Graphical abstract
Keywords: Ginseng, Clinical trial, Bibliometrics, Data analysis
Abstract
Background
Ginseng has a long history of widespread use and remarkable effects as traditional medicine, adjuvant and dietary supplement. The therapeutic value, diverse functionalities and rapid development of ginseng have driven a significant increase in the number of ginseng clinical trials, ranging from its use in various ailments, formulation to safety concerns. Despite the persistent interest in ginseng clinical research, the medical effectiveness of ginseng is inconclusive and there is a lack of bibliometric analysis of the hundreds of ginseng clinical trials.
Aim of Review
This review aims to provide an extensive overview of ginseng clinical trials over the past 40 years (1979-2018) in combination with a qualitative and quantitative analysis. The annual clinical trial analysis of time distribution, country and institution network analysis for space cooperation, statistical analysis for various functions, as well as efficiency and effect size were performed for global ginseng clinical trials. Besides, preparation categories, administration routes, and the safety of ginseng clinical trials were also investigated.
Key Scientific Concepts of Review
The 40-year journey of ginseng clinical trials has experienced emerging, boom, and stable or transitional stages. The global network of ginseng clinical trials has relevant regional distribution in Asia, North America and Europe. South Korea makes a great contribution to building up large research clusters and strong cooperation links. Universities are the key contributors to ginseng clinical trials. The development of ginseng products could be focused on the clinical trial in diseases with higher effectiveness or effect size, such as sexual function and cognitive & behavior and require rigorous investigations and evidence to evaluate safety. More attention should be paid to different effects from different preparations. We believe this review will provide new insights into the understanding of global ginseng clinical trials and identifies potential future perspectives for research and development of ginseng.
Introduction
Ginseng is a time-honored herb internationally that belongs to the Araliaceae family, and it is commonly referred as the ginseng family. There are several species that are under the same name ginseng, such as Panax ginseng C. A. Mey. (P. ginseng) (Asian ginseng), Panax quinquefolius L. (PQ) (American ginseng), and Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Siberian ginseng). According to the processing methods, ginseng can also be classified into the following types: fresh ginseng (less than 4 years old, eaten fresh), white ginseng (4–6 years old, peeled and dried in an oven or air), sun ginseng (SUG) (white ginseng steamed at a high temperature and pressure), and red ginseng (RG) (6 years old, unpeeled and steamed) [1], [2].
Tracing back to thousands of years ago, China was the first country to use and record ginseng accurately and reliably. In Shennong’s Herbal Classic, the earliest existing monograph of Chinese medicine, ginseng was recorded as traditional medicine for calming the mind, stopping palpitations and brightening the eyes. Ginseng has also attracted attentions of the western countries and is approved by the German Commission E and the World Health Organization (WHO) as an adaptogen and tonic, as well as an anti-fatigue and anti-stress herb [3]. It is well known for consisting of high contents of ginsenosides (e.g. Rb1, Rb2, Rc, Rd, Re, and Rg1), which endorse ginseng with diverse bioactivities, including ameliorating cardiovascular functions [4], regulating glucose metabolism [5], benefiting cognitive and behaviors [6], and improving sexual functions [7].
The therapeutic value, diverse application and rapid development of ginseng have driven the increase of ginseng clinical trials significantly [8]. Despite the persistent interest in ginseng clinical research, the medical effectiveness is inconclusive and there is a lack of systematic analysis for the hundreds of ginseng clinical trials through qualitative and quantitative analysis in this field. Bibliometric analysis is widely used to investigate academic research shift and burst, explore current research frontiers and hotspots, and predict future research focuses and achievements [9], [10], [11]. It has been applied to understand the global and longitudinal trends of ginseng research [12], [13]. Meanwhile, CiteSpace is one of the most common and powerful software designed for the detection, analysis and visualization of the patterns and trends in scientific and systematic literatures [14], [15].
In this sense, the combination of bibliometrics and CiteSpace can deal with the large and complex ginseng clinical data, as well as provide special perspectives and meaningful conclusions. In this review, we intend to provide an extensive overview and analysis of global ginseng clinical trials over the past 40 years (1979–2018): Section I (Data collection and analysis) introduces the methodology and software used to analyze ginseng clinical trials; Section II (Time distribution of ginseng clinical trials) analyzes the annual number of ginseng clinical trials and summaries these clinical trials into three stages; Section III (Space cooperation of ginseng clinical trials) visualizes the country and institution network and discusses a landscape of the global trend in ginseng clinical trials; Section IV (Function classification of ginseng clinical trials) divides the clinical trials into 8 different function classifications and analyzes the efficiency and effect size of ginseng clinical trials; Section V (Ginseng preparations in clinical trials) describes the preparations and administration routes of ginseng; Section VI (Ginseng safety in clinical trials) presents the situations of side effects in ginseng clinical trials; finally, some concluding remarks are provided in the Conclusion and perspectives. We believe this study will shed light on the understanding of global ginseng clinical trials and identify potential future perspectives of ginseng research and development.
Data collection and analysis
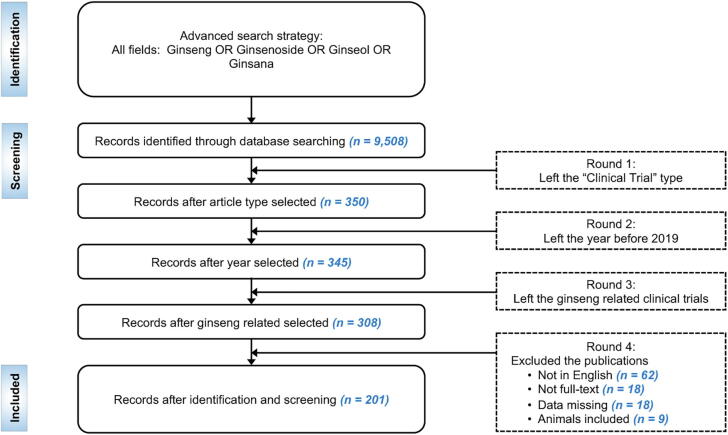
The following search strategies were applied based on the PubMed advanced search tutorial: (((Ginseng) OR (Ginsenoside)) OR (Ginseol)) OR (Ginsana) in full fields. “Clinical Trial” was selected, which met the inclusion criteria: 1) published before 2019, 2) related to ginseng individual or compound herbal medicines, 3) written in English, 4) involved human clinical trials, 5) provided sufficient data for analysis. In addition, there were no criteria set for gender, age or ethnicity. In other words, the clinical trials that do not meet these criteria were excluded. Fig. 1 presents the flow chart in details.
Fig. 1.
The flow-chart summary of the searching process.
CiteSpace software (Version 5.3.R4) was used to analyze the time distribution and space cooperation. The parameters were imported into the software as follows: 1) Time slicing: 1979–2018, 2) Years per slice: 1 year as the length of a single time slice, 3) Node type: country/institution, 4) Links: cosine was used for the connection strength and scope was used for the within slices, 5) Selection criteria: select all items from each time slice, 6) Pruning and Visualization: pathfinder, pruning sliced networks, cluster view-static and show merged network were selected. Function classification was analyzed by SPSS software (Version 21) for statistical analysis, including Fisher’s exact probability test, Chi-square test and Bonferroni correction.
