at week 6.
Table 1.
Eligibility criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note. aeCO = exhaled carbon monoxide; AA = African American.
Procedures
Participants randomized to e-cigarette (i.e. JUUL) chose their preferred e-liquid flavor of those commercially available at the time of the study (all 5% nicotine): Virginia tobacco, classic menthol, cool mint, or mango. Those in the e-cigarette condition also received education and training for JUUL use and brief action planning to create a plan for complete switching from trained research assistants. Participants completed follow-ups at week 1 (phone), 2 (in-person), 4 (phone), and 6 (in-person). At baseline and week 2, participants received a two- and four-week supply of pods in their preferred flavor. For the current investigation, only baseline and week 6 data are presented when relevant measures were taken. Study procedures were reviewed and approved by the university’s Institutional Review Board.
Measures
Participant characteristics.
Participants completed self-report measures of age, gender, yearly household income, education, employment status, number of years smoking, and menthol cigarette use.
Group classification
Use trajectory.
Based on criteria in the parent trial,15 exclusive e-cigarette users reported exclusive e-cigarette use and had eCO < 6 ppm. Dual users reported e-cigarette use and cigarette smoking regardless of eCO; those that reported exclusive e-cigarette use but showed eCO ≥ 6 ppm were also classified as dual users. Exclusive smokers reported exclusive cigarette smoking regardless of eCO.
Exhaled carbon monoxide (eCO).
Carbon monoxide, a cardiovascular toxicant, was measured at baseline and week 6 to biochemically confirm exclusive e-cigarette use (< 6 ppm).22 Cigarettes produce CO while e-cigarettes do not. Therefore, following recommendations of the recent SRNT white paper on biochemical verification,22 CO was used to biochemically verify exclusive e-cigarette use.
Outcome measures
Cigarette, e-cigarette, and total nicotine dependence.
Cigarette dependence was assessed for all participants at baseline and week 6 using a 16-item dependence symptoms scale. This measure has been used in nationally representative surveys and has shown strong psychometric properties.16 Items included “I find myself reaching for a cigarette without thinking about it” and “I would find it hard to stop using cigarettes for a week.” For the first 15 items, response options ranged from 1 (“not true of me at all”) to 5 (“extremely true of me”). For the final item (In the past 12 months, did you find it difficult to keep from smoking cigarettes in places where it was not permitted?) response options were yes (1)/no (0). Total scores were calculated and ranged from 15 to 76 with higher scores indicating greater cigarette dependence. Cronbach’s alpha was 0.91 and 0.95 at baseline and week 6, respectively. E-cigarette dependence was measured using an identical measure with cigarette-related terms replaced with e-cigarette-related terms.16 E-cigarette dependence was assessed at week 6 among participants who reported e-cigarette use since their previous visit. Cronbach’s alpha was 0.92. To examine participants’ combined dependence on cigarettes and e-cigarettes, a total nicotine dependence score17 was calculated at baseline and week 6 by summing e-cigarette and combustible cigarette dependence scores for each time point (possible range 30 to 152). Cronbach’s alpha was 0.91 at both baseline and week 6 for the combined measure.
Cigarettes per day and e-cigarette use.
At baseline and week 6, participants reported the number of cigarettes they smoked and number of e-cigarette sessions each day during the past seven days using Timeline Follow Back (TLFB).18,19 Cigarettes per day (CPD) and sessions per day were calculated by dividing the total number of cigarettes smoked/sessions in the past seven days by the number of smoking/vaping days.
Cigarette craving.
Craving for cigarettes was measured at baseline and week 6 using the 5-item adapted Questionnaire of Smoking Urges-Brief (QSU-Brief20). Items were rated on a 1 (“strongly disagree”) to 7 (“strongly agree”) scale with higher ratings indicating stronger cravings. Example items include, “I have a strong desire for a cigarette right now” and “I could control things better right now if I could smoke”. Total scale scores were calculated and ranged from 5 to 35. Cronbach’s alpha was 0.85 at both time points.
Nicotine withdrawal.
