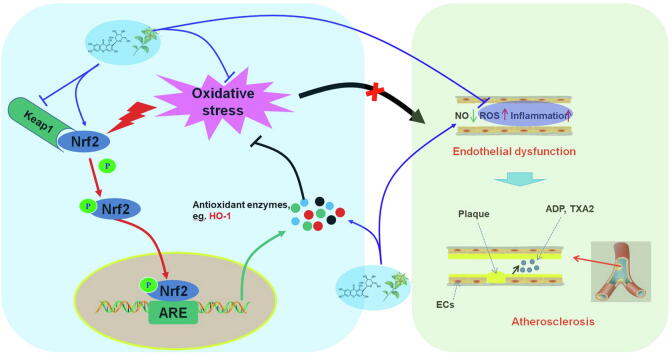
Graphical abstract
Keywords: Atherosclerosis, Nrf2/HO-1 signaling, Vascular endothelial cells, Oxidative stress, Herbal medicine, Molecular mechanism
Abbreviations: 7-HMR, (−)-7(S)-hydroxymatairesinol; ADH, andrographolide; AGE, advanced glycation end product; Akt, protein kinase B; AMP, Athyrium Multidentatum; Ang, Angiotensin; ApoE, apolipoprotein E; APV, aqueous extracts of Prunella Vulgaris; ARE, antioxidant reaction elements; AS, atherosclerosis; ASD-IV, Astragaloside IV; ASP, Angelica sinensis polysaccharide; ASTP, Astragalus polysacharin; BAECs, bovine artery endothelial cells; BBR, Berberine; BITC, benzyl isothiocyanate; C3G, Cyanidin-3-O-glucoside; CINM, Cinnamaldehyde; CNC, Cap'n'collar; CREB, cAMP-response element binding protein; CVDs, cardiovascular diseases; CVRF, cardiovascular risk factors; DMY, Dihydromyricetin; ECC, (−)-Epicatechin; ECs, endothelial cells; EGCG, epigallocatechin-3-O-gallate; eNOS, endothelial NO synthase; ERK, extracellular regulated protein kinases; ET, endothelin; EXS, Xanthoceras sorbifolia; FFA, Fatty Acids; Gau A, Glaucocalyxin A; GPx, Glutathione peroxidase; GSD Rg1, Ginsenoside Rg1; GTE, Ganoderma tsugae extracts; HAMS, human anthocyanin medicated serum; Hcy, Homocysteine; HG, high glucose; HIF-1, Hypoxia-inducible factor 1; HO-1, heme oxygenase; HUVECs, human umbilical vein endothelial cells; HXC, Huoxue capsule; ICAM, intercellular adhesion molecule; IL, interleukin; Keap1, kelch-like epichlorohydrin-related proteins; KGRE, extracts of KGR; KRG, Korean red ginseng; LWDH, Liuwei-Dihuang pill; MA, maslinic acid; MAPKK, mitogen-activated protein kinase kinase; MAPKs, mitogen-activated protein kinases; MCGA3, 3-O-caffeoyl-1-methylquinic acid; MCP-1, monocyte chemotactic protein 1; MMPs, matrix metalloproteinases; NAF, Nepeta Angustifolia; NF-κB, nuclear factor kappa-B; NG, naringenin; NQO1, NAD(P)H: quinone oxidoreductase; Nrf2, nuclear factor erythroid-2 related factor 2; OA, Oleanolic acid; OMT, Oxymatrine; OX-LDL, oxidized low density lipoprotein; PA, Palmitate; PAA, Pachymic acid; PAI-1, plasminogen activator Inhibitor-1; PEITC, phenethyl isocyanate; PKC, protein kinase C; PI3K, phosphatidylinositol 3 kinase; PT, Pterostilbene; RBPC, phenolic extracts derived from rice bran; ROS, reactive oxygen species; Sal B, salvianolic acid B; SAL, Salidroside; SchB, Schisandrin B; SFN, sulforaphane; SMT, Samul-Tang Tang; SOD, superoxide dismutase; TCM, traditional Chinese medicine; TNF, tumor necrosis factor; TrxR1, thioredoxin reductase-1; TXA2, Thromboxane A2; US, uraemic serum; VA, Vanillic acid; VCAM, vascular cell adhesion molecule; VEC, vascular endothelial cells; VEI, vascular endothelial injury; XAG, xanthoangelol; XXT, Xueshuan Xinmaining Tablet; Z-Lig, Z-ligustilide
Abstract
Introduction
Recently, Nrf2/HO-1 has received extensive attention as the main regulatory pathway of intracellular defense against oxidative stress and is considered an ideal target for alleviating endothelial cell (EC) injury.
Objectives
This paper aimed to summarized the natural monomers/extracts that potentially exert protective effects against oxidative stress in ECs.
Methods
A literature search was carried out regarding our topic with the keywords of “atherosclerosis” or “Nrf2/HO-1” or “vascular endothelial cells” or “oxidative stress” or “Herbal medicine” or “natural products” or “natural extracts” or “natural compounds” or “traditional Chinese medicines” based on classic books of herbal medicine and scientific databases including Pubmed, SciFinder, Scopus, the Web of Science, GoogleScholar, BaiduScholar, and others. Then, we analyzed the possible molecular mechanisms for different types of natural compounds in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. In addition, perspectives for possible future studies are discussed.
Results
These agents with protective effects against oxidative stress in ECs mainly include phenylpropanoids, flavonoids, terpenoids, and alkaloids. Most of these agents alleviate cell apoptosis in ECs due to oxidative stress, and the mechanisms are related to Nrf2/HO-1 signaling activation. However, despite continued progress in research on various aspects of natural agents exerting protective effects against EC injury by activating Nrf2/HO-1 signaling, the development of new drugs for the treatment of atherosclerosis (AS) and other CVDs based on these agents will require more detailed preclinical and clinical studies.
Conclusion
Our present paper provides updated information of natural agents with protective activities on ECs against oxidative stress by activating Nrf2/HO-1. We hope this review will provide some directions for the further development of novel candidate drugs from natural agents for the treatment of AS and other CVDs.
Introduction
Cardiovascular disease (CVD) is a common disease that seriously threatens the life and health of humans. CVD is more common in elderly individuals over 50 years old and is characterized by high morbidity, disability and mortality [1]. At present, greater than 17 million people die of CVD every year worldwide [2]. In China, Europe, the United States and other developed countries, the incidence and mortality of CVD rank first [3], [4]. CVD is responsible for greater than 31% of deaths worldwide [5]. Importantly, with the acceleration of the aging of the social population, this situation will become increasingly serious, and morbidity and mortality will continue to increase. As the common pathological basis of various CVDs (such as hypertension, coronary heart disease and acute myocardial infarction), atherosclerosis (AS) also attracts much attention. In short, AS is a degenerative and proliferative systemic disease that mainly involves large- and medium-sized arteries [6], [7]. The basic features of AS are lipid deposition in the intima of the artery, which forms many uneven atherosclerotic plaques, making the vascular lumen smaller and less elastic and thickening and hardening the wall [8]. In addition, during the formation of plaques, internal hemorrhage, plaque rupture and calcification, thrombosis and artery porridge tumors will also occur, which will eventually affect the blood supply of the artery and cause ischemia or necrosis of surrounding tissues or organs [9], [10]. AS is a complex process, and its pathogenesis remains unclear. Clinicopathological examination revealed severe structural and functional impairment of endothelial cells in CVD patients. In addition, the pathogenesis of many cardiovascular risk factors (CVRFs), such as hypertension, diabetes mellitus and hypercholesterolemia, is closely related to endothelial injury [11], [12], [13]. These results suggest that vascular endothelial cells (VECs) play an important role in the occurrence and development of AS. Therefore, protecting VECs from injury is one of the feasible directions for treating AS and discovering anti-AS candidate drugs.
For thousands of years, natural medicines have been widely used to prevent and treat various diseases due to their remarkable efficacy and high safety. In recent years, with the progression and development of society, these natural medicines have also become popular worldwide, and herbal medicine has been widely accepted in many countries as a supplement and alternative therapy. Currently, increasing scientific evidence has shown that a large number of natural drugs have achieved good results in the treatment of AS, such as Allium sativum [14], Angelica gigas [15], Artemisia annua [16] and Cinnamomum cassia [17]. Furthermore, some monomers from natural herbal medicines exhibit promising anti-AS properties, such as alkaloids, flavonoids, terpenoids, and phenylpropanoids [18], [19], [20], [21]. Therefore, natural agents derived from herbal medicines are undoubtedly invaluable resources for identifying candidate drugs to treat AS based on VEC protection. The phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) signaling pathway plays an important role in the regulation of various cell functions (including metabolism, growth, proliferation, survival, transcription and protein synthesis) and has become the focus of current research. Studies have found that endothelial cell (EC) apoptosis induced by many factors is mediated by PI3K/Akt signaling [22]. Similarly, the nuclear factor erythroid-2 related factor 2 (Nrf2)/heme oxygenase (HO-1) signaling pathway, as an indispensable signaling pathway in the oxidative stress response, is involved in anti-inflammatory, antioxidant, apoptosis and other processes and is one of the important targets for the treatment of AS [23]. PI3K/Akt is the upstream gene that regulates Nrf2, so the two signaling pathways are often studied in combination. Consequently, in this review, we summarized the natural agents contained in herbal medicines showing protective properties inVECs via the activation of Nrf2/HO-1 signaling to provide a reference for follow-up studies on herbal medicines for treating AS and related diseases.
Vascular endothelial cells and atherosclerosis
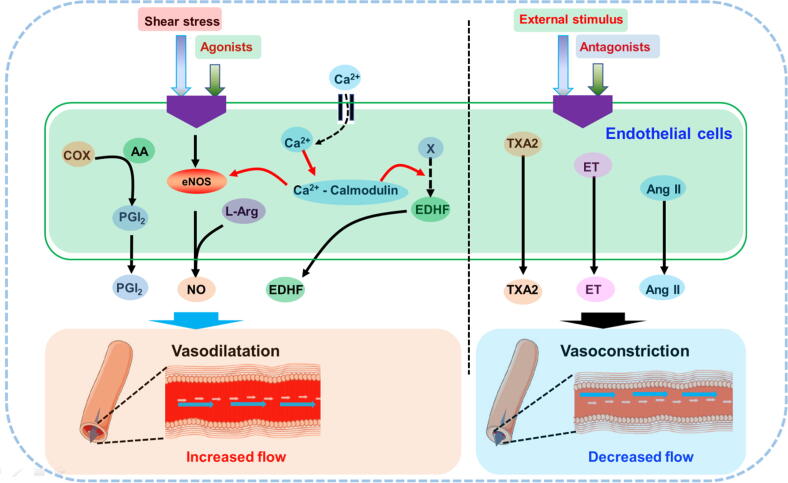
Vascular endothelial cells are specialized epithelial cells that form the lining of blood vessels between plasma and vascular tissue. These cells not only complete the metabolic exchange of plasma and tissue fluid and can also synthesize and secrete a variety of biologically active substances to ensure the normal contraction and relaxation of blood vessels, maintain vascular tension, adjust blood pressure, and balance blood coagulation and anticoagulation, such as to maintain the normal flow of blood and long-term patency of blood vessels and control vascular permeability. Vascular blood flow results in mechanotransduction, mainly including shear stress, stretch stress and wall hydrostatic pressure, which play important roles in maintaining endothelial function and homeostasis. Among these, shear stress is considered the most important factor for the development of AS, which has crucial effects on vasodilatation. Shear stress results in the activation of endothelial NO synthase (eNOS) in vascular endothelial cells, which further induces the production of NO via mechanoreceptors on the surface of endothelial cells and a series of complex molecular signaling pathways, leading to vasodilatation. In addition, shear stress can lead to rapid Ca2+ influx into the cytoplasm via Ca2+ iron channels, which will further activate eNOS and NO production (Fig. 1). In addition, ECs predominantly show vasoconstriction via the release of many media, such as thromboxane A2 (TXA2), endothelin (ET) and angiotensin (Ang) II, under various external stimuli. Therefore, vascular endothelial cells play an important role in maintaining vascular homeostasis [24], [25], [26], [27], [28]. However, when endothelial cells are subjected to a series of pathological stimuli, their structure and function are damaged, which is vascular endothelial injury (VEI). Once VEI occurs, cells in the blood, such as white blood cells, are recruited to accumulate and combine with fibrin tissue to form a fibrin network [29], [30].
Fig. 1.
Endothelial cells play important role in maintaining vascular homeostasis. COX, cyclooxygenase; AA, arachidonic acid; PGI2, prostaglandin I2; EDHF, endothelium-derived hyperpolarizing factor; TXA2, Thromboxane A2; ET, endothelin; Ang II, Angiotensin II.
Normally, small endothelial damage is perfectly repaired by these fibrins. However, under pathological conditions, sustained and extensive damage to the vascular endothelium cannot be repaired, and vascular integrity is damaged, leading to vascular rupture and bleeding. When an increasing number of monocytes adhere simultaneously, AS plaques form at the wound of the inner skin. Damaged endothelial cells also produce a large number of growth factors and active substances to promote the migration and proliferation of smooth muscle cells, secrete extracellular matrix, form fibrous caps, and increase the instability of AS plaques. Unstable plaques are prone to rupture and promote clot formation. Some of these thrombi are washed away with the blood flow. Small thrombi are absorbed and dissipated, and large thrombi block capillaries, vein vessels and even the middle artery and other blood vessels, causing cerebral infarction, myocardial infarction, pulmonary embolism and other diseases. In addition, damage to the structure and function of endothelial cells will lead to weakened vascular barrier function, making lipids and monocytes in the blood more likely to deposit in the intima of blood vessels, further promoting the formation of foam cells and AS plaques and accelerating the occurrence of AS. Therefore, endothelial injury is often considered the key early link in the onset of AS [31], [32], [33].
With the development of science and technology, our understanding of the process behind endothelial injury is improving. As early as the last century, some scholars noted that AS is a chronic inflammatory disease of endothelial cells caused by various stress factors, such as homocysteine, Ang Ⅱ, viruses, mechanical damage, immune complexes, type oxidized low-density lipoprotein (ox-LDL), the structure and function of damage, and excessive chronic inflammatory reactions in blood vessels [34]. In recent years, studies have found that many factors, such as oxidative stress, the renin-angiotensin system, ox-LDL, homocysteine, hyperglycemia and inflammatory factors, can cause endothelial cell activation, dysfunction, loss of integrity and secretion dysfunction [35], [36], [37]. Excessive oxidative stress increases ROS levels in endothelial cells; induces the expression of numerous adhesion molecules, such as monocyte chemotactic protein 1 (MCP-1), intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin; promotes the adhesion of monocytes to vascular endothelial cells; and causes tissue infiltration [38]. In addition, ROS increase intracellular calcium concentrations, consume more ATP, increase mitochondrial burden, and induce cell apoptosis [39], [40].
NO, an important vasodilator, is important for maintaining vasodilation and systole [41]. On the one hand, pathological stimulation reduces eNOS levels in vascular endothelial cells, reduces the synthesis and secretion of NO, and stimulates the production of hypoxia-inducible factor 1 (HIF-1) and endothelin-1 (ET-1). On the other hand, NO bioavailability decreases and plasminogen activator inhibitor-1 (PAI-1) secretion increased in endothelial cells. These effects can cause the vasodilatory response to weaken or even disappear, increase platelet aggregation, induce the enhancement of the endothelial procoagulant effect, and promote the proliferation and migration of smooth muscle cells, thus promoting thrombosis [42], [43]. In addition, the nuclear factor kappa-B (NF-κB) signaling pathway of endothelial cells is activated and produces a wide range of proinflammatory factors, such as tumor necrosis factor (TNF) α, interleukin (IL)-1β, IL-8, IL-6, and matrix metalloproteinases (MMPs), which contribute to endothelial dysfunction and lipid plaque formation in the intima [44]. In addition, these inflammatory factors also promote the production of ROS and adhesion molecules in endothelial cells, further exacerbating endothelial injury [45]. Therefore, endothelial cell injury is generally characterized by increased production of reactive oxygen species (ROS), apoptosis, persistent local inflammation, and increased adhesion of monocytes.
Collectively, the inhibition of vascular endothelial injury seems to be a feasible strategy for the treatment of AS. In fact, some clinical drugs have protective effects on endothelial cells due to reducing oxidative stress and homocysteine and inhibiting the formation of ox-LDL, such as vitamin E, statins, coenzyme Q10, and probucol [46], [47], [48]. However, AS treatment typically requires long-term medication and therefore is expensive for the patient. In addition, during long-term drug treatment, single-component chemicals may cause side effects, such as hepatorenal toxicity and myositis, and an increased risk of bleeding [49], [50].
