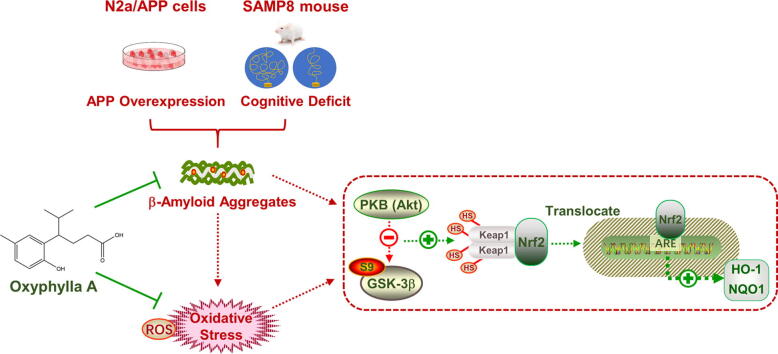
Graphical abstract
Abbreviations: AD, Alzheimer’s disease; SAMP8 mice, senescence-accelerated mouse prone 8; APP, amyloid precursor protein; Aβ, amyloid beta; NFTs, neurofibrillary tangles; Nrf2, erythroid-derived 2-related factor 2; ARE, antioxidant response element; HO-1, heme oxygenase-1; NQO1, NAD(P)H:quinone oxidoreductase1; Keap1, Keleh-like ECH-associated protein; GSK3, glycogen synthase kinase 3; AOE, ethanolic extract of Alpinia oxyphylla; PD, Parkinson’s disease; PHF, paired helical filaments; ROS, reactive oxygen species; ARE, antioxidant responsive element; pRL-TK, Renilla luciferase reporter plasmid; RLU, relative luciferase units; MWM, Morris Water Maze
Keywords: Alzheimer’s disease, Oxidative stress, oxyphylla A, SAMP8, Amyloid beta proteins
Abstract
Introduction
Alzheimer’s disease (AD) is a progressive brain disorder, and one of the most common causes of dementia and amnesia. Due to the complex pathogenesis of AD, the underlying mechanisms remain unclear. Although scientists have made increasing efforts to develop drugs for AD, no effective therapeutic agents have been found.
Objectives
Natural products and their constituents have shown promise for treating neurodegenerative diseases, including AD. Thus, in-depth study of medical plants, and the main active ingredients thereof against AD, is necessary to devise therapeutic agents.
Methods
In this study, N2a/APP cells and SAMP8 mice were employed as in vitro and in vivo models of AD. Multiple molecular biological methods were used to investigate the potential therapeutic actions of oxyphylla A, and the underlying mechanisms.
Results
Results showed that oxyphylla A, a novel compound extracted from Alpinia oxyphylla, could reduce the expression levels of amyloid precursor protein (APP) and amyloid beta (Aβ) proteins, and attenuate cognitive decline in SAMP8 mice. Further investigation of the underlying mechanisms showed that oxyphylla A exerted an antioxidative effect through the Akt-GSK3β and Nrf2-Keap1-HO-1 pathways.
Conclusions.
Taken together, our results suggest a new horizon for the discovery of therapeutic agents for AD.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by progressive decline in memory and learning, which leads to a highly compromised quality of life [1]. Apart from the cognitive deficits, studies have emphasized that muscle atrophy and motor impairment also occur in patients suffering from AD [2], [3]. Although considerable effort has been made over the past few years, the pathogenesis of AD still remains unclear. Senile plaques and neurofibrillary tangles (NFTs) are two primary hallmarks of sporadic AD [4], [5]. Amyloid beta (Aβ) peptide and abnormal phosphorylated tau protein are responsible for the formation of senile plaques and NFTs, respectively. Aβ peptides consist of amyloid monomers, which are derived from amyloid precursor proteins (APP). APP undergoes a proteolytic process via β-secretase and γ-secretase, the APP cleaving enzyme, ultimately leading to the formation of Aβ fragments and neurological damage [6], [7]. The accumulation of abnormally phosphorylated tau protein could lead to the formation of NFTs (sometimes presenting as paired helical filaments (PHF)), and finally to progression of the pathological process of AD [8]. Thus, Aβ and NFTs, and the underlying mechanisms thereof, are potential therapeutic targets for AD treatment. Moreover, studies have also shown that increased oxidative stress and activation of associated pathways contribute to the pathogenesis of AD. Researchers found that patients with AD exhibited an obvious change in oxidative stress biomarkers and mediators [9]. Nuclear factor erythroid-derived 2-related factor 2 (Nrf2) is a master transcription factor involved in the regulation of redox-related genes. When activated, the antioxidant response element (ARE) in promoters of redox-related genes cause an antioxidant cascade reaction [10], [11]. Several endogenous antioxidant enzymes, such as heme oxygenase-1 (HO-1) and NAD(P)H:quinone oxidoreductase1 (NQO1), can be further modulated by Nrf2 in the cascade reactions of oxidant stress [12]. Nrf2 activity is also tightly linked with Keleh-like ECH-associated protein (Keap1) [13]. Meanwhile, as a small kinase, glycogen synthase kinase 3 (GSK3) plays a well-established role in the pathogenesis of AD. Several studies demonstrated that GSK3, especially GSK3β, is associated with the pathology of both Aβ and phosphorylated tau in AD [14]. There is evidence that the inhibition of GSK3 by lithium had beneficial effects in transgenic mouse models overexpressing APP intracellular domain [15]. Recent studies have also found that suppression of GSK3β activity improved cognitive deficits and reduced the oxidative stress response [13]. However, due to the multiple phosphorylated sites of GSK3 (>20) [14], no effective disease-modifying treatment impeding or reversing the progress of AD is currently available. Thus, anti-oxidant stress might be a potential target for therapeutic interventions.
In the history of AD research, animal models have proven to be among the most important research tools, and have long been utilized. Senescence-accelerated mouse prone 8 (SAMP8) mice were derived from an AKR/J breeding colony [16]. Abiogenetic cognitive deficits and memory loss at certain ages were found in SAMP8 mice [16], [17]. Studies showed age-associated pathological changes, such as phosphorylation of tau protein, Aβ accumulation and blood–brain barrier dysfunction in the hippocampus of SAMP8 mice. These pathological alterations may result in behavioral and learning disturbances, as well as memory loss [18]. Therefore, the evidence suggests that SAMP8 mice could be a useful model of AD.
Oxyphylla A [19] is a novel compound extracted from Alpinia oxyphylla (known as Yi Zhi in Chinese). Alpinia oxyphylla has been used historically in traditional Chinese herbal formulas as a treatment of brain-related disorders, such as memory loss and learning dysfunction caused by neurodegenerative disease [20], [21]. In recent years, pharmacological studies have demonstrated the potential neuroprotective effects of ethanolic extracts of Alpinia oxyphylla (AOE) in models of Parkinson’s disease (PD) [22]. Our previous study showed that oxyphylla A could protect against neuronal damage both in vivo and in vitro [19], [23], [24]. In this study, we aimed to further investigate the therapeutic effect of oxyphylla A on cognitive deficits in vivo, and the mechanisms underlying the regulation of amyloid proteins in vitro.
Material and methods
Animals and treatment
Eight-month-old SAMP8 mice were selected for this study of the therapeutic effects of oxyphylla A on cognitive deficits. SAMR1 mice of the same age were used as the negative control. The animals were purchased from Zhishan (Beijing) Institute of Healthcare Research Co., Ltd. All animals were maintained in standard cages in an individually ventilated cage (IVC) system, and maintained under a 12 h:12 h light/dark cycle. Sufficient food and water were provided, and the temperature was maintained at 25 °C. The mice were divided into five groups: SAMR1, SAMP8, Donepezil-treated (1 mg/kg) and oxyphylla A-treated (10 or 20 mg/kg) groups. Donepezil was employed as the positive control. All drugs were dissolved in 20% PEG and 80% distilled water, and the animals were treated once a day for 6 weeks via oral gavage. Body weight was monitored and recorded weekly, while the survival rate was recorded daily during the 6-week study period. All animals were sacrificed under anesthesia (intraperitoneal injection of 40 mg/kg sodium pentobarbital) by cervical dislocation after 6 weeks of drug administration. The brains of the animals were dissected and different parts (cortex, hippocampus) were isolated on ice. Then, the brain tissues were placed directly into a −80 ℃ refrigerator before further experiments. The animal experiments in this study were in compliance with the ARRIVE Guidelines.