At baseline and week 6, nicotine withdrawal was measured using the Minnesota Nicotine Withdrawal Scale21, an 8-item measure listing symptoms of nicotine withdrawal. Participants indicated the extent to which they experienced these symptoms in the last 24 hours. Items included “angry, irritable, frustrated” and “depressed mood, sad”. Response options ranged from 0 (“none”) to 4 (“severe”). Total scores were calculated and ranged from 0 to 32 with higher scores indicating greater withdrawal. Cronbach’s alpha was 0.82 at both time points.
Cotinine.
Cotinine, the main proximate metabolite of nicotine, was measured at baseline and week 6 from urine samples by ultraperformance liquid chromatography-tandem mass spectrometer and normalized for creatinine.
Data analytic plan
Because the treatment group (and not AOC) was exposed to e-cigarettes, an a priori decision was made to assess outcomes only among the treatment group and to not include the AOC group among the exclusive smokers group at week 6. Descriptive statistics are presented for participant baseline characteristics. Change scores were calculated for all outcome measures by subtracting baseline scores from week 6 scores (week 6 – baseline). One-way analyses of covariance (ANCOVAs) controlling for baseline scores on the target variable were utilized to compare change scores for all outcomes by use trajectory at week 6 (exclusive e-cigarette use, dual use, and exclusive cigarette smoking). For those variables where the overall ANCOVA was significant, post-hoc pairwise comparisons were conducted using Tukey’s HSD method. Next, paired samples t-tests were conducted to assess changes in all outcomes from baseline to week 6 within each use trajectory group. In this final step, participants were excluded from analyses if they had incomplete data for cigarette dependence at either time point or e-cigarette dependence at week 6. This resulted in exclusion of four exclusive e-cigarette users, six dual users, and one exclusive cigarette smoker. Analyses were conducted using IBM SPSS version 26. A type I error rate of 5% was used for all comparisons. The analysis was not pre-registered and results should be considered preliminary.
Results
Participants
Overall, 114 participants provided data at baseline and week 6. We examined whether the 11 participants that did not complete week 6 follow up differed from those that did complete in terms of baseline cigarette dependence, craving, withdrawal, cigarettes per day, and cotinine and found no statistical difference between the completers and non-completers (all ps >.300). At week 6, participants were classified as exclusive e-cigarette users, dual users, or exclusive smokers based on responses to the 7-day TLFB and eCO measure. Of the overall sample, 32 were categorized as exclusive e-cigarette users, 66 were dual users, and 16 were exclusive smokers at week 6.
Participant characteristics.
Overall, participants had a mean age of 44.6 years and just over half the sample were male (58.8%). The sample was 52.6% AA and 47.4% Latinx. The majority of participants had less than or equal to a high school degree/GED (55.3%) and approximately 47% were employed full- or part-time. In terms of smoking history, participants reported smoking for an average of 17.3 years and over half were menthol smokers (56.1%). Complete participant characteristics are presented in Table 2.
Table 2.
Participant characteristics by use trajectory group – n(%)/M(SD)
| Characteristic | Overall N = 114 |
Exclusive EC usersa n = 32 |
Dual usersb n = 66 |
Exclusive smokersc n = 16 |
p-value |
|---|---|---|---|---|---|
| Age | 44.6 (12.9) | 44.4 (13.8) | 43.9 (13.0) | 48.0 (10.6) | .517 |
| Gender | .623 | ||||
| Male | 67 (58.8) | 16 (50.0) | 40 (60.6) | 11 (68.8) | |
| Female | 46 (40.4) | 16 (50.0) | 25 (37.9) | 5 (31.3) | |
| Transgender/Gender nonconforming |
1 (0.9) | 0 (0.0) | 1 (1.5) | 0 (0.0) | |
| Yearly household income | $26,923.3 ($26,165.6) | $30,476.6 ($32,599.4) | $26,230.2 ($24,689.5) | $22,346.3 ($15,326.2) | .583 |
| Education | .344 | ||||
| ≤ High school/GED | 63 (55.3) | 21 (65.6) | 33 (50.0) | 9 (56.3) | |
| > High school/GED | 51 (44.7) | 11 (34.4) | 33 (50.0) | 7 (43.8) | |
| Employment status | .485 | ||||
| Full- or part-time | 53 (46.5) | 12 (37.5) | 33 (50.0) | 8 (50.0) | |
| Other | 61 (53.5) | 20 (62.5) | 33 (50.0) | 8 (50.0) | |
| Years smoking | 17.3 (13.1) | 13.6 (12.0) | 16.8 (12.0) | 26.7 (15.7) | .004 |
| Menthol/non-menthol | .975 | ||||
| Non-menthol | 41 (36.0) | 11 (34.4) | 25 (37.9) | 5 (31.3) | |
| Menthol | 64 (56.1) | 18 (56.3) | 36 (54.5) | 10 (62.5) | |
| Both | 9 (7.9) | 3 (9.4) | 5 (7.6) | 1 (6.3) |
Note.