Time distribution of ginseng clinical trials
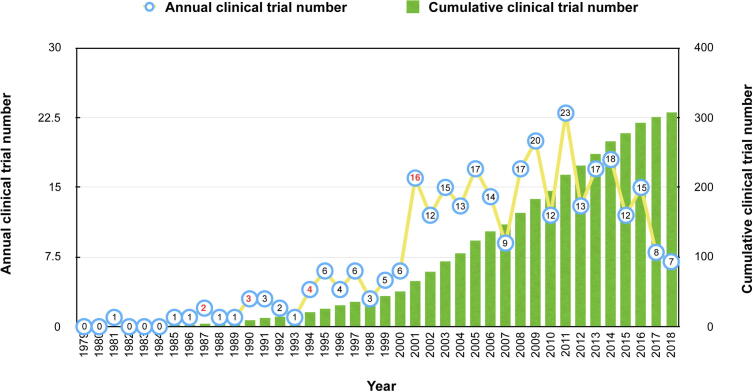
As shown in Fig. 1, 308 ginseng clinical trials were identified in the time distribution during the past 40 years (from 1979 to 2018). The average annual number of ginseng clinical trials was near to 8. The first clinical trial of ginseng was published in 1981. The second one was completed in 1985 and then ginseng clinical trials were conducted in each subsequent year. It was shown that there were decreases in 14 years compared to the last year (1988, 1992, 1993, 1996, 1998, 2002, 2004, 2006, 2007, 2010, 2012, 2015, 2017, and 2018), which accounted for 35% of the entire period. However, there was an overall growth trend according to the cumulative clinical trial number, with a distinct surge in 2001 and 2011. Moreover, the chain growth rates in the year 1987, 1990, 1994, and 2001 were twice or more than twice, with a significant increase. In 2012, it was the first time that the chain growth rate was near to negative 0.5 (Fig. 2).
Fig. 2.
The annual and cumulative clinical trial number of ginseng from 1979 to 2018. The blue circle represents the annual clinical trial number. The blue circle with red number represents the chain growth rate of the annual clinical trial number ≥1. The green column represents the cumulative clinical trial number. The yellow line represents the fluctuation.
From this aspect, the duration of the past 40 years can be preliminarily divided into three stages according to the tendency. Stage I (1979–2000) can be regarded as the emerging stage with muted growth. It suggests the initial development of ginseng clinical trials. Stage II (2001–2011) is called the boom stage, due to a rapid and apparent increase of ginseng clinical trials. Practically, the number peaked in 2011, which implied that there was a considerable interest in the ginseng clinical trial field at that time. Stage III (2012–2018) is recognized as the downtrend. This implicates that this field may have entered into a stable or transitional period.
Space cooperation of ginseng clinical trials
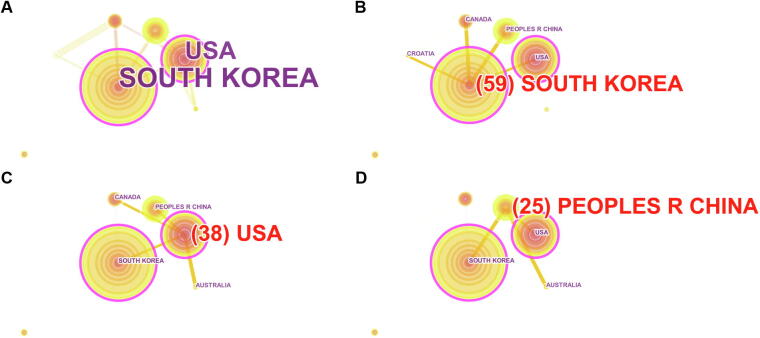
Country and institution network visualize a landscape of the global trend of research cooperation involved in the ginseng clinical trials. After the screening and exclusion, 201 clinical trials were selected for this analysis. Fig. 3A presents the network for all the countries with 7 nodes. The diameter of each line circle represents the intensity of the clinical trial number of the country. The thickness of the connecting line represents the intensity of country cooperation. Apparently, South Korea occupies the largest global share with nearly one third of the ginseng clinical trials (59) (Fig. 3B), which is followed by the USA (38) (Fig. 3C) and China (25) (Fig. 3D). The top 3 countries cooperate closely with each other and with other countries, such as Canada, Croatia, and Australia. Nevertheless, England displays isolated nodes with no connections. In Fig. 3B-3D, it shows that South Korea is connected with 4 countries, whereas the USA is connected with 4 countries, and China is connected with 3 countries.
Fig. 3.
Country relationship networks of ginseng clinical trials. (A) Network for all the countries. (B) South Korea network. (C) The USA network. (D) The People's Republic of China network.
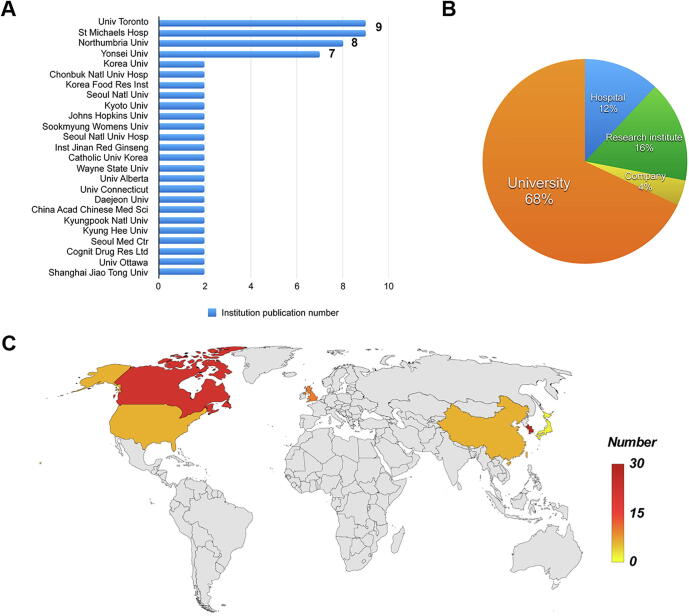
The number of clinical trials conducted in institutions was also analyzed. In total, 25 different institutions are shown in Fig. 4A, according to that the clinical trial number is equal to or greater than two. The University of Toronto and St Michaels Hospital have the largest number of clinical trials, which is followed by North Umbria University and Yonsei University. In addition, there are 4 classifications for institutions: University (17), Research institute (4), Hospital (3), and Company (1), as shown in Fig. 4B. In terms of the countries of the institutions, they are from South Korea (29), Canada (22), the UK (10), China (6), the USA (6), and Japan (2). All the institutions are in the colored countries (Fig. 4C). The institution with more clinical trials is presented in darker red color. Obviously, South Korea can be identified as the most productive country.
Fig. 4.
Institution relationship networks of ginseng clinical trials. (A) The bar chart shows the clinical trial numbers for each of the institutions. (B) The pie chart shows 4 institution classifications with percentages of the total number of clinical trials. (C) The map shows the geographic distribution of the global institutions.
