Abstract
Among cases of SARS-CoV-2 infections that result in serious conditions or death, many have pre-existing conditions such as hypertension and are on renin–angiotensin–aldosterone system (RAAS) inhibitors. The angiotensin-converting-enzyme-2 (ACE2), a key protein of the RAAS pathway, also mediates cellular entry of SARS-CoV-2. RAAS inhibitors might affect the expression levels of ace2, which could impact patient susceptibility to SARS-CoV-2. However, multi-organ-specific information is currently lacking and no species other than rodents have been examined. To address this knowledge gap, we treated adult zebrafish with the RAAS inhibitors aliskiren, olmesartan, and captopril for 7 consecutive days and performed qRT-PCR analysis of major RAAS pathway genes in the brain, gill, heart, intestine, kidney, and liver. Both olmesartan and captopril significantly increased ace2 expression in the heart, gill, and kidney. Olmesartan also increased ace2 expression in the intestine. Conversely, aliskiren significantly decreased ace2 expression in the heart. Discontinuation of compound treatments for 7 days did not return ace2 expression to baseline levels. While potential risks or benefits of antihypertensive RAAS inhibitors to SARS-CoV-2 infections in humans remain uncertain, this study provides new insights regarding the impact of RAAS inhibitors on organ-specific ace2 expression in another vertebrate model, thereby providing comparative data and laying scientific groundwork for future clinical decisions of RAAS inhibitor use in the context of COVID-19.
Subject terms: Gene expression, Hypertension, Pharmacogenetics, Animal physiology
Introduction
As a global pandemic, the Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected more than 140 million people and led to more than 3 million deaths worldwide. SARS-CoV-2 is an RNA virus that spreads and mutates rapidly. Although continued safety guidance and vaccination efforts have played an immense role in controlling the disease, it is likely to remain as a global pandemic for the foreseeable future. The clinical manifestations of SARS-CoV-2 are predominantly respiratory symptoms but some hospitalized patients also suffer from cardiac dysfunctions including myocardial injury, heart failure, and dysrhythmias1. Furthermore, studies have shown that hypertension is associated with increased risk of developing COVID-19 complications and increased mortality from COVID-192.
The angiotensin-converting-enzyme-2 (ACE2) is a coreceptor for cellular entry of SARS-CoV23. It is also a key component of the renin angiotensin system (RAAS). ACE2 negatively regulates the Angiotensin II receptor 1 activity by decreasing the ligand Angiotensin II thereby exerting organ-protective effects4,5. Many patients with hypertension and cardiovascular comorbidities are commonly prescribed with anti-hypertensive RAAS inhibitors, such as the angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin II receptor blockers (ARBs). Interest has grown on understanding whether the use of ACE-Is and ARBs can provide potential benefit or harm to COVID-19 patients. So far, clinical studies remain inconclusive regarding the relationship between RAAS inhibitors and outcomes in COVID-19 patients6. Animal studies aimed at determining how RAAS inhibitors might affect tissue ace2 levels have primarily used rodents and focused on restricted tissues (e.g., heart and kidney)7, leaving it unclear how these drugs may exert their effects in a broader set of organs and species. The impact of discontinuation of RAAS inhibitors on ace2 expression has not been evaluated.
In this study, we examined the effects of a panel of antihypertensive drugs (Table 1) on the transcript levels of ace2 and other RAAS pathway genes across six organs (brain, gill, heart, kidney, intestine, and liver) in adult zebrafish. Previous analysis of single cell transcriptomic data of zebrafish embryos has detected major RAAS pathway genes similar to humans8. Zebrafish as a vertebrate possess a high degree of genetic, physiological, and morphological similarity to humans9–11. Approximately 71% genes and 84% disease-associated genes are shared between zebrafish and humans12. Drugs can be conveniently delivered systemically through direct dissolution in the tank water. Our results have uncovered drug- and organ-specific effects on ace2 transcript levels and provide a critical comparative dataset in a new species.
Table 1.