Exclusive EC users = exclusive e-cigarette use at week 6 and eCO < 6 ppm;
Dual users = dual e-cigarette and cigarette use at week 6;
Exclusive smokers = exclusive cigarette smoking at week 6. ANOVAs and Chi-Squared tests were used to test for significant difference between use groups. Bolded values indicate statistically significant omnibus tests. Pairwise comparisons: Significant between group differences emerged in years smoking such that exclusive smokers reported smoking significantly longer than exclusive e-cigarette users and dual users.
Between group changes in outcomes by use trajectory (exclusive e-cigarette users, dual users, and combustible smokers)
Group differences emerged by use trajectory for cigarette dependence (p < .001), craving (p < .001), withdrawal (p < .001), and CPD (p < .001). Specifically, exclusive e-cigarette users showed the greatest reduction in cigarette dependence followed by dual users and exclusive smokers, though cigarette dependence scores did not reduce to zero. Exclusive e-cigarette users and dual users showed significantly greater reductions in withdrawal and craving compared to exclusive smokers. Exclusive e-cigarette users showed the greatest reduction in CPD followed by dual users and exclusive smokers. See Table 3 for complete data.
Table 3.
Comparison of between group change scores by use trajectory
| Exclusive EC users n = 32 |
Dual users n = 66 |
Exclusive smokers n = 16 |
F | Unadjusted p | *Adjusted p | ||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||||
| Cigarette dependence | −32.38a | 15.35 | −18.48b | 15.55 | −14.67c | 16.21 | 9.64 | <.001 | <.001 |
| Withdrawal | −6.25a | 6.55 | −3.18a | 7.63 | 0.60b | 7.64 | 4.64 | .012 | <.001 |
| Craving | −11.44a | 7.90 | −9.59a | 8.31 | −6.00b | 12.22 | 1.88 | .158 | <.001 |
| Cigarettes per day | −11.19a | 5.54 | −9.39b | 7.75 | −3.38c | 7.65 | 6.47 | .002 | <.001 |
| Cotinine (ng/mL) | −287.62 | 2077.33 | 135.70 | 1649.62 | −7.31 | 659.61 | 0.67 | .513 | .263 |
Note. Bolded values indicate statistically significant omnibus tests. Non-matching (i.e., a and b) superscripts within each row indicate significant post-hoc pairwise comparisons in the adjusted model. *adjusted models include baseline measure of target outcome as covariate in the model.
Within group comparison of outcomes by use trajectory
Exclusive e-cigarette and dual users showed significant reductions from baseline to week 6 across all measures (all p < .01) except cotinine (all p > .05). Exclusive smokers displayed significant reductions in cigarette dependence from baseline to week 6 (p = .004). However, no other comparisons were statistically significant (all p > .05). See Table 4 for complete data.
Table 4.