Morris water maze
The Morris water maze (MWM) equipment (SLY‑WMS Morris Water Maze System) was purchased from Beijing Sunny Instruments Co., Ltd. (Beijing, China). The equipment included a black circular pool and self-contained camera mounted on the top of the pool. The pool is 120 cm in diameter and 40 cm in height. Before every experiment, the pool is filled with non-toxic white-colored water (24–26 °C, 30 cm in depth). Four equal quadrants were created and a white escape platform was placed in one of the quadrants. The platform is 8 cm in diameter and located 1 cm below the surface of the colored water. The experiment was performed in a quiet, temperature-controlled (25 ℃) room. The MWM was performed twice (weeks 3 and 6). Mice first performed a navigation test for 5 consecutive days. The details of the training and testing are provided in the supplementary material.
Grip force test
Grip strength was measured by a grip strength meter (Yiyan Technology Ltd., Shandong, China) every 3 weeks. The mice in each group were positioned horizontally so that they could grasp the grip bar. Then, the grip bars were pulled back gradually until the mice released the bar. After repeating this process three times, the peak force of the paws was measured. The average result of the three measurements was analyzed.
Rota-rod test
The rota-rod test was performed every 3 weeks. Before the test, all mice were trained on the instrument (Yiyan Technology Ltd., Shandong, China) at 5 rpm for 5 min, for adaptation. After training, the mice were tested and the trial sustained for 10 min. The rotation speed was 22 rpm. The mice were allowed to run until they were exhausted and dropped off the rod three times within 5 min, or after 10 min had elapsed. The total distance was analyzed.
Cell culture and treatments
N2a/APP cells, also called NaS cells, were kindly gifted by Dr. Jiahong Lu (University of Macau) and cultured in full DMEM medium (supplemented with 10% (v/v) fetal bovine serum, 1% (v/v) PS, and 1% (v/v) G418). N2a/APP cells were established by transfecting and expressing human Swedish mutant APP695 gene stably in the mouse neuroblastoma N2a cell line. A wild-type N2a cell line (N2a/WT) was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured according to the protocol of the ATCC. All cells were cultured in a humidified incubator under conditions of 37 °C and 5% CO2.
N2a/WT cells were considered as the control group and the N2a/APP cells were treated with either 0.1% (v/v) DMSO or drug. For the drug treatment, cells were seeded in plates and treated with various concentrations of oxyphylla A when they reached 70% confluence. In the following experiments, cells were treated with LY294002 for 2 h and then incubated with oxyphylla A for another 24 h. The control group was treated with DMSO only. Both oxyphylla A and LY294002 were dissolved in DMSO to obtain stock solution (200 mM for oxyphylla A and 10 mM for LY294002). Details of the reagents used for cell culture and drug treatment are provided in the supplementary material.
Cell viability and toxicity
Thiazolyl bluet tetrazolium bromide (MTT) assay and lactate dehydrogenase activity (LDH) assay were used to measure cell viability and toxicity. N2a/WT and N2a/APP cells (8 × 103 cells/well) were seeded in a 96-well plate for 12 h. After incubation with oxyphylla A (12 μM ∼ 400 μM) for 24 h, the supernatant was removed for cytotoxicity analysis by LDH assay, while the remaining cells were used for cell viability analysis by MTT. The absorbance at 570 nm for MTT and 490 nm for LDH was measured using a Spectra Max M5 instrument (Molecular Devices, Sunnyvale, CA, USA). The optical density (OD) of each treatment group was compared with the control group. Details of the kit used for the MTT and LDH assays are provided in the supplementary material.
Determination of intracellular reactive oxygen species (ROS)
To detect intracellular ROS, probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Beyotime Biotechnology, Shanghai, China) was applied for further testing. N2a/WT and N2a/APP cells (12 × 103 cells/well) were seeded in a 12-well plate for 12 h. After treated with oxyphylla A for 24 h, cells from all groups were incubated with DMEM containing 10 μM DCFH-DA for 30 min before flow cytometry analysis. The increase in fluorescence was measured by the BD Accuri C6 Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and at least 10,000 events were acquired for further calculation. All data were analyzed by FlowJo software (FlowJo, LLC; BD Bioscience, NJ, USA) and the fluorescence intensity was normalized to the control group (N2a/WT group).
Preparation of brain tissue, whole cell, cell cytoplasmic and nuclear protein
For protein extraction from whole cells and mouse brain tissues, all samples were washed in PBS three times and incubated with RIPA lysis buffer for 30 min on ice (containing 1% phenylmethylsulphonyl fluoride and 1% protease inhibitor cocktail), and then homogenized and centrifuged. Then, the supernatant was collected and stored under 4 ℃ for further western blot analysis, as described below. Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Shanghai, China) was used in for subcellular protein extraction according to the manufacturer’s protocol. BCA protein kit assay was used to determine the protein concentrations. Details of the reagents used for protein extraction are provided in the supplementary material.
Western blot
Proteins were separated on either 10% or 12% Tris-HCl polyacrylamide gels (Bio-Rad, Hercules, CA, USA) according to the molecular weight of proteins by gel electrophoresis, and transferred onto a polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with 5% BSA in TBST, followed by incubation with specific primary antibodies (diluted in TBST, 1:1000) overnight at 4 °C, and then incubated with corresponding HRP-conjugated, anti-rabbit/mouse secondary antibodies (diluted in TBST, 1:5000) for 1 h at room temperature. ECL Select Western Blotting Detection Regent kit (GE Healthcare, Chicago, IL, USA) was used in the protein band detection process. The Bio-Rad ChemiDoc XRS Imaging System was used to scan the membranes, and the band intensity was analyzed by Bio-Rad Quantity One Software (Bio-Rad, CA, USA).
RNA extraction, reverse transcription and q-PCR
Total RNA was extracted from the mouse brain using TRIzol regent (Life Technologies, Eugene, OR, USA) according to the manufacturer’s protocol. Total RNA extraction from cells was done using the RNAprep Pure Cell/Bacteria Kit (TIANGEN, Beijing, China) according to the manufacturer’s protocol. Single-strand cDNA was reverse-transcribed from the extracted RNA using the SuperScript™ III First-Strand Synthesis System following the manufacturer’s instructions. q-PCR was performed on an Agilent Mx3005P QPCR System (Agilent Technologies, Santa Clara, CA, USA) using SYBR® Premix Ex Taq™ II (Tli RNaseH Plus; Takara Bio Inc., Shiga, Japan). More specific information on the reactions is provided in the supplementary material [25]. Forward (F-) and reverse (R-) primers are listed in the supplementary material.
Immunofluorescence assay
N2a/WT and N2a/APP cells (6 × 103 cells/well) were seeded in a 24-well plate for 12 h. After incubation with oxyphylla A for 24 h, cells from all groups were fixed with 3.7% PFA for 15 min at room temperature. Then, the PFA was removed and 0.3% (v/v) Triton X-100 (diluted in PBS) was added for permeabilization for 20 min. After permeabilization, cells were blocked with 2% BSA (diluted in PBS) for 1 h. Primary antibody (Nrf2, 1: 500; Proteintech, Rosemont, IL, USA) was added to 2% BSA and incubated with the cells at 4 ℃ overnight. The cells were further incubated with secondary antibody (Alexa Fluro 488 Rabbit Anti-Goat IgG, 1:1000; Cell Signaling Technology, Danvers, MA, USA) for 1 h. DAPI (diluted in PBS, 1:1000) was applied for nuclear staining. Images were taken using the IX73 florescence microscope (Olympus, Tokyo, Japan).
Luciferase reporter gene assay
In brief, pARE-luc reporter plasmid was transfected into cells after the cells had reached 60–70% confluence. Lipo8000 Transfection Reagent was used in the transfection process according to the manufacturer’s protocol. Co-transfection with Renilla luciferase reporter plasmid (pRL-TK) was done to control transfection efficiency. After transfection for 24 h, the transfected cells were subjected to further treatment. The luciferase activities of the cell samples were measured and analyzed by the Dual-Luciferase Reporter Assay. The source and information of the pARE-luc reporter plasmid are listed in the supplementary material.
Ethics statement
All experiments involving animals were conducted according to the ethical policies of, and procedures approved by, the Research Ethics Committee of Institute of Chinese Medical Sciences, University of Macau (UMARE-007–2016 and UMARE-AMEND-042).