Description of the anti-hypertensive drugs evaluated in this study.
| Drug | Pharmaceutical class | Mechanism of action | IC50 | MW (g/mol) | Therapeutic indication |
|---|---|---|---|---|---|
| Aliskiren | Direct renin inhibitor | Renin inhibitor blocking the conversion of angiotensinogen to angiotensin I | 1.5 nM | 609.79 | Hypertension |
| Captopril | Angiotensin converting enzyme inhibitor | Blocks the conversion of angiotensin I to angiotensin II | 6 nM | 217.29 | Hypertension, congestive heart failure, diabetic nepropathy |
| Olmesartan Medoxomil | Angiotensin II receptor antagonist | Selective binding to angiotensin I receptor for competitive blocking of angiotensin II | 66.2 μM | 558.59 | Hypertension, heart failure |
| Amlodipine besylate | Calcium channel blocker | Block the voltage-dependent L-type calcium channels to inhibit the influx of calcium | 1.9 nM | 567.05 | Angina, coronary artery disease, hypertension |
Results
Quantitative analysis of RAAS pathway gene expression in six organs reveals tissue-specific enrichment of ace2 transcripts
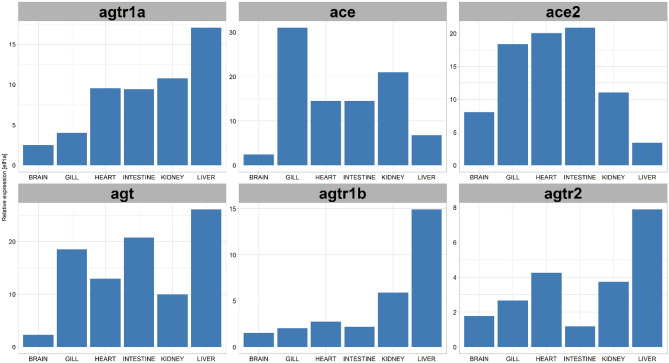
Major genes of the RAAS pathway and available small molecules that inhibit RAAS signaling are schematized (Fig. 1). To determine tissue-specific mRNA expression of RAAS genes in untreated conditions, adult zebrafish organs including the brain, gill (physiologically similar to the lung), heart, intestine, kidney, and liver were extracted from aged AB wildtype fish of 2–3 years old, to better mimic the life stage of COVID patients who might be taking these drugs (Fig. 2). After tissue homogenization and isolation of RNA, qRT-PCR was performed for angiotensinogen (agt), angiotensin II receptor type 1a (agtr1a), angiotensin II receptor type 1b (agtr1b), angiotensin II receptor type 2 (agtr2), angiotensin converting enzyme (ace), and Angiotensin converting enzyme 2 (ace2) (Fig. 3). The elongation factor 1 alpha (elf1a1) was used as the control housekeeping gene to quantify the relative expression of RAAS genes. There was no significant difference in RAAS gene expression levels between sexes for all the different organs (Fig. S1). For ace2, the gill, heart, and intestine showed higher expression levels of nearly 1.5-fold relative to elf1a1. The kidney, brain, and liver also showed ace2 expression but at lower levels compared to elf1a1. The liver showed high expression of agt and the angiotensin receptors agtr1a and agtr1b. Trace amounts of agtr2 expression was detected across all organs but showed lower levels relative to elf1a1. Together, while ace2 expression is detected in all organs examined, it appears to be enriched in the gill, heart, and intestine.
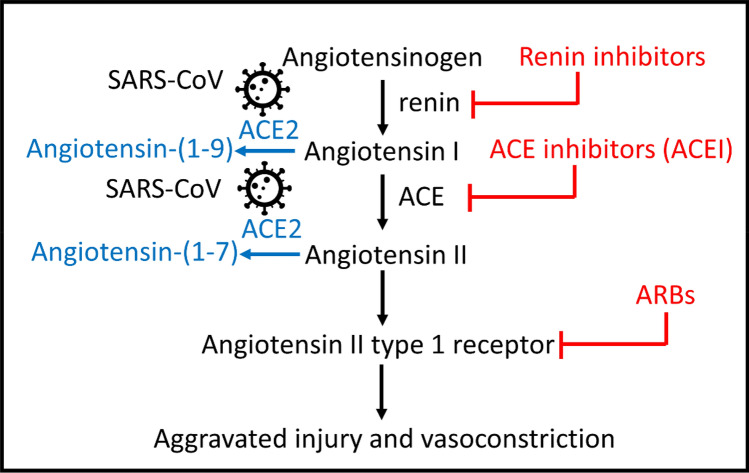
Figure 1.