Comparison of within group outcomes by use trajectory at week 6
| Baseline |
Week 6 |
Mean ∆ | SD | p | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Exclusive EC users | |||||||
| Cigarette dependence | 50.90 | 12.84 | 18.52 | 7.97 | −32.38 | 15.35 | <.001 |
| Withdrawal | 15.09 | 7.88 | 8.84 | 7.46 | −6.25 | 6.55 | <.001 |
| Craving | 16.97 | 7.64 | 5.53 | 1.30 | −11.44 | 7.90 | <.001 |
| Cigarettes per day | 11.19 | 5.54 | 0.00 | 0.00 | −11.19 | 5.54 | <.001 |
| Cotinine | 1362.39 | 2111.63 | 1074.77 | 839.81 | −287.62 | 2077.33 | .439 |
| Duals users | |||||||
| Cigarette dependence | 46.16 | 14.00 | 27.69 | 14.01 | −18.48 | 15.55 | <.001 |
| Withdrawal | 14.33 | 7.79 | 11.15 | 7.74 | −3.18 | 7.63 | .001 |
| Craving | 17.83 | 8.10 | 8.23 | 5.10 | −9.56 | 8.31 | <.001 |
| Cigarettes per day | 12.40 | 7.88 | 3.01 | 3.41 | −9.39 | 7.75 | <.001 |
| Cotinine | 1145.56 | 1029.60 | 1281.26 | 1708.76 | 135.70 | 1649.62 | .506 |
| Exclusive smokers | |||||||
| Cigarette dependence | 52.20 | 16.62 | 37.53 | 17.13 | −14.67 | 16.21 | .004 |
| Withdrawal | 12.53 | 4.53 | 13.13 | 5.29 | 0.60 | 7.64 | .766 |
| Craving | 16.93 | 11.69 | 10.93 | 8.56 | −6.00 | 12.22 | .089 |
| Cigarettes per day | 14.75 | 11.50 | 11.36 | 11.97 | −3.38 | 7.65 | .097 |
| Cotinine | 1274.52 | 955.07 | 1267.21 | 820.29 | −7.31 | 659.61 | .966 |
Note. Bolded values indicate statistically significant omnibus tests. Scale ranges: Cigarette dependence = 15-76; Withdrawal = 0-32; Craving = 5-35.
Total nicotine dependence by time and group
Dual users showed a significant increase in total nicotine dependence from baseline to week 6 (p < .001). In contrast, cigarette smokers showed a significant decrease in total nicotine dependence (comprised only of cigarette dependence in this case) from baseline to week 6 (p = .004). Exclusive e-cigarette users did not show significant changes in total nicotine dependence (p = .123; Figure 1). Exclusive e-cigarette users and dual users had comparable e-cigarette dependence at week 6. However, dual users experienced a significantly lesser reduction in cigarette dependence compared to exclusive e-cigarette users, driving their increase in total nicotine dependence.
Figure 1. Total nicotine dependence by time and use group.
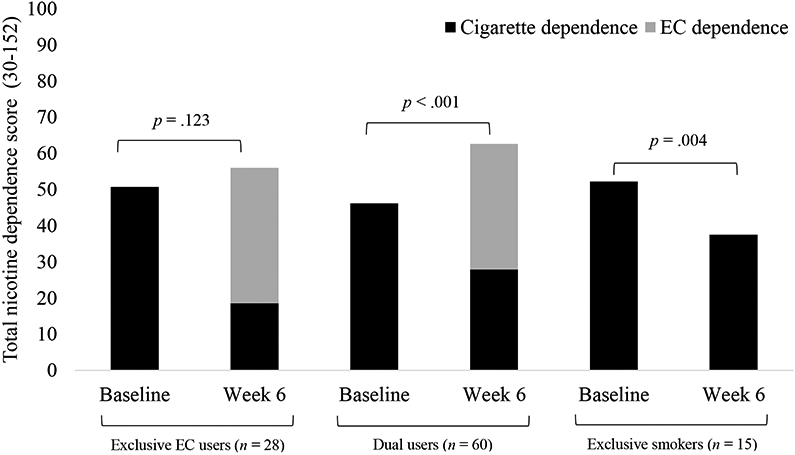
Note. EC = electronic cigarette. Participants with incomplete data for cigarette dependence at either timepoint or e-cigarette dependence at week 6 were excluded from analyses. Number excluded: exclusive EC users = 4, dual users = 6, exclusive smokers = 1.
Discussion
Consistent with our hypotheses, smokers who completely switched to e-cigarettes experienced the greatest reduction in cigarette dependence from baseline to week 6. Cigarette smokers who completely switched to e-cigarettes and those that were dual users, showed significant reductions in withdrawal and cigarettes per day while maintaining cotinine levels. Furthermore, exclusive e-cigarette users showed no significant changes in total nicotine dependence from baseline to week 6. Together these data provide evidence that smokers who are successful at completely replacing cigarettes with e-cigarettes transfer their dependence and maintain nicotine levels. Interestingly, even those that switched completely to e-cigarettes showed some continued cigarette dependence. Dependence is comprised of various facets including amount and frequency of use, but also aspects such as behavioral rituals, latency to discomfort, and stimulus control.23-25 Some of these components may take longer to decrease and eventually disappear than others that disappear immediately with discontinued use, such as amount and frequency.