Statistical analysis
Statistical analyses were performed, and figures generated, using GraphPad Prism statistical software (version 7.0; GraphPad Software Inc., San Diego, CA, USA). All data are expressed as the mean ± SD, and statistical significance was determined by one-way ANOVA with Dunnett’s test applied for multiple comparisons. Differences were accepted as significant at P < 0.05.
Results
Cytotoxic effects of oxyphylla A on N2a/APP and N2a/WT cells
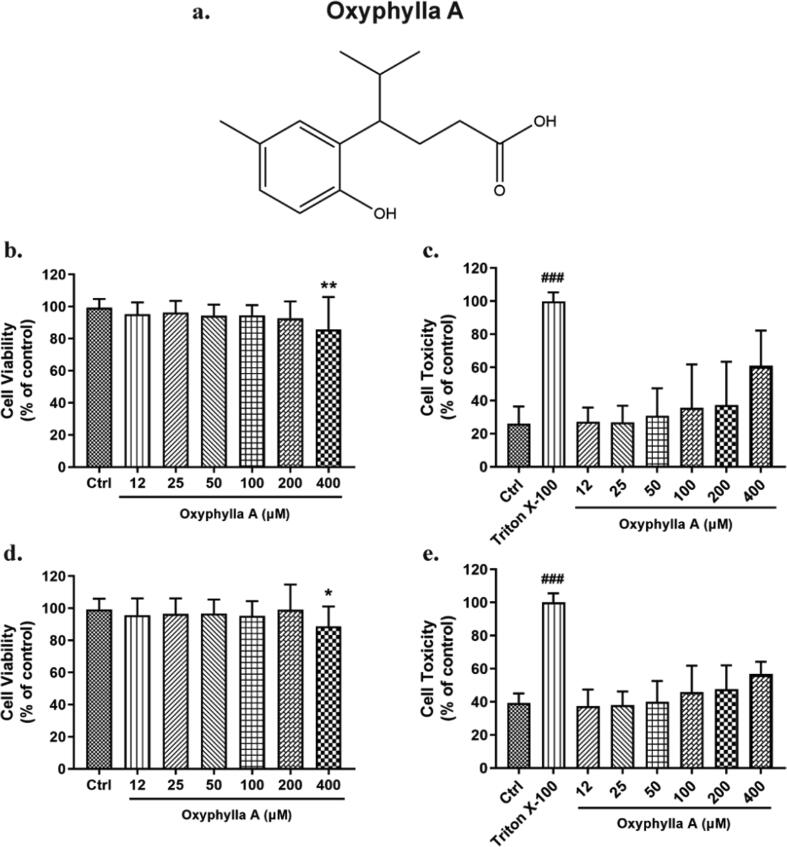
Before further exploration of the underlying mechanisms of oxyphylla A, the cytotoxic effects of various concentrations of oxyphylla A were evaluated in N2a/WT and N2a/APP cells. Cells were incubated with oxyphylla A (12–400 μM) for 24 h, and cell viability and cell toxicity were assessed by MTT and LDH assay. A concentration-dependent cytotoxic effect of oxyphylla A was found on N2a/WT and N2a/APP cells. The results showed that 400 μM oxyphylla A decreased cell viability slightly (Fig. 1 b, d). On the other hand, the results of LDH assay revealed a similar cytotoxic effect of oxyphylla A between N2a/WT and N2a/APP cells (Fig. 1 c, e). These results indicated that oxyphylla A was relatively safe from 12 to 200 μM on N2a/WT and N2a/APP cells.
Fig. 1.
Cell viability and cell toxicity of oxyphylla A on N2a/WT and N2a/APP cells. Cells in different groups were incubated with various concentrations of oxyphylla A (12–400 μM) for 24 h. MTT and LDH were employed to assess cell viability and toxicity. (a) The chemical structure of oxyphylla A. (b) and (c) Statistical analysis of the cell viability and toxicity of oxyphylla A on N2a/WT cells. (d) and (e) Statistical analysis of the cell viability and toxicity of oxyphylla A on N2a/APP cells. The data are expressed as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, compared to the control group; ###P < 0.001, compared to the Triton X-100 group.
Oxyphylla A attenuated the expression of amyloid proteins (APP, Aβ1-40 and Aβ1-42) in N2a/APP cells
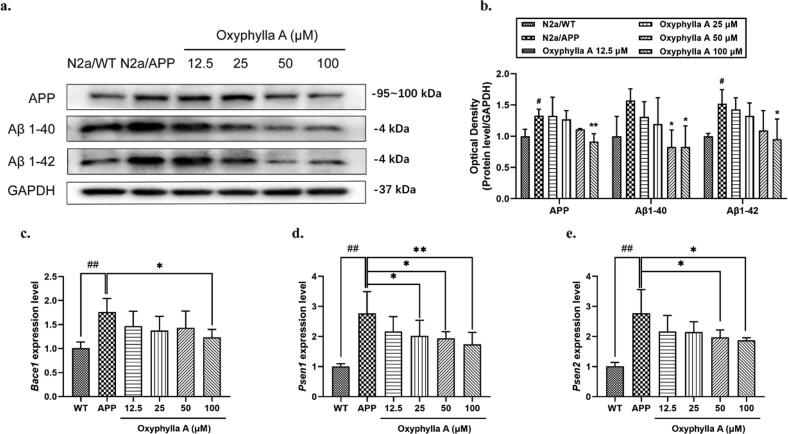
Aβ is the essential hallmark of the development of AD, and we hypothesized that oxyphylla A exerts its neuroprotective effects through regulating amyloid proteins expression. To test this hypothesis, N2a/APP and N2a/WT cells were further investigated. The N2a/APP cell is a mouse neuroblastoma cell line that stably expresses the human APP695 gene. After treatment with various concentrations of oxyphylla A (12.5–100 μM) for 24 h, cells were collected and protein was extracted for western blot. We chose the concentration of oxyphylla A according to our pilot experiments and previous results from our lab. The results showed that the expression levels of APP, Aβ1-40 and Aβ1-42 in N2a/APP cells were decreased by oxyphylla A in a dose-dependent manner (Fig. 2 a, b). Compared to the vehicle group, 100 μM of oxyphylla A enhanced the down-regulation of APP, Aβ1-40 and Aβ1-42. Further, we extracted mRNA to determine if oxyphylla A can affect genes associated with the proteolytic enzymes of amyloid proteins. As shown in Fig. 2, 100 μM of oxyphylla A significantly decreased the mRNA levels of Bace1, which encodes for the beta site APP-cleaving enzymes, Psen1 and Psen2, which encode for presenilin proteins constituted of the gamma-secretase intramembrane protease complex in N2a/APP cells (Fig. 2 c, d, e). These results indicated that oxyphylla A exerted its neuroprotective effects by down-regulating amyloid proteins and related genes in an in vitro AD model.
Fig. 2.
Effects of oxyphylla A on the expression levels of APP, Aβ1-40 and Aβ1-42. (a) N2a/APP and N2a/WT cells were incubated with oxyphylla A (12.5–100 μM) and DMSO, respectively, for 24 h. Western blot was used to detect the expression levels of APP, Aβ1-40 and Aβ1-42. (b) Statistical analysis of the expression levels of APP, Aβ1-40 and Aβ1-42. (c)–(e) Relative mRNA levels of Bace1, Psen1 and Psen2. The data are expressed as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, compared to the N2a/APP group; #P < 0.05, ##P < 0.01, compared to the N2a/WT group.