Nrf2/HO-1 signaling, oxidative stress and atherosclerosis
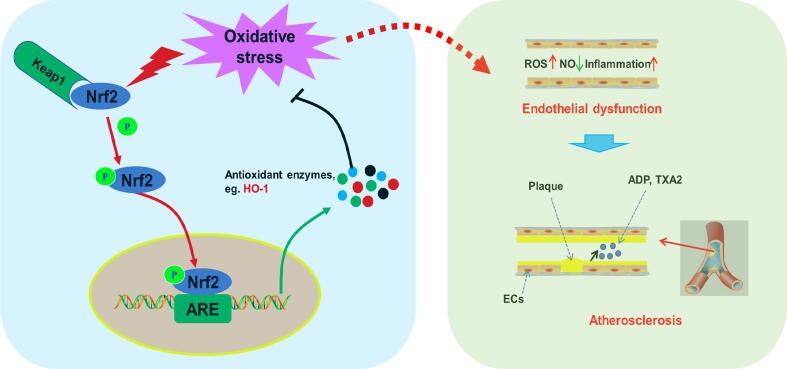
Nrf2 is a member of the Cap'n'collar (CNC)-BZIP transcription factor family. Nrf2 contains six highly conserved domains and is a key factor in regulating the oxidative stress response in cells. Under normal physiological conditions, Nrf2 binds to Kelch-like epichlorohydrin-related proteins (Keap1) to form complexes. These complexes are anchored by actin in the cytoplasm and are in a low activity state. Under oxidative stress or other pathological stimuli, the cysteine residue of Keap1 is modified to induce phosphorylation of Nrf2, which is released from the complex and translocates to the nucleus, forming heterodimers (Nrf2-Maf) with the Maf protein and Jun bZip transcription factors in the nucleus. The sequence of antioxidant reaction elements (AREs) in the nucleus can accurately identify NRF2-MAF and bind to the Neh4 and Neh5 domains of Nrf2. Then, with the help of the cAMP-response element binding protein (CREB), transcription activators, etc., Nrf2-mediated transcription process is initiated to regulate the downstream gene expression; activate a series of antioxidant enzymes and phase II antioxidant enzymes, such as HO-1, NAD(P)H:quinone oxidoreductase (NQO1), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px); remove ROS and other harmful substances; and facilitate antioxidative stress, anti-inflammatory, anti-apoptosis and other cell protection mechanisms. In addition to Keap1, various protein kinases, such as mitogen-activated protein kinases (MAPKs), protein kinase C (PKC) and PI3K, induce phosphorylation of Nrf2 and participate in Nrf2 transcription [51], [52]. In an apolipoprotein E (ApoE)-ko mouse model, Nrf2 plays an important role in lowering serum total cholesterol and reducing atherosclerotic plaque [53], [54]. Moreover, Nrf2 deficiency in macrophages can promote the formation of foam cells and aggravate AS. Reduced Nrf2 expression damages the function of endothelial progenitor cells, induces aging and increases the risk of CVD [55], [56]. HO-1, a phase II antioxidant enzyme regulated by Nrf2, is an important inducible stress response protein that converts hemoglobin to CO, Fe2+, and biliverdin and reduces the above products to bilirubin, thereby playing antioxidant, anti-inflammatory, antiapoptotic and antithrombotic roles [57]. Studies have reported that HO-1 overexpression inhibits AS in ApoE KO mice; in contrast, HO-1 deficiency causes the accelerated development of AS lesions [58], [59], [60].
ECs are an important endocrine organ and are generally considered to participate in the pathological process of several diseases, particularly CVDs, such as AS, heart failure and hypertension. [61]. Importantly, increasing evidence has suggested that EC activation and dysfunction are initial steps in the development of AS, and inhibiting EC injury seems to be a feasible strategy for the treatment or prevention of CVDs [62]. Oxidative stress is a predominant factor for EC activation and dysfunction, and apoptosis is a major mechanism of oxidative stress-induced endothelial cell injury [63]. HO-1 is a rate-limiting enzyme of heme that plays a crucial role in the degradation and metabolism of heme. Currently, accumulating studies have revealed that HO-1 can exhibit antioxidative potential to protect ECs from oxidative stress-induced injury and further inhibit vessel remodeling and endothelial dysfunction. Activation and transcription of Nrf2 plays a pivotal role in HO-1 expression [64], [65]. Therefore, Nrf2/HO-1 signaling activation would be beneficial for protecting ECs from oxidative stress-induced injury and controlling AS [65], [66] (Fig. 2).
Fig. 2.
Nrf2/HO-1 signaling, oxidative stress and atherosclerosis.Nrf2, nuclear factor erythroid 2 related factor 2; HO-1, Heme Oxygenase 1; ROS, reactive oxygen species; ADP, adenosine diphosphate; TXA2, Thromboxane A2.
Protective effects of herbal medicine and its monomers on vascular endothelial cells related to Nrf2/HO-1
Accumulating reports have comprehensively investigated the effects of herbal medicines on AS, and it has been suggested that extracts or monomers derived from natural herbal medicines possess promising protective activities on VECs via activation of Nrf2/HO-1 signaling. In the following section, the protective effects of herbal medicine and its monomers on vascular endothelial cells related to Nrf2/HO-1 are described (Table 1, Table 2, Table 3, Table 4, Fig. 3).
Table 1.
Protective effects of herbal medicine formulas on vascular endothelial cells.
| Extracts | Compositions | Animal/cells | Dose/Concentration | Effects | Related molecular targets |
Refs. | |
|---|---|---|---|---|---|---|---|
| Up-regulation | Down-regulation | ||||||
| XXT | The roots of Ligusticum chuanxiong, the roots of Salvia miltiorrhiza, Whitmania pigra, the roots of Ilex pubescens, Bovis calculus, Moschus, the flowers of Sophora japonica, the folium of Panax ginseng, Borneolum, Bufonis venenum | H2O2 (200 μM) induced -HUVECs | 50,100,200 µg /mL | Increasing cell survival; Decreasing oxidative stress | Nrf2, HMOX, GCLM, NQO1 | ROS | 67 |
| LWDH | The roots of Rehmannia glutinosa, the fructus of Cornus officinalis, the barks of Paeonia szechuanica, the roots of Dioscorea poopsita, Poria, the roots of Alisma orientale | Eahy 926 cells | 5%, 10%, 20% LWDH Medicated serum | Increasing cell proliferation | HO-1, p-ERK, Nrf2 | 68 | |
| SMT | Angelica gigas, Ligusticum officinale, Rehmannia glutinosa, Paeonia lactiflora | TNF-α (50 ng/mL) induced HUVECs | 10,30,50 µg/mL | Inhibiting NF-κB translocation and activation, and expression of CAMs; Inhibiting the adhesion of monocytes; Decreasing vascular inflammation | HO-1, Nrf2, NO | ROS, ICAM-1, VCAM-1, E-selectin; NF-κB-p65; p-IκBα | 69 |
| Paeotang | The roots of Glycyrrhiza glabra, the rhizome of Zingiber officinale, the twigs of Cinnamomum zeylanuicum, the roots of Salvia miltiorrhiza, the seeds of Prunus persica, the barks of Paeonia szechuanica, Poria cocos, the roots of Cynanchum wilfordii | TNFα (10 ng/mL) induced HUVECs | 10,20,50 µg/mL | Decreasing oxidative stress; Inhibiting the adhesion of monocytes; Decreasing vascular inflammation | HO-1, Nrf2 | ROS, ICAM-1, VCAM-1, E-selectin, MMP-2, MMP-9, NF-κB-p65; p-IκB, Keap1 | 70 |
| HXC | The roots of Astragalus membranaceu, the seeds of Prunus persica, the flowers of Carthamus tinctorius, the roots of Ligusticum chuanxiong, the roots of Achyranthes bidentata, the roots of Paeonia veitchii, the roots of Angelica sinensis, the roots of Rehmannia glutinosa, the fructus of Citrus aurantium, the roots of Platycodon grandiflorum, the seeds of Ziziplus jujuba, the roots of Glycyrrhiza glabra | H2O2 (0.2 mM) induced bEND.3 cells | 150,300,750 µg/mL | Increasing cell survival; Decreasing oxidative stress | HO-1, p-Akt, Nrf2 | 71 | |
HXC, Huoxue capsule; LWDH, Liuwei Dihuang Pill; SMT, Samul-Tang; XXT, Xueshuan Xinmaining Tablet.
Table 2.
Protective effects of herbal medicine extracts on vascular endothelial cells.
| Extracts | Animal/cells | Dose/Concentration | Effects | Related molecular targets |
Refs. | |
|---|---|---|---|---|---|---|
| Up-regulation | Down-regulation | |||||
| GTE | PM2.5 (500, 1000 μg/mL) Induced HUVECs |
100 μg/ml | Decreasing oxidative stress; Reducing DNA damage; Reducing cell death and apoptosis; Improving vascular permeability | GSH, GR, SOD-1, HO-1, CAT | ROS, MDA, Caspase-3, Caspase-7, VEGFA | 73 |
| KRG | H2O2 (100 μM) induced HUVECs | 0.5,2 mg/mL | Decreasing oxidative stress; Reducing cell death | HO-1, Nrf2 | ROS | 74 |
| APV | HG (25 mM) induced - HUVECs | 10,30,50 µg/ml | Inhibiting the adhesion of monocytes; Decreasing vascular inflammation | eNOS, HO-1, Nrf2, p-Akt | ICAM-1, VCAM-1, E-selectin, ROS, NF-κB-p65, p-IκBα, Keap 1, MMP-2, MMP-9 | 75 |
| TSAR | ox-LDL (100 μg/mL) induced HUVECs | 10,40,160 µg/mL | Increasing survival of HUVECs | NO | ET, LDH | 76 |
| EXS | TNFα (10 ng/mL) induced HUVECs | 1,10,50 μg/mL | Decreasing oxidative stress; Inhibiting the adhesion of monocytes; Decreasing vascular inflammation | SOD, HO-1, Nrf2 | ICAM-1, VCAM-1, E-selectin, NF-κB-p65, p-IκBα | 77 |
| RBPC | H2O2 (200 μM) induced HUVECs | 25,50,100, 200 µg/mL | Decreasing oxidative stress; Decreasing vascular inflammation | Nrf2, NQO1, HO-1, eNOS | NOX4, ICAM1, CD39, CD73 | 78 |
| NAF | HG (25 mM) induced HUVECs | 50,10,150,200 µg/mL | Decreasing oxidative stress; Decreasing vascular inflammation | HO-1, Nrf2 | ICAM-1, VCAM-1, E-selectin, NF-κB-p65, p-IκBα, ROS | 79 |
APV, Prunella vulgaris; EXS, Xanthoceras sorbifolia; GDE, Ganoderma tsugae; GR, Glutathione Reductase; NAF, Nepeta angustifolia; RBPC, Rice Bran Phenolic Compounds; SMT, Samul-Tang; TSAR, Total saponins of Anemone raddeana Regel.
Table 3.
Protective effects of polysaccharides on vascular endothelial cells.
| Extracts | Animal/cells | Dose/Concentration | Effects | Related molecular targets |
Refs. | |
|---|---|---|---|---|---|---|
| Up-regulation | Down-regulation | |||||
| ASTP | H2O2 (200 μM) induced HUVECs | 100, 200, 300 μM | Increasing cell survival; Reducing cell apoptosis | HO-1, KLF2, Nfr2, p-MEK, p-ERK | ROS, P53, p21, Bax, Caspase 9, Caspase 3 | 80 |
| AMCP | H2O2(300 μM) Induced HUVECs |
25–150 μg/mL | Reducing cell apoptosis | NO, SOD, CAT, MMP, PI3K, Akt, FOXO3a, Nrf2, HO-1, Bcl-2 | ROS, MDA, Bax | 81 |
| ASP | ox-LDL (200 μg/mL) induced HUVECs | 10,20,40 µg/mL | Increasing cell survival | eNOS, NO, VEGF, Akt | LDH, | 82 |
AMP, polysaccharide from Athyrium Multidentatum (Doll.) Ching; ASP, Angelica Sinensis Polysaccharide; ASTP, Astragalus polysaccharide; EXS, Xanthoceras sorbifolia.
Table 4.
Protective effects of monomers from herbal medicine on vascular endothelial cells.
| Classification/Monmers | Chemical Structure | Animal/cells | Dose/Concentration | Effects | Related molecular targets |
Ref | |
|---|---|---|---|---|---|---|---|
| Up-regulation | Down-regulation | ||||||
| Phenylpropanoids | |||||||
| CINM | 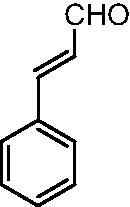 |
HG (30 mM) induced-HUVECs; HG (30 mM)- C57BL/6J mouse aortic rings | 10 μM | Protecting the endothelium relaxation; Decreasing oxidative stress | NO, Nrf2, HO-1, NQO1, CAT, GPx-1 | ROS, Nitrotyrosine | 86 |
| H2O2 (350 μM) and TNF-α (10 ng/mL) induced- HUVECs | 20 µM | Inhibiting the adhesion of monocytes; Increasing cell survival; Reducing cell apoptosis; Decreasing vascular inflammation | HO-1, Nrf2, p-p38 | VCAM-1, ROS, c-PARP, NF-κB-p65 | 87 | ||
| Ferulic Acid | 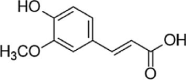 |
10 Gy of 60Co source (1.64 Gy/min) induced-HUVECs | 0.2,1,5μM | Increasing cell survival; Decreasing oxidative stress | GSH, NADPH, Nrf2, GCLC, GCLM, NQO1, HO-1 | ROS | 88 |
| Caffeic acid | 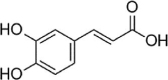 |
HG (25 mM) induced-HUVECs | 10 nM | Increasing cell survival; Decreasing oxidative stress; Inhibiting the adhesion of monocytes | SOD, TAA, GSH, Nrf2, HO-1 | ROS, NF-κB-p65, E-selectin | 89 |
| MCGA3 | 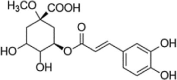 |
t-BHP (1 mM) induced-HUVECs; H2O2 (0.1 mM) induced-HUVECs |
0–200 μM | Increasing cell survival; Decreasing oxidative stress | HO-1, Ferritin, GCL, GR, GST, GSH, GSSG, Nrf2, p-JNK, p-ERK | ROS/LDH | 90 |
| Echinacoside | 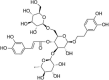 |
HG (30 mM) induced-HUVECs | 200 μM | Decreasing oxidative stress | eNOS, HO-1, p-Akt, Nrf 2 | ROSFyn | 91 |
| Sal B | 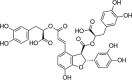 |
t-BHP (800 μM) induced EA.hy926 cells | 50–400 μM | Reducing oxidative stress; activating Keap1-Nrf2-ARE signaling | HO-1, NQO1, Nrf2 | LDH, Keap1 | 92 |
| SchB | 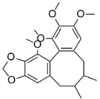 |
LPS (1 μg/mL) induced-HUVECs | 10, 20, 40 μM | Inhibiting the adhesion of monocytes; Decreasing vascular inflammation | Nrf2, HO-1 | TNF-α, IL-8, VCAM-1, ICAM-1, NF-κB-p65, p-IκBα | 93 |
| Sauchinone | 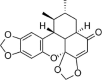 |
HG (25 mM) induced-HUVECs | 5,10,20,50 μM | Decreasing vascular inflammation | HO-1, Nrf2 | ROS, VCAM-1, ICAM-1, NF-κB-p65, p-IκBα | 94 |
| 7-HMR | 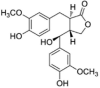 |
TNF-α (20 ng/mL) induced-RAECs | 100 μM | Decreasing vascular inflammation; Decreasing oxidative stress | SOD1, Nrf2, HO-1 | IL-6, VCAM-1, NLRP3, iNOS, NF-κB-p65, ROS, Keap1, p-ERK | 95 |
| Curcumin | 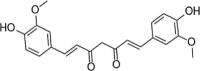 |
Co60 γ rays (Theratron 780, 0.6 Gy/min) induced- HUVECs | 5 µM | Inhibiting the adhesion of monocytes; Reducing DNA damage; Decreasing vascular inflammation | Nrf2, GSH | ICAM-1, VCAM-1, E-selectin, NF-κB-p65, IL-6, IL-8, MCP-1, 8-OHdG, TBARS | 97 |
| Flavonoids | |||||||
| Anthocyan | 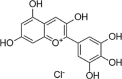 |
H2O2(400 μmol/L) induced-bEND.3 cells | 50,100 μg/mL | Reducing cell apoptosis; Decreasing oxidative stress | NO, SOD, GSH-PX, HO-1, Nrf2 | ROS, MDA, TNF-α, IL-6, LDH | 101 |
| Hyperoxia (O2 32%) induced-HUVECs | 20% | Increasing cell survival | HO-1, Nrf2, NQO1, p-ERK1/2 | 102 | |||
| C3G | 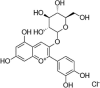 |
PA (100 μM) induced-HUVECs | 20,50 μM | Improving insulin resistance and vascular inflammation; Increasing vasodilatory activity | Nrf2, HO-1, p-Akt, p-eNOS | ET-1, PAI-1, p-IRS-1, p-IKK-β, p-JNK | 103 |
| TNF-α (20 ng/mL) induced-HUVECs | 20,40 μM | Increasing cell survival; Decreasing vascular inflammation and oxidative stress | SOD, GSH, HO-1, NQO1, Nrf2, p-ERK | GSSG, NF-κB-p65 | 104 | ||
| PA (100 μM) induced-HUVECs | 20,50 μM | Increasing cell survival; Inhibiting monocytes adhesion; Decreasing vascular inflammation and oxidative stress | Nrf2, HO-1, NQO1, GSH | ROSVCAM-1, E-selectin, NF-κB-p65, Bch1 | 105 | ||