While all groups maintained their cotinine levels, exclusive e-cigarette users and dual users showed greater reductions in craving and withdrawal compared to smokers. These data are consistent with studies showing that e-cigarette use is associated with reduced withdrawal symptoms compared to cigarette smoking.26-28 This finding also indicates that switching from smoking to exclusive e-cigarette use could facilitate complete cessation by decreasing factors which maintain use like uncomfortable withdrawal symptoms and urges to use.
Research has consistently shown that compared to combustible smoking, exclusive e-cigarette use is associated with reduced harm.5,6,29,30 Studies have shown that exclusive e-cigarette users and even dual users experience reductions in NNAL, carbon monoxide, and respiratory symptoms as they transition from traditional cigarette smoking to e-cigarettes.5,8,15,31-35 Smokers in this sample were able to switch to e-cigarettes when they maintained their nicotine levels and dependence, highlighting the importance of hitting the “sweet spot” of reinforcement value in order to help smokers switch to products associated with reduced harm. Smokers will be most likely to switch to a product that rivals cigarettes in terms of appeal and reinforcement while showing reduced toxicity, thereby minimizing harm from tobacco use.9
While dual users showed similar trends as exclusive e-cigarette users in terms of reductions in cigarette dependence, withdrawal, craving, and cigarettes per day, they also showed a significant increase in total nicotine dependence, due largely to similar e-cigarette dependence but a smaller magnitude of change in cigarette dependence relative to exclusive e-cigarette users. These findings are consistent with a recent study also showing increases in total nicotine dependence among dual users.17 This increased nicotine dependence among dual users is a concern because of health effects resulting from exposure to toxicants in both cigarettes and e-cigarettes and worry that overall consumption, particularly consumption of cigarettes, will increase as nicotine dependence rises. Health effects were beyond the scope of this paper and should be carefully monitored, although it is important to note that use of cigarettes decreased significantly among dual users from 12.4 (7.8) CPD at baseline to 3.0 (3.4) CPD at week 6 (p < .001). This would be expected to translate into reduced exposure to cigarette-related toxicants by way of reduced cigarette consumption. While the literature remains mixed, some recent data suggest dual e-cigarette and cigarette use is associated with poorer physical health outcomes,36,37 while other studies show harm reduction among dual users.32,33,35 In the current sample, dual users did not show increased nicotine levels despite increased dependence.
Interestingly, exclusive smokers showed reductions in total nicotine dependence (comprised only of cigarette dependence in this case) from baseline to week 6 despite not switching. Our data showed that at week 2, all but one of these participants was categorized as a dual user or exclusive e-cigarette user based on self-report, suggesting that almost all attempted to switch to e-cigarettes. While not statistically significant, this group experienced a reduction in CPD from baseline to week 6 supporting the idea that mere exposure and a switch attempt decreases cigarette dependence through short-term reduction in CPD.
The current study has important limitations that should be considered when interpreting these findings. The study included a relatively short follow-up period. While the observed trends are important, it remains unknown whether the changes will maintain or change over time. Future studies should incorporate longer-term follow-ups. Additionally, the sample was comprised exclusively of AA and Latinx smokers from two U.S. cities. Therefore, findings may not generalize to other populations or other geographical areas. Analyses regarding total nicotine dependence were exploratory. For unmeasured observations (i.e., participants reporting no e-cigarette/cigarette use), we assumed a dependence score of zero and used a simple sum score to examine total nicotine dependence. These analyses should be treated as hypothesis-generating. Sample sizes by use trajectory were relatively small; findings from the current study should be confirmed in larger studies. However, significant differences were found despite modest group sizes, suggesting robust effects. Finally, measures of dependence and biochemically verified use status were not assessed at weeks 2 or 4 of the current study. Future research should include comprehensive assessments at all timepoints to better understand the process of changes in dependence, withdrawal, craving, and tobacco use throughout switch attempts.