Among these countries, South Korea has the most ginseng clinical trials and the closest cooperation with other countries. Meanwhile, the institutions from South Korea are ranked the first on the global country map, which is consistent with the trend described in a study in 2010 [12]. Therefore, South Korea makes a great contribution to the ginseng clinical trials by playing a leader role in working together to build up large research clusters and strong collaboration links. As a whole, Asia, North America and Europe are identified as the active research areas with the power of research distribution. The ginseng clinical trial global network has relevant regional distribution, which may be linked to the existence of different origins of ginseng species. In Asia, China is famous for Northeast ginseng and Siberian ginseng. Besides, Korean red ginseng (KRG) is also popular globally. In North America and Europe, PQ is the predominant ginseng [1]. In relation to institution types, it should be emphasized that universities are the key contributors to the number of clinical trials, and this phenomenon also exists in other research areas, such as global regulatory T-Cell research [16], global molecular modeling of cyclodextrins research [17], and global liposome research [18].
Researchers from China have published thousands of ginseng clinical research in the CNKI database (a key national research and information publishing institution in China). However, no institutes from China are ranked in the world top 10 ginseng clinical trial institutes. Consequently, it is necessary to enhance cooperation among countries and institutions, especially Chinese universities. Regional cooperation plays a vital role in enhancing more research outputs, and could effectively reduce the cost of clinical trials and improve research qualities and efficiencies [19], [20]. In addition, the governmental and financial supports, and the development of information and communication technologies are also indispensable. Therefore, we believe that the cooperation network will become more complicated with faster development and more communication between countries and institutions.
Function classification of ginseng clinical trials
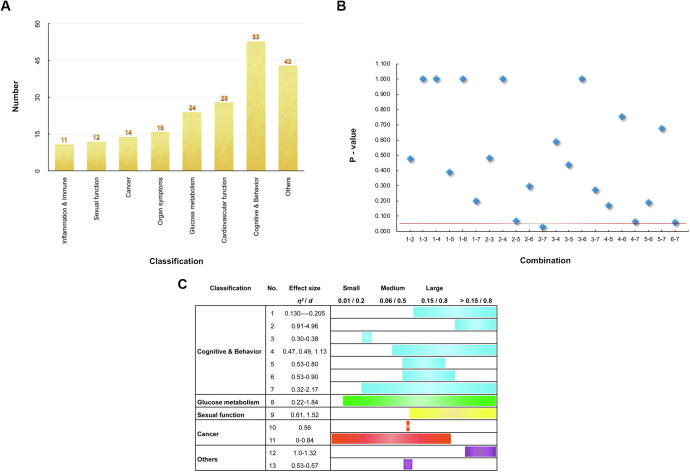
Two authors extracted the data independently in a predefined and standardized manner. The authors discussed the discrepancies and met to achieve a consensus on the data (title, objective, trial design, trial length, sample size, patients entered, function classification, medicine details (name, formulation, ingredients/position, manufacturer), intervention process (administration route, dosage), and main results (effects, side effect, and effect size)). All the clinical trials were sorted according to the clinical functions: inflammation & immune (11), sexual function (12), cancer (14), organ symptoms (16), glucose metabolism (24), cardiovascular function (28), cognitive & behavior (53), and others (43) (Fig. 5A).
Fig. 5.
The analysis of function classifications, efficiency and effect size of ginseng clinical trials. (A) The bar chart shows 8 function classifications with their total clinical trial numbers. (B) The scatter chart shows P values between two classifications, including 21 combinations: 1–2 (Inflammation & Immune-Sexual function), 1–3 (Inflammation & Immune-Cancer), 1–4 (Inflammation & Immune-Organ symptoms), 1–5 (Inflammation & Immune-Glucose metabolism), 1–6 (Inflammation & Immune-Cardiovascular function), 1–7 (Inflammation & Immune-Cognitive & Behavior), 2–3 (Sexual function-Cancer), 2–4 (Sexual function-Organ symptoms), 2–5 (Sexual function-Glucose metabolism), 2–6 (Sexual function-Cardiovascular function), 2–7 (Sexual function-Cognitive & Behavior), 3–4 (Cancer- Organ symptoms) and 3–5 (Cancer-Glucose metabolism), 3–6 (Cancer-Cardiovascular function), 3–7 (Cancer-Cognitive & Behavior), 4–5 (Organ symptoms-Glucose metabolism), 4–6 (Organ symptoms-Cardiovascular function), 4–7 (Organ symptoms-Cognitive & Behavior), 5–6 (Glucose metabolism-Cardiovascular function), 5–7 (Glucose metabolism-Cognitive & Behavior), and 6–7 (Cardiovascular function-Cognitive & Behavior). The blue dots represent the P-value, whereas the red line represents the P-value that is equal to 0.05. (C) The bar chart shows the effect size in different classifications. The blue column represents Cognitive & Behavior. The green column represents Glucose metabolism. The yellow column represents Sexual function. The red column represents Cancer. The purple column represents Others.
Cognitive & Behavior
The application of ginseng in the past serves as a platform for its modern clinical trials. In the ancient, ginseng was used to tranquilize mind and promote intelligence. Now, it can ameliorate mental health, improve social behavior, and benefit memory performance, such as secondary memory, quality of memory, and working memory, as shown in Supplementary Table 1. Moreover, the modern clinical effects of ginseng on improving cognitive function and behavioral symptoms in patients with moderately severe Alzheimer’s disease (AD) have also been proved [6].
Cardiovascular function
The current evidence does not support the use of ginseng to prevent or reduce cardiovascular risks. Ginseng contributes to the improvement in cardiac function and is beneficial for cardiovascular health, especially chronic heart failure (CHF) [21], and hypertension [22], as shown in Supplementary Table 2. Therefore, the pharmacological properties and molecular mechanisms of ginseng should be further investigated in the area of cardiovascular research.
Glucose metabolism
It has been confirmed by several clinical trials that ginseng can regulate glucose metabolism [23], [24]. As shown in Supplementary Table 3, all the clinical trials have similar conclusions, suggesting that ginseng may have beneficial effects on the prevention of diabetes. However, due to the inconsistent standardization of methodology and limited data from clinical trials, a definitive conclusion could not be made, which was consistent with the conclusion from a study in 2011 [25]. Thus, future studies must be focused on standardized methodologies, powerful calculation procedures and rigorous treatment regimens to reach a conclusive agreement.
Organ symptoms
Ginseng has been reported to ameliorate the symptoms occurring in organs, as shown in Supplementary Table 4. For example, ginsenoside Rb1 (GS-Rb1) can reduce oxidative stress and inflammation in chronic kidney disease (CKD) patients [26]. Shenmai injection (SMI) containing ginseng possesses a better protective effect on mitigating pulmonary gas exchange dysfunction in patients who were scheduled for lower extremity surgery [27]. Combined Ninjin-to can improve gastrointestinal motility and mucosal blood flow [28]. While KRG can protect subjects from contracting acute respiratory illness (ARI) [29]. In addition, KRG is regarded as a complementary therapy for chronic hepatitis B and an adjuvant for patients undergoing bile acid dissolution therapy for gallstones [30], [31]. Taken together, ginseng has considerable and potential clinical applications in treating organ symptoms.
Cancer
Ginseng has an excellent potential as an adjuvant for patients with cancer and cancer-related symptoms. There are 12 ginseng clinical trials in cancer, shown in Supplementary Table 5. Among all types of cancer, non-small cell lung cancer (NSCLC) accounted for the most studied cancer with a 25% share [32], [33], [34]. It is shown that ginseng is used frequently in combination with chemotherapy for adjuvant effects, and this reduces the side effects of the anti-cancer drugs for NSCLC patients. In addition, ginseng can improve cancer-related fatigue (CRF), as well as the overall quality of life [35], [36].