The RAAS pathway and its relationship to SARS-CoV(1 and 2) viruses. Upon entry to cells, the SARS-CoV is known to bind to its functional receptor, angiotensin converting enzyme 2 (ACE2). In normal physiology, renin cleaves angiotensinogen, produced by the liver which yields angiotensin I. The angiotensin converting enzyme (ACE) converts angiotensin I to angiotensin II and the angiotensin II binds to the angiotensin II type 1 receptor which leads to vasoconstriction, aggravated tissue injury and hormonal production. ACE2 cleaves angiotensin II into angiotensin (1–7) to attenuate the effects of vasoconstriction. Another function of ACE2 involves cleaving angiotensin I to angiotensin-(1–9) for the hydrolysis of peptides such as apelin-1. Aliskiren is a direct renin inhibitor that blocks the conversion of angiotensinogen to angiotensin I. Captopril is an ACE-I that blocks the conversion of angiotensin I to angiotensin II. Olmesartan is an ARB ACE-I: Angiotensin Converting Enzyme Inhibitor, ARB: Angiotensin Receptor Blocker, ACE: Angiotensin Converting Enzyme-1, ACE2: Angiotensin Converting Enzyme-2.
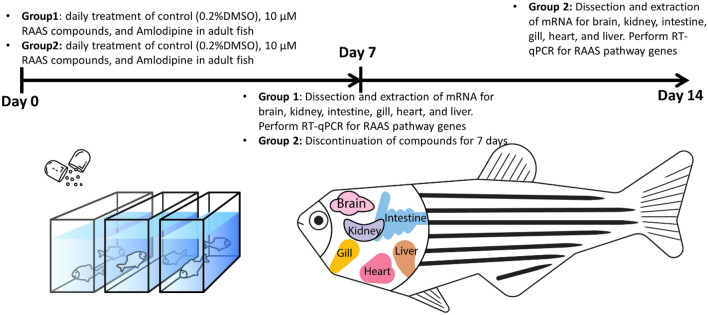
Figure 2.
A schematic diagram for the experimental design for drug treatment and discontinuation. To determine mRNA expression of RAAS genes following one week of chronic antihypertensive drug administration, Group 1 consisted of zebrafish that were treated with 0.2% DMSO (control), aliskiren, olmesartan, captopril, and amlodipine daily for 7 days, then dissected for qRT-PCR analysis. Additionally, Group 2 consisted of zebrafish that were treated identically as Group 1 for 7 days, but then drug administration was discontinued for 7 more days before dissection and qRT-PCR analysis. The extracted organs include the brain, kidney, intestine, gill, liver, and heart. Six biological replicates (3 males and 3 females) were used for each compound (or vehicle) in each group.
Figure 3.
RAAS pathway gene expression displays tissue-specific enrichment. The relative expression levels of agtr1a, ace, ace2, agt, agtr1b, and agtr2 to elf1a1 in different organs of wild type adult zebrafish (n = 6). agt: Angiotensinogen, agtr1b: Type-1B angiotensin II receptor, agtr2: Type-2 Angiotensin II Receptor, agtr1a: Type-1A angiotensin II receptor, ace: Angiotensin-converting enzyme 1, ace2: Angiotensin-converting enzyme 2.