In conclusion, in this 6-week switching study exclusive e-cigarette and dual users showed reduced cigarette dependence, withdrawal, craving, and CPD while maintaining their cotinine levels. There is some concern over the high levels of nicotine in many pod-based e-cigarettes; however, the current study showed that smokers attempting to switch to a pod-based e-cigarette titrated their nicotine intake to maintain but not exceed their baseline nicotine levels. Together, these data suggest that pod-based e-cigarettes have similar reinforcement value as cigarettes and likely facilitate switching in part by transitioning dependence from an extremely harmful product to a similar level of dependence on a less harmful one and may be an important step in facilitating complete cessation by reducing withdrawal symptoms.
Acknowledgments:
Thank you to Tricia Snow, Brian Hernandez, and Michael Arnold at Kansas City, MO. Thank you to the research assistants of Dr. Pulvers’ Laboratory in San Diego, CA: Ana Leon, Jennifer Mosley-Garcia, Amanda Dean, Crystal Marez, Dalia Hipolito, Mirella Orozco, Justin Sanchez, Juan Alva, John Le, Madison Garrett, Nathan Au-Yeung, Jeremy Mills-Shimell, Shyla Everett, Alexis Osuna, Daniell Derry, and Flavia Ponce.
Supported by NIH 5SC3GM122628 (Pulvers), ClinicalTrials.gov Identifier: NCT03511001. ELSL supported on a CTSA award to the University of Kansas Medical Center NIH TL1TR002368. EIB supported on NIH T32-DA043469. KP, NN, and MR were supported by funding from the NIH (SC3GM122628; PI: Pulvers). Ahluwalia funded in part by P20GM130414, a NIH funded Center of Biomedical Research Excellence (COBRE).
Footnotes
Conflict of interest declaration: None
References
- 1.Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General. In: Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control; 2014. [Google Scholar]
- 2.Wang TW, Asman K, Gentzke AS, et al. Tobacco Product Use Among Adults - United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(44):1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owusu D, Huang J, Weaver SR, et al. Patterns and trends of dual use of e-cigarettes and cigarettes among US adults, 2015–2018. Preventive medicine reports. 2019;16:101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner KE How to think—not feel—about tobacco harm reduction. Nicotine and Tobacco Research. 2019;21(10):1299–1309. [DOI] [PubMed] [Google Scholar]
- 5.Goniewicz ML, Smith DM, Edwards KC, et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA network open. 2018;1(8):e185937–e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Round EK, Chen P, Taylor AK, Schmidt E Biomarkers of tobacco exposure decrease after smokers switch to an e-cigarette or nicotine gum. Nicotine and Tobacco Research. 2019;21(9):1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle RG, Richter S, Helgertz S Who is using and why: Prevalence and perceptions of using and not using electronic cigarettes in a statewide survey of adults. Addictive Behaviors Reports. 2019;10:100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pulvers K, Emami AS, Nollen NL, et al. Tobacco consumption and toxicant exposure of cigarette smokers using electronic cigarettes. Nicotine and Tobacco Research. 2018;20(2):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams DB, Glasser AM, Pearson JL, Villanti AC, Collins LK, Niaura RS Harm minimization and tobacco control: reframing societal views of nicotine use to rapidly save lives. Annual review of public health. 2018;39:193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajek P, Pittaccio K, Pesola F, Myers Smith K, Phillips-Waller A, Przulj D Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e-cigarette products. Addiction. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voos N, Goniewicz ML, Eissenberg T What is the nicotine delivery profile of electronic cigarettes? Expert opinion on drug delivery. 2019;16(11):1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leavens E, Smith T, Natale N, Carpenter M Electronic cigarette dependence and demand among pod mod users as a function of smoking status. Psychology of Addictive Behaviors. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harlow AF, Stokes A, Brooks DR Socioeconomic and racial/ethnic differences in e-cigarette uptake among cigarette smokers: longitudinal analysis of the population assessment of tobacco and health (PATH) study. Nicotine and Tobacco Research. 2019;21(10):1385–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovenco DPPMPH. Different Smokes for Different Folks? E-Cigarettes and Tobacco Disparities. American Journal of Public Health. 2019;109(9):1162–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulvers K, Nollen NL, Rice M, et al. Effect of Pod e-Cigarettes vs Cigarettes on Carcinogen Exposure Among African American and Latinx Smokers: A Randomized Clinical Trial. JAMA Network Open. 2020;3(11):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strong DR, Pearson J, Ehlke S, et al. Indicators of dependence for different types of tobacco product users: Descriptive findings from Wave 1 (2013–2014) of the Population Assessment of Tobacco and Health (PATH) study. Drug and alcohol dependence. 2017;178:257–266. [DOI] [PubMed] [Google Scholar]