Sexual function
As presented in Supplementary Table 6, ginseng has different effects on different genders. On one hand, ginseng can treat infertility [37], and erectile dysfunction (ED) [38] in male patients. On the other hand, it is proved to improve sexual arousal, enhance sexual life, satisfy sexual desire, reduce vaginal dryness, and increase the frequency of sexual intercourse, orgasm and clitoral sensation in menopausal and postmenopausal women [39], [40]. Although notable improvements and positive effects were observed, the clinical trial data remains insufficient to make a consistent conclusion. As a result, ginseng is only treated as an alternative medicine or supplement in clinical trials, related to sexual function. All in all, clinical trials should be conducted to produce sufficient, novel and strong evidence, and ginseng with alternative effects in sexual function should be further developed, which has also been suggested by some evidence-based systematic reviews and meta-analysis [41], [42].
Inflammation & Immune
Among all the clinical trials of ginseng, the function of inflammation & immune has the smallest number of clinical trials, as shown in Supplementary Table 7. Importantly, it was shown that the ginseng and one of the active constituents of ginseng displayed the same effects. Regarding to inflammation, it was demonstrated that Ren Shen Yang Rong Tang (RSYRT) (a formulation product with ginseng) could decrease chronic inflammation [43], and ginsenoside compounds might inhibit inflammatory responses [44]. For the immune effects, RG [45] and ginseng polysaccharide (Y-75) [46] were reported to enhance immune function. For this reason, activity markers should be used more and more significantly in the further research on ginseng, and animal studies may provide important evidence to screen some potential markers for the study of clinical benefits of ginseng [47], [48].
Others
In addition to the above 7 classifications, ginseng clinical trials have been studied in diverse research areas, and were shown to exert a large number of functions, such as cellular DNA protection [49], menopausal syndrome transition [50], skin anti-aging function [51], CD4+ T cell slow depletion (in human immunodeficiency virus type 1-infected patients) [52], alcohol hangover symptom relief [53] and so on (Supplementary Table 8).
Efficiency and effect size analysis of ginseng clinical trials
Although most clinical trials of ginseng show obvious or potential effects, 20% of the clinical trials fail to achieve consistent outcomes in the several areas, including electrocardiograph values, blood pressure (BP), platelet function, arterial stiffness, renal function, glucose and insulin levels, impaired glucose tolerance, postprandial hypoglycemic, cognitive improvement, mood, stress, exercise performance, driving performance, fatigue, CRF, breast cancer survivors (BCSs), immune, human immunodeficiency virus, fibromyalgia, and ischemic stroke. The results were not consistent, either effective or ineffective (Table 1). Fisher’s exact probability test was shown pictorially among the 7 classifications with p < 0.05 (exact significance (2-sided) = 0.037), indicating that there was a significant difference. This value makes it necessary to use the four-fold table test to compare two classifications. The P values showing the differences between the two classifications, namely 21 combinations, are shown in Fig. 5B. The P values with p > 0.007 were considered to be insignificant between two classifications, according to the Bonferroni correction.
Table 1.
The count, excepted count and percentage of each classification for the effective and ineffective.
| No. | Classification | Item |
Effect |
Total | |
|---|---|---|---|---|---|
| Ineffective | Effective | ||||
| 1 | Inflammation & Immune | Count | 1 | 10 | 11 |
| Expected Count | 2.3 | 8.7 | 11.0 | ||
| % within Classification | 9.1% | 90.9% | 100.0% | ||
| 2 | Sexual function | Count | 0 | 12 | 12 |
| Expected Count | 2.5 | 9.5 | 12.0 | ||
| % within Classification | 0.0% | 100.0% | 100.0% | ||
| 3 | Cancer | Count | 2 | 12 | 14 |
| Expected Count | 2.9 | 11.1 | 14.0 | ||
| % within Classification | 14.3% | 85.7% | 100.0% | ||
| 4 | Organ symptoms | Count | 1 | 15 | 16 |
| Expected Count | 3.3 | 12.7 | 16.0 | ||
| % within Classification | 6.3% | 93.8% | 100.0% | ||
| 5 | Glucose metabolism | Count | 7 | 17 | 24 |
| Expected Count | 5.0 | 19.0 | 24.0 | ||
| % within Classification | 29.2% | 70.8% | 100.0% | ||
| 6 | Cardiovascular function | Count | 4 | 24 | 28 |
| Expected Count | 5.8 | 22.2 | 28.0 | ||
| % within Classification | 14.3% | 85.7% | 100.0% | ||
| 7 | Cognitive & Behavior | Count | 18 | 35 | 53 |
| Expected Count | 11.1 | 41.9 | 53.0 | ||
| % within Classification | 34.0% | 66.0% | 100.0% | ||
| 8 | Total | Count | 33 | 125 | 158 |
| Expected Count | 33.0 | 125.0 | 158.0 | ||
| % within Classification | 20.9% | 79.1% | 100.0% | ||
According to the analysis, there is a significant difference in all the classifications of the ginseng clinical trial effects. Sexual function has the highest effective rate, which means that ginseng could potentially be investigated in this area. However, it may be limited due to the sample size. The effective rate is ranked as Sexual function > Organ symptoms > Inflammation & Immune > Cancer > Cardiovascular function > Glucose metabolism > Cognitive & Behavior, so this provides a complete picture on the therapeutic value of ginseng clinical trials, which is beneficial for drug development. In contrast, a comparison that shows no significant difference suggests that the differences in clinical trial effects are focused on the integrated pattern, and are not mutually independent. Hence, the effects of ginseng clinical trials should be conducted at larger size and multi-classifications.
Effect size is used to evaluate the power of effect, including Cohen d and η2. Meanwhile, d = 0.2, d = 0.5 and d = 0.8 correspond to the small, medium and large effect sizes, respectively. Similarly, η2 = 0.01, 0.06, 0.15 are considered to be small, medium, and large in effect sizes, respectively. Among all the clinical trials, only 13 of them [36], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65] provided this information, as shown in Fig. 5C.
Most clinical trials were conducted in the medium-large range. In other words, these clinical trials have an intermediate level effect of power. However, the classification Cognitive & Behavior is in the large range mostly, which possesses potential effect of power for ginseng medical applications. Only 6% of all clinical trials presented the d or η2 parameters. Notably, very few clinical trials analyze the effect size. It is important to emphasize that this information should not be ignored. Moreover, the 6th edition of the American Psychological Association (APA) guideline reported in 2010 that it is necessary to record and report the effect size. Therefore, we suggest that the effect size may be a potential parameter to evaluate the effects of clinical trials and to help ginseng development.