Treatment with RAAS inhibitors increases ace2 expression in a drug- and organ- specific manner
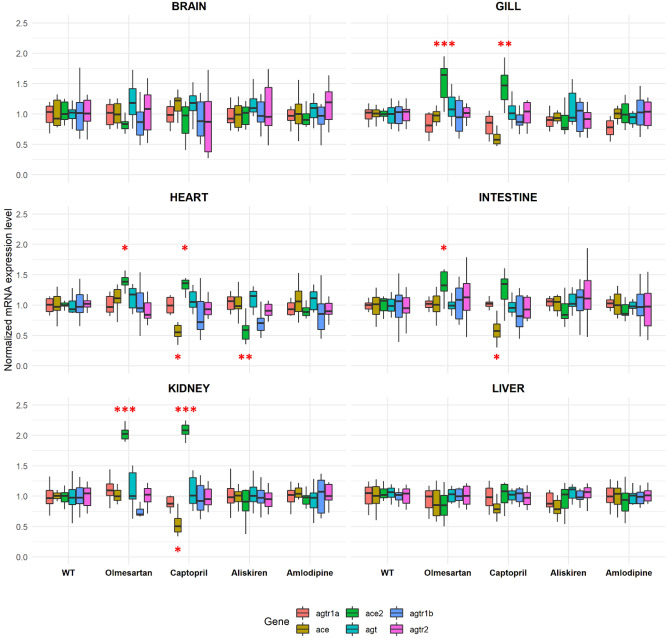
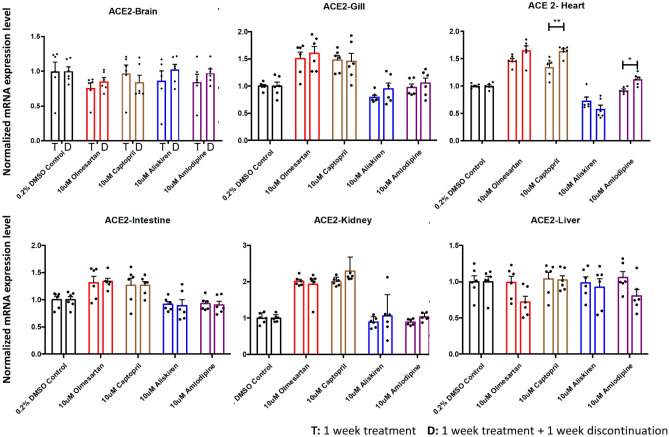
The transcript levels of the RAAS pathway genes were examined after 7 days of RAAS inhibitor or vehicle (0.2% DMSO) treatment (group 1) (Fig. 2) in aged AB wildtype zebrafish of 2–3 years old. Data were normalized to vehicle control for each gene-organ combination (Fig. 4). In addition to RAAS inhibitors, we also used amlodipine (a non-RAAS antihypertensive) as a comparison. Daily monitoring showed no physiological abnormalities, and all samples were included after the completion of 7-day treatment. Olmesartan treatment significantly increased ace2 expression in the gill, heart, intestine, and kidney (p < 0.001 for gill, p = 0.012 for heart, p = 0.043 for intestine, p < 0.001 for kidney, unpaired t-test). Captopril increased ace2 expression in the gill, heart, and kidney (p = 0.0087 for gill, p = 0.026 for heart, p < 0.001 for kidney, unpaired t test). Conversely, aliskiren treatment significantly decreased ace2 expression in the heart (p = 0.021). In addition, captopril decreased ace expression in the heart, intestine, and kidney (p = 0.012 for heart, p = 0.037 for intestine, p = 0.026 for kidney, unpaired t test). The interaction coefficient between drug treatment, gene, and organ identified a total of 11 significant combinations at the 95% confidence interval (Supplemental Fig. 2). Thus, RAAS inhibitors increase ace2 expression in a drug- and organ-specific manner: the ARB Olmesartan and ACE-I captopril commonly increase ace2 expression in the gill, heart, and kidney. Olmesartan also increases ace2 expression in the intestine. However, the renin inhibitor aliskiren decreases ace2 expression in the heart.
Figure 4.
Tissue-specific up-regulation of ace2 expression by RAAS inhibitors. The ace2 expression was significantly increased in captopril and olmesartan treated groups compared to the DMSO control in the gill, heart, and kidney (n = 6 per gene-organ-drug combination, *p < 0.05, **p < 0.01, ***p < 0.001 unpaired t test). Olmesartan also increased ace2 expression in the intestine (n = 6, p < 0.05, unpaired t test). Aliskiren treatment significantly decreased the ace2 expression in the heart; captopril treatment decreased ace expression in the heart, intestine, and the kidney (n = 6, p < 0.05, unpaired t test). Olm: olmesartan, Cap: captopril, Ali: aliskiren, Amlo: amlodipine.
The expression of ace2 is largely unchanged upon discontinuation of RAAS inhibitors for seven days
For a second group of zebrafish (group 2), the fish were treated with 7 days of RAAS inhibitors, amlodipine, or vehicle control. The compounds were then discontinued for 7 days, followed by organ dissection and qRT-PCR analysis (Fig. 2) to determine whether discontinuation would have an effect on gene expression levels. When comparing the ace2 expression between treatment (group 1) and treatment plus discontinuation (group 2), we found no significant ace2 expression differences in the brain, gill, intestine, kidney, and liver. The elevated ace2 expression in the gill, kidney, and the heart following 7-day olmesartan and captopril treatment was not significantly altered after 7-day discontinuation of these compounds. In contrast, discontinuing captopril and amlodipine treatment for 7 days significantly elevated ace2 expression in the heart compared to 7-day treatment with these compounds (p = 0.0013 for captopril, p = 0.0419 for amlodipine, unpaired t test) (Fig. 5). The decrease in ace2 expression with 7-day aliskiren treatment in the heart did not change following 7-day discontinuation. Together, 7-day discontinuation of RAAS inhibitors does not significantly alter ace2 transcript levels.