- 17.Martínez Ú, Martínez-Loredo V, Simmons VN, et al. How does smoking and nicotine dependence change after onset of vaping? A Retrospective analysis of dual users. Nicotine and Tobacco Research. 2020;22(5):764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris KJ, Golbeck AL, Cronk NJ, Catley D, Conway K, Williams KB Timeline follow-back versus global self-reports of tobacco smoking: A comparison of findings with nondaily smokers. Psychology of Addictive Behaviors. 2009;23(2):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12(2):101. [Google Scholar]
- 20.Toll BA, Katulak NA, McKee SA Investigating the factor structure of the Questionnaire on Smoking Urges-Brief (QSU-Brief). Addictive behaviors. 2006;31(7):1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes JR, Hatsukami D Signs and symptoms of tobacco withdrawal. Archives of general psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 22.Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine and Tobacco Research. 2020;22(7):1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiffman S, Waters AJ, Hickcox M The nicotine dependence syndrome scale: a multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6(2):327–348. [DOI] [PubMed] [Google Scholar]
- 24.Piper ME, Piasecki TM, Federman EB, et al. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68). Journal of consulting and clinical psychology. 2004;72(2):139. [DOI] [PubMed] [Google Scholar]
- 25.Glover ED, Nilsson F, Westin Å, Glover PN, Laflin MT, Persson B Developmental history of the Glover-Nilsson smoking behavioral questionnaire. American journal of health behavior. 2005;29(5):443–455. [DOI] [PubMed] [Google Scholar]
- 26.Hughes JR, Peters EN, Callas PW, et al. Withdrawal Symptoms From E-Cigarette Abstinence Among Former Smokers: A Pre–Post Clinical Trial. Nicotine and Tobacco Research. 2020;22(5):734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes JR, Peters EN, Callas PW, et al. Withdrawal Symptoms From E-Cigarette Abstinence Among Adult Never-Smokers: A Pilot Experimental Study. Nicotine and Tobacco Research. 2020;22(5):740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rostron BL, Schroeder MJ, Ambrose BK Dependence symptoms and cessation intentions among US adult daily cigarette, cigar, and e-cigarette users, 2012-2013. BMC public health. 2016;16(1):814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manzoli L, Flacco ME, Ferrante M, et al. Cohort study of electronic cigarette use: effectiveness and safety at 24 months. Tobacco control. 2017;26(3):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baig SA, Giovenco DP Behavioral heterogeneity among cigarette and e-cigarette dual-users and associations with future tobacco use: Findings from the Population Assessment of Tobacco and Health Study. Addictive Behaviors. 2020:106263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P, Benowitz NL Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine & Tobacco Research. 2017;19(2):160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czoli CD, Fong GT, Goniewicz ML, Hammond D Biomarkers of exposure among “dual users” of tobacco cigarettes and electronic cigarettes in Canada. Nicotine and Tobacco Research. 2019;21(9):1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McRobbie H, Phillips A, Goniewicz ML, et al. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prevention Research. 2015;8(9):873–878. [DOI] [PubMed] [Google Scholar]
- 34.Shahab L, Goniewicz ML, Blount BC, et al. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: a cross-sectional study. Annals of internal medicine. 2017;166(6):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piper ME, Baker TB, Benowitz NL, Kobinsky KH, Jorenby DE Dual users compared to smokers: demographics, dependence, and biomarkers. Nicotine and Tobacco Research. 2019;21(9):1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parekh T, Pemmasani S, Desai R Risk of Stroke With E-Cigarette and Combustible Cigarette Use in Young Adults. Am J Prev Med. 2020;58(3):446–452. [DOI] [PubMed] [Google Scholar]
- 37.Bhatta DN, Glantz SA Association of E-Cigarette Use With Respiratory Disease Among Adults: A Longitudinal Analysis. Am J Prev Med. 2020;58(2):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]