| ECC |  |
H2O2 (100 μM) induced-HUVECs | 10 μM | Decreasing oxidative stress | HO-1 | ROS | 106 |
| EGCG |  |
microcystin-LR (40 μM) induced-HUVECs | 50 μM | Reducing cell apoptosis; Inhibiting mitochondrial dysfunction | MMP, Nrf2, HO-1 | Cyt c, Caspase 3, Caspase 9 | 107 |
| Ang II (400 nmol/L) induced-HUVECs | 200 μM | Increasing cell survival; Reducing cell apoptosis; Decreasing oxidative stress; Inhibiting mitochondrial dysfunction | SOD, CAT, GST, GPx, MMP, Nrf2 | ROS, MDA, Cyt c, Caspase-3, Caspase-9, NOX | 108 | ||
| IL-1β (2 ng/mL) induced-HUVECs | 100 μM | Decreasing oxidative stress; Decreasing vascular inflammation | HO-1, SOD, Nrf2f | ROS, NF-κB-p65, p-IκBα, COX-2, PGE2, GPx | 109 | ||
| PM2.5(200 μg/mL) induced-HUVECs | 50,100,200 μg/mL | Increasing cell survival; Decreasing oxidative stress | HO-1, Nrf2 | ROS | 110 | ||
| Endothelial cells of porcine aorta | 30 μM | Increase bilirubin production | HO-1, Nrf2 | 111 | |||
| DHMT |  |
Ox-LDL (70 μg/mL) induced-HUVECs | 40 μM | Increasing cell survival; Reducing cell apoptosis; Decreasing oxidative stress | MMP, SOD, CAT, GSH-Px, Bcl2, Nrf2, HO-1, p-ERK, p-Akt | ROS, MDA, Caspase 3, Caspase 9, Bax, LOX-1, Cyt c | 112 |
| PA (300 μM) induced-HUVECs | 0.1, 0.5, 1 μM | Increasing cell survival; Reducing pyroptosis; Decreasing vascular inflammation | Nrf2, HO-1, NQO1 | ROS, Caspase1, IL-1β, NLRP3, p20, ICAM-1 | 113 | ||
| Kaempferol |  |
ox-LDL (100 μg/mL) induced-HUVECs | 50 μg/mL | Increasing cell survival; Reducing cell apoptosis; Decreasing oxidative stress; Decreasing vascular inflammation | SOD, p-AMPK, Nrf2, HO-1, Bcl-2 | TNF-α, IL-1β, IL-6, ROS, Caspase3, ICAM-1, VCAM-1, E-selectin | 114 |
| Fisetin | 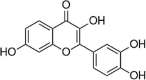 |
H2O2(300 μM) induced-HUVECs | 5,10,25 µM | Increasing cell survival; Decreasing oxidative stress; Reducing cell death | HO-1, Nrf2, HO, ARE | 115 | |
| Myricitrin | 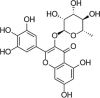 |
H2O2 (600 μM) induced- ECV-304 cells | 16,32,64 μM | Reducing cell apoptosis; Decreasing oxidative stress | SOD, NO, Bcl-2, ERK | ROS, LDH, MDA, Bax, Caspase-9, Caspase-3, p-ERK | 116 |
| Rutin | 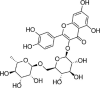 |
H2O2(200 μM) induced-HUVECs | 1,3,10,30 µM | Decreasing oxidative stress | Nrf2, GCLC | TrxR1, NF-κB-p65, HIF | 114 |
| Eriodictyol | 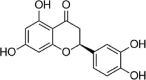 |
H2O2 (300 μM) induced-HUVECs | 10 μM | Decreasing oxidative stress; Reducing cell death | HO-1, HO, p-ERK, Nrf2, ARE | ROS | 118 |
| Apigenin |  |
AGEs (500 μg/mL) induced-HUVECs | 10 μM | Decreasing oxidative stress; Decreasing vascular inflammation | Nrf2, GCLM, GCLC, HO-1 | ROS, MCP-1, IL-6, ICAM1, TGF-β1, P22phox, RAGE, p-ERK, NF-κB-p65 | 119 |
| Naringenin |  |
HG (33 mM)/FFA(1 mM) induced-HUVECs | 0–100 μM | Reducing cell apoptosis | HO-1, p-Akt, p-ERK, p-JNK, Nrf2 | 120 | |
| Baicalin |  |
HG (22 mM) induced-HUVECs | 50 μM | Reducing cell apoptosis; Decreasing oxidative stress; Decreasing vascular inflammation | Bcl2, Nrf2, NQO1, NQO2, HO-1, CAT, SOD, p-Akt, p-GSK3B | Caspase3, Bax, IL-1β, IL6, IL8, TNF-α, n-Fyn | 121 |
| Genistein |  |
ox-LDL (100 mg/mL) induced-HUVECs | 1,10 μM | Increasing cell survival; Decreasing vascular inflammation | HO-1, Nrf2, HO | MCP-1, VCAM-1, ICAM-1 | 122 |
| Equol | 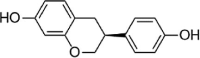 |
t-BHP (50 μM)/ thapsigargin (1 μM)/ PA (500 μM) induced- HUVECs apoE-/- mice | 1, 10, 100 nM | Alleviating AS plaque formation; Increasing cell survival; Reducing cell apoptosis; Attenuating ER stress | Nrf2, NQO1 | Caspase3, p-elF2α, p-PERK, GRP78, CHOP, ATF6 | 123 |
| Phloretin | 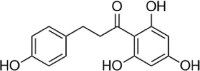 |
PA (100 μM) induced- HUVECs | 1, 10, 50 μM | Decreasing oxidative stress | MMP, SOD, Gpx-1, p-LKB1, p-AMPK, Nrf2, HO-1 | ROS, MDA | 124 |
| Euxanthone | 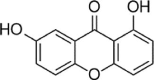 |
ox-LDL (100 μg/mL) induced-HUVECs | 5,10 μM | Increasing cell survival; Reducing cell apoptosis; Decreasing oxidative stress; Decreasing vascular inflammation | Bcl2, MMP, SOD, CAT, GSH-Px, Nrf2, HO-1, NQO1, p-ERK, p-P38, p-JNK | MDA, ROS, Caspase 3, PARP, Bax, Cyc c, Keap1, MCP-1, IL-1β, TNF-α | 125 |
| XAG | 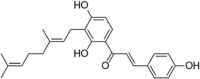 |
ox-LDL (100 μg/mL) induced-HUVECs | 5 μM | Increasing cell survival; Reducing cell apoptosis; Decreasing oxidative stress | Bcl2, CAT, SOD, GSH-Px, Nrf2, HO-1, NQO1 | MDA, ROS, Caspase 3, PARP, Bax, Keap1 | 126 |
| Terpenoids | |||||||
| PMA | 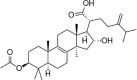 |
ox-LDL (70 μg/mL) induced-HUVECs | 10 μmol/L | Reducing cell apoptosis; Decreasing oxidative stress | SOD, Nrf2, HO-1, Caspase 3, Bcl2 | MDA, ROS, Bax | 129 |
| Celastrol | 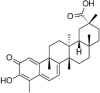 |
Ang II (400 nM) induced-HUVECs | 50 nM | Increasing cell survival; Reducing cell apoptosis; Decreasing oxidative stress; Decreasing vascular inflammation | SOD, GSH-Px, p-ERK, Nrf2 | MDA, ROS, IL-6, TNF-α, VCAM, NADPH oxidase, NOX2, AT1 | 130 |
| Oleanolic acid | 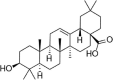 |
ox-LDL (200 μg/mL) induced-HUVECs HFD-quails | 5,10,20 μmol/L 25, 50 mg/kg |
Increasing cell survival; Decreasing oxidative stress; Alleviating AS plaque formation | HO-1, Nrf2 | ROS, LOX-1, MADPH | 131 |
| Maslinic acid | 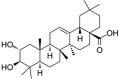 |
LPS ((1 μg/mL) induced-HUVECs | 2,5,10,20 μM | Decreasing oxidative stress; Decreasing vascular inflammation | NO, HO-1, Nrf2, ARE | COX-2, iNOS, PGE2, NF-κB-p65, IL-1β, p-STAT1 | 132 |
| ASD IV | 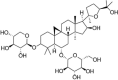 |
ox-LDL (100 μg/mL) induced-HUVECs | 10,20,50 μM | Increasing cell survival; Reducing cell apoptosis; Inhibiting cell migration; Decreasing vascular inflammation | Nrf2, HO-1 | MDA, ROS, NADPH oxidase, TNF-α, IL-6 | 133 |
| GSD Rg1 | 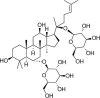 |
PM2.5 (400 μg/mL) induced-HUVECs | 2.5,10,40 μg/mL | Increasing cell survival; Decreasing oxidative stress | HO-1, Nrf2 | MDA, ROS | 134 |
| ADH | 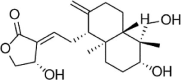 |
TNF-α (1 ng/mL) induced-EA.hy926 cells | 7.5 μM | Inhibiting the adhesion of monocytes; Decreasing vascular inflammation | GSH, GCLM, HO-1, p-Akt, p-ERK, Nrf2, c-Jun | ROS, ICAM-1 | 135 |
| CoCl2 (200 μM) induced-EA.hy926 cells | 1.8,3.75,7.5 μM | Decreasing vascular inflammation | HO-1, PHD, Nrf2 | ROS, HIF-1α, ET-1, p-38 | 136 | ||
| Gau A | 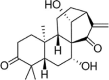 |
H2O2 (100 μM) induced-HUVECs | 0.1,0.5,1,10 μM | Decreasing vasoconstriction | eNOS | ET, iNOS | 137 |
| ZAD |  |
Ox-LDL (150 μg/mL) induced-HUVECs | 20 μg/mL | Increasing cell survival; Decreasing oxidative stress; Decreasing vascular inflammation | SOD, /Nrf2, HO-1, NQO1 | MDA, ROS, LOX-1, IL-1β, MCP-1, TNF-α, Keap1 | 138 |
| Lycopene | Strain (20% in length, 1 Hz) induced-HUVECs | 3 μM | Decreasing oxidative stress | HO-1, Nrf2, p-Akt | ROS, ET-1, p22, NADPH oxidase, p-ERK | 139 | |
| Alkaloids | |||||||
| Oxymatrine |  |
HCS (3 mM) induced-HUVECs | 0.1, 0.3,0.6 µM | Decreasing oxidative stress; Increasing cell survival; Reducing cell apoptosis | NO, SOD, MMP/, Bcl2, Nrf2, p-Akt, p-eNOS | MDA, ROS, LDH, Bax, Caspase 9, Caspase 3 | 141 |
| Corynoline |  |
LPS (10 ng/ml) induced-HUVECs | 1,2,4µM | Increasing cell survival; Decreasing vascular inflammation | HO-1, Nrf2 | TNF-α, IL-6, IL-8, VCAM-1, ICAM-1, NF-κB-p65, p-IκBα | 142 |
| Berberine |  |
H2O2 (200 μM) induced-HUVECs | 5,10 μM | Reducing cell apoptosis | Nrf2, HO-1 | ROS | 143 |
| BITC | 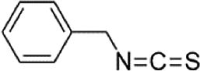 |
Ox-LDL (40 µg/mL) induced-HUVECs | 2,5,10 µM | Inhibiting the adhesion of monocytes; Decreasing vascular inflammation; Decreasing oxidative stress | HO-1, GCLC, GCLM, GSH, Nrf2, ARE-driven | ROS, ICAM-1, VCAM-1, E-selectin/, NF-κB-p65, p-IκBα | 144 |
| PEITC | 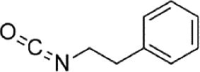 |
Ox-LDL (40 µg/mL) induced-HUVECs | 2,5,10 µM | Inhibiting the adhesion of monocytes; Decreasing vascular inflammation; Decreasing oxidative stress | HO-1, GCLC, GCLM, GSH, Nrf2, ARE-driven | ROS, ICAM-1, VCAM-1, E-selectin, NF-κB-p65, p-IκBα | 145 |
| Lotusine | 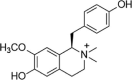 |
H2O2 (400 μM) induced-ECV304 | 100 μM | Increasing cell survival | NOS, NO | 145 | |
| Liensinine | 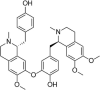 |
H2O2 (400 μM) induced-ECV304 | 0.1 μM | Increasing cell survival | NOS, NO | 145 | |
| Isoliensinine |  |
H2O2 (400 μM) induced-ECV304 | 0.1 μM | Increasing cell survival | NOS, NO | 145 | |
| Neferine |  |
H2O2 (400 μM) induced-ECV304 | 0.1 μM | Increasing cell survival | NOS, NO | 145 | |
| SFN | 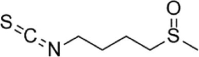 |
Ang II (400 nM) induced-HUVECs | 2 μM | Increasing cell survival; Reducing cell apoptosis; Inhibiting mitochondrial dysfunction; Decreasing oxidative stress | MMP, SOD, CAT, GSH, Nrf2, HO-1 | ROS, MDA, Cyt c, LDH, Caspase 3, Caspase 9, NADPH oxidase, GSSG | 146 |
| Stilbenes | |||||||
| Pterostilbene | 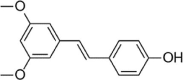 |
US induced-HUVECs | 5,10,50 µM | Decreasing oxidative stress; Increasing cell survival; Decreasing vascular inflammation | SOD, CAT, eNOS, HO-1, Nrf2 | MDA, NADPH oxidase, iNOS,IL-1β, TNF-α, MCP-1, VCAM-1, Keap1, NOX | 148 |
| AS-rat H2O2(300 μM) induced-HUVECs | Decreasing oxidative stress; Increasing cell survival; Reducing cell apoptosis; Reducing AS plaque size; Inhibiting of vascular wall apoptosis | Nrf2, p-AMPK | STAT3 | 149 | |||
| Resveratrol |  |
H2O2 (300 μM) induced- HUVECs | 5,10,20 µM | Decreasing cell apoptosis; Activating PI3K-Akt-mTOR pathway; Decreasing oxidative stress; | SOD, GSH, p-Akt, p-mTOR, p-Nrf2 | ROS, MDA, LDH, Caspase-3, | 150,151 |
| Quinones | |||||||
| Miltirone |  |
ox-LDL (120 μg/mL) induced-EA.hy926 cells | 0.5, 1, 2 µM | Increasing cell survival; Decreasing oxidative stress | Nrf2, ARE, HO-1, NQO1, SOD, GST, p-ERK, p-JNK | ROS, Keap1 | 152 |
| Tanshinone IIA |  |
HG (20 mM) induced-HUVECs | 10,30,50 μg/mL | Improving oxidative stres; Reducing lipid peroxidation and MDA; Enhancing activity of antioxidant enzyme; Increasing release of NO via regulation of Rho/Rho kinase system | NOS, SOD | ROS, ROCKI | 153 |
| Others | |||||||
| Withaferin A |  |
HUVECs EA.hy926 | 1 µM | Increasing survival of HUVECs | HMOX1, HO-1, Nrf2 | Keap1 | 154 |
| Z-Ligustilide | 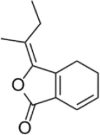 |
TNF-α (50 ng/ml) induced-HUVECs | 1, 3, 10 μM | Inhibiting the adhesion of monocytes; Decreasing vascular inflammation | NO, Nrf2, HO-1 | ROS, ICAM-1, VCAM-1, E-selectin, NF-κB-p65, p-IκBα | 155 |
| t-BHP (200 μM) induced-EA.hy 926cells; HFD- Ldlr−/−mice (C57BL/6JNju) |
100μΜ, 20 mg/kg | Increasing cell survival; Decreasing oxidative stress; Alleviating AS plaque formation | GSH, Nrf2, GCLM, HO-1, NQO1, SOD1, SOD2, CAT, GCLC, ARE-driven | GSSG, RO, Keap1 | 156 | ||
| Salidroside | 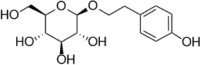 |
H2O2 (300 μM) induced-HUVECs | 0.1,1,10 μM | Increasing cell survival; Decreasing oxidative stress | SOD, CAT, Nrf2, HO-1, NQO1 | ROS, MDA | 158 |
| AGEs (200 μg/mL) induced-HUVECs | 0.1, 1, 10 μM | Protecting the endothelium relaxation; Decreasing oxidative stress; Decreasing vascular inflammation | NO, Nrf2, HO-1, NF-κB-p65 | ROS, Keap1 | 159 | ||
| Vanillic acid | 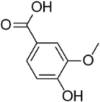 |
PA (100 μM) induced-HUVECs | 1,10,20 µM | Decreasing oxidative stress | CAT, SOD, MMP, HO-1, Nrf2, p-LKB1, p-AMPK, SIRT1, PGC-1α | ROS, MDA | 160 |
| PCA | 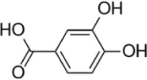 |
PA (100 µM) induced-HUVECs | 100 µM | Decreasing oxidative stress; Increasing the mitochondrial density | SOD, HO-1, Gpx-1, p-LKB1, p-AMPK, Nrf2, PGC-1α | ROS, MDA | 161 |
| Salicin | 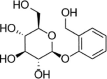 |
TNF-α (10 ng/mL) induced-HUVECs; H2O2 (100 μM) induced-HUVECs |
50 and 100 μM | Inhibiting cellular senescence; preventing cell cycle arrest; Decreasing oxidative stress | Nrf2 | SAβ-Gal, P21, ROS, PAI-1, P53 | 162 |
| Allicin | 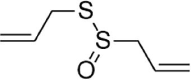 |
LPS (1 μg/mL) induced-HUVECs | 40 μg/mL | Increasing cell survival; Reducing cell apoptosis; Inhibiting mitochondrial dysfunction; Inhibiting the adhesion of monocytes; Decreasing oxidative stress; Decreasing vascular inflammation | SOD, CAT, GST, GPX, MMP, LXRα, Nrf2 | ROS, MDA, Cyt c, TNF-α, IL-8, NF-κB-p65 | 163 |
| STZ-induced mice; HG (30 mM) induced- HUVECs |
30 mg/kg/d 10 μg/mL |
Increasing cell survival; Reducing cell apoptosis; Decreasing vascular inflammation | Bcl2, Nrf2 | TNF-α, VCAM-1, Inos, MMP-2, MCP-1, Caspase 3, Bax, NF-κB-p65 | 164 | ||
AMP, polysaccharide from Athyrium Multidentatum (Doll.) Ching; ASP, Angelica Sinensis Polysaccharide; ASTP, Astragalus polysaccharide; EXS, Xanthoceras sorbifolia 7-HMR, (−)-7(S)-hydroxymatairesinol; ADH, Andrographolide; ASD IV, Astragaloside IV; BITC, Benzyl isothiocyanate; C3G, Cyanidin-3-O-glucoside; CFA, Caffeic acid; CINM, Cinnamaldehyde; DHMT, Dihydromyricetin; ECC, (−)-epicatechin; EGCG, Epigallocatechin-3-gallate; ET, endothelin; FAD, Ferulic Acid; FFA, free fatty acids; Gau A, glaucocalyxin A; GSD Rg1, Ginsenoside Rg1; HCS, Homocysteine; HFD, High fat diet; HG, high glucose; MCGA3, 3-O-caffeoyl-1-methylquinic acid; MSA, Maslinic Acid; OLA, Oleanolic acid; PA, palmitic acid; PCA, Protocatechuic acid; PEITC, phenethyl isocyanate; PMA, Pachymic acid; RAECs, rat aortic endothelial cells; Sal B, Salvianolic acid B; SchB, Schisandrin B; SFN, (−)-Sulforaphane; STZ, Streptozotocin; t-BHP, tert-butyl hydroperoxide; US, uraemic serum; VNA, Vanillic acid; XAG, Xanthoangelol; ZAD, Zedoarondiol.