Figure 5.
Comparison of ace2 expression levels between treatment and discontinuation. The expression levels were compaired between the 1-week treatment group and 1-week treatment plus 1-week discontinuation group for different organs. The captopril and amlodipine treatment increased ace2 mRNA expression in the heart 7 days after discontinuation compared to 7-day treatment (n = 6 per group; *p < 0.05, **p < 0.01, unpaired t test). Other organs showed no significant difference between treatment and discontinuation. T: 1 week treatment D: 1 week treatment + 1 week discontinuation.
Discussion
In this study, we have determined that different RAAS inhibitors have varying effects on the ace2 mRNA expression across different organs. We have also shown that adult zebrafish express all the major components of RAAS pathway genes that have been used in this study. Many of these gene expression profiles across zebrafish organs closely resembled that of the human organs. For instance, the angiotensin receptor type 1 (AGTR1) RNA in humans is predominantly expressed in the liver, kidney, adrenal glands, and adipose tissues13. For the zebrafish, the liver showed the highest expression of both agtr1a and agtr1b followed by kidney, heart, and intestine. The zebrafish kidney showed the highest ace2 expression which is also the case of humans13. The RAAS gene expression in the brain showed minimal changes for both treatment and discontinuation. This could be because the antihypertensive drugs used in this study have very low bioavailability in the brain. While there are studies that show decreased ACE activity with oral captopril in the cerebral spinal fluid14, convincing evidence on whether these drugs cross the blood brain barrier remains unclear.
The gill, kidney, heart, and intestine showed significantly higher ace2 expression when treated with an ACE-I and an ARB. It is known that ACE inhibitors such as captopril activate the ACE2/angiotensin-(1–7) /Mas receptor axis which could lead to the increase of ace2 expression at the transcript level15,16. In a study conducted on Lewis rats it was shown that lisinopril, another commonly used ACE inhibitor, caused an increase in plasma Ang-(1–7), and increased cardiac ace2 mRNA but did not affect the ACE2 protein expression17. Interestingly, ace2 expression was decreased in the heart with 7-day aliskiren treatment. As a direct renin inhibitor, the pharmacological action of aliskiren is known to affect the AngII/ Ang1-7 signal axis18. In a diabetic neuropathy model of Sprague Dawley rats, it was shown that chronic administration of aliskiren decreased ACE2 expression in the kidneys and this decreased expression was also observed in another hypertension rat model18,19. As many patients who experience COVID symptoms have pre-existing cardiovascular comorbidities, it is common that these patients use RAAS inhibitors chronically. In our zebrafish model we have observed that the 7-day discontinuation did not alter the ace2 expression in most organs examined compared to the 7-day treatment groups. In this case, an abrupt discontinuation of RAAS inhibitors, particularly if already being used for other indications, would not be beneficial in managing COVID symptoms.
One side of the ongoing debate on use of RAAS inhibitors in the COVID-19 setting is toward continuing the medication based on the large cohort studies that find no association between the use of RAAS inhibitors and susceptibility to SARS-CoV-2 infection20–22. Studies are also trying to understand whether the increase in ace2 can potentially be linked to protective benefits. Clinical trials have been conducted on initiating losartan, another commonly used ARB, for COVID-19 hospitalized patients (NCT04312009). The downregulation of ACE2 with COVID-19 could lead to an increase in ACE activity resulting in damage to the alveolus and lead to acute respiratory failure which could warrant the use of RAAS inhibitors23. A meta-analysis of evaluating patients with COVID-19 showed a significantly lower risk of severe adverse events among patients who received ACE-Is or ARBs with implications on the protective benefits21. In another study it has been shown that the RAAS imbalance through angiotensin II or ACE2 blockage shows clinical manifestations closely resembling COVID-1924.