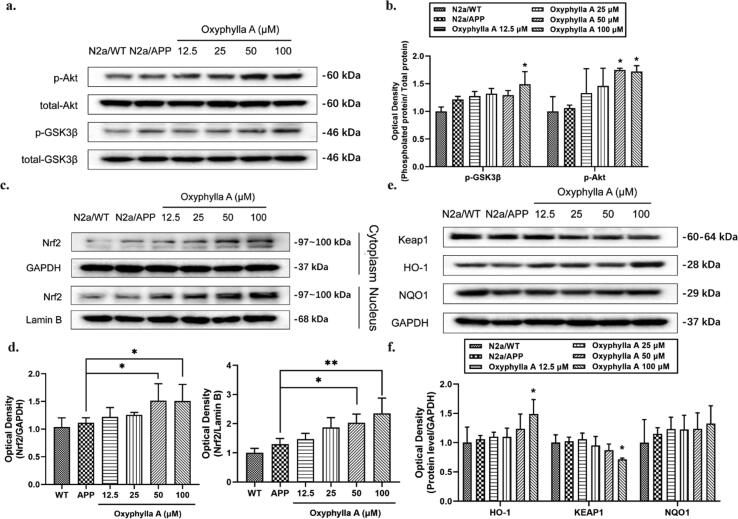
Oxyphylla A activated Nrf2 through the Akt/GSK3β pathway in N2a/APP cells
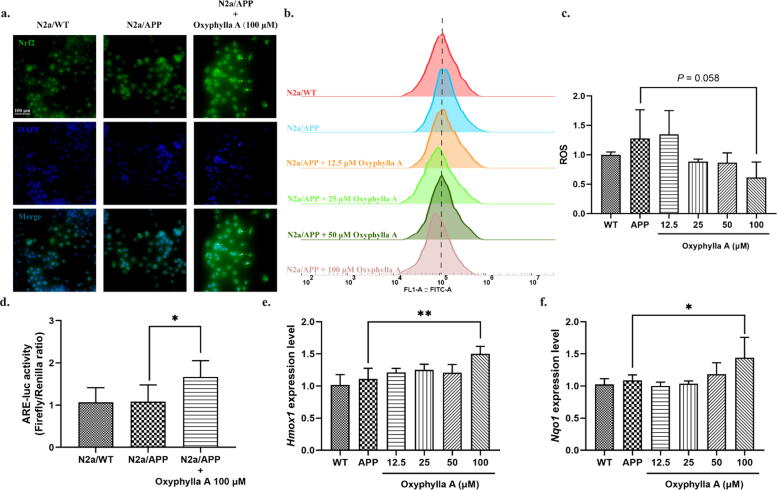
In some previous studies, oxidative stress proved to be a crucial mechanism underlying the pathogenesis of AD. Nrf2, a mediator of anti-oxidative stress, could act as a key regulator of antioxidation. Akt/GSK3β was also found to be associated with the activation of Nrf2. Thus, we next investigated if the neuroprotective effects of oxyphylla A involved the Akt/GSK3β and Nrf2/Keap1/HO-1 pathways. After treating N2a/APP cells with oxyphylla A, the ROS level was examined by flow cytometry, while phosphorylated and total protein levels of Akt, GSK3β, KEAP1, NQO1, HO-1, cytoplasmic and nuclear Nrf2 were determined by Western blotting. Our results showed that oxyphylla A reduced the ROS level (P = 0.0588) (Fig. 4 b, c) in N2a/APP cells. Further, the results of western blot showed that oxyphylla A up-regulated p-Akt (ser473), p-GSK3β (ser9), cytoplasmic and nuclear Nrf2 expression in N2a/APP cells in a concentration-dependent manner (Fig. 3 a–d). On the other hand, as a master regulator of the antioxidant response, Keap1 was significantly decreased by oxyphylla A (Fig. 3 e, f). Meanwhile, HO-1 and NQO1 which are the downstream target genes of Nrf2, were increased by oxyphylla A (Fig. 3 e, f). Furthermore, immunofluorescence staining indicated that, after treating N2a/APP cells with 100 μM oxyphylla A, Nrf2 translocated from the cytoplasm to nucleus (Fig. 4 a). The results of luciferase reporter gene assay showed that oxyphylla A could activate the antioxidant responsive element (ARE), which was responsible for the activation of downstream antioxidant enzymes (Fig. 4 d). Consistent results were observed in the changes of mRNA levels of Hmox1 and Nqo1 (encoding for HO-1 and NQO1, respectively) (Fig. 4 e, f). These results suggested that oxyphylla A might exert its neuroprotective effects through the Akt-GSK3β and Nrf2/Keap1/HO-1 pathways.
Fig. 4.
Effects of oxyphylla A on the activity of the Akt/GSK3β and Nrf2/Keap1/HO-1 pathways in N2a/APP cells. N2a/APP cells were incubated with various concentrations of oxyphylla A, while N2a/WT cells were incubated with DMSO for 24 h. (a) Representative immunofluorescence images of the translocation of Nrf2 from cytoplasm to nucleus. (b) and (c) Effects of oxyphylla A on ROS levels. (d) Firefly luciferase reporter plasmids carrying pARE-luc promoters were transfected into N2a/APP cells before oxyphylla A treatment. The Renilla luciferase reporter plasmid (pRL-TK) was used to normalize the transfection efficiency. Relative luciferase units (RLU) were statistically analyzed. (e) and (f) Relative mRNA levels of Hmox1 and Nqo1. The data are expressed as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, compared to the N2a/APP group.
Fig. 3.
Effects of oxyphylla A on the activity levels of the Akt/GSK3β and Nrf2/Keap1/HO-1 pathways in N2a/APP cells. N2a/APP cells were incubated with various concentrations of oxyphylla A, while N2a/WT cells were incubated with DMSO for 24 h. Western blot was applied to detect the expression levels of total and phosphorylated proteins. (a)–(d) Western blot results and statistical analysis of the expression levels of p- GSK3β (ser9), p-Akt (ser473), Nrf2, Keap1, HO-1 and NQO1. The data are expressed as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, compared to the N2a/APP group.
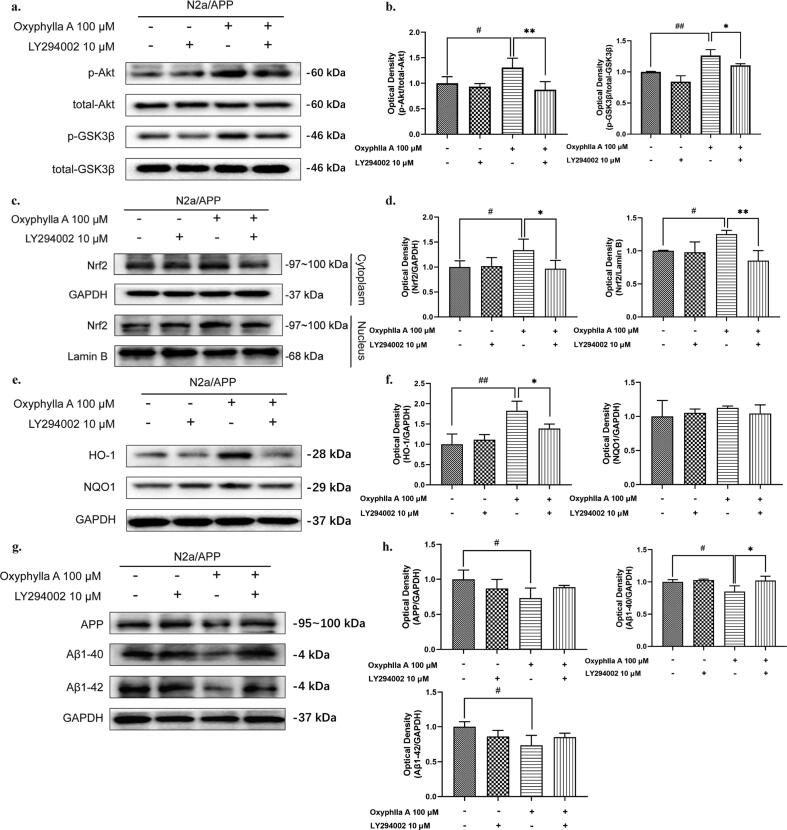
Inhibition of the Akt/GSK3β pathway attenuated the activating effects of oxyphylla A on Nrf2 and reversed its down-regulation of APP, Aβ1-40 and Aβ1-42 in N2a/APP cells
To further confirm whether oxyphylla A activated the Nrf2/Keap1/HO-1 pathway through the Akt/GSK3β pathway, we investigated whether the Akt pathway inhibitor modulated the antioxidant effect of oxyphylla A in N2a/APP cells. The western blot results showed that pre-incubation of LY294002 for 2 h followed by 24 h-treatment of oxyphylla A partially abolished the antioxidant effect of oxyphylla A in N2a/APP cells. Compared to the oxyphylla A-treated group, the increased expression levels of p-Akt (ser473), p-GSK3β (ser9), HO-1, cytoplasmic and nuclear Nrf2 were reversed by LY294002 (Fig. 5 a–f). Similar results were also observed in terms of the expression levels of APP, Aβ1-40, Aβ1-42 and the luciferase reporter gene assay of ARE (Fig. 5 g, h and Fig. S 3). These data indicated that oxyphylla A activated the Nrf2/Keap1/HO-1 pathway partially through the Akt/GSK3β pathway.
Fig. 5.