Ginseng preparations in clinical trials
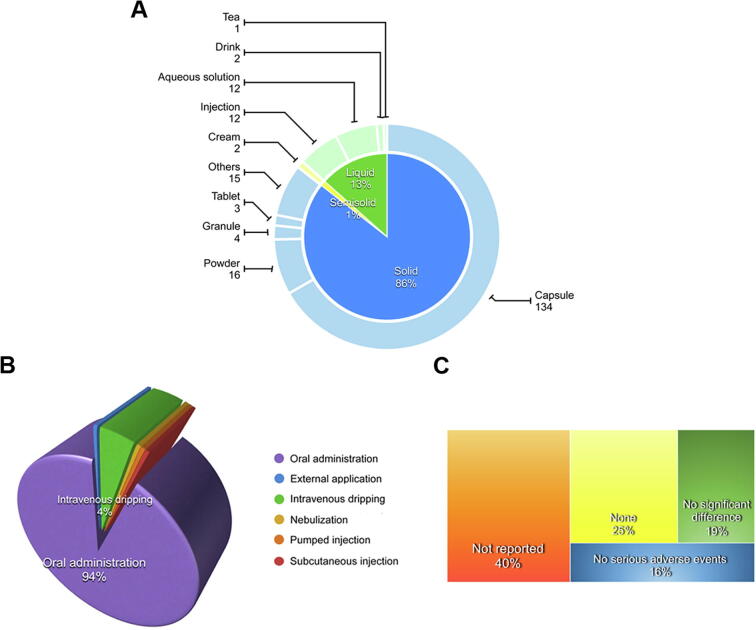
Fig. 6A and 6B show the preparations and administration routes in a pie chart and a 3D pie chart, respectively. The inner-circle is divided into three dosage forms, namely solid (172), semi-solid (2), and liquid (27). The solid dosage form includes capsule (67%), powder (8%), granule (2%), tablet (1%) and others (7%). On the other hand, the liquid dosage form includes 4 types, injection (6%), drink (1%), tea (1%), and aqueous solution (6%). The least commonly used dosage form is the semisolid cream (1%). Moreover, oral administration was shown to be the most common administration route, which was used by 188 clinical trials and the capsule is also widely used in ginseng clinical trials. Besides, Chinese medicine injection is also used but with a few shares (9).
Fig. 6.
The analysis of preparation categories, administration routes and side effects of ginseng clinical trials. (A) The pie chart shows the percentages of clinical trial number and the total number of clinical trials for different ginseng preparations. (B) The 3D pie chart shows the percentages of clinical trial number for different administration routes. (C) The tree diagram shows the percentages of clinical trials tested for side effect.
For P. ginseng, drink preparation does not have a significant impact on the BP parameters [66]. However, capsule [67] and powder [68] preparations have opposite effects. Considering the major active components of ginseng, each capsule of ginseng extract (100 mg) is standardized to contain 4% ginsenosides. 1 g powder consists of ginsenosides Rb1 (3.77 mg), Rg1 (0.57 mg), Re (1.86 mg), Rf (12.3 mg), Rb2 (1.24 mg), Rh1 (41.5 mg), Rc (0.99 mg) and Rg3 (100 mg). Nevertheless, ginsenoside profiles are limited for the drink preparation.
Based on the available clinical trials, different preparations have different and even opposite effects on BP, and not all the preparations provide information about the constituents and ratio of major active components of ginseng. Therefore, we suggest that ginseng preparations and its major active components should be reported in a standard way to achieve the accurate therapeutic effects of ginseng.
Ginseng safety in clinical trials
The tree diagram (Fig. 6C) shows the situation for recording side effects in ginseng clinical trials. Clinical adverse effects are categorized as no serious adverse events (16%; mild or slight side effects but not serious), no significant difference (19%; no statistically significant difference between groups), none (25%; no side effects), and not reported (40%; missing data for this part).
In total, 35% of clinical trials reported side effects of ginseng. The adverse effects included both physiological and psychological symptoms in humans. The mild and slight symptoms include dizziness, facial flushing, headaches, transient anxiety, diarrhea, insomnia, hypoglycemia, nausea, dyspepsia, stomach discomfort, body rash and pruritus, palpitation, urticarial condition, vomiting, fever, nervousness, and constipation with no regularities.
Most of the ginseng clinical trials have an important safety concern. 60% of the ginseng clinical trials were examined to be safe. However, it was shown that 40% of the clinical trials did not report any side effects in details, especially for Chinese medicine injection, and 50% of the clinical trials were lack of safety evaluation, such as Shenmai injection [69], Shenfu Injection [70], and Ginseng polysaccharides Injection [34]. With limited clinical trial information for safety evaluation, it is hard to make a definitive conclusion for the safe use of ginseng. Therefore, clinical trials must be designed specifically to test the adverse effects or toxicity in order to provide complete data for safety considerations, which can also enhance the significance of increasing quality of research. Meanwhile, safety is also the key factor for international development of Chinese medicine.
Conclusion and perspectives
In the past 40 years, the journey of ginseng clinical trials has experienced into three stages: emerging stage, boom stage, and stable or transitional stage. The global network of ginseng clinical trials has relevant regional distribution in Asia, North America and Europe. In particular, South Korea makes a great contribution to building up large research clusters and strong cooperation links with other countries. Moreover, universities are the key contributors to ginseng clinical trials, which contribute more than research institutes, hospitals or companies. There are 7 main function classifications of ginseng clinical trials. In terms of efficiency, ginseng shows potential medical effectiveness in various ailments, with the highest effectiveness in sexual function and the highest effect size in cognitive & behavior. Interestingly, different ginseng preparations display different and even opposite effects with the same condition.
Despite that ginseng plays an important role in both East and West of the world, the clinical efficacy of ginseng remains to be established. Based on the analysis of ginseng clinical trials, the development of ginseng products could be focused on the clinical trial in diseases with higher effectiveness or effect size, such as sexual function and cognitive & behavior. Meanwhile, more attention should be paid to the different effects from different preparations. Moreover, 35% of the clinical trials reported some side effects of ginseng, so it is warranted that ginseng products require more rigorous investigations and evidence to evaluate its safety, especially for the safety evaluation of herbal injections. In particular, ginseng clinical trials should be analyzed via a set of standardized ways and an unbiased assessment of their qualities. The CONSORT (Consolidated Standards of Reporting Trials) statement is a useful standardization for the design of clinical trials. Furthermore, the effect size, preparations with its major active components, and side effects should be used as potential parameters to evaluate the quality of ginseng clinical trials.
Although we conducted a global whole picture of ginseng clinical trials, the search strategy was limited to clinical outcomes published in English. Besides, the screening criteria did not include the evaluation of the quality of these ginseng clinical trials. Thus, the future direction should be focused on the evaluation of the quality of the ginseng clinical trials, which can provide better clinical trial analysis for ginseng based on more stringent principles and regulations.
Compliance with Ethics Requirements
This review does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors have declared no conflict of interest.
Acknowledgements
This work was supported by the grants of the Science and Technology Development Fund, Macau SAR (File No. 0013/2019/AFJ and SKL-QRCM(UM)-2020-2022), Guangxi Science and Technology Research Project (GuiKeAA18242040), and the Research Fund of the University of Macau (File No. MYRG2019-00143-ICMS).
Biographies

Weijie CHEN obtained her bachelor degree from China Pharmaceutical University in 2015, and master degree from University of Macau in 2017. She is a PhD candidate in Institute of Chinese Medical Sciences in University of Macau currently. She focuses on ginseng research, molecular dynamics, and data analysis.

Peifen YAO obtained her bachelor degree from Shandong University of Traditional Chinese Medicine in 2008, and master degree from Sun Yat-sen University in 2011. After then, she became a Ph.D candidate in University of Macau. Her research primarily focuses on the quality control and international standards of medicinal herbs.