Although we have chosen three compounds with mechanism of actions that target different parts of the RAAS pathway, the ace2 expression could also be different based on the chemical structure, pharmacokinetics, and receptor affinities. Olmesartan medoxomil is a prodrug that requires hydrolysis to the active form. Olmesartan medoxomil undergoes hydrolysis to its active form by esterases during absorption in the gastrointestinal tract25. Valsartan and lisnopril, other commonly used RAAS inhibitors, are active drugs that do not undergo extensive metabolism and are excreted unchanged in the urine. Losartan, another ARB, has nearly tenfold greater selectivity compared to olmesartan26. Whether these differences in molecular effects have implications in the clinical setting is not clear. As we have tried to mimic the study to be a chronic treatment model, the treatment and discontinuation duration was chosen as 7 days based on other studies that involved continued drug treatment for pharmacological studies in zebrafish27,28. While this is certainly not a direct comparison to the long duration that many hypertension patients have been taking chronically for years, based on the pharmacokinetic properties of the RAAS inhibitors used in the study, the direct uptake of the drugs in the water bath should reach steady state at the target tissues during the treatment duration.
In conclusion, our study uncovers that RAAS inhibitors can influence the RAAS pathway gene expression in an organ-specific manner in zebrafish. The organs that were most sensitive to changes in ace2 expression include the gill (physiologically similar to the lung), heart, intestine, and kidney, which are all known target sites of COVID-19 leading to clinical manifestations. Furthermore, the expression levels after 7 days of discontinuation did not show remarkable changes in gene expression. Although more studies need to be done to understand how these gene expression profiles translate at the protein level, our study provides new insights into the transcript level modulation of RAAS pathway genes with RAAS inhibitor treatment. This basic knowledge lays foundation for deciding the use of RAAS inhibitors in the context of COVID-19.
Methods
Zebrafish husbandry and ethics statement
For all experiments, the wild type of the AB strain adult zebrafish was used in this study. All experimental protocols and procedures were approved by the University of California San Francisco Laboratory Animal Resource Center and Institutional Animal Care and Use Program. All study procedures were performed in compliance with the ARRIVE guidelines. The zebrafish were raised on a 14:10 h light/dark cycle and maintained in the zebrafish facility in accordance with the University of California San Francisco Institutional Animal Care and Use Committee standards.
Sample setup, drug treatment, and discontinuation
The adult zebrafish were housed in 1-L tanks separated based on what compound they receive and the treatment group (group 1) and treatment plus discontinuation group (group 2). For each group, three aged males and females between 2–3 years old were selected to control for sex and age distribution per compound (or vehicle control). Each tank was filled with 500 mL of system water along with the dissolved compounds. All samples were treated with a concentration of 10 μM olmesartan medoxomil, captopril, aliskiren hemifumarate, or amlodipine besylate. The vehicle control was treated with 0.2% DMSO. The tanks were changed to fresh system water and compounds daily. The compounds olmesartan medoxomil, captopril, aliskiren hemifumarate, amlodipine besylate were obtained from Sigma-Aldrich (cat #144,689–63-4, 62,571–86-2, 173,334–58-2, A5605).
Extraction of total RNA and cDNA synthesis from adult zebrafish organs
The adult zebrafish were treated with 2 µg/mL of tricaine for sedation and the brain, kidney, heart, intestine, liver, and gill were dissected. Total RNAs were prepared from isolated adult tissues of zebrafish using Trizol reagent (Invitrogen cat no 15596026) by homogenization and purified using RNeasy Mini Kit (Qiagen cat no 74104). cDNAs were synthesized from 1 µg of purified RNA using SuperScript® IV First-Strand Synthesis System for RT-PCR (Invitrogen cat no 18091050) and used as templates.
Quantitative polymerase chain reaction (qPCR) analysis
The primers for the qPCR were designed with the NCBI primer blast. The primer sequences and the ensemble ID for each gene were listed in Table 2. The PCR product size was designed to span 120 to 200 bp with low self 3′-complementarity score. Different primer designs were initially validated with gel electrophoresis to determine the optimal forward and reverse pair with specific amplification of the desired sized products. qPCR was performed using Applied Biosystems SYBR Green PCR Master Mix (Thermofisher cat no 4367659) and the ABI7900HT (Applied Biosystems machine cat no 4329001). Cycling conditions were 95 °C for 10 min, [95 °C for 15 s, 60 °C for 1 min 40 cycles], 20 °C for 2 min. Each sample was run with triplicates along with the positive control (elf1a1) in the top row of the MicroAmp Optical 384-Well Reaction Plate (Applied Biosystems cat no 4309849). The Ct values were exported and ΔCt values were calculated to compare relative expression of the genes of interest to the elf1a1 control.