The role of Akt in the antioxidative effects of oxyphylla A on N2a/APP cells. N2a/APP cells were incubated with oxyphylla A for 24 h, with or without pretreatment with the Akt pathway inhibitor LY294002 for 2 h. Western blot was applied to assess the expression levels of total and phosphorylated proteins. (a)–(h) Western blot results and statistical analysis of the expression levels of p-Akt, p- GSK3β, Nrf2, HO-1, NQO1, APP, Aβ1-40 and Aβ1-42 in N2a/APP cells. The data are expressed as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, compared to the N2a/APP group with treatment of LY294002 and oxyphylla A; #P < 0.05, ##P < 0.01, compared to the N2a/APP group with treatment of 0.1% (v/v) DMSO.
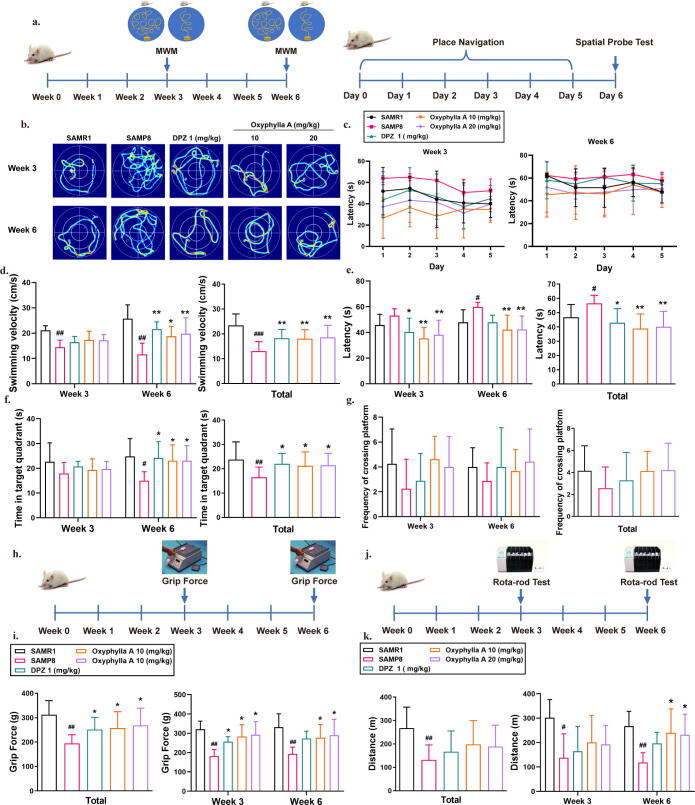
Oxyphylla A ameliorated cognitive deficits and improved muscle strength and endurance in SAMP8 mice
To evaluate the therapeutic effect of oxyphylla A on cognitive deficits, the Morris water maze (MWM) was used. As shown in the experimental diagrams, the MWM was performed in weeks 3 and 6. During the 6-day behavioral test, navigation trials were performed from day 1 to day 5, with the spatial probe test performed on the last day (Fig. 6 a). Fig. 6 shows representative patterns of the different groups by week. The SAMP8 group showed obvious deficits in spatial memory. The results also showed a significant decrease in the latency to find the target platform, and erratic swimming behavior in comparison with the oxyphylla A mouse groups (Fig. 6 b). Statistical analysis showed that the oxyphylla A group (10 mg/kg, 20 mg/kg) had a shorter latency to find the platform and faster swimming velocity compared to the SAMP8 group (Fig. 6 c, d). Moreover, mice in the oxyphylla A group showed better performance in the spatial probe test. On day 6, SAMP8 mice treated with oxyphylla A spent more time in the target quadrant and platform area compared to the vehicle group (Fig. 6 f, g). The results of the navigation and spatial probe tests indicated that oxyphylla A started to exert a therapeutic effect in week 3, which persisted to week 6 (Fig. 6 c–f). These results indicated that oxyphylla A had improved impaired cognitive function.
Fig. 6.
Behavioral analysis of the effects of oxyphylla A on SAMP8 mice in the MWM, grip force and rota-rod tests. (a) Experimental paradigm of the MWM. Five groups were devised in this study: the SAMR1, SAMP8, Donepezil-treated (1 mg/kg) and oxyphylla A-treated (10 or 20 mg/kg) groups. The animals were treated with different drugs by oral gavage, once a day for 6 weeks. The behavioral tests were performed in weeks 3 and 6. (b) Representative swimming patterns of all groups. (c) Latencies to find the target platform on different days in the navigation test. (d) Average swimming velocity. (e) Statistical analysis of latencies in the navigation test. (f) Frequencies of crossing the platform in the spatial probe test. (g) Time spent in the target quadrant during the spatial probe tests. (h) Experimental paradigm of the grip force test. (i) Statistical analysis of muscle strength in all groups. (j) Experimental paradigm of the Rota-rod test. (k) Statistical analysis of muscle endurance in all groups. The data are expressed as mean ± SD, n = 6–8 per group. *P < 0.05, **P < 0.01, compared to the SAMP8 group; #P < 0.05, ##P < 0.01, compared to the SAMR1 group.
Studies have proven that loss of muscle mass and movement impairment are related to cognitive deficits in AD penitents. Therefore, in this study, the grip force and rota-rod tests were applied to assess muscle strength and endurance. The results of the grip force test showed that oxyphylla A (10 mg/kg, 20 mg/kg) improved the strength of the fore limb, which in turn indicates that oxyphylla A was beneficial in terms of reversing the decline in muscle strength (Fig. 6 h, j). In the rota-rod test, compared to the SAMP8 group, the oxyphylla A group mice were capable of staying on the rod for longer, and walked a greater distance (Fig. 6 i, k). This suggests that oxyphylla A (10 mg/kg, 20 mg/kg) positively influenced the decreased muscle endurance of the SAMP8 mice.
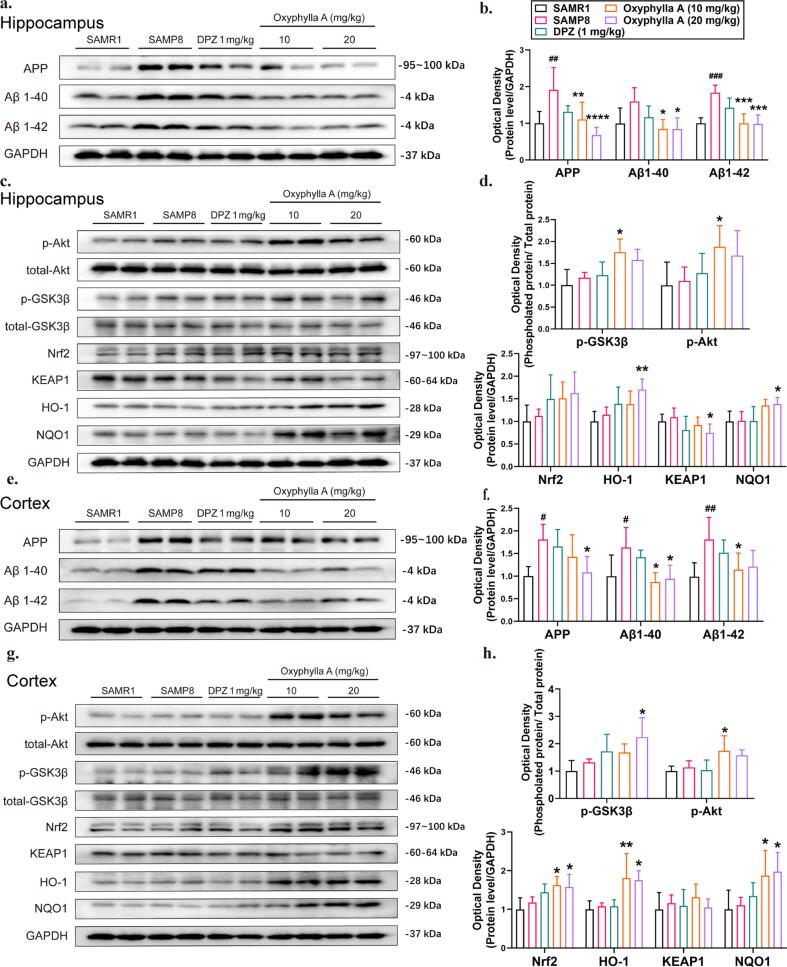
Oxyphylla A attenuated amyloid proteins, and activated the Nrf2/Keap1/HO-1 and Akt/GSK3β pathways in SAMP8 mice
Based on the above in vitro results, we examined whether oxyphylla A could reduce the expression level of amyloid proteins in vivo. SAMR1 and SAMP8 mice were used in this investigation. The protein levels of APP, Aβ1-40 and Aβ1-42 were analyzed by western blot, and were shown to be significantly decreased by oxyphylla A (10 and 20 mg/kg) compared to the vehicle group (Fig. 7 a–d). Furthermore, as shown in the Fig. 7, compared to the vehicle group, oxyphylla A (10 and 20 mg/kg) increased the expression level of Nrf2 and its downstream target proteins, HO-1 and NQO1, in both the hippocampus and cortex. Meanwhile, the regulatory protein of Nrf2, the level of Keap1 was significantly decreased by oxyphylla A (20 mg/kg) in the cortex (Fig. 7 a–d). According to our in vitro study, Nrf2 could be regulated by the Akt/GSK3β pathway. Similarly, oxyphylla A increased the levels of phosphorylated Akt (ser473) and GSK3β (ser9) in the cortex and hippocampus of SAMP8 mice (Fig. 7 e–h). All of these data were consistent with our previous in vitro study.