Chi Teng VONG received her bachelor and master degrees from King’s College London, UK in 2008 and 2009, and obtained doctoral degree from University of Macau in 2017. She has been working as a research assistant at King’s College London for 3 years, and is currently working as a postdoctoral fellow at University of Macau. Her research interests are focused on the drug discovery and development for inflammatory diseases including Type 2 Diabetes and Inflammatory Bowel Disease.

Xiuzhu LI received her bachelor degree from South China Agricultural University of Food Science and Engineering in 2012. Now she is a master candidate of Institute of Chinese Medical Sciences in University of Macau. Currently, her research primarily focuses on the evaluation of clinical trials of herbal medicines.

Zhejie CHEN obtained obtained his bachelor degree of Pharmaceutical Engineering from Xihua University in 2015, and Master degree of Pharmaceutics from Chengdu University of Traditional Chinese Medicine in 2018. Since then, he became a Ph.D candidate of Institute of Chinese Medical Sciences in University of Macau.

Jianbo XIAO obtained his PhD in nutritional science from Okayama Prefectural University, Japan (2009). He worked as Humboldtian at University of Wuerzbug, Germany (2013-2015), prior to join University of Macau in 2015. Dr. Xiao has published over 100 papers on international journals. Prof. Xiao has been selected as 2016 and 2017 Clarivate Analytics Highly Cited Researcher (HCR) in agricultural science. Dr. Xiao is currently the associate editor of Phytochemical Analysis (Wiley) and Journal of Berry Research (IOS), the editorial boards of several international journals. He was the chairman of several international conferences.

Shengpeng WANG received his bachelor degree from Chengdu University of Traditional Chinese Medicine in 2010 and obtained his doctoral degree from University of Macau in 2016. He is currently an Assistant Professor of Institute of Chinese Medical Sciences in University of Macau. Dr. WANG primarily focuses on drug discovery from Chinese medicine for prevention and treatment of cancer and inflammatory bowel disease, as well as development of novel delivery systems and health products of Chinese medicine.

Yitao WANG is the founding Director of the Institute of Chinese Medical Sciences and Chair Professor at University of Macau as well as the Director of the State Key Laboratory of Quality Research in Chinese Medicine. He is also the Chief Scientist of Chinese Academy of Chinese Medical Sciences and the Director of International Research Centre of Medicinal Administration at Peking University. He is known internationally for his pioneering contributions to the modernization of Chinese medicine, with an emphasis on systematic evaluation and quality control of Chinese medicine. He has a long-standing interest in efficacy, safety, stability and controllability evaluation of Chinese medicine.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2020.07.016.
Contributor Information
Shengpeng Wang, Email: swang@um.edu.mo.
Yitao Wang, Email: ytwang@um.edu.mo.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Yun T.K. Brief introduction of Panax ginseng CA Meyer. J Korean Med Sci. 2001;16(Suppl):S3. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J., Yao Q., Chen C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7(3):293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riaz M., Rahman N.U., Zia-Ul-Haq M., Jaffar H.Z.E., Manea R. Ginseng: A dietary supplement as immune-modulator in various diseases. Trends Food Sci Technol. 2019;83:12–30. [Google Scholar]
- 4.Zhang Y, Chen M, Wang F, Rong Y, Lu B. Effect of Shengmai injection on cardiac function and inflammatory reaction in patients with acute coronary syndrome. Heart 2010;96(Suppl 3):A129–A. [DOI] [PubMed]
- 5.Bang H.J., Kwak J.H., Ahn H.Y., Shin D.Y., Lee J.H. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J Med Food. 2014;17(1):128–134. doi: 10.1089/jmf.2013.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakoot M., Salem A., Helmy S. Effect of Memo®, a natural formula combination, on Mini-Mental State Examination scores in patients with mild cognitive impairment. Clin Interv Aging. 2013;8:975. doi: 10.2147/CIA.S44777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi Y.D., Park C.W., Jang J., Kim S.H., Jeon H.Y., Kim W.G., et al. Effects of Korean ginseng berry extract on sexual function in men with erectile dysfunction: A multicenter, placebo-controlled, double-blind clinical study. Int J Impot Res. 2013;25(2):45. doi: 10.1038/ijir.2012.45. [DOI] [PubMed] [Google Scholar]
- 8.Vogler B.K., Pittler M.H., Ernst E. The efficacy of ginseng: A systematic review of randomised clinical trials. Eur J Clin Pharmacol. 1999;55(8):567–575. doi: 10.1007/s002280050674. [DOI] [PubMed] [Google Scholar]
- 9.Mazloumian A. Predicting scholars' scientific impact. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uddin S., Hossain L., Abbasi A., Rasmussen K. Trend and efficiency analysis of co-authorship network. Scientometrics. 2011;90(2):687–699. [Google Scholar]
- 11.Cobo M.J., López Herrera A.G., Herrera Viedma E., Herrera F. Science mapping software tools: Review, analysis, and cooperative study among tools. J Am Soc Inform Sci Technol. 2011;62(7):1382–1402. [Google Scholar]
- 12.Kim S.K., Park J.H. Trends in ginseng research in 2010. J Ginseng Res. 2011;35(4):389. doi: 10.5142/jgr.2011.35.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu W., Choi H.K., Huang L. State of Panax ginseng research: A global analysis. Molecules. 2017;22(9):1518. doi: 10.3390/molecules22091518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C. The citespace manual. Retrieved Octubre 2014;12.