Table 2.
Summary of RAAS pathway genes, RT-qPCR primer sequence, and Ensembl ID used for the RT-qPCR analysis.
| Gene Name | Gene symbol | Function | Primer Sequences (5′- > 3′) | Transcript ID | Product length |
|---|---|---|---|---|---|
| Angiotensin II receptor, type 1a | agtr1a | G protein-coupled receptor for angiotensin II | F: CATCCGTGGGACCCATTTCA | ENSDART00000021528.7 | 154 |
| R: GCAGTAGCACGTGAGGATGA | |||||
| Angiotensin II receptor, type 1b | agtr1b | G protein-coupled receptor for angiotensin II | F: TTCATGCCGTTTGGCTCAGA | ENSDART00000066834.5 | 200 |
| R: GGTCCTCGCTCATTGCTGAT | |||||
| Angiotensin converting enzyme | ace | Metallopeptidase involved in the conversion of angiotensin I to angiotensin II | F: GAGCCAATCCTGGCTTCCAT | ENSDART00000114637.4 | 133 |
| R: CCGATGACGCTGAGAGTGAC | |||||
| Angiotensin converting enzyme 2 | ace2 | Transmembrane protein catalyzing angiotensin II hydrolysis. Main entry point for coronavirus | F: CTGATGCCTGTCTTCCAGCA | ENSDART00000003712.8 | 141 |
| R: TTTCATCCCAACCCTGCTCC | |||||
| Angiotensinogen | agt | Precursor molecule for angiotensin I and the substrate for renin produced in the liver | F: GGCTTCGACACCTCAAGGAA | ENSDART00000010918.5 | 192 |
| R:ACACCACCTTGTTGAGTACCTTA | |||||
| Angiotensin II receptor, type 2 | at2 | G protein-coupled receptor. Regulation of aldosterone secretion | F: GTTCACGAACATCAGAACTCCC | ENSDART00000051532.5 | 188 |
| R: TGAGGCTGTAAAAGGCAGGG | |||||
| Elongation factor 1 alpha 1 | elf1a1 | Housekeeping gene for RT-qCPR | F: CTTCTCAGGCTGACTGTGC | Adopted from McCurley et al29 | – |
| R: CCGCTAGCATTACCCTCC |
Statistical analysis
The mRNA expression from the qRT-PCR was processed by the 2−ΔΔct method in comparison to the elf1a1 housekeeping gene. The histograms in the study are represented as means ± standard error of the mean (SEM). The box and whisker plot are represented as medians with first and third quartile ranges. The gene-organ-drug interaction was examined with the R ‘interactions’ package and multiple regression model. The comparison between drug treatment and discontinuation was compared with an unpaired t-test. The 95% confidence interval of the gene-drug-organ interaction was analyzed with the R program.
Supplementary Information
Acknowledgements
We thank Michael Munchua and Vivian Yuan for excellent animal care and maintenance of the zebrafish facility and the Guo lab members for helpful discussions throughout the study design and analysis. This work was supported by R01 NS120219 (to S.G.). G.J.K was a recipient of the Luis Zeh Fellowship, and A.M. was a recipient of the NSF Predoctoral Fellowship.
Author contributions
S.G. and G.J.K. conceived the project. G.J.K. and A.M. performed the experiments. G.J.K. and F.J analyzed data. F.J. and S.G. provided resources, supervision, and funding acquisition. G.J.K. and S.G. wrote the paper with the contributions from all authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gha-hyun J. Kim, Email: Jeffrey.kim@ucsf.edu
Su Guo, Email: Su.guo@ucsf.edu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-03244-5.