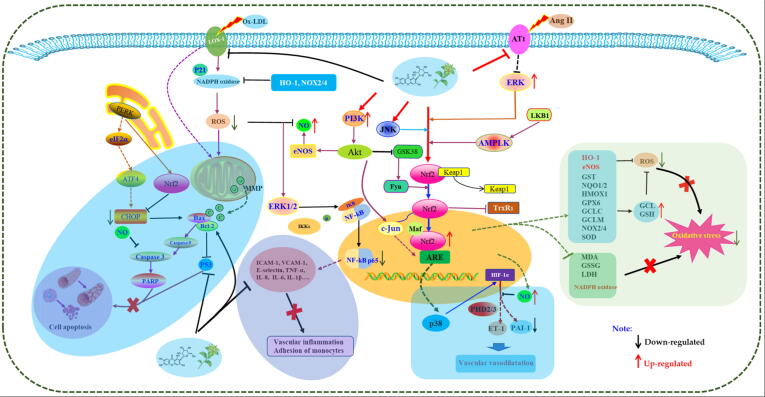
Fig. 3.
The possible molecular mechanisms for herbal medicines to protect vascular endothelial cells via activation of Nrf2/HO-1 signaling.
Extracts from herbal medicines
Herbal medicine formulas
In 2015, using H2O2-induced human umbilical vein endothelial cells (HUVECs), Xiong et al. found that Xueshuan Xinmaining tablet (XXT), a traditional Chinese medicine formula commonly used for the treatment of cardiovascular diseases in the clinic, exerted a protective effect on H2O2-stimulated HUVECs and reduced ROS levels in HUVECs, and the potential mechanisms were correlated with the regulation of Nrf2 and downstream protein expression (HMOX, GCLM, NQO1) [67]. In 2016, Lv et al. studied the effects of another classic traditional Chinese medicine (TCM) formula named the Liuwei-Dihuang pill (LWDH) on Eahy926 cells and found that serum of patients treated with LWDH promote the proliferation of Eahy926 cells and upregulate ERK phosphorylation and HO-1 and Nrf2 expression in cells [68]. Later, based on the TNFα-induced HUVEC model, researchers investigated two herbal medicine formulas named Samul-Tang Tang (SMT) (also named Si-Wu decoction) and Paeotang. Choi et al. reported that SMT could inhibit NF-κB translocation and activation, the expression of CAMs, monocyte adhesion, and ROS levels in TNF-α-stimulated HUVECs; the potential molecular mechanisms are related to the upregulation of Nrf2, HO-1 and NO [69]. Similarly, Kim et al. found that under TNF-α stimulation, the levels of ROS, MMPs and adhesion molecules in HUVECs were abnormally elevated; however, Paeotang intervention could improve these changes by increasing HO-1 and Nrf2 expression [70]. In 2019, Zhao et al. studied the treatment effect of Huoxue capsule (HXC) on H2O2 stimulated bEND.3 cells, and the results suggested that HXC increases the survival of H2O2-stimulated bEND.3 cells, upregulates HO-1 expression, and promotes Akt and Nrf2 phosphorylation and Nrf2 nuclear translocation [71] (Table 1).
Plant extracts
In 2009, Wei et al. studied the effect of Ganoderma tsugae extracts (GTE) on bovine artery endothelial cells (BAECs) and found that GTE could promote the translocation of Nrf2 to the nucleus in BAECs and induce the expression of Ho-1 and thioredoxin reductase-1 (TrxR1). Finally, they identified a major active ingredient in GTE, namely F5-2, which a peptidoglycan-like compound [72]. In addition, it has been reported that long-term GTE therapy upregulates GSH, SOD-1 and HO-1; decreases vascular permeability; and alleviates the genotoxicity and apoptosis caused by PM2.5 in endothelial cells [73]. Korean red ginseng (KRG) is a food and drug with various pharmacological activities. Yang et al. found that extracts of KGR (KGRE) had a significant inhibitory effect on H2O2-induced apoptosis in HUVECs and reduced ROS levels in cells. In-depth studies found that the mechanism of action was related to the regulation of the Nrf2/Ho-1 pathway [74]. Prunella vulgaris is a known herbal medicine in China with the functions of clearing heat, dispersing swelling and dissipating binds. In 2012, Huang reported that aqueous extracts of Prunella vulgaris (APV) inhibit the adhesion of HL-60 cells to HUVECs under high glucose (HG) stimulation. Further mechanistic investigations revealed that APV reduces ROS and the expression of ICAM-1, VCAM-1, and E-selectin. In addition, APV also suppresses the activation of NF-κB p65 and induce the phosphorylation of Akt and the activation of HO-1, eNOS and Nrf2 [75]. Han et al. investigated the effects of the total saponins of Anemone raddeana Regel (TSAR) on ox-LDL-induced HUVECs, and the results revealed that TSAR increases the survival of ox-LDL-induced HUVECs, decreases LDH, and increases NO and ET [76]. Another study by Yoon et al. reported that extracts of Xanthoceras sorbifolia (EXS) improve the oxidative stress in HUVECs induced by TNF-α in cytoplasmic fractions and Ho-1 protein levels in nuclear fractions and inhibits the nuclear translocation of NF-κB and the binding of IκBα [77]. Similarly, phenolic extracts derived from rice bran (RBPC) also have a protective effect on H2O2-stimulated HUVECs. The mechanism is related to the downregulation of ICAM1, CD39, CD73, and NOX4 expression and upregulation of Nrf2, NQO1, Ho-1, and eNOS expression, indicating that RBPC alleviates endothelial dysfunction through its antioxidant and anti-inflammatory activities [78]. In 2019, Huang et al. showed that Nepeta angustifolia (NAF) has a protective effect on HG-induced HUVEC injury by reducing IκBα phosphorylation, inhibiting ROS production, increasing HO-1 protein levels and inducing Nrf2 translocation to the nucleus [79] (Table 2).
Polysaccharides
Polysaccharides are polysugar macromolecular carbohydrates that are bonded by multiple monosaccharides and glycosidic bonds. Polysaccharides are widely distributed in nature and have a variety of pharmacological activities, such as antitumor, immunoregulatory, and anti-inflammatory activities. Currently, an increasing number of studies have reported that polysaccharides derived from natural medicines could be beneficial for the treatment or prevention of CVDs. Astragalus polysaccharide (ASTP) reduces ROS generation and the expression of Baz, Caspase-9 and Caspase-3, increases HO-1 and Nrf2 expression, inhibits cell apoptosis, and effectively protects HUVECs from H2O2 stimulation. KLF2, a transcription regulatory protein, primes Nrf2 for activation in endothelial cells, and the authors also found that ASTP can increase KLF2 expression [80]. Similarly, polysaccharides from Athyrium multidentatum (AMP) increase the survival rate of H2O2-damaged HUVECs, reduce ROS production, and enhance SOD and CAT activity. In addition, pretreatment with AMP increases the Bcl-2/Bax ratio and reduces the mitochondrial membrane potential. Furthermore, AMP increases FOXO3a levels, upregulates the expression of PI3K and Akt genes, and increases the expression levels of Nrf2 and Ho-1 genes [81]. Currently, another study by Liu et al. revealed that Angelica sinensis polysaccharide (ASP) increases the survival of ox-LDL-induced HUVECs; increases eNOS, NO, VEGF and Akt; and decreases LDH in ox-LDL-induced HUVECs [82] (Table 3).
Monomers from herbal medicines
Phenylpropanoids
Phenylpropanoids, a class of secondary metabolite compounds produced in natural plants that contain a representative phenyl group, possess a wide spectrum of bioactivities, including antitumor, anti-inflammatory, antibacterial, antioxidant and cardiovascular activities [83], [84]. Cinnamaldehyde (CINM), a classical and simple phenylpropanoid, is a major constituent of Cinnamomum cassia [85]. In 2015, Wang et al. demonstrated that CINM reduces ROS production and increases NO levels in endothelial cells of aortae treated with HG. In addition, CINM preconditioning increases the activity of HG-induced HUVECs and upregulates the expression of Nrf2 and its downstream genes, such as HO-1, NQO1, glutathione peroxidase (GPx)-1 and CAT [86]. Furthermore, in another recent study, CINM exhibits an inhibitory effect on H2O2-induced apoptosis in HUVECs, and the protective effect of CINM on HUVECs could be blocked by a HO-1 inhibitor. In addition, CINM also activates Nrf2, and this effect could be blocked by siRNA against Nrf2. These results suggest that CINM exerts protective effects on H2O2-induced HUVECs through the Nrf2/HO-1 signaling pathway [87]. Ferulic acid and caffeic acid are two other classical and simple phenylpropanoids that comprehensively exist in natural plants, and the two compounds possess similar structures. In the cell models of 60Co-induced HUVECs and HG-induced HUVECs, respectively, the two compounds increase the survival of HUVECs and reduce the ROS levels in cells. The possible mechanisms of both compounds corresponding to their protective effects on HUVECs are related to the activation of Nrf2/HO-1 [88], [89]. In addition, a previous study showed that 3-O-caffeoyl-1-methylquinic acid (MCGA3), a new antioxidant obtained from bamboo leaves, protects HUVECs induced with t-BHP or H2O2 from ROS-induced endothelial injury and upregulates HO-1 expression via the nuclear translocation of Nrf2 [90]. Echinacoside is a compound derived from the medicinal plants Cistanche and Echinacea. Research has found that this compound can protect HUVECs induced by HG by reducing ROS production, reducing Fyn protein levels in the nucleus, and inducing Nrf2 nuclear translocation and HO-1 expression. However, these effects can be blocked by Nrf2 siRNA. The author also found that Akt inhibitor intervention could also block the regulation of echinacoside on Nrf2, suggesting that the Akt pathway is involved in the protection of endothelial injury of echinacoside [91]. In 2020, Guo et al. reported that salvianolic acid B (Sal B), an active compound isolated from the known TCM of Salvia miltiorrhiza, possessed protective effects against t-BHP-induced vascular endothelial cell EA.hy926 injury via activation of the Keap1-Nrf2-ARE pathway [92]. Inflammatory mediators can cause endothelial cell damage and destroy the integrity of the vasculature. Lin et al. found that schisandrin B (SchB) inhibits the release of TNF-α and IL-8 in HUVECs induced by LPS, reduces the binding of VCAM-1 and ICAM-1 in the cells and simultaneously inhibits NF-κB P65 and IκBα phosphorylation. However, the anti-inflammatory effect of SchB are blocked by an Nrf2 inhibitor [93]. Sauchinone is a lignan extracted from Saururus chinensis, and previous work suggested that this natural monomer improves the vascular inflammatory reaction by suppressing cell adhesion molecules, such as VCAM-1 and ICAM-1, in high glucose-induced HUVECs. A further study revealed that sauchinone inhibited the degradation of IκB and the nuclear translocation of NF-κB p65 as well as the activation of HO/Nrf2 in HG-induced HUVECs [94]. Similarly, (−)-7(S)-hydroxymatairesinol (7-HMR) inhibits TNF-α-induced inflammatory mediators, such as the expression of VCAM-1, IL-6, and iNOS, and further studies have found that its anti-inflammatory mechanism is related to the promotion of Keap1 degradation and upregulation of HO-1 and Nrf2 [95]. Ionizing radiation causes chronic oxidative stress, leading to cell inflammation and vascular damage, which is one of the potential risk factors for CVD [96]. Curcumin is a known natural compound with many bioactivities, particularly antitumor, antioxidant and anti-inflammatory activities. Soltani et al. found that curcumin (nanoformulation) exerted a protective effect on HUVEC damage caused by ionizing radiation and inhibited the adhesion between endothelial cells and monocytes, suggesting that curcumin represents a potential preventive agent against inflammation and vascular damage induced by ionizing radiation [97] (Table 4).
Flavonoids
Flavonoids are widely distributed in natural plants and herbs with greater than 4000 natural flavonoids found in plants and berries [98], [99]. Flavonoids are often used as drugs to treat various diseases, such as cancer and cardiovascular diseases, as well as aging. Humans also obtain flavonoids in their diet as supplements to the body in everyday life. In recent years, flavonoids have also been used to prevent and treat AS-related diseases.
Anthocyanins are common compounds existing in natural plants with promising bioactivities and are also mainly responsible for the blue, purple, and red color of the fruits, flowers, and leaves [100]. Anthocyanin is a representative anthocyanin in plants that enhances the activity of H2O2-stimulated HUVECs, reduces the release of ROS and inflammatory factors, and increases the expression of Nrf2 and Ho-1 proteins, demonstrating that anthocyanin may play an antioxidant role by upregulating Nrf2/HO-1[101]. In 2012, a study reported that HUVECs pretreated with human anthocyanin medicated serum (hAMS) seemed to be more resistant to mild hyperoxia stimulation, suggesting that hAMS has a protective effect against mild hyperoxia-induced cell damage. Further mechanistic studies found that hAMS significantly increased the nuclear levels of the transcription factor Nrf2, and HO-1 and NQO-1 expression showed similar trends. In addition, hAMS also induces phosphorylation of ERK1/2. However, the selective pharmacological inhibitor of mitogen-activated protein kinase kinase (MAPKK), PD98059, severely inhibits the protective effect of hAMS and Nrf2 localization, indicating that the MAPK cascade is directly involved in the activation of the Nrf2/ARE pathway in hAMS [102]. Cyanidin-3-O-glucoside (C3G) is another active anthocyanin that protects HUVECs from TNF-α-induced damage by improving the antioxidant system and activating the Nrf2/ARE pathway [103]. In 2016, Deborah et al. also found that C3G improves the oxidative stress status of HUVECs exposed to palmitate (PA), significantly increases Nrf2 nuclear accumulation, decreases Bach1 nuclear accumulation, and induces HO-1 and NQO-1 gene expression. In addition, when the NF-κB pathway was inhibited with wedelolactone (an IKK inhibitor), the upregulation of PA on E-selectin and VCAM-1 was significantly reduced, indicating that NF-κB was important in PA-induced endothelial dysfunction. Fortunately, C3G pretreatment significantly inhibited PA activation of the NF-κB proinflammatory pathway [104]. Furthermore, in another study of this compound, pretreatment with C3G also effectively inhibited the phosphorylation of Akt and eNOS by PA and maintained NO levels in cells. However, Nrf2 siRNA transfection inhibited gene regulation by C3G, while the inhibition of IKK phosphorylation by C3G was weakened [105]. All these above results confirm that C3G is a natural Nrf2 inducer that can be used as a potential therapeutic strategy for AS to protect vascular endothelial cells from various stress injuries.
Flavanol is another characteristic flavonoid widely distributed in natural plants with various bioactivities. (−)-Epicatechin (ECC) is an effective free radical scavenger that has a protective effect on H2O2-induced oxidative stress in HUVECs, and the mechanism is related to an increase in HO-1 expression [106]. Increasing evidence has indicated that green tea is beneficial for the prevention of coronary atherosclerosis, and epigallocatechin-3-O-gallate (EGCG) is reported to be one of the bioactive agents in green tea for preventing coronary atherosclerosis. EGCG is one of the most abundant catechins in green tea, so it is considered the main active ingredient in green tea to improve endothelial cell function and reduce cardiovascular risk. EGCG can play antioxidative, anti-inflammatory and antiapoptotic roles to address a variety of stimuli, such as HUVEC damage induced by PM2.5, H2O2, angiotensin II, and microcystin-LR. The mechanism seems to be related to the upregulation of Nrf2, HO-1 and related genes, indicating that activation of the Nrf2/HO1 signaling pathway is an important mechanism by which EGCG protects against endothelial cell damage [107], [108], [109], [110], [111].