Fig. 7.
Effects of oxyphylla A on the expression levels of amyloid proteins in SAMP8 mice. The animals in all groups were treated with various drugs by oral gavage, once a day for 6 weeks. When the experiment ended, all animals were sacrificed and different brain regions (hippocampus and cortex) were collected for western blot. (a–(d) Western blot results and statistical analysis of APP, Aβ1-40 and Aβ1-42 expression levels in the hippocampus and cortex of SAMP8 mice. (e)– (h) Western blot results and statistical analysis of p-Akt, p- GSK3β, Nrf2, Keap1, HO-1 and NQO1 expression levels in the hippocampus and cortex of SAMP8 mice. The data are expressed as the mean ± SD, n = 6 per group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, compared to the SAMP8 group; #P < 0.05, ##P < 0.01, ###P < 0.001, compared to the SAMR1 group.
Discussion
In this study, the neuroprotective effects and possible mechanisms of action of oxyphylla A were investigated in vivo and in vitro. Our data firstly showed that the expression levels of APP, Aβ1-40 and Aβ1-42 were decreased by oxyphylla A in N2a/APP cells. Further investigation of the mechanisms in vitro showed that the neuroprotective effects of oxyphylla A were partially meditated by the suppression of oxidant stress via activation of Nrf2 through the Akt/GSK3β pathway. Similar results were obtained in SAMP8 mice. We also found in behavioral tests that oxyphylla A could ameliorate cognitive deficits, restore motor functions and improve muscle strength in SAMP8 mice. Taken together, our findings suggested that oxyphylla A could be used as a therapeutic intervention for AD and associated neurodegenerative conditions.
In the study of AD, animal models are essential for understanding the pathogenesis and mechanisms. Moreover, animal models make it possible to test therapeutic strategies that cannot be readily used for humans. Due to the impossibility of rodents spontaneously developing AD for research purposes, different transgenic mouse lines harboring human genes such as Psen1, APP, APOE ε4, and ob (leptin), etc., have been employed in this research field [26], [27], [28], [29]. However, senescence-accelerated-prone mice were established through phenotypic selection. SAMP8 mice, as a SAMP substrain, can exhibit serious age-associated symptoms including the deposition of amyloid β-peptide, cognitive decline and increased oxidative stress [16], [30]. Thus, we used SAMP8 mice as a model of spontaneous AD in the present study. Considering that the onset of impairment in learning ability and loss of memory started from 8 months old, with disease severity increasing thereafter [31], [32], 8-month-old SAMP8 mice were selected for this experiment. Our data showed that oxyphylla A could significantly ameliorate cognitive deficits in the MWM test. In the acquisition phase, the SAMP8 mice in the oxyphylla A groups showed a shorter latency to find the target platform compared to the vehicle group, in both week 3 and week 6. However, a statistically significant difference in swimming velocity between the oxyphylla A and vehicle groups was only observed in week 6. These results suggested that oxyphylla A could improve the impaired spatial memory of SAMP8 mice, and was as effective as donepezil (positive control). Similar results in terms of the duration of time that the animal stayed in the target quadrant were obtained in the subsequent spatial probe trial. However, no significant difference was observed between the oxyphylla A and vehicle groups in terms of the frequency of crossing the platform, either in week 3 or week 6. This may due to the individual differences. In addition, our swimming velocity and time spent in target quadrant data showed that oxyphylla A exhibited more positive effects in week 6. This suggested that after oral intake of oxyphylla A, more than 3 weeks is required to observe improvement in AD symptoms in SAMP8 mice. As it has been accepted widely that the MWM task is a hippocampus-dependent task [33], [34], the hippocampus might be involved in the therapeutic effects of oxyphylla A. More specifically, failing to recall spatial cues reflects a deterioration of spatial memory, which might be caused by hippocampal dysfunction [35]. Therefore, further study is required to investigate the relationship between the therapeutic effects of oxyphylla A and hippocampal dysfunction. Other than cognitive deficits, 15–50% patients suffering from AD develop motor symptoms such as decreased locomotor ability [36], [37]. In our study, muscle strength was assessed by the grip force test and muscle endurance by the rota-rod test. The data showed that, compared to the SAMR1 group, muscle strength was decreased in the SAMP8 group and restored in the oxyphylla A treatment groups. Moreover, oxyphylla A improved the decline in locomotor activity in the SAMP8 group. These results indicated that oxyphylla A might be beneficial with respect to motor symptoms. Since motor symptoms and signs could reflect the development in neurodegenerative diseases such as AD, further study of the role of oxyphylla A in motor dysfunctions, as mentioned above, may be worthwhile.
As one of the cardinal hallmarks of AD, the aggregation of amyloid plaques is a key factor underlying dementia and cognitive dysfunction during progression of the disease. Amyloid β proteins exert destructive actions in the brain, including reducing synaptic plasticity, inhibiting long-term potentiation in the hippocampus and producing ROS, etc. [38], [39]. Thus, we investigated whether oxyphylla A exerted its neuroprotective effects through attenuating Aβ and oxidative stress. For this purpose, we used a mouse neuroblastoma (N2a) cell that was stably transfected with human APP695 genes to reproduce the overexpression of APP. Our results showed that oxyphylla A significantly reduced the expression levels of APP, Aβ1-40 and Aβ1-42. In addition, oxyphylla A down-regulated the mRNA level of Bace1, as well as Psen1 and Psen2, significantly. Bace1 is responsible for encoding β-secretase enzyme 1, which catalyzes APP proteolysis and Aβ fragment formation. On the other hand, Psen1 and Psen2 encode presenilin proteins, which are catalytic components of γ-secretase enzyme responsible for final cleavage of APP to generate Aβ. The data showed that oxyphylla A mediated the down-regulated expression of Bace1, Psen1 and Psen2. These results suggested that this natural small molecule might exert its therapeutic effects partially through inhibiting the generation of Aβ. Further investigations should be conducted at the protein levels of β-secretase and γ-secretase enzymes, and other associated mechanisms.