- 15.Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inform Sci Technol. 2006;57(3):359–377. [Google Scholar]
- 16.Yin Z., Chen D., Li B. Global regulatory T-cell research from 2000 to 2015: A bibliometric analysis. PLoS ONE. 2016;11(9) doi: 10.1371/journal.pone.0162099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Q., Zhang W., Wang R., Wang Y., Ouyang D. Research advances in molecular modeling in cyclodextrins. Curr Pharm Des. 2017;23(3):522–531. doi: 10.2174/1381612822666161208142617. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X., Zhao G. Global liposome research in the period of 1995–2014: A bibliometric analysis. Scientometrics. 2015;105(1):231–248. [Google Scholar]
- 19.Zhang J., Han R., Chen W., Zhang W., Li Y., Ji Y., et al. Analysis of the literature and patents on solid dispersions from 1980 to 2015. Molecules. 2018;23(7):1697. doi: 10.3390/molecules23071697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howells J. The location and organisation of research and development: New horizons. Res Policy. 1990;19(2):133–146. [Google Scholar]
- 21.Wei H., Wu H., Yu W., Yan X., Zhang X. Shenfu decoction as adjuvant therapy for improving quality of life and hepatic dysfunction in patients with symptomatic chronic heart failure. J Ethnopharmacol. 2015;169:347–355. doi: 10.1016/j.jep.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Rhee M.Y., Cho B., Kim K.I., Kim J., Kim M.K., Lee E.K., et al. Blood pressure lowering effect of Korea ginseng derived ginseol K-g1. Am J Chinese Med. 2014;42(03):605–618. doi: 10.1142/S0192415X14500396. [DOI] [PubMed] [Google Scholar]
- 23.Vuksan V., Sievenpiper J.L. Herbal remedies in the management of diabetes: Lessons learned from the study of ginseng. Nutrit, Metabolism Cardiovascular Dis. 2005;15(3):149–160. doi: 10.1016/j.numecd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Lee H.J., Lee Y.H., Park S.K., Kang E.S., Kim H.J., Lee Y.C., et al. Korean red ginseng (Panax ginseng) improves insulin sensitivity and attenuates the development of diabetes in Otsuka Long-Evans Tokushima fatty rats. Metabolism. 2009;58(8):1170–1177. doi: 10.1016/j.metabol.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Sievenpiper J.L., Djedovic V., Cozma A.I., Ha V., Jayalath V.H., Jenkins D.J., et al. The effect of ginseng (the genus panax) on glycemic control: A systematic review and meta-analysis of randomized controlled clinical trials. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X., Lu Q., Wu J., Li Y., Sun J. Impact of extended ginsenoside Rb1 on early chronic kidney disease: A randomized, placebo-controlled study. Inflammopharmacology. 2017;25(1):33–40. doi: 10.1007/s10787-016-0296-x. [DOI] [PubMed] [Google Scholar]
- 27.Jin J., Shen H., Shan Y., Chen L., Zhao X., Wang L., et al. Effect of two administration routes of Shenmai Injection on pulmonary gas exchange function after tourniquet-induced ischemia-reperfusion. Chinese J Integrat Med. 2017;23(1):18–24. doi: 10.1007/s11655-016-2475-4. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y., Katagiri F., Inoue S., Itoh H., Takeyama M. Effects of Ninjin-to on levels of calcitonin gene-related peptide and substance P in human plasma. Biol Pharm Bull. 2004;27(12):2032–2034. doi: 10.1248/bpb.27.2032. [DOI] [PubMed] [Google Scholar]
- 29.Lee C.S., Lee J.H., Oh M., Choi K.M., Jeong M.R., Park J.D., et al. Preventive effect of Korean red ginseng for acute respiratory illness: A randomized and double-blind clinical trial. J Korean Med Sci. 2012;27(12):1472–1478. doi: 10.3346/jkms.2012.27.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi S.H., Yang K.J., Lee D.S. Effects of complementary combination therapy of Korean red ginseng and antiviral agents in chronic hepatitis B. J Alternat Compl Med. 2016;22(12):964–969. doi: 10.1089/acm.2015.0206. [DOI] [PubMed] [Google Scholar]
- 31.Lee J.K., Kang H.W., Kim J.H., Lim Y.J., Koh M.S., Lee J.H. Effects of Korean red ginseng as an adjuvant to bile acids in medical dissolution therapy for gallstones: A prospective, randomized, controlled, double-blind pilot trial. Food Funct. 2013;4(1):116–120. doi: 10.1039/c2fo30196b. [DOI] [PubMed] [Google Scholar]
- 32.Jiang S., Liu H., Liu Z., Liu N., Liu R., Kang Y.R., et al. Adjuvant effects of fermented red ginseng extract on advanced non-small cell lung cancer patients treated with chemotherapy. Chinese J Integrat Med. 2017;23(5):331–337. doi: 10.1007/s11655-015-2146-x. [DOI] [PubMed] [Google Scholar]
- 33.Yance D.R., Jr, Sagar S.M. Targeting angiogenesis with integrative cancer therapies. Integrat Cancer Therap. 2006;5(1):9–29. doi: 10.1177/1534735405285562. [DOI] [PubMed] [Google Scholar]
- 34.Ma J., Liu H., Wang X. Effect of ginseng polysaccharides and dendritic cells on the balance of Th1/Th2 T helper cells in patients with non-small cell lung cancer. J Tradit Chin Med. 2014;34(6):641–645. doi: 10.1016/s0254-6272(15)30076-5. [DOI] [PubMed] [Google Scholar]
- 35.Yennurajalingam S., Tannir N.M., Williams J.L., Lu Z., Hess K.R., Frisbee Hume S., et al. A double-blind, randomized, placebo-controlled trial of Panax ginseng for cancer-related fatigue in patients with advanced cancer. J Natl Compr Canc Netw. 2017;15(9):1111–1120. doi: 10.6004/jnccn.2017.0149. [DOI] [PubMed] [Google Scholar]
- 36.Barton D.L., Liu H., Dakhil S.R., Linquist B., Sloan J.A., Nichols C.R., et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: A randomized, double-blind trial, N07C2. J Natl Cancer Inst. 2013;105(16):1230–1238. doi: 10.1093/jnci/djt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park H.J., Choe S., Park N.C. Effects of Korean red ginseng on semen parameters in male infertility patients: A randomized, placebo-controlled, double-blind clinical study. Chinese J Integrat Med. 2016;22(7):490–495. doi: 10.1007/s11655-015-2139-9. [DOI] [PubMed] [Google Scholar]
- 38.Kim T.H., Jeon S.H., Hahn E.J., Paek K.Y., Park J.K., Youn N.Y., et al. Effects of tissue-cultured mountain ginseng (Panax ginseng CA Meyer) extract on male patients with erectile dysfunction. Asian J Androl. 2009;11(3):356. doi: 10.1038/aja.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh K.J., Chae M.J., Lee H.S., Hong H.D., Park K. Effects of Korean red ginseng on sexual arousal in menopausal women: Placebo-controlled, double-blind crossover clinical study. J Sexual Med. 2010;7(4):1469–1477. doi: 10.1111/j.1743-6109.2009.01700.x. [DOI] [PubMed] [Google Scholar]
- 40.Ito T.Y., Polan M.L., Whipple B., Trant A.S. The enhancement of female sexual function with ArginMax, a nutritional supplement, among women differing in menopausal status. J Sex Marital Ther. 2006;32(5):369–378. doi: 10.1080/00926230600834901. [DOI] [PubMed] [Google Scholar]
- 41.Durg S., Shivaram S.B., Bavage S. Withania somnifera (Indian ginseng) in male infertility: An evidence-based systematic review and meta-analysis. Phytomedicine. 2018;50:247–256. doi: 10.1016/j.phymed.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Jang D.J., Lee M.S., Shin B.C., Lee Y.C., Ernst E. Red ginseng for treating erectile dysfunction: A systematic review. Br J Clin Pharmacol. 2008;66(4):444–450. doi: 10.1111/j.1365-2125.2008.03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsiao P.J., Lin K.S., Chiu C.C., Chen H.W., Huang J.S., Kao S.Y., et al. Use of traditional Chinese medicine (Ren Shen Yang Rong Tang) against microinflammation in hemodialysis patients: An open-label trial. Complement Therap Med. 2015;23(3):363–371. doi: 10.1016/j.ctim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Xia Z., Liu X., Zhan L., He Y., Luo T., Xia Z. Ginsenosides compound (shen-fu) attenuates gastrointestinal injury and inhibits inflammatory response after cardiopulmonary bypass in patients with congenital heart disease. J Thoracic Cardiovascular Surgery. 2005;130(2):258–264. doi: 10.1016/j.jtcvs.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 45.Kim B.G., Shin K.S., Yoon T.J., Yu K.W., Ra K.S., Kim J.M., et al. Fermentation of Korean red ginseng by Lactobacillus plantarum M-2 and its immunological activities. Appl Biochem Biotechnol. 2011;165(5–6):1107–1119. doi: 10.1007/s12010-011-9328-6. [DOI] [PubMed] [Google Scholar]