Flavanol is also beneficial for the protection of ECs under various stimuli. Dihydromyricetin (DMY) is a natural flavanol isolated from Ampelopsis grossedentata. Studies have found that DMY activates Akt and ERK1/2, induces Nrf2/HO-1 signaling, restores cell membrane potential, inhibits endothelial cell apoptosis and caspase-3 activity, and protects HUVECs from ox-LDL-induced oxidative damage [112]. Pyroptosis is a caspase-1-dependent form of inflammatory cell death that leads to DNA fragmentation, cell swelling, lysis, and release of contents. Studies have shown that pyroptosis plays an important role in the pathological instability of AS. Hu et al. found that DWY can reverse PA-induced pyroptosis in HUVECs and inhibit caspase-1 activation, IL-1β release, and NLRP3 inflammatory corpuscle activation. However, siRNA knockout of Nrf2 removes the pyropastic inhibition of DHM, indicating that the Nrf2 signaling pathway plays an important role in DHM's improvement of PA-induced pyroptosis of vascular endothelial cells [113]. In 2018, Kang and Jing noted that pretreatment with different concentrations of kaempferol alleviates the endothelial injury induced by ox-LDL, reduces HUVEC apoptosis and enhances cell vitality by promoting the activation of the AMPK/Nrf2/HO-1 signaling pathway [114]. In addition, Lee et al. reported that fisetin (3,7,3′, 4′-tetrahydroxyflavone), a flavonoid obtained from vegetables or fruits, reduces H2O2-induced HUVEC death by increasing Nrf2 nuclear translocation and HO-1 expression, suggesting that Nrf2 and HO-1 may be involved in the endothelial protection of fisetin and that siRNA targeting PKC and P38 MAPK can attenuate fisetin-induced HO-1 expression [115]. Myricitrin is a flavonol glycoside. In 2013, Sun et al. reported the protective effects of myricitrin against oxidative stress-induced apoptosis of vascular ECs, and the related mechanisms were involved in inhibiting the phosphorylation of ERK and apoptosis proteins [116]. Rutin is a known flavanol diglycoside with various bioactivities. In 2017, Sthijins et al. reported that rutin protects against H2O2-induced oxidative stress damage in HUVECs via Nrf2-mediated adaptation [117].
Flavone is the most representative type of flavonoid with a wide spectrum of pharmacological activities. In 2015, eriodictyol treatment protected HUVECs from oxidative stress induced by H2O2 by increasing HO-1 mRNA and protein expression in cells, but Nrf2 siRNA and extracellular regulated protein kinase (ERK) inhibitors blocked the upregulation of eriodictyol by HO-1. Interestingly, eriodictyol also induces Nrf2 nuclear translocation and ARE activity [118]. A recent study found that apigenin, a known natural flavonoid that widely exists in plants, can intervene in the ERK/Nrf2 pathway to regulate cellular inflammation and the antioxidant system, exhibiting great potential for preventing age-related endothelial dysfunction similar to AS [119]. In addition, naringenin (NG) has a strong angioprotective effect, inhibiting HUVEC apoptosis induced by HG or free fatty acids (FFAs) by inducing HO-1 expression. As a key regulator of HO-1 expression, Nrf2 is also activated by NG. However, PI3K inhibitors reduced NG-induced HO-1 expression, suggesting that NG stimulates Nrf2 and protects blood vessels by activating the PI3K pathway. In addition, they found that JNK was also involved in this important work [120]. Hyperglycemia is believed to aggravate AS and increase the occurrence of CVD mainly because it leads to endothelial dysfunction, resulting in endothelial repair defects and angiogenesis disorders. Chen et al. found that HG could induce oxidative stress in vascular endothelial cells both in vivo and in vitro. Baicalin, a known flavonoid isolated from Scutellaria baicalensis, has a protective effect on ECs under hyperglycemic conditions, and the mechanisms are related to downregulating reactive oxygen species (ROS) and inflammation via activation of the Akt/GSK3B/Fyn-mediated Nrf2 pathway [121].
Isoflavone is another common type of flavonoid that often exists in plants of the Leguminosae family. Genistein, the main component of soybean isoflavone, plays an important role in preventing AS. Zhang et al. established a damage model of ox-LDL-induced HUVECs and evaluated the protective effect of genistein. The results showed that the flavone component could significantly reduce the secretion and mRNA transcription of ox-LDL-induced MCP-1, VCAM-1 and ICAM-1. However, HO-1 inhibitor intervention reversed this trend. In addition, Nrf2 siRNA significantly reduces the promoting effect of genistein on HO-1 expression [122]. In addition, equol, a metabolic product of daidzein, possesses various bioactivities. Zhang et al. reported that this compound alleviates AS plaque formation, increases cell survival and reduces cell apoptosis, and the possible mechanism is related to attenuating ER stress by activating the Nrf2 signaling pathway [123].
Rhizogenin is a dihydrochalcone-type flavonoid that is mainly distributed in the pericarp and roots of plants. This compound has been demonstrated to reduce intracellular ROS and MDA levels and increase SOD and Gpx-1 activity. In-depth mechanistic studies have found that rhizogenin activates Nrf2 through the AMPK pathway to weaken the oxidative stress induced by PA in HUVECs [124]. In addition, euxanthone was reported to inhibit Keap1, activate Nrf2, increase HO-1 and NQO-1 expression, reduce mitochondrial membrane potential loss, and rescue HUVEC apoptosis caused by ox-LDL [125]. In addition, in 2019, Yan et al. reported that xanthoangelol (XAG) extracted from Angelica keiskei Koidzumi also prevents ox-LDL-induced EC injury by activating Nrf2/ARE signaling [126] (Table 4).
Terpenoids
Terpenes are promising natural agents for medical use and are widely distributed in various plants. Previous studies have indicated that terpenes, in particular triterpenes, could be used to treat cardiovascular diseases by reducing oxidative stress and activating the Nrf2/HO-1 pathway [127], [128].
Pachymic acid (PMA) is a triterpene obtained from Poria cocos. Currently, a study reported that PA inhibits ox-LDL-induced cell death and apoptosis in HUVECs and increases the expression of Caspase-3 and Bas and Bcl-2, and further investigations revealed that PMA reduces oxidative stress damage via activation of the Nrf2/HO-1 signaling pathway [129]. Another study in 2017 found that celastrol activates Nrf2, inhibits the expression of the Nox2/AT1 receptor, upregulates ERK1/2 phosphorylation, and inhibits the production of ROS in HUVECs, potentially attenuating angiotensin II-induced HUVEC damage [130]. LOX-1 is a major receptor for the binding, phagocytosis and breakdown of ox-LDL by vascular endothelial cells and plays a key role in the process of AS. Oleanolic acid (OA), a well-known bioactive pentacyclic triterpenoid, reduces ox-LDL-induced ROS production, promotes HO-1 expression and Nrf2 nuclear transfer in cells, and protects HUVECs from oxidative damage. However, LOX-1 silencing inhibited the expression of Nrf2 and HO-1 induced by OA, suggesting that LOX-1 is involved in the endothelial protection of OA. OA treatment improved lipid and antioxidant status and significantly reduced AS area in Coturnix coturnix fed a high-fat diet. Similarly, OA pretreatment alleviates the decrease in HUVEC viability and increase in ROS caused by ox-LDL, inhibits the expression of LOX-1 and NADPH oxidase subunits, and further improves the expression of Nrf2 and HO-1. However, LOX-1 silencing eliminated the effects of ox-LDL on cell activity, ROS production and gene expression. In summary, the potential mechanism of the protective effect of OA endothelial cells involves the regulation of LOX-1 activity, including the inhibition of NADPH oxidase subunit expression and the increase in Nrf2 and HO-1 expression [131]. In addition, a recent study reported that a natural compound of the triterpenoid group from olives, maslinic acid (MA), induces the nuclear translocalization of Nrf2 in LPS-stimulated HUVECs, increasing the combination of Nrf2 and ARE and reducing the generation of IL-1α. In addition, 20 μM MA inhibits the expression of iNOS and COX-2 and reduces NF-κB activity and STAT-1 phosphorylation. In addition, inhibition of HO-1 by RNA interference reverses the MA-induced reduction in iNOS/NO expression [132]. Astragaloside IV (ASD-IV) is a triterpene glycoside isolated from the known traditional Chinese medicine Astragalus membranaceus with various bioactive effects. In 2019, Zhu et al reported that ASD-IV enhances the activity and migration of HUVECs with ox-LDL intervention in a concentration-dependent manner and inhibits the release of LDH, ROS production and NADPH oxidase. ASD-IV also increases Nrf2 and HO-1 mRNA expression and decreases TNF-α and IL-6 mRNA expression. In general, ASD-IV prevents ox-LDL-induced endothelial cell injury by reducing apoptosis, oxidative stress, and inflammation [133]. Ginsenoside Rg1 (GSD Rg1), another known triterpene glycoside, is one of the active ginsenosides isolated from Panax ginseng and would be beneficial for the treatment of cardiovascular diseases. In 2013, it was reported that GSD Rg1 significantly antagonized the decrease in HUVEC activity induced by PM2.5 and reduced the production of intracellular ROS and MDA by increasing the expression of HO-1 and Nrf2 and promoting the nuclear transfer of Nrf2 [134].
In addition to triterpenes, some diterpenes are also reported to be useful for protecting ECs from oxidative stress via activation of Nrf2/HO-1 signaling. In 2014, Lu et al. found that andrographolide (ADH) inhibited TNF-α-induced ROS generation, Src phosphorylation, NADPH oxidase activation, and ICAM-1 expression in EA.hy926 cells; increased GSH content; and induced HO-1 and GCLM gene expression. Interestingly, in subsequent studies, they found that LY294002, an inhibitor of the PI3K/Akt pathway, inhibited Nrf2 nuclear translocation and c-jun phosphorylation and increased GSH content while attenuating ADH-induced HO-1 and GCLM gene expression. In addition, Nrf2 or c-jun knockout also weakened HO-1 and GCLM expression. These results indicate that the PI3K/Akt/Nrf2/HO-1 pathway participates in the improvement of TNF-α-induced endothelial dysfunction through a cascade reaction [135]. In another study, ADH also showed a significant protective effect against hypoxia-induced EA.hy926 cell injury, which also reduced ROS production in cells and increased HO-1 expression. In addition, ADH inhibited hypoxia-induced HIF-1α gene expression via PHD2/3 and Nrf2/HO-1 in EA.hy926 cells. ET-1, one of the downstream target genes of HIF-1α, is involved in the pathogenesis of various CVDs. Preconditioning of ADH also inhibited ET-1 secretion. An in-depth study found that SB203580, an inhibitor of P38 MAPK, inhibited the expression of HIF-1A and ET-1 induced by CoCl2 (hypoxia-mimetic agent) [136]. Glaucocalyxin A (Gau A) is a diterpene isolated from Isodon japonica. A study by Xia et al. (2014) reported that Gau A has protective effects on HUVECs stimulated by H2O2, and further studies revealed that this compound reduces endothelin (ET-1) and iNOS mRNA and increase eNOS mRNA expression [137].
In addition, zedoarondiol (ZAD), a sesquiterpene lactone, increases SOD, decreases ROS and MDA, improves the cell survival rate and inhibits LDH activity in ox-LDL-induced ECs. It also inhibits the release of inflammatory cytokines, such as IL-1β, MCP-1, and TNF-α. The mechanism is mainly related to downregulation of LOX-1 and Keap1 expression, induction of Nrf2 nuclear translocation, and upregulation of HO-1 and NQO1 expression [138]. Lycopene is a tetraterpene found in tomato. Sung et al. found that lycopene suppresses cyclic strain-induced ET-1 expression in HUVECs via inhibition of ROS generation and induction of HO-1 expression [139] (Table 4).
Alkaloids
Alkaloids derived from plants are important natural agents for promoting healthcare and disease prevention and remain one of the most important resources for the identification of candidate drugs [140]. Homocysteine (Hcy), a sulfur-containing amino acid, is closely related to the development of CVDs and is considered an important risk factor for CVDs. The most important reason is that Hcy causes endothelial injury through a series of complex mechanisms, participates in the occurrence and development of AS, and ultimately leads to CVDs. In 2019, Wu et al. found that Hcy exhibits obvious cytotoxicity and induces HUVEC apoptosis. In addition, Hcy increases the levels of ROS, LDH and MDA and reduces SOD levels in HUVECs. Importantly, oxymatrine (OMT), a known alkaloid isolated from Sophora flavescens, protects HUVECs from Hcy-induced damage, reverses the Hcy-induced MMP reduction, increases the Bcl-2/Bax ratio, and inhibits the expression of caspase-9 and caspase-3. Furthermore, OMT also increases Nrf2 levels and the phosphorylation of Akt and eNOS. Collectively, these results suggested that OMT prevents Hcy-induced endothelial injury by activating the Akt-Enos-Nrf2 signaling pathway [141]. Corynoline is an alkaloid isolated from Corydalis bungeana and has significant anti-inflammatory effects. Corynoline inhibits LPS-induced TNF-α and IL-8 production and reduces the expression of VCAM-1 and ICAM-1 in HUVECs. In addition, corynoline inhibits the LPS-induced phosphorylation of NF-κB p65 and IκBα and increases the expression of Nrf2 and HO-1. In addition, the inhibitory effects of corynoline on the release of TNF-α and IL-8 are decreased by blocking Nrf2, and these results suggest that the anti-inflammatory activity shown by corynoline is realized by activating Nrf2 signaling [142]. Berberine (BBR) is a versatile alkaloid extracted from Coptis chinensis with various bioactivities, such as anti-inflammatory, antitumor, antidiabetic and antibacterial activities. It has been reported that BBR increases the activity of HUVECs stimulated by H2O2, effectively inhibits cell apoptosis and ROS production, promotes the nuclear translocation of Nrf2 and upregulates HO-1 expression. In addition, Nrf2 siRNA treatment not only significantly inhibits Nrf2/Ho-1 activation but also significantly reverses the protective effect of BBR on HUVECs [143]. Isothiocyanates are small organosulfur molecules with an —N C S group and are reported to be beneficial for decreasing the risk of AS. In 2013, Huang et al. evaluated the protective effects of BITC (benzyl isothiocyanate) and PEITC (phenethyl isocyanate) against ox-LDL-induced EC damage and found that the corresponding molecular mechanisms are related to upregulating Nrf2-dependent antioxidation and suppressing NF-κB activation [144]. In 2015, Zhang et al. studied the protective effects of four alkaloids in seeds of Nelumbo nucifera on H2O2-induced HUVECs, and the results showed that four alkaloids, lotusine, liensinine, isoliensinine and neferine, increase HUVEC survival by increasing the production of NOS and NO in HUVECs [145]. In addition, another paper by Zhang et al. in 2020 revealed that sulforaphane (SFN) attenuates angiotensin (Ang) II-induced HUVEC injury by modulating ROS-mediated mitochondrial signaling via Nrf2 activation [146] (Table 4).
Stilbenoids
Stilbenoids are a class of small molecules with many bioactivities [147]. Pterostilbene (PT) is a stilbenoid that weakens the inhibition of HUVEC proliferation induced by uremic serum (US). PT dose-dependently eliminates the decrease in SOD and CAT activity induced by US; prevents the increase in MDA, H2O2, superoxide anion levels and NAD(P)H oxidase activity in HUVECs; and improves intracellular oxidative stress. In addition, PT significantly inhibits the expression of the proinflammatory cytokines IL-1β, TNF-α, MCP-1 and VCAM-1 as well as iNOS. Further studies found that PT dose-dependently downregulates Keap1 levels and upregulates total Nrf2 and HO-1 expression. However, Snpp (HO-1 inhibitor) eliminates PT's inhibitory effect on US-induced cytotoxicity, oxidative stress and the inflammatory response. PT is a potential anti-AS drug with antioxidant, anti-inflammatory and anti-cytotoxicity pharmacological activities that protects against US-mediated endothelial cell injury [148]. Similarly, Tang et al. also found that PT reduces AS in rats fed a high-fat diet, reduces aortic plaque size, reduces macrophage infiltration, and inhibits oxidative stress and vascular wall apoptosis in vivo. PT also inhibits H2O2-induced HUVEC apoptosis in vitro, and the mechanism is related to the regulation of the Nrf2 pathway [149]. Resveratrol is a versatile stilbenoid that exists in grapes and P. cuspidatum and has many bioactivities, such as antioxidative, antiaging, cardiovascular protective and antitumor effects. Wang et al. reported that resveratrol protects H2O2-induced HUVECs from oxidative stress-induced damage by decreasing apoptosis and activating PI3K-Akt-mTOR [150], [151] (Table 4).
Quinones
Miltirone is a phenanthraquinone in Salvia miltiorrhiza that significantly improves the survival rate of ox-LDL-induced EA.hy926 cells in a concentration-dependent manner, inhibits the formation of intracellular ROS, and improves morphological changes. In addition, miltirone induces Nrf2 phosphorylation and nuclear accumulation, upregulates the expression of some downstream Nrf2 genes (such as HO-1 and NQO1), accelerates the degradation of Keap1, and activates the ARE. However, siRNA silencing of Nrf2 blocked miltirone’s induction of Nrf2 and its downstream genes. More importantly, miltirone's protective effect on ox-LDL-induced cell death was weakened after Nrf2 was knocked down, confirming the important role of Nrf2 in oxidative stress-induced EA.Hy926 cell death. Similarly, siRNA targeting HO-1 also attenuated miltirone's protective effect. In conclusion, these results suggest that miltirone has a significant protective effect on ox-LDL-induced cell injury by mediating the Nrf2/HO-1-dependent pathway [152]. Tanshinone IIA is another active component in S. miltiorrhiza. Currently, increasing evidence has also revealed that this compound has protective effects on undulatory high glucose-induced HUVECs, and the possible mechanisms are related to decreasing oxidative stress, reducing lipid peroxidation and MDA, enhancing the activity of antioxidant enzymes, and increasing the release of NO via regulation of the Rho/Rho kinase system [153] (Table 4).
Others
In addition to the compounds mentioned above, there are many other natural agents with potential protective effects on ECs via Nrf2/HO-1 signaling. Withaferin A (WA), a steroid isolated from Withania somnifera, has a wide range of pharmacological activities, including anti-inflammatory, antitumor and antiangiogenic activities. WA increases EC viability, inhibits ROS production and improves endothelial dysfunction in rat aortic rings in vitro. Mechanistic studies showed that WA increases the nuclear translocation of Nrf2, the expression of HO-1 in HUVECs and endothelial cell lines (EA.hy926), and the upregulation of HMOX1 [154].