Since Aβ is one of the most crucial biomarkers of the pathogenesis of AD, adverse effects such as oxidative stress induced by Aβ are of concern [40]. Therefore, we further investigated whether the neuroprotective effect of oxyphylla A involved anti-oxidative stress and signaling pathways related to oxidative stress regulation. From our flow cytometry data, we found out that 100 μM oxyphylla A decreased the ROS level compared to the vehicle group (P = 0.058). The results of Western blot indicated that oxyphylla A increased the expression levels of Nrf2, both in cytoplasm and the nucleus. Nrf2 is well known as a crucial mediator of cellular detoxification and antioxidant defense. In addition, we found that oxyphylla A promoted the translocation of Nrf2 from cytoplasm into the nucleus, and then increased binding between Nrf2 and ARE to activate target genes such as HO-1 (Hmox1) and NQO1 (Nqo1), in both mRNA and protein [41]. Moreover, our further indicated that the Akt-GSK3β pathway might be involved in the regulation of these antioxidative processes. When seeking to confirm whether oxyphylla A attenuated oxidative stress through the Akt-GSK3β pathway, we found that the Akt inhibitor, LY294002, partially abolished the antioxidative effects of oxyphylla A. Except for the in vitro results, similar outcomes were obtained in SAMP8 mice. Taken together, these results suggested that oxyphylla A exerts its neuroprotective effects by attenuating the expression of Aβ and oxidative stress, where these processes are associated with the Akt-GSK3β pathway. Further investigation is needed to validate the current finding that Akt is a regulator of the antioxidative effects of oxyphylla A. As GSK3βhas proven to be another key factor in this pathway, and the phosphorylated form of GSK3β could be another interesting research target There are several different phosphorylated sites responsible for the inactive or active status of GSK3β [14]. As a complication, GSK3β plays different roles in other signaling pathways. GSK3β has been shown to coimmunoprecipitate with Presenilin-1, which is a critical gene involved in the generation of Aβ and early onset AD [42], [43]. In both the pathological and physiological state, active GSK3β has the ability to phosphorylate over 20 sites on Tau protein, leading to the formation of NFTs [44], [45]. Several studies have shown that inhibition of GSK3β can induce the activation of Nrf2 under oxidative stress conditions in AD [46], [47], [48]. Therefore, the GSK3β inhibitor has promise in the development of new therapeutic strategies for AD. Whether oxyphylla A exerts its neuroprotective effects directly through other phosphorylated sites of GSK3β remains unknown and requires further study. Oxidative stress contributes greatly to AD pathogenesis in the nervous system. Nrf2 has been found to have protective effects against AD pathogenesis [46]. Even though there is evidence of low expression of Nrf2 in AD animal models and patients, paradoxical findings of increased NQO1, p62 and HO-1 in AD have been provided by other researchers [49], [50]. These contradictory results might be due to differences in stages of AD and brain regions among studies [51]. To study the neuroprotective effects of Nrf2, transgenic technology using lentiviral vector (LV) has been applied to introduce human genes encoding Nrf2 into an AD mouse model, and has been proven to significantly ameliorate cognitive deficits in APP/PS1 mice [52]. In the present study, we demonstrated that oxyphylla A up-regulated the expression of Nrf2, as well as its targets genes such as HO-1 and NQO1, both in vitro and in vivo. In future studies, we may use the LV to deliver antisense Nrf2 genes into SAMP8 mice, and will further investigate the antioxidative effects of oxyphylla A in vivo. Last but not least, natural products for treating neurodegenerative diseases have attracted increasing attention in recent years. Researchers have made some noteworthy breakthroughs. Some natural compounds (lignans, flavonoids, tannins and polyphenols) have been found to decrease the symptoms and pathogenesis of various neurodegenerative diseases, including AD [53], [54]. Our comprehensive study of oxyphylla A provides new insight into how this novel chemical affects AD in experimental models, and its potential as a therapeutic agent for the management of AD.
Conclusions
In conclusion, as a novel compound extracted from a natural product, oxyphylla A exhibits promising neuroprotective properties both in vitro and in vivo AD models. Our data revealed that oxyphylla A attenuates the expression of Aβ and ameliorates cognitive deficits. Further analysis showed that oxyphylla A exerts its antioxidative effect through the Nrf2-Keap1 and Akt-GSK3β pathways. As natural products and their active constituents become more popular during the development and discovery of new drugs, our results suggest a new horizon for the discovery of disease-modifying therapeutic strategies for AD and, potentially, other neurodegenerative conditions.
Compliance with ethics requirements
All Institutional and National Guidelines for the care and use of animals (fisheries) were followed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research was supported by The Science and Technology Development Fund, Macau SAR (No. 0058/2019/A1 and 0016/2019/AKP); University of Macau (No. MYRG2019-00105-ICMS); National Natural Science Foundation of China (No. 81803398).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.09.002.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Lane C.A., Hardy J., Schott J.M. Alzheimer's disease. Eur J Neurol. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 2.Wirths O., Bayer T.A. Motor impairment in Alzheimer's disease and transgenic Alzheimer's disease mouse models. Genes Brain Behav. 2008;7(Suppl 1):1–5. doi: 10.1111/j.1601-183X.2007.00373.x. [DOI] [PubMed] [Google Scholar]
- 3.Schirinzi T., Di Lorenzo F., Sancesario G.M., Di Lazzaro G., Ponzo V., Pisani A., et al. Amyloid-mediated cholinergic dysfunction in motor impairment related to Alzheimer's disease. J Alzheimers Dis. 2018;64(2):525–532. doi: 10.3233/JAD-171166. [DOI] [PubMed] [Google Scholar]
- 4.Shin R.W., Ogomori K., Kitamoto T., Tateishi J. Increased tau accumulation in senile plaques as a hallmark in Alzheimer's disease. Am J Pathol. 1989;134(6):1365–1371. [PMC free article] [PubMed] [Google Scholar]
- 5.Behrouz N., Defossez A., Delacourte A., Mazzuca M. The immunohistochemical evidence of amyloid diffuse deposits as a pathological hallmark in Alzheimer's disease. J Gerontol. 1991;46(6):B209–B212. doi: 10.1093/geronj/46.6.b209. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien R.J., Wong P.C. Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci. 2011;34(1):185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheltens P., Blennow K., Breteler M.M.B., de Strooper B., Frisoni G.B., Salloway S., et al. Alzheimer's disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen J.T., Sigurdsson E.M. Tau immunotherapy for Alzheimer's disease. Trends Mol Med. 2015;21(6):394–402. doi: 10.1016/j.molmed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Bonda D.J., Wang X., Perry G., Nunomura A., Tabaton M., Zhu X., et al. Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology. 2010;59(4-5):290–294. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Bahn G., Jo D.-G. Therapeutic approaches to Alzheimer's disease through modulation of NRF2. Neuromolecular Med. 2019;21(1):1–11. doi: 10.1007/s12017-018-08523-5. [DOI] [PubMed] [Google Scholar]
- 11.Li C., Tang B., Feng Y.u., Tang F., Pui-Man Hoi M., Su Z., et al. Pinostrobin exerts neuroprotective actions in neurotoxin-induced Parkinson's disease models through Nrf2 induction. J Agric Food Chem. 2018;66(31):8307–8318. doi: 10.1021/acs.jafc.8b02607. [DOI] [PubMed] [Google Scholar]
- 12.Cui YuanBo, Ma ShanShan, Zhang ChunYan, Li DongPeng, Yang B.o., Lv PengJu, et al. Pharmacological activation of the Nrf2 pathway by 3H–1, 2-dithiole-3-thione is neuroprotective in a mouse model of Alzheimer disease. Behav Brain Res. 2018;336:219–226. doi: 10.1016/j.bbr.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Farr S.A., Ripley J.L., Sultana R., Zhang Z., Niehoff M.L., Platt T.L., et al. Antisense oligonucleotide against GSK-3beta in brain of SAMP8 mice improves learning and memory and decreases oxidative stress: Involvement of transcription factor Nrf2 and implications for Alzheimer disease. Free Radic Biol Med. 2014;67:387–395. doi: 10.1016/j.freeradbiomed.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel P., Woodgett J.R. Glycogen synthase kinase 3: a kinase for all pathways? Curr Top Dev Biol. 2017;123:277-+ doi: 10.1016/bs.ctdb.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Ryan K.A., Pimplikar S.W. Activation of GSK-3 and phosphorylation of CRMP2 in transgenic mice expressing APP intracellular domain. J Cell Biol. 2005;171(2):327–335. doi: 10.1083/jcb.200505078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butterfield D., Poon H. The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer's disease. Exp Gerontol. 2005;40(10):774–783. doi: 10.1016/j.exger.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Farr S.A., Poon H.F., Dogrukol-Ak D., Drake J., Banks W.A., Eyerman E., et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84(5):1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 18.Banks W.A., Farr S.A., Morley J.E., Wolf K.M., Geylis V., Steinitz M. Anti-amyloid beta protein antibody passage across the blood-brain barrier in the SAMP8 mouse model of Alzheimer's disease: an age-related selective uptake with reversal of learning impairment. Exp Neurol. 2007;206(2):248–256. doi: 10.1016/j.expneurol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G., Zhang Z., Quan Q., Jiang R., Szeto S.S.W., Yuan S., et al. Discovery, synthesis, and functional characterization of a novel neuroprotective natural product from the fruit of Alpinia oxyphylla for use in Parkinson's disease through LC/MS-based multivariate data analysis-guided fractionation. J Proteome Res. 2016;15(8):2595–2606. doi: 10.1021/acs.jproteome.6b00152. [DOI] [PubMed] [Google Scholar]