- 46.Cho Y.J., Son H.J., Kim K.S. A 14-week randomized, placebo-controlled, double-blind clinical trial to evaluate the efficacy and safety of ginseng polysaccharide (Y-75) J Trans Med. 2014;12(1):283. doi: 10.1186/s12967-014-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z., Chen A., Sun H., Ye Y., Fang W. Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in mice. Vaccine. 2007;25(1):161–169. doi: 10.1016/j.vaccine.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 48.Ahn J.Y., Choi I.S., Shim J.Y., Yun E.K., Yun Y.S., Jeong G., et al. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 2006;36(1):37–45. doi: 10.1002/eji.200535138. [DOI] [PubMed] [Google Scholar]
- 49.Szeto Y.T., Sin Y.S.P., Pak S.C., Kalle W. American ginseng tea protects cellular DNA within 2 h from consumption: Results of a pilot study in healthy human volunteers. Int J Food Sci Nutr. 2015;66(7):815–818. doi: 10.3109/09637486.2015.1088937. [DOI] [PubMed] [Google Scholar]
- 50.Yakoot M., Salem A., Omar A.M. Effectiveness of a herbal formula in women with menopausal syndrome. Complement Med Res. 2011;18(5):264–268. doi: 10.1159/000333430. [DOI] [PubMed] [Google Scholar]
- 51.Hwang E., Park S.Y., Jo H., Lee D.G., Kim H.T., Kim Y.M., et al. Efficacy and safety of enzyme-modified Panax ginseng for anti-wrinkle therapy in healthy skin: A single-center, randomized, double-blind, placebo-controlled study. Rejuvenation Res. 2015;18(5):449–457. doi: 10.1089/rej.2015.1660. [DOI] [PubMed] [Google Scholar]
- 52.Sung H., Kang S.M., Lee M.S., Kim T.G., Cho Y.K. Korean red ginseng slows depletion of CD4+ T cells in human immunodeficiency virus type 1-infected patients. Clin Diagn Lab Immunol. 2005;12(4):497–501. doi: 10.1128/CDLI.12.4.497-501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee M.H., Kwak J.H., Jeon G., Lee J.W., Seo J.H., Lee H.S., et al. Red ginseng relieves the effects of alcohol consumption and hangover symptoms in healthy men: A randomized crossover study. Food Funct. 2014;5(3):528–534. doi: 10.1039/c3fo60481k. [DOI] [PubMed] [Google Scholar]
- 54.Teik D.O.L., Lee X.S., Lim C.J., Low C.M., Muslima M., Aquili L. Ginseng and ginkgo biloba effects on cognition as modulated by cardiovascular reactivity: A randomised trial. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0150447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steiner G.Z., Yeung A., Liu J., Camfield D.A., De Blasio F.M., Pipingas A., et al. The effect of Sailuotong (SLT) on neurocognitive and cardiovascular function in healthy adults: A randomised, double-blind, placebo controlled crossover pilot trial. BMC Complement Alternative Med. 2015;16(1):15. doi: 10.1186/s12906-016-0989-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ellis J.M., Reddy P. Effects of Panax ginseng on quality of life. Ann Pharmacother. 2002;36(3):375–379. doi: 10.1345/aph.1A245. [DOI] [PubMed] [Google Scholar]
- 57.Ko H.J., Kim I., Kim J.B., Moon Y., Whang M.C., Lee K.M., et al. Effects of Korean red ginseng extract on behavior in children with symptoms of inattention and hyperactivity/impulsivity: A double-blind randomized placebo-controlled trial. J Child Adolesc Psychopharmacol. 2014;24(9):501–508. doi: 10.1089/cap.2014.0013. [DOI] [PubMed] [Google Scholar]
- 58.Lee N., Lee S.H., Yoo H.R., Yoo H.S. Anti-fatigue effects of enzyme-modified ginseng extract: A randomized, double-blind, placebo-controlled trial. J Alternative Complement Med. 2016;22(11):859–864. doi: 10.1089/acm.2016.0057. [DOI] [PubMed] [Google Scholar]
- 59.Kim H.G., Cho J.H., Yoo S.R., Lee J.S., Han J.M., Lee N.H., et al. Antifatigue effects of Panax ginseng CA Meyer: A randomised, double-blind, placebo-controlled trial. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartz A., Bentler S., Noyes R., Hoehns J., Logemann C., Sinift S., et al. Randomized controlled trial of Siberian ginseng for chronic fatigue. Psychol Med. 2004;34(1):51–61. doi: 10.1017/s0033291703008791. [DOI] [PubMed] [Google Scholar]
- 61.Sievenpiper J.L., Arnason J.T., Leiter L.A., Vuksan V. Null and opposing effects of Asian ginseng (Panax ginseng CA Meyer) on acute glycemia: Results of two acute dose escalation studies. J Am Coll Nutr. 2003;22(6):524–532. doi: 10.1080/07315724.2003.10719331. [DOI] [PubMed] [Google Scholar]
- 62.Cai T., Wagenlehner F.M., Mazzoli S., Meacci F., Mondaini N., Nesi G., et al. Semen quality in patients with Chlamydia trachomatis genital infection treated concurrently with prulifloxacin and a phytotherapeutic agent. J Androl. 2012;33(4):615–623. doi: 10.2164/jandrol.111.013961. [DOI] [PubMed] [Google Scholar]
- 63.Barton D.L., Soori G.S., Bauer B.A., Sloan J.A., Johnson P.A., Figueras C., et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: A randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer. 2010;18(2):179. doi: 10.1007/s00520-009-0642-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gurley B.J., Gardner S.F., Hubbard M.A., Williams D.K., Gentry W.B., Cui Y., et al. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly. Drugs Aging. 2005;22(6):525–539. doi: 10.2165/00002512-200522060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gurley B.J., Gardner S.F., Hubbard M.A., Williams D.K., Gentry W.B., Cui Y., et al. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin Pharmacol Ther. 2002;72(3):276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 66.Shah S.A., Occiano A., Nguyen T.A., Chan A., Sky J.C., Bhattacharyya M., et al. Electrocardiographic and blood pressure effects of energy drinks and Panax ginseng in healthy volunteers: A randomized clinical trial. Int J Cardiol. 2016;218:318–323. doi: 10.1016/j.ijcard.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Caron M.F., Hotsko A.L., Robertson S., Mandybur L., Kluger J., White C.M. Electrocardiographic and hemodynamic effects of Panax ginseng. Ann Pharmacother. 2002;36(5):758–763. doi: 10.1345/aph.1A411. [DOI] [PubMed] [Google Scholar]
- 68.Jovanovski E., Bateman E.A., Bhardwaj J., Fairgrieve C., Mucalo I., Jenkins A.L., et al. Effect of Rg3-enriched Korean red ginseng (Panax ginseng) on arterial stiffness and blood pressure in healthy individuals: A randomized controlled trial. J Am Soc Hypertens. 2014;8(8):537–541. doi: 10.1016/j.jash.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Liu Q., Wu H., Wang J., Li X. Effects of Shenmai injection on the values of CO, SV, and EF in patients undergoing off-pump coronary artery bypass graft: A randomized, clinical trial. Medicine. 2018;97(10) doi: 10.1097/MD.0000000000010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng C., Min S. Cardioprotection of Shenfu Injection against myocardial ischemia/reperfusion injury in open heart surgery. Chinese J Integrat Med. 2008;14(1):10–16. doi: 10.1007/s11655-008-0010-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.