Z-Ligustilide (Z-Lig) is a bioactive phthalide derivative isolated from Cnidium officina and Angelica sinensis. Studies have shown that Z-Lig can reduce ICAM-1, VCAM-1 and E-selectin expression in HUVECs to suppress HL-60 monocyte adhesion. In addition, TNF-α-induced ROS production and the NF-κB signaling pathway activation were significantly inhibited. In addition, Z-Lig significantly induces HO-1 expression by promoting Nrf2 nuclear translocation and endothelial NO synthesis, reduces vascular inflammation, and activates the endothelial defense system [155]. In addition, Z-Lig has a good inhibitory effect on the local inflammatory response caused by AS [156]. Similarly, a 2019 study also suggested that Z-Lig increases the activity of antioxidant enzymes, significantly reduces the formation of AS plaques and lipid peroxidation in the aorta, and inhibits AS development in HFD-FedLdlr−/− mice. The main mechanism involves upregulating HO-1 and NQO1 expression by activating Nrf2 while reducing the production of ROS. In vitro experiments also found that Z-Lig increases NRF2 and ARE-driven enzyme expression and reduces the cytotoxicity in EA-hy926 cells caused by T-BHP [157]. Salidroside (SAL) is one of the main active ingredients of Rhodiola rosea. Studies have revealed that this natural ingredient has a wide range of pharmacological effects, such as enhancing immunity and protecting the cardiovascular system. A 2016 study found that SAL reduces ROS and MDA, increases the activities of SOD and CAT, and alleviates H2O2-induced HUVEC injury. Further mechanistic studies found that SAL can inhibit oxidative stress injury by inducing the nuclear translocation of Nrf2 and activating the expression of the antioxidant enzyme genes HO-1 and NQO1 regulated by Nrf2 [158]. Zhang et al. also reported that SAL has a protective effect on vascular endothelial dysfunction induced by advanced glycation end products (AGEs), and the mechanism is also related to activation of Nrf2/HO-1, reduction of Keap1 expression, reduction of ROS formation, restoration of NO levels and suppression of NF-κB pathway activation [159]. Vanillic acid (VA) is present in a variety of grains and fruits, and studies have found that VA reduces PA-induced ROS and MDA, recovers MMP levels, enhances intracellular antioxidant enzyme activity, and protect cells from oxidative damage. The mechanism of this protective effect is related to the upregulation of the LKB1/AMPK signaling pathway, which activates P-Nrf2 and HO-1 and promotes SIRT1 and PGC1α expression. In addition, AMPK inhibitors reduce VA-induced enhancement of Nrf2 and HO-1, suggesting that VA activates the Nrf2/HO-1 pathway to exert its protective effects on ECs [160]. Protocatechuic acid (PCA), a natural phenolic acid substance, is the main metabolite of anthocytes and the effective active ingredient in Salvia miltiorrhiorum. It has been reported that PCA has an obvious protective effect on PA-induced oxidative damage in HUVECs. The molecular mechanism involves increasing AMPK phosphorylation by activating LKB1, which promotes P-NRF2 and HO-1 expression, while lowering ROS and MDA levels and increasing the activity of antioxidant enzymes (including SOD, GP-1, and HO-1) in HUVECs. Moreover, PCA also reversed the reduction in pGC-1α expression caused by PA and significantly increased mitochondrial density [161]. EC senescence is considered to be one of the key risk factors for vascular diseases and is of great significance for the prevention and treatment of AS. Li et al. found that the inflammatory factor TNF-α promotes premature senescence of rat nucleus pulposus cells through the PI3K/Akt signaling pathway. Guo et al. also found that TNF-α induces HUVEC senescence and increases the activity of SAb-Gal (a biomarker of senescence). Salicin, a natural ingredient from willow bark or the stems and roots of Alangium chinense, inhibits cell senescence and cycle arrest caused by TNF-α. In addition, salicin reduces the expression of P21 and PAI-1 induced by TNF-α and promotes the nuclear translocation of NRF2. After NRF2 is knocked out using small RNA transfection, these beneficial effects of salicin are eliminated, suggesting that the antiaging effect of salicin is mediated by Nrf2 [162]. Clinical studies have suggested that garlic has the potential to protect vascular endothelial function and health. Allicin, the main ingredient in garlic, has also drawn attention as a natural agent that can protect the cardiovascular system. Previous studies have revealed that allicin reduces LPS-induced apoptosis in HUVECs, inhibits ROS, and reduces lipid peroxidation. In addition, allicin improves mitochondrial dysfunction, increases MMPs and mitochondrial ATP, and reduce the release of cytochrome C. Allicin also reduces the LPS-induced inflammatory response, increases LXR expression and inhibits the release of TNF-α and IL-8 via the activation of Nrf2 [163], [164] (Table 4).
Conclusion and perspectives
In our present paper, we summarized the potential natural monomers/extracts derived from herbal medicines that have protective effects on ECs against oxidative stress-induced injury. These agents mainly include phenylpropanoids, flavonoids, terpenoids, alkaloids, stilbenes, and quinones. Most of these agents alleviate cell apoptosis in ECs due to oxidative stress induced by ox-LDL, H2O2, homocysteine (Hcy), high glucose (HG), and Ang II, and the predominant mechanisms are related to activation of Nrf2/HO-1 signaling (Fig. 3). However, despite continued progress on various aspects of natural agents with protective effects against EC injury by activating Nrf2/HO-1 signaling, the development of new drugs for treating AS and other CVDs from these agents will require more detailed investigations in both preclinical and clinical venues.
First, some agents mentioned in our paper have only been assessed in preliminary pharmacological studies, and further studies on molecular mechanisms will be performed in the future. Consequently, continuing investigations should be performed to explore the molecular mechanisms by which these candidate molecules protect ECs from oxidative stress-induced injury by targeting Nrf2/HO-1 signaling. Second, there are not yet adequate data on the pharmacokinetics and clinical research of these reported natural extracts/monomers, and few studies on toxicity and its target organs have been reported. Thus, more work should be devoted to investigating the PK profiles and systemic side effects and toxicities of these candidate agents in the future. Additionally, further clinical investigations are encouraged to evaluate the actual therapeutic effects of candidate drugs in humans. Last but perhaps the most important, CVDs commonly require a longer course of treatment, and oral medications remain the first choice for AS and other CVDs. However, most of the agents mentioned above, such as curcumin, exhibit low bioavailability from oral administration. Therefore, appropriate drug delivery systems and preparations should be developed for these candidate drugs; in addition, structural modification based on molecular docking targeted to Nrf2/HO-1 would be beneficial for designing better molecules with high bioactivities and drug-like properties as well as low toxicities.
Collectively, our present paper provides updated information of natural agents with protective activities on ECs against oxidative stress by activating Nrf2/HO-1. We hope this review will provide some directions for the further development of novel candidate drugs from natural agents for the treatment of AS and other CVDs.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the Project of State Administration of Traditional Chinese Medicine of Sichuan Province of China (No. 2021MS460, No. 2020HJZX001), the Sichuan Science and Technology Program (No. 2020YFS0523), and the Xinglin scholar discipline promotion talent program of Chengdu University of traditional Chinese medicine (No. BSH2018006).
Biographies

Dr. Wei Peng, World’s Top 2% Scientists 2020 (Pharmacology & Pharmacy), obtained his Ph.D. Degree at Chengdu university of Traditional Chinese Medicine (CDUTCM) in July 2017. Currently, Dr. Peng is an Associate Professor in the School of Pharmacy, CDUTCM, and has published over 40 research papers (among these, over 30 as first or corresponding authors) with total citation over 1000. His current main interests are focused on the natural products with anti-oxidative stress, antitumor, anti-inflammatory & immunological activities and their potential molecular mechanisms. In addition, Dr. Peng also focuses on new strategies for treating tumor, inflammatory & immunological disorders and the new pathogenesis of these diseases.

Dr. Chunjie Wu obtained his Ph.D. Degree at Sichuan University in July 2005. Currently, Dr. Wu is a Professor in the School of Pharmacy, Chengdu University of Traditional Chinese Medicine, and has published over 100 research papers. His current main interests are focused on the Pharmaceutical preparations, bioactive natural products and their potential molecular mechanisms.

Qing Zhang received her master's degree from Chengdu University of Traditional Chinese Medicine (CDUTCM) in July 2020 and will continue her doctoral studies at CDUTCM in september of the same year. At present, Dr. Zhang has participated in the development of more than 20 research papers (including 9 as the first author or co-first author), and 1 paper on high-power hot topics. The main research direction is to find natural products and their potential molecular mechanisms for the treatment of tumors, rheumatoid arthritis, neurological diseases and cardiovascular diseases.

Dr. Jia Liu is studying for her Master's Degree at Chengdu University of Traditional Chinese Medicine. At present, she has participated in the publication of more than 10 research papers (among these, over 4 as first or co-first author). She is currently working on natural products with anti-inflammatory and immunological activities and their underlying molecular mechanisms. Moreover, she focuses on new strategies and research directions for treating inflammatory and immune diseases.

Dr. Hu-xinyue Duan is a graduate student majoring in science of Chinese materia medica at Chengdu university of Traditional Chinese Medicine (CDUTCM). Her current interests are focused on the traditional Chinese medicine with anti-inflammatory & immunological activities and their potential molecular mechanisms.

Dr. Ruo-Lan Li will obtain her master’s Degree at Chengdu university of Traditional Chinese Medicine (CDUTCM) and has published over 5 research paper. Her current main interests are focused on the natural products with antioxidant and their potential molecular mechanisms. In addition, Dr. Li also focuses on new strategies for treating neurodegenerative diseases and the new pathogenesis of this diseases.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Wei Peng, Email: pengwei@cdutcm.edu.cn.
Chunjie Wu, Email: wucjcdtcm@163.com.
References
- 1.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., et al. Heart disease and stroke statistics-2017 update: a report from the american heart association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aryan L., Younessi D., Zargari M., Banerjee S., Agopian J., Rahman S., et al. The role of estrogen receptors in cardiovascular disease. Int J Mol Sci. 2020;21:4314. doi: 10.3390/ijms21124314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang G., Wang Y., Zeng Y., Gao G., Liang X., Zhou M., et al. Rapid health transition in China 1990–2010: findings from the global burden of disease study 2010. Lancet. 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend N., Wilson L., Bhatnagar P., Wickramasinghe K., Rayner M., Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37:3232–3245. doi: 10.1093/eurheartj/ehw334. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;35:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47:C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 7.Glass C.K., Witztum J.L. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 8.Crea F., Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017;136:1155–1166. doi: 10.1161/CIRCULATIONAHA.117.029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavurma M.M., Rayner K.J., Karunakaran D. The walking dead: macrophage inflammation and death in atherosclerosis. Curr Opin Lipidol. 2017;28:91–98. doi: 10.1097/MOL.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirii H., Niwa T., Yamada Y., Wada H., Saito K., Iwakura Y., et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoEdeficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Ma K.L., Gong Y.X., Wang G.H., Hu Z.B., Liu L., et al. Platelet microparticles mediate glomerular endothelial injury in early diabetic nephropathy. J Am Soc Nephrol. 2018;29:2671–2695. doi: 10.1681/ASN.2018040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo J., Wang Z., Wu J., Liu M., Li M., Sun Y., et al. Endothelial SIRT6 is vital to prevent hypertension and associated cardiorenal injury through targeting Nkx3.2-GATA5 Signaling. Circ Res. 2019;124:1448–1461. doi: 10.1161/CIRCRESAHA.118.314032. [DOI] [PubMed] [Google Scholar]
- 13.Ashry N.A., Abdelaziz R.R., Suddek G.M. The potential effect of imatinib against hypercholesterolemia induced atherosclerosis, endothelial dysfunction and hepatic injury in rabbits. Life Sci. 2020;243:117275. doi: 10.1016/j.lfs.2020.117275. [DOI] [PubMed] [Google Scholar]
- 14.Varshney R., Budoff M. Garlic and heart disease. J Nutr. 2016;146:416S–421S. doi: 10.3945/jn.114.202333. [DOI] [PubMed] [Google Scholar]
- 15.Si Y., Guo S., Fang Y., Qin S., Li F., Zhang Y., et al. Celery seed extract blocks peroxide injury in macrophages via notch1/NFkappaB pathway. Am J Chin Med. 2015;43:443–455. doi: 10.1142/S0192415X15500287. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Huang Z.Q., Wang C.Q., Wang L.S., Meng S., Zhang Y.C., et al. Artemisinin inhibits extracellular matrix metalloproteinase inducer (EMMPRIN) and matrix metalloproteinase-9 expression via a protein kinase Cdelta/p38/extracellular signal-regulated kinasepathway inphorbolmyristate acetate-induced THP-1 macrophages. Clin Exp Pharmacol Physiol. 2011;38:11–18. doi: 10.1111/j.1440-1681.2010.05454.x. [DOI] [PubMed] [Google Scholar]
- 17.Jin Y.H., Kim S.A. 2-Methoxycinnamaldehyde inhibits the TNF alpha-induced proliferation and migration of human aorticsmooth muscle cells. Int J Mol Med. 2017;39:191–198. doi: 10.3892/ijmm.2016.2818. [DOI] [PubMed] [Google Scholar]
- 18.Anwar S., Bhandari U., Panda B.P., Dubey K., Khan W., Ahmad S. Trigonelline inhibits intestinal microbial metabolism of choline and its associated cardiovascular risk. J Pharm Biomed Anal. 2018;159:100–112. doi: 10.1016/j.jpba.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud A.M., Hernández Bautista R.J., Sandhu M.A., Hussein O.E. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid Med Cell Longev. 2019;2019:5484138. doi: 10.1155/2019/5484138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng H.M., Koutsidis G., Lodge J.K., Ashor A., Siervo M., Lara J. Tomato and lycopene supplementation and cardiovascular risk factors: a systematic review and meta-analysis. Atherosclerosis. 2017;257:100–108. doi: 10.1016/j.atherosclerosis.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Kozłowska A., Szostak-Wegierek D. Flavonoids–food sources and health benefits. Rocz Panstw Zakl Hig. 2014;65:79–85. [PubMed] [Google Scholar]
- 22.Sheu M.L., Ho F.M., Yang R.S., Chao K.F., Lin W.W., Lin-Shiau S.Y., et al. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol. 2005;25:539–545. doi: 10.1161/01.ATV.0000155462.24263.e4. [DOI] [PubMed] [Google Scholar]
- 23.Fiorelli S., Porro B., Cosentino N., Di Minno A., Manega C.M., Fabbiocchi F., et al. Activation of Nrf2/HO-1 pathway and human atherosclerotic plaque vulnerability:an in vitro and in vivo study. Cells. 2019;8:356. doi: 10.3390/cells8040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunting S., Moncada S., Vane J.R. Antithrombotic properties of vascular endothelium. Lancet. 1977;310:10756. doi: 10.1016/s0140-6736(77)91906-7. [DOI] [PubMed] [Google Scholar]
- 25.Fukao H., Matsuo O. Antithrombotic regulation in human endothelial cells by fibrinolytic factors. Semin Thromb Hemost. 2000;26:338. doi: 10.1055/s-2000-9800. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K., Joshi D., Timalsina S., Schwartz M.A. Early events in endothelial flow sensing. Cytoskeleton. 2021 doi: 10.1002/cm.21652. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Lupu F., Kinasewitz G., Dormer K. The role of endothelial shear stress on haemodynamics, inflammation, coagulation and glycocalyx during sepsis. J Cell Mol Med. 2020;24(21):12258–12271. doi: 10.1111/jcmm.15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baratchi S., Khoshmanesh K., Woodman O.L., Potocnik S., Peter K., McIntyre P. Molecular sensors of blood flow in endothelial cells. Trends Mol Med. 2017;23(9):850–868. doi: 10.1016/j.molmed.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 30.Rao R.M., Yang L., Garcia-Cardena G., Luscinskas F.W. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 31.Fan J., Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2003;10:63–71. doi: 10.5551/jat.10.63. [DOI] [PubMed] [Google Scholar]