References
- 1.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Y, Zhou N, Zha W, Lv Y. Hypertension is a clinically important risk factor for critical illness and mortality in COVID-19: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2021;31:745–755. doi: 10.1016/j.numecd.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamorano Cuervo N, Grandvaux N. ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. Elife. 2020;9:e61390. doi: 10.7554/eLife.61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel VB, Zhong J-C, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circ. Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem. J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Kimmenade RRJ, et al. The effects of ACE2 expression mediating pharmacotherapy in COVID-19 patients. Netherlands Hear. J. Mon. J. Netherlands Soc. Cardiol. Netherlands Hear. Found. 2021;29:20–34. doi: 10.1007/s12471-021-01573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kriszta G, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on angiotensin-converting enzyme 2 levels: A comprehensive analysis based on animal studies. Front. Pharmacol. 2021;12:619524. doi: 10.3389/fphar.2021.619524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postlethwait JH, et al. The SARS-CoV-2 receptor and other key components of the renin–angiotensin–aldosterone system related to COVID-19 are expressed in enterocytes in larval zebrafish. Biol. Open. 2021;10:058172. doi: 10.1242/bio.058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo S. Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish? Genes. Brain. Behav. 2004;3:63–74. doi: 10.1046/j.1601-183X.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 10.van Rooijen E, Fazio M, Zon LI. From fish bowl to bedside: The power of zebrafish to unravel melanoma pathogenesis and discover new therapeutics. Pigment Cell Melanoma Res. 2017;30:402–412. doi: 10.1111/pcmr.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin JT, Fishman MC. From Zebrafish to human: Modular medical models. Annu. Rev. Genom. Hum. Genet. 2002;3:311–340. doi: 10.1146/annurev.genom.3.031402.131506. [DOI] [PubMed] [Google Scholar]
- 12.Howe K, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consortium, Gte. The Genotype-Tissue Expression (GTEx) project. Nat. Genet.45, 580–585 (2013). [DOI] [PMC free article] [PubMed]
- 14.Geppetti P, et al. Acute oral captopril inhibits angiotensin converting enzyme activity in human cerebrospinal fluid. J. Hypertens. 1987;5:151–154. doi: 10.1097/00004872-198704000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Wysocki J, Lores E, Ye M, Soler MJ, Batlle D. Kidney and lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: Implications for COVID-19. J. Am. Soc. Nephrol. 2020;31:1941–1943. doi: 10.1681/ASN.2020050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SimõeseSilva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1–7) and Mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 2013;169:477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrario CM, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 18.Ding W, et al. Aliskiren inhibits angiotensin II/angiotensin 1–7(Ang II/Ang1–7) signal pathway in rats with diabetic nephropathy. Chin. J. Cell. Mol. Immunol. 2018;34:891–895. [PubMed] [Google Scholar]
- 19.Hsu C-N, et al. Aliskiren administration during early postnatal life sex-specifically alleviates hypertension programmed by maternal high fructose consumption. Front. Physiol. 2016;7:299. doi: 10.3389/fphys.2016.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta N, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baral R, et al. Association between renin–angiotensin–aldosterone system inhibitors and clinical outcomes in patients with COVID-19: A systematic review and meta-analysis. JAMA Netw. Open. 2021;4:e213594–e213594. doi: 10.1001/jamanetworkopen.2021.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of covid-19. N. Engl. J. Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhry F, et al. Manipulation of ACE2 expression in COVID-19. Open Hear. 2020;7:e001424. doi: 10.1136/openhrt-2020-001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rysz S, et al. COVID-19 pathophysiology may be driven by an imbalance in the renin–angiotensin–aldosterone system. Nat. Commun. 2021;12:2417. doi: 10.1038/s41467-021-22713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma S-F, et al. Hydrolysis of Angiotensin II receptor blocker prodrug olmesartan medoxomil by human serum albumin and identification of its catalytic active sites. Drug Metab. Dispos. 2005;33:1911–1919. doi: 10.1124/dmd.104.002410. [DOI] [PubMed] [Google Scholar]
- 26.Miura S, Karnik SS, Saku K. Review: Angiotensin II type 1 receptor blockers: Class effects versus molecular effects. J. Renin. Angiotensin. Aldosterone. Syst. 2011;12:1–7. doi: 10.1177/1470320310370852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrunich-Rutherford ML. Chronic fluoxetine treatment of juvenile zebrafish (Danio rerio) does not elicit changes in basal cortisol levels and anxiety-like behavior in adulthood. PeerJ. 2019;7:e6407–e6407. doi: 10.7717/peerj.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dang M, Henderson RE, Garraway LA, Zon LI. Long-term drug administration in the adult zebrafish using oral gavage for cancer preclinical studies. Dis. Model. Mech. 2016;9:811–820. doi: 10.1242/dmm.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCurley AT, Callard GV. Characterization of housekeeping genes in zebrafish: Male–female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 2008;9:102. doi: 10.1186/1471-2199-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.