- 20.Shi S.-H., Zhao X.u., Liu A.-J., Liu B., Li H., Wu B.o., et al. Protective effect of n-butanol extract from Alpinia oxyphylla on learning and memory impairments. Physiol Behav. 2015;139:13–20. doi: 10.1016/j.physbeh.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 21.He B., Xu F., Yan T., Xiao F., Wu B., Wang Y., et al. Tectochrysin from Alpinia Oxyphylla Miq. alleviates Abeta1-42 induced learning and memory impairments in mice. Eur J Pharmacol. 2019;842:365–372. doi: 10.1016/j.ejphar.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z.-J., Cheang L.C.V., Wang M.-W., Li G.-H., Chu I.K., Lin Z.-X., et al. Ethanolic extract of fructus Alpinia oxyphylla protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Cell Mol Neurobiol. 2012;32(1):27–40. doi: 10.1007/s10571-011-9731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Li G., Law H.C.H., Chen H., Lee S.-Y. Determination of oxyphylla A enantiomers in the fruits of Alpinia oxyphylla by a chiral high-performance liquid chromatography-multiple reaction monitoring-mass spectrometry method and comparison of their in vivo biological activities. J Agric Food Chem. 2020;68(40):11170–11181. doi: 10.1021/acs.jafc.0c04031. [DOI] [PubMed] [Google Scholar]
- 24.Zhou H., Li S., Li C., Yang X., Li H., Zhong H., et al. Oxyphylla A promotes degradation of alpha-synuclein for neuroprotection via activation of immunoproteasome. Aging Dis. 2020;11(3):559–574. doi: 10.14336/AD.2019.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Esquerda-Canals G., Montoliu-Gaya L., Güell-Bosch J., Villegas S. Mouse models of Alzheimer's disease. J Alzheimers Dis. 2017;57(4):1171–1183. doi: 10.3233/JAD-170045. [DOI] [PubMed] [Google Scholar]
- 27.Grootendorst J., Bour A., Vogel E., Kelche C., Sullivan P.M., Dodart J.-C., et al. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav Brain Res. 2005;159(1):1–14. doi: 10.1016/j.bbr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Paz-Filho G., Wong M.L., Licinio J. Leptin levels and Alzheimer disease. JAMA. 2010;303(15):1478 doi: 10.1001/jama.2010.436. author reply -9. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Alloza M., Robbins E.M., Zhang-Nunes S.X., Purcell S.M., Betensky R.A., Raju S., et al. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24(3):516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Hall A.M., Roberson E.D. Mouse models of Alzheimer's disease. Brain Res Bull. 2012;88(1):3–12. doi: 10.1016/j.brainresbull.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morley J.E. The SAMP8 mouse: a model of Alzheimer disease? Biogerontology. 2002;3(1–2):57–60. doi: 10.1023/a:1015207429786. [DOI] [PubMed] [Google Scholar]
- 32.Markowska A.L., Spangler E.L., Ingram D.K. Behavioral assessment of the senescence-accelerated mouse (SAM P8 and R1) Physiol Behav. 1998;64(1):15–26. doi: 10.1016/s0031-9384(98)00011-0. [DOI] [PubMed] [Google Scholar]
- 33.Morris R.G.M., Garrud P., Rawlins J.N.P., O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 34.Morris R.G., Schenk F., Tweedie F., Jarrard L.E. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur J Neurosci. 1990;2(12):1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 35.Yanai S., Endo S. Early onset of behavioral alterations in senescence-accelerated mouse prone 8 (SAMP8) Behav Brain Res. 2016;308:187–195. doi: 10.1016/j.bbr.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 36.Scarmeas N., Hadjigeorgiou G.M., Papadimitriou A., Dubois B., Sarazin M., Brandt J., et al. Motor signs during the course of Alzheimer disease. Neurology. 2004;63(6):975–982. doi: 10.1212/01.wnl.0000138440.39918.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scarmeas N., Albert M., Brandt J., Blacker D., Hadjigeorgiou G., Papadimitriou A., et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64(10):1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malenka R.C., Bear M.F. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Chang E.H., Savage M.J., Flood D.G., Thomas J.M., Levy R.B., Mahadomrongkul V., et al. AMPA receptor downscaling at the onset of Alzheimer's disease pathology in double knockin mice. Proc Natl Acad Sci U S A. 2006;103(9):3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Alloza M., Dodwell S.A., Meyer-Luehmann M., Hyman B.T., Bacskai B.J. Plaque-derived oxidative stress mediates distorted neurite trajectories in the Alzheimer mouse model. J Neuropathol Exp Neurol. 2006;65(11):1082–1089. doi: 10.1097/01.jnen.0000240468.12543.af. [DOI] [PubMed] [Google Scholar]
- 41.Fakhri S., Pesce M., Patruno A., Moradi S.Z., Iranpanah A., Farzaei M.H., et al. Attenuation of Nrf2/Keap1/ARE in Alzheimer's disease by plant secondary metabolites: a mechanistic review. Molecules. 2020;25(21):4926. doi: 10.3390/molecules25214926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uemura K., Kuzuya A., Shimozono Y., Aoyagi N., Ando K., Shimohama S., et al. GSK3 beta activity modifies the localization and function of presenilin 1. J Biol Chem. 2007;282(21):15823–15832. doi: 10.1074/jbc.M610708200. [DOI] [PubMed] [Google Scholar]
- 43.Hernández F., Gómez de Barreda E., Fuster-Matanzo A., Lucas J.J., Avila J. GSK3: a possible link between beta amyloid peptide and tau protein. Exp Neurol. 2010;223(2):322–325. doi: 10.1016/j.expneurol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Hanger D.P., Anderton B.H., Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15(3):112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Huang L.P., Lin M.Q., Zhong X.Q., Yang H.Y., Deng M.Z. Galangin decreases p-tau, A(42) and -secretase levels, and suppresses autophagy in okadaic acid-induced PC12 cells via an Akt/GSK3/mTOR signaling-dependent mechanism. Mol Med Rep. 2019;19(3):1767–1774. doi: 10.3892/mmr.2019.9824. [DOI] [PubMed] [Google Scholar]
- 46.Gameiro I., Michalska P., Tenti G., Cores A., Buendia I., Rojo A.I., et al. Discovery of the first dual GSK3 beta inhibitor/Nrf2 inducer. A new multitarget therapeutic strategy for Alzheimer's disease. Sci Rep-Uk. 2017;7 doi: 10.1038/srep45701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Espada S., Rojo A.I., Salinas M., Cuadrado A. The muscarinic M1 receptor activates Nrf2 through a signaling cascade that involves protein kinase C and inhibition of GSK-3beta: connecting neurotransmission with neuroprotection. J Neurochem. 2009;110(3):1107–1119. doi: 10.1111/j.1471-4159.2009.06208.x. [DOI] [PubMed] [Google Scholar]
- 48.Zou Y., Hong B., Fan L., Zhou L., Liu Y., Wu Q., et al. Protective effect of puerarin against beta-amyloid-induced oxidative stress in neuronal cultures from rat hippocampus: involvement of the GSK-3beta/Nrf2 signaling pathway. Free Radic Res. 2013;47(1):55–63. doi: 10.3109/10715762.2012.742518. [DOI] [PubMed] [Google Scholar]
- 49.SantaCruz K.S., Yazlovitskaya E., Collins J., Johnson J., DeCarli C. Regional NAD(P)H:quinone oxidoreductase activity in Alzheimer's disease. Neurobiol Aging. 2004;25(1):63–69. doi: 10.1016/s0197-4580(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 50.Tanji K., Maruyama A., Odagiri S., Mori F., Itoh K., Kakita A., et al. Keap1 is localized in neuronal and glial cytoplasmic inclusions in various neurodegenerative diseases. J Neuropath Exp Neur. 2013;72(1):18–28. doi: 10.1097/NEN.0b013e31827b5713. [DOI] [PubMed] [Google Scholar]
- 51.Johnson D.A., Johnson J.A. Nrf2–a therapeutic target for the treatment of neurodegenerative diseases. Free Radic Biol Med. 2015;88(Pt B):253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanninen K., Heikkinen R., Malm T., Rolova T., Kuhmonen S., Leinonen H., et al. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106(38):16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bian Y., Zhao C., Lee S.M. Neuroprotective potency of saffron against neuropsychiatric diseases, neurodegenerative diseases, and other brain disorders: from bench to bedside. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.579052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ansari N., Khodagholi F. Natural products as promising drug candidates for the treatment of Alzheimer's disease: molecular mechanism aspect. Curr Neuropharmacol. 2013;11(4):414–429. doi: 10.2174/1570159X11311040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.