- 32.Incalza M.A., D'Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. VascPharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Kim S.M., Huh J.W., Kim E.Y., Shin M.K., Park J.E., Kim S.W., et al. Endothelial dysfunction induces atherosclerosis: Increased aggrecan expression promotes apoptosis in vascular smooth muscle cells. BMB Rep. 2019;52:145–150. doi: 10.5483/BMBRep.2019.52.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 35.Gao S., Zhao D., Wang M., Fan Zhao, Han X.Y., Qi Y., et al. Association between circulating oxidized LDL and atherosclerotic cardiovascular disease: a meta-analysis of observational studies. Can J Cardiol. 2017;33:1624–1632. doi: 10.1016/j.cjca.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Ganguly P., Alam S.F. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laakso M., Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 38.Clapp B., Hingorani A.D., Kharbanda R.K., Mohamed-Ali V., Stephens J.W., Vallance P., et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64:172–178. doi: 10.1016/j.cardiores.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Ochoa C.D., Wu R.F., Terada L.S. ROS signaling and ER stress in cardiovascular disease. Mol Aspects Med. 2018;63:18–29. doi: 10.1016/j.mam.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pignatelli P., Menichelli D., Pastori D., Violi F. Oxidative stress and cardiovascular disease: new insights. Kardiol Pol. 2018;76:713–722. doi: 10.5603/KP.a2018.0071. [DOI] [PubMed] [Google Scholar]
- 41.Tousoulis D., Kampoli A.M., Tentolouris C., Papageorgiou N., Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- 42.Joseph L. Nitric oxide signaling and atherothrombosis redux evidence from experiments of nature and implications for therapy. Circulation. 2018;137:233–236. doi: 10.1161/CIRCULATIONAHA.117.032901. [DOI] [PubMed] [Google Scholar]
- 43.Guber S., Ebrahimian T., Heidari M., Eliopoulos N., Lehoux S. Endothelial nitric oxide synthase overexpressing human early outgrowth cells inhibit coronary artery smooth muscle cell migration through paracrine functions. Sci Rep. 2018;8:877. doi: 10.1038/s41598-017-18848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang X., Hou X., Yang Y., Liu H., Guo R.W., Yang Z.H., et al. The feedback loop of “EMMPRIN/NF-κB” worsens atherosclerotic plaque via suppressing autophagy in macrophage. J Mol Cell Cardiol. 2018;114:129–140. doi: 10.1016/j.yjmcc.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., et al. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muqri F., Helkin A., Maier K.G., Gahtan V. Thrombospondin-5 and fluvastatin promote angiogenesis and are protective against endothelial cell apoptosis. J Cell Biochem. 2020 doi: 10.1002/jcb.29686. [DOI] [PubMed] [Google Scholar]
- 47.Sabbatinelli J., Orlando P., Galeazzi R., Silvestri S., Cirilli I., Marcheggiani F., et al. Ubiquinol ameliorates endothelial dysfunction in subjects with mild-to-moderate dyslipidemia: a randomized clinical trial. Nutrients. 2020;12:1098. doi: 10.3390/nu12041098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y., Hu K., Bu H., Si Z., Sun H., Chen L., et al. Probucol protects circulating endothelial progenitor cells from ambient PM2.5 damage via inhibition of reactive oxygen species and inflammatory cytokine production in vivo. Exp Ther Med. 2018;16:4322–4328. doi: 10.3892/etm.2018.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stroes E.S., Thompson P.D., Corsini A., Vladutiu G.D., Raal F.J., Ray K.K., et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gross P.L., Weitz J.I. New antithrombotic drugs. Clin Pharmacol Ther. 2009;86:139–146. doi: 10.1038/clpt.2009.98. [DOI] [PubMed] [Google Scholar]
- 51.Cui W., Leng B., Wang G.P. Klotho protein inhibits H2O2-induced oxidative injury in endothelial cells via regulation of PI3K/AKT/Nrf2/HO-1 pathways. Can J Physiol Pharmacol. 2019;97:370–376. doi: 10.1139/cjpp-2018-0277. [DOI] [PubMed] [Google Scholar]
- 52.Zhao C., Zhang Y., Liu H., Li P., Zhang H., Cheng G. Fortunellin protects against high fructose-induced diabetic heart injury in mice by suppressing inflammation and oxidative stress via AMPK/Nrf-2 pathway regulation. Biochem Biophys Res Commun. 2017;490:552–559. doi: 10.1016/j.bbrc.2017.06.076. [DOI] [PubMed] [Google Scholar]
- 53.Barajas B., Che N., Yin F., Rowshanrad A., Orozco L.D., Gong K.W., et al. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler Thromb Vasc Biol. 2011;31:58–66. doi: 10.1161/ATVBAHA.110.210906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freigang S., Ampenberger F., Spohn G., Heer S., Shamshiev A.T., Kisielow J., et al. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 55.Ruotsalainen A.K., Inkala M., Partanen M.E., Lappalainen J.P., Kansanen E., Mäkinen P.I., et al. The absence of macrophage Nrf2 promotes early atherogenesis. Cardiovasc Res. 2013;98:107–115. doi: 10.1093/cvr/cvt008. [DOI] [PubMed] [Google Scholar]
- 56.Wang R., Liu L., Liu H., Wu K., Liu Y., Bai L., et al. Reduced NRF2 expression suppresses endothelial progenitor cell function and induces senescence during aging. Aging (Albany NY) 2019;11:7021–7035. doi: 10.18632/aging.102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiao H., Sai X., Gai L., Huang G., Chen X., Tu X., et al. Association between heme oxygenase 1 gene promoter polymorphisms and susceptibility to coronary artery disease: a HuGE review and meta-analysis. Am J Epidemiol. 2014;179:1039–1048. doi: 10.1093/aje/kwu024. [DOI] [PubMed] [Google Scholar]
- 58.Juan S.H., Lee T.S., Tseng K.W., Liou J.Y., Shyue S.K., Wu K.K., et al. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;104:1519–1525. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- 59.Yet S.F., Layne M.D., Liu X., Chen Y.H., Ith B., Sibinga N.E.S., et al. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. FASEB J. 2003;17:1759–1761. doi: 10.1096/fj.03-0187fje. [DOI] [PubMed] [Google Scholar]
- 60.Kishimoto Y., Kondo K., Momiyama Y. The protective role of heme oxygenase-1 in atherosclerotic diseases. Int J Mol Sci. 2019;20:3628. doi: 10.3390/ijms20153628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eelen G., de Zeeuw P., Simons M., Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ Res. 2015;116(7):1231–1244. doi: 10.1161/CIRCRESAHA.116.302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gimbrone M.A., Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 64.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiorelli S., Porro B., Cosentino N., Di Minno A., Manega C.M., Fabbiocchi F., et al. Activation of Nrf2/HO-1 pathway and human atherosclerotic plaque vulnerability: an in vitro and in vivo study. Cells. 2019;8:356. doi: 10.3390/cells8040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J., Cai W., Fan Z., Yang C., Wang W., Xiong M., et al. MicroRNA-24 inhibits the oxidative stress induced by vascular injury by activating the Nrf2/Ho-1 signaling pathway. Atherosclerosis. 2019;290:9–18. doi: 10.1016/j.atherosclerosis.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 67.Xiong L.X., Xie J.S., Song C.X., Liu J.P., Zheng J.T., Liu C.G., et al. The activation of Nrf2 and its downstream regulated genes mediates the antioxidative activities of Xueshuan xinmaining tablet in human umbilical vein endothelial cells. Evid Based Complement Alternat Med. 2015;2015:187265. doi: 10.1155/2015/187265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lv X.H., Sun J.Y., Cai Z.G., Zhang R.P., San A.Y. Effects of serum containing Liuwei Dihuang Pill on ERK/ NRF2-HO-1 signaling pathway in Eahy926 cells. J Guangdong Pharm Univ. 2016;32:618–621. [Google Scholar]
- 69.Choi E.S., Lee Y.J., Seo C.S., Yoon J.J., Han B.H., Park M.C., et al. Vascular protective role of Samul-Tang in HUVECs: involvement of Nrf2/HO-1 and NO. Evid Complement Alternat Med. 2016;2016:9580234. doi: 10.1155/2016/9580234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim D.H., Lee S.M., Lee Y.J., Yoon J.J., Tan R., Yu Y.C., et al. Effect of Paeotang on tumor necrosis factor α-induced vascular inflammation in human umbilical vein endothelial cells. Chin J Integr Med. 2017;24:1–10. doi: 10.1007/s11655-017-2759-3. [DOI] [PubMed] [Google Scholar]
- 71.Zhao J., Yuan R.H., Wang H.F., Deng G.R., Zhang L.P., Cao Q.W., et al. Huoxue capsule activates THE PI3K/Akt/Nrf2/ HO-1 pathway to exert antioxidant damage on vascular endothelial cells. J Yunnan Univ Trad Chin Med. 2019;42:321–330. [Google Scholar]
- 72.Wei Y.S., Wung B.S., Lin Y.C., Hsieh C.W. Isolating a cytoprotective compound from Ganoderma tsugae: effects on induction of Nrf-2-related genes in endothelial cells. Biosci Biotechnol Biochem. 2009;73:1757–1763. doi: 10.1271/bbb.90098. [DOI] [PubMed] [Google Scholar]
- 73.Tseng C.Y., Chung M.C., Wang J.S., Chang Y.J., Chang J.F., Lin C.H., et al. Potent in vitro protection against PM2.5-caused ROS generation and vascular permeability by long-term pretreatment with ganoderma tsugae. Am J Chin Med. 2016;44:355–376. doi: 10.1142/S0192415X16500208. [DOI] [PubMed] [Google Scholar]
- 74.Yang H., Lee S.E., Jeong S.I., Park C.S., Jin Y.H., Park Y.S. Up-regulation of heme oxygenase-1 by korean red ginseng water extract as a cytoprotective effect in human endothelial cells. J Ginseng Res. 2011;35:352–359. doi: 10.5142/jgr.2011.35.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hwang S.M., Lee Y.J., Yoon J.J., Lee S.M., Kim J.S., Kang D.G., et al. Prunella vulgaris suppresses HG-induced vascular inflammation via Nrf2/HO-1/eNOS activation. Int J Mol Sci. 2012;13:1258–1268. doi: 10.3390/ijms13011258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han L., Liu E., Li M., Huang F. The protective effect of total saponins on vascular endothelial cell injury. Hubei J TCM. 2014;36:06–11. [Google Scholar]
- 77.Yoon J.J., Hyuk H.B., Sik C.E., Seung N., Hye J.D., Jung L.Y., et al. Involvement of heme oxygenase-1 induction in anti-vascular inflammation effects of Xanthoceras sorbifolia in human umbilical vein endothelial cells. J Tradit Chin Med. 2018;38:803–814. [PubMed] [Google Scholar]
- 78.Saji N., Francis N., Blanchard C.L., Schwarz L.J., Santhakumar A.B. Rice bran phenolic compounds regulate genes associated with antioxidant and anti-Inflammatory activity in human umbilical vein endothelial cells with induced oxidative stress. Int J Mol Sci. 2019;20:4715. doi: 10.3390/ijms20194715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang S., Liu Z.M., Liu H.F., Lee D.S., Wang J.L., Yuan R.Y., et al. Nepeta angustifolia attenuates responses to vascular inflammation in high glucose-induced human umbilical vein endothelial cells through heme oxygenase-1 induction. J Ethnopharmacol. 2019;231:187–196. doi: 10.1016/j.jep.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 80.Li D.T., Liu Y., Xu R., Jia X., Li X., Huo C., et al. Astragalus polysaccharide alleviates H2O2-triggered oxidative injury in human umbilical vein endothelial cells via promoting KLF2. Artif Cells Nanomed Biotechnol. 2019;47:2188–2195. doi: 10.1080/21691401.2019.1621886. [DOI] [PubMed] [Google Scholar]
- 81.Liang J., Sheng J.W., Jiang J.R., Wang Y., Shen X.Y., Liu D.M., et al. Chemical characteristics and cytoprotective activities of polysaccharide fractions from Athyrium Multidentatum (Doll.), Ching. Int J Biol Macromol. 2020;S0141–8130:33198–33199. doi: 10.1016/j.ijbiomac.2020.05.053. [DOI] [PubMed] [Google Scholar]
- 82.Liu L., Cai Z.Y., Diao Y.H., Li H., Shen W.Y. Protective effect of angelica sinensis polysaccharide on oxidized low density lipoprotein induced vascular endothelial cells injury. Chin J Clin Pharm. 2020;36:309. [Google Scholar]
- 83.Alves-Silva J.M., Zuzarte M., Marques C., Girão H., Salgueiro L. Protective effects of phenylpropanoids and phenylpropanoid-rich essential oils on the cardiovascular system. Mini Rev Med Chem. 2019;19(17):1459–1471. doi: 10.2174/1389557519666190620091915. [DOI] [PubMed] [Google Scholar]
- 84.Vanholme B., Houari I.E., Boerjan W. Bioactivity: phenylpropanoids' best kept secret. Curr Opin Biotechnol. 2019;56:156–162. doi: 10.1016/j.copbio.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 85.Liu J., Zhang Q., Li R., Wei S., Gao Y., Pu X. The traditional uses, Botany, phytochemistry, and pharmacology of Cinnamomi Ramulus: a review. J Pharm Pharmacol. 2020;72(3):319–342. doi: 10.1111/jphp.13189. [DOI] [PubMed] [Google Scholar]
- 86.Wang F., Pu C.H., Zhou P., Wang P.J., Liang D.P., Wang Q.L., et al. Cinnamaldehyde prevents endothelial dysfunction induced by high glucose by activating Nrf2. Cell Physiol Biochem. 2015;36:315–324. doi: 10.1159/000374074. [DOI] [PubMed] [Google Scholar]
- 87.Kim N.Y., Trinh N.T., Ahn S.G., Kim S.A. Cinnamaldehyde protects against oxidative stress and inhibits the TNF-α-induced inflammatory response in human umbilical vein endothelial cells. Int J Mol Med. 2020;46:449–457. doi: 10.3892/ijmm.2020.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma Z.C., Hong Q., Wang Y.G., Tan H.L., Xiao C.R., Liang Q.D., et al. Ferulic acid protects human umbilical vein endothelial cells from radiation induced oxidative stress by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Biol Pharm Bull. 2010;33:29–34. doi: 10.1248/bpb.33.29. [DOI] [PubMed] [Google Scholar]
- 89.Fratantonio D., Speciale A., Canali R., Natarelli L., Ferrari D., Saija A., et al. Low nanomolar caffeic acid attenuates high glucose-induced endothelial dysfunction in primary human umbilical-vein endothelial cells by affecting NF-κB and Nrf2 pathways. BioFactors. 2017;43:54–62. doi: 10.1002/biof.1312. [DOI] [PubMed] [Google Scholar]
- 90.Kweon M.H., Park I.Y., Sung H.C., Mukhtar H. The novel antioxidant 3-O-caffeoyl-1-methylquinic acid induces Nrf2-dependent phase II detoxifying genes and alters intracellular glutathione redox. Free Radic Biol Med. 2006;40:1349–1361. doi: 10.1016/j.freeradbiomed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Xue J.H., Zhu C.Z., Song A.Q. Echinacoside protects against high glucose-induced oxidative stress in vascular endothelial cells through Nrf2/HO-1 dependent pathway. J Am Coll Cardiol. 2017;70:C22–C23. [Google Scholar]
- 92.Guo B.W., Yi Y.L., Miao J.Q., Zhang C.Y., Qiao Y.B., Li Q.S. Protective effects of salvianolic acid B against t-BHP-induced vascular endothelial cell EA.hy926 injury through Keap1-Nrf2-ARE pathway. Cent South Pharm. 2020;18:4. [Google Scholar]
- 93.Lin Q.N., Qin X., Shi M., Qin Z., Meng Y.B., Qin Z.Y., et al. Schisandrin B inhibits LPS-induced inflammatory response in human umbilical vein endothelial cells by activating Nrf2. Int Immunopharmacol. 2017;49:142–147. doi: 10.1016/j.intimp.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 94.Li B., Lee Y.J., Kim Y.C., Yoon J.J., Lee S.M., Lee Y.P., et al. Sauchinone from Saururus chinensis protects vascular inflammation by heme oxygenase-1 induction in human umbilical vein endothelial cells. Phytomedicine. 2014;21:101–108. doi: 10.1016/j.phymed.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 95.Yang D., Xiao C.X., Su Z.H., Huang M.W., Qin M., Wu W.J., et al. (-)-7(S)-hydroxymatairesinol protects against tumor necrosis factor-αmediated inflammation response in endothelial cells by blocking the MAPK/ NF-κB and activating Nrf2/HO-1. Phytomedicine. 2017;32:15–23. doi: 10.1016/j.phymed.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 96.Borghini A., Gianicolo E.A., Picano E., Andreassi M.G. Ionizing radiation and atherosclerosis: current knowledge and future challenges. Atherosclerosis. 2013;230:40–47. doi: 10.1016/j.atherosclerosis.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 97.Soltani B., Bodaghabadi N., Mahpour G., Ghaemi N., Sadeghizadeh M. Nanoformulation of curcumin protects HUVEC endothelial cells against ionizing radiation and suppresses their adhesion to monocytes: potential in prevention of radiation-induced atherosclerosis. Biotechnol Lett. 2016;38:2081–2088. doi: 10.1007/s10529-016-2189-x. [DOI] [PubMed] [Google Scholar]
- 98.Wen L., Jiang Y., Yang J., Zhao Y., Tian M., Yang B. Structure, bioactivity, and synthesis of methylated flavonoids. Ann N Y Acad Sci. 2017;1398:120–129. doi: 10.1111/nyas.13350. [DOI] [PubMed] [Google Scholar]
- 99.Peng W., Qin R.X., Li X.L., Zhou H. Polygonum cuspidatum Sieb. et Zucc.: a review of its botany, phytochemistry, pharmacology, and potential applications. J Ethnopharmacol. 2013;148:729–745. doi: 10.1016/j.jep.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Fang J. Bioavailability of anthocyanins. Drug Metab Rev. 2014;46:508–520. doi: 10.3109/03602532.2014.978080. [DOI] [PubMed] [Google Scholar]
- 101.Chen H., Hu Y. Protective effect of anthocyanin on vascular endothelial injury induced by H2O2 via Nrf2/Ho-1 signaling pathway. Clin J Trad Chin Med. 2019;31:5. [Google Scholar]
- 102.Cimino F., Speciale A., Anwar S., Canali R., Ricciardi E., Virgili F., et al. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013;8:391–399. doi: 10.1007/s12263-012-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fratantonio D., Cimino F., Molonia M.S., Ferrari D., Saija A., Virgili F., et al. Cyanidin-3-O-glucoside ameliorates palmitate-induced insulin resistance by modulating IRS-1 phosphorylation and release of endothelial derived vasoactive factors. Biochim Biophys Acta, Mol Cell Biol Lipids. 2017;1862:351–357. doi: 10.1016/j.bbalip.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 104.Speciale A., Anwar S., Canali R., Chirafisi J., Saija A., Virgili F., et al. Cyanidin-3-O-glucoside counters the response to TNF-alpha of endothelial cells by activating Nrf2 pathway. Mol Nutr Food Res. 2013;57:1979–1987. doi: 10.1002/mnfr.201300102. [DOI] [PubMed] [Google Scholar]
- 105.Fratantonio D., Speciale A., Ferrari D., Cristani M., Saija A., Cimino F. Palmitate-induced endothelial dysfunction is attenuated by cyanidin-3-O-glucoside through modulation of Nrf2/Bach1 and NF-κB pathways. Toxicol Lett. 2015;239:152–160. doi: 10.1016/j.toxlet.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 106.Ruijters E.J.B., Weseler A.R., Kicken C., Guido R.M.M.H., Aalt Bast. The flavanol (-)-epicatechin and its metabolites protect against oxidative stress in primary endothelial cells via a direct antioxidant effect. Eur J Pharmacol. 2013;715:147–153. doi: 10.1016/j.ejphar.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 107.Shi J., Zhang M., Zhang L.B., Deng H. Epigallocatechin-3-gallate attenuates microcystin-LR-induced apoptosis in human umbilical vein endothelial cells through activation of the NRF2/HO-1 pathway. Environ Pollut. 2018;239:466–472. doi: 10.1016/j.envpol.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 108.Zhou X.L., Liang L.W., Zhao Y., Zhang H. Epigallocatechin-3-gallate ameliorates angiotensin II-induced oxidative stress and apoptosis in human umbilical vein endothelial cells through the activation of Nrf2/caspase-3 signaling. J Vasc Res. 2017;54:299–308. doi: 10.1159/000479873. [DOI] [PubMed] [Google Scholar]
- 109.Toniolo A., Buccellati C., Trenti A., Trevisi L., Carnevali S., Sala A., et al. Antiinflammatory and antioxidant effects of H2O2 generated by natural sources in IL1β-treated human endothelial cells. Prostaglandins Other Lipid Mediat. 2015;121:190–198. doi: 10.1016/j.prostaglandins.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 110.Yang G.Z., Wang Z.J., Bai F., Qin X.J., Cao J., Lv J.Y., et al. Epigallocatechin-3-gallate protects HUVECs from PM2.5-induced oxidative stress injury by activating critical antioxidant pathways. Molecules. 2015;20:6626–6639. doi: 10.3390/molecules20046626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng Y.Y., Morris A., Sunkara M., Layne J., Toborek M., Hennig B. Epigallocatechin-gallate stimulates NF-E2-related factor and heme oxygenase-1 via caveolin-1 displacement. J Nutr Biochem. 2012;23:163–168. doi: 10.1016/j.jnutbio.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luo Y., Lu S., Dong X., Xu L.J., Sun G.B., Sun X.B. Dihydromyricetin protects human umbilical vein endothelial cells from injury through ERK and Akt mediated Nrf2/HO-1 signaling pathway. Apoptosis. 2017;22:1013–1024. doi: 10.1007/s10495-017-1381-3. [DOI] [PubMed] [Google Scholar]
- 113.Hu Q., Zhang T., Yi L., Zhou X., Mi M.T. Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells. BioFactors. 2018;44:123–136. doi: 10.1002/biof.1395. [DOI] [PubMed] [Google Scholar]
- 114.Kang G.L., Jing Z.L. Kaempferol alleviates ox-LDL-mediated endothelial cell injury via regulating AMPK/Nrf2/HO-1 signaling pathway. Chin J Immunol. 2018;34:4–13. [Google Scholar]
- 115.Lee S.E., Jeong S.I., Yang H., Park C.S., Jin Y.H., Park Y.S. Fisetin induces Nrf2-Mediated HO-1 expression through PKC-δ and p38 in human umbilical vein endothelial cells. J Cell Biochem. 2011;112:2352–2360. doi: 10.1002/jcb.23158. [DOI] [PubMed] [Google Scholar]
- 116.Sun G.B., Qin M., Luo Y., Pan R.L., Meng X.B., Wang M., et al. Protect effects and the underlying mechanisms of myricitrin against vascular endothelial cells apoptosis induced by oxidative stress. Acta Pharmaceutica Sin. 2013;48:615–620. [PubMed] [Google Scholar]
- 117.Sthijns M.M.J.P.E., Schiffers P.M., Janssen G.M., Lemmens K.J.A., Ides B., Vangrieken P., et al. Rutin protects against H2O2-triggered impaired relaxation of placental arterioles and induces Nrf2-mediated adaptation in Human Umbilical Vein Endothelial Cells exposed to oxidative stress. Biochim Biophys Acta, Mol Cell Biol Lipids. 1861;2017:1177–1189. doi: 10.1016/j.bbagen.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 118.Lee S.E., Yang H., Son G.W., Park H.R., Park C.S., Jin Y.H., et al. Eriodictyol protects endothelial cells against oxidative stress-induced cell death through modulating ERK/Nrf2/ARE-dependent heme oxygenase-1 expression. Int J Mol Sci. 2015;16:14526–14539. doi: 10.3390/ijms160714526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou Q., Cheng K.W., Gong J., Li E.T.S., Mf Wang. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem Pharmacol. 2019;166:231–241. doi: 10.1016/j.bcp.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 120.Feng J., Luo J., Deng L., Zhong Y., Wen X., Cai Y., et al. Naringenin-induced HO-1 ameliorates high glucose or free fatty acidsassociated apoptosis via PI3K and JNK/Nrf2 pathways in human umbilical vein endothelial cells. Int Immunopharmacol. 2019;75:105769. doi: 10.1016/j.intimp.2019.105769. [DOI] [PubMed] [Google Scholar]
- 121.Chen G., Chen X.J., Niu C., Huang X.Z., An N., Sun J., et al. Baicalin alleviates hyperglycemia-induced endothelial impairment via Nrf2. J Endocrinol. 2018;18:0457. doi: 10.1530/JOE-18-0457. [DOI] [PubMed] [Google Scholar]
- 122.Zhang H.Q., Zheng F.L., Zhao J.H., Guo D.X., Chen X.L. Genistein inhibits ox-LDL-in duced VCAM-1, ICAM-1 and MCP-1 expression of HUVECs through heme oxygenase-1. Arch Med Res. 2013;44:13–20. doi: 10.1016/j.arcmed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 123.Zhang T., Hu Q., Shi L.Y., Qin L., Zhang Q.Y., Mi M. Equol attenuates atherosclerosis in apolipoprotein E-Deficient mice by inhibiting endoplasmic reticulum stress via activation of Nrf2 in endothelial cells. PLoS ONE. 2016;11:e0167020. doi: 10.1371/journal.pone.0167020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang Q., Han L., Li J., Xu H., Liu X.F., Wang X.Y., et al. Activation of Nrf2 by phloretin attenuates palmitic acid-induced endothelial cell oxidative stress via AMPK-dependent signaling. J Agric Food Chem. 2019;67:120–131. doi: 10.1021/acs.jafc.8b05025. [DOI] [PubMed] [Google Scholar]
- 125.Li S.N., Sun Y., Han Z.W., Bu X.C., Yu W.J., Wang J.Y. Cytoprotective effects of euxanthone against ox-LDL-induced endothelial cell injury is mediated via Nrf2. Life Sci. 2019;223:174–184. doi: 10.1016/j.lfs.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 126.Yan R., Yan J.F., Chen X.Z., Yu Y.F., Sun T. Xanthoangelol prevents Ox-LDL–induced endothelial cell injury by activating Nrf2/ARE signaling. J Cardiovasc Pharmacol. 2019;74:162–171. doi: 10.1097/FJC.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 127.Tholl D. Biosynthesis and biological functions of terpenoids in plants. Adv Biochem Eng Biotechnol. 2015;148:63–106. doi: 10.1007/10_2014_295. [DOI] [PubMed] [Google Scholar]
- 128.Bergman M.E., Davis B., Phillips M.A. Medically useful plant terpenoids: biosynthesis, occurrence, and mechanism of action. Molecules. 2019;24:3961. doi: 10.3390/molecules24213961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu F., Liu Z.Z. Pachymic acid inhibits OX-LDL-induced injury in human umbilical vein endothelial cells via activating Nrf2/HO-1 signaling pathway. Chin J Immunol. 2020;36:03–09. [Google Scholar]
- 130.Li M., Liu X., He Y.P., Zheng Q.Y., Wang M., Wu Y., et al. Celastrol attenuates angiotensin II mediated human umbilical vein endothelial cells damage through activation of Nrf2/ERK1/2/Nox2 signal pathway. Eur J Pharmacol. 2017;797:124–133. doi: 10.1016/j.ejphar.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 131.Jiang Q.X., Wang D.Y., Han Y.T., Han Z.W., Zhong W.Z., Wang C.B. Modulation of oxidized-LDL receptor-1 (LOX1) contributes to the antiatherosclerosis effect of oleanolic acid. Int J Biochem Cell Biol. 2015;69:142–152. doi: 10.1016/j.biocel.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 132.Lee W., Kim J., Park E.K., Ba J.S. Maslinic acid ameliorates inflammation via the downregulation of NF-κB and STAT-1. Antioxidants (Basel) 2020;9:106. doi: 10.3390/antiox9020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu Z.S., Li J.Y., Zhang X.R. Astragaloside IV protects against oxidized low density lipoprotein (ox-LDL)-induced endothelial cell injury by reducing oxidative stress and inflammation. Med Sci Monit. 2019;25:2132–2140. doi: 10.12659/MSM.912894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li C.P., Qin G., Shi R.Z., Zhang M.S., Lv J.Y. Ginsenoside Rg1 reduces toxicity of PM(2.5) on human umbilical vein endothelial cells by upregulating intracellular antioxidative state. Environ Toxicol Pharmacol. 2013;35:21–29. doi: 10.1016/j.etap.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 135.Lu C.Y., Yang Y.S., Li C.C., Liu K.L., Li C.K., Chen H.W. Andrographolide inhibits TNFα-induced ICAM-1 expression via suppression of NADPH oxidase activation and induction of HO-1 and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1 pathways in human endothelial cells. Biochem Pharmacol. 2014;91:40–50. doi: 10.1016/j.bcp.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 136.Lin H.C., Su S.L., Lu C.Y., Lin A.H., Lin W.C., Liu C.S., et al. Andrographolide inhibits hypoxia-induced HIF-1α-driven endothelin 1 secretion by activating Nrf2/HO-1 and promoting the expression of prolyl hydroxylases 2/3 in human endothelial cells. Environ Toxicol. 2017;32:918–930. doi: 10.1002/tox.22293. [DOI] [PubMed] [Google Scholar]
- 137.Xia L., Chu C.J., Yan B., Xu N.Y., Zhang J. The protective effects of Glaucocalyxin A on vascular endothelial cells. Guiding J Trad Chin Med Pharm. 2014;20:6. [Google Scholar]
- 138.Mao H.M., Tao T.Q., Wang X.R., Liu M., Song D.D., Liu X.H., et al. Zedoarondiol attenuates endothelial cells injury induced by oxidized low-density lipoprotein via Nrf2 activation. Cell Physiol Biochem. 2018;48:1468–1479. doi: 10.1159/000492257. [DOI] [PubMed] [Google Scholar]
- 139.Sung L.C., Chao H.H., Chen C.H., Tsai J.C., Liu J.C., Hong H.J., et al. Lycopene inhibits cyclic strain-induced endothelin-1 expression through the suppression of reactive oxygen species generation and induction of heme oxygenase-1 in human umbilical vein endothelial cells. Clin Exp Pharmacol Physiol. 2015;42:632–639. doi: 10.1111/1440-1681.12412. [DOI] [PubMed] [Google Scholar]
- 140.Qiu S., Sun H., Zhang A.H., Xu H.Y., Yan G.L., Han Y., et al. Natural alkaloids: basic aspects, biological roles, and future perspectives. Chin J Nat Med. 2014;12:401–406. doi: 10.1016/S1875-5364(14)60063-7. [DOI] [PubMed] [Google Scholar]
- 141.Wu B., Yue H., Zhou G.H., Zhu Y.Y., Wu T.H., Wen J.F., et al. Protective effects of oxymatrine on homocysteine-induced endothelial injury: involvement of mitochondria-dependent apoptosis and Akt-eNOS-No signaling pathways. Eur J Pharmacol. 2019;864:172717. doi: 10.1016/j.ejphar.2019.172717. [DOI] [PubMed] [Google Scholar]
- 142.Liu B., Su K., Wang J.X., Wang J.J., Xin Z.Y., Li F., et al. Corynoline exhibits anti-inflammatory effects in lipopolysaccharide(LPS)-stimulated human umbilical vein endothelial cells through activating Nrf2. Inflammation. 2018;41:1640–1647. doi: 10.1007/s10753-018-0807-6. [DOI] [PubMed] [Google Scholar]
- 143.Wang Z., Ding H., Yang R. Protective effects of berberine on H2O2-induced endothelial cell death through Nrf2/HO-1 pathway. Chin J ECC. 2019;17:28. [Google Scholar]
- 144.Huang C.S., Lin A.H., Liu C.T., Tsai C.W., Chang I.S., Chen H.W., et al. Isothiocyanates protect against oxidized LDL-induced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NF-κB activation. Mol Nutr Food Res. 2013;57:1918–1930. doi: 10.1002/mnfr.201300063. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Y.L., Yang G.M., Li P., Pan Y. Protective effects of four alkaloids of embryo loti on H2O2-induced oxidative damage of vascular endothelial cells. Chin J Biochem Med. 2015;3:35. [Google Scholar]
- 146.Zhang M., Xu Y., Jiang L. Sulforaphane attenuates angiotensin II-induced human umbilical vein endothelial cell injury by modulating ROS-mediated mitochondrial signaling. Hum Exp Toxicol. 2020;39:734–747. doi: 10.1177/0960327119893414. [DOI] [PubMed] [Google Scholar]
- 147.Akinwumi B.C., Bordun K.M., Anderson H.D. Biological activities of stilbenoids. Int J Mol Sci. 2018;19:792. doi: 10.3390/ijms19030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen Z.W., Miu H.F., Wang H.P., WuZN Wang WJ, Ling Y.J., et al. Pterostilbene protects against uraemia serum-induced endothelial cell damage via activation of Keap1/Nrf2/HO-1 signaling. Int Urol Nephrol. 2018;50:559–570. doi: 10.1007/s11255-017-1734-4. [DOI] [PubMed] [Google Scholar]
- 149.Tang T., Duan Z., Xu J., Liang J., Zhang S., Zhang H., et al. Pterostilbene reduces endothelial cell injury in vascular arterial walls by regulating the Nrf2-mediated AMPK/STAT3 pathway in an atherosclerosis rat model. Exp Ther Med. 2020;19:45–52. doi: 10.3892/etm.2019.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Y.T., Zhou H.Y., Lv X.P. Resveratrol protects against hydrogen peroxide induced injury in human umbilical vein endothelial cells. Nat Med J Chin. 2013;93:15. [PubMed] [Google Scholar]
- 151.Liu Y.G., Luo J.H. Protective effect of resveratrol on human umbilical vein endothelial cell injury induced by hydrogen peroxide. Chin Trad Patent Med. 2011;33:7. [Google Scholar]
- 152.Zhang L., Zhang H., Li X.Y., Jia B.J., Yang Y.Y., Zhou P., et al. Miltirone protects human EA.hy926 endothelial cells from oxidized low-density lipoprotein-derived oxidative stress via a heme oxygenase-1 and MAPK/Nrf2 dependent pathway. Phytomedicine. 2016;23:1806–1813. doi: 10.1016/j.phymed.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 153.Zhang J., Wang D.X., He S.H., Lu J.S. Protective effect of tanshinone Ⅱ A on undulatory high glucose injuried human umbilical vein endothelial cell. Chin J Clin Pharmacol. 2016;32:4–10. [Google Scholar]
- 154.Heyninck K., Sabbe L., Chirumamilla C.S., Szarc Vel Szic K., Vander Veken P., Lemmens K.J.A. Withaferin A induces heme oxygenase (HO-1) expression in endothelial cells via activation of the Keap1/Nrf2 pathway. Biochem Pharmacol. 2016;109:48–61. doi: 10.1016/j.bcp.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 155.Choi E.S., Yoon J.J., Han B.H., Jeong D.H., Lee Y.J., Kang D.G., et al. Ligustilide attenuates vascular inflammation and activates Nrf2/HO-1 induction and NO synthesis in HUVECs. Phytomedicine. 2018;38:12–23. doi: 10.1016/j.phymed.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 156.Lei W., Deng Y.F., Hu X.Y., Ni J.N., Jiang M., Bai G. Phthalides, senkyunolide A and ligustilide, show immunomodulatory effect in improving atherosclerosis, through inhibiting AP-1 and NF-κB expression. Biomed Pharmacother. 2019;117:109074. doi: 10.1016/j.biopha.2019.109074. [DOI] [PubMed] [Google Scholar]
- 157.Zhu Y., Zhang Y.J., Huang X., Xie Y., Qu Y., Long H.Y., et al. Z-Ligustilide protects vascular endothelial cells from oxidative stress and rescues high fat diet-induced atherosclerosis by activating multiple NRF2 downstream genes. Atherosclerosis. 2019;284:110–120. doi: 10.1016/j.atherosclerosis.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 158.Zhu Y., Zhang Y.J., Liu W.W., Shi A.W., Gu N. Salidroside suppresses HUVECs cell injury induced by oxidative stress through activating the Nrf2 signaling pathway. Molecules. 2016;21:1033. doi: 10.3390/molecules21081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang P., Li Y.M., Guo R., Zang W.F. Salidroside protects against advanced glycation end products-induced vascular endothelial dysfunction. Med Sci Monit. 2018;24:2420–2428. doi: 10.12659/MSM.906064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ma W.F., Duan X.C., Han L., Zhang L.L., Meng X.M., Li Y.L., et al. Vanillic acid alleviates palmitic acid-induced oxidative stress in human umbilical vein endothelial cells via adenosine monophosphate-activated protein kinase signaling pathway. J Food Biochem. 2019;43:e12893. doi: 10.1111/jfbc.12893. [DOI] [PubMed] [Google Scholar]
- 161.Han L., Yang Q., Ma W.F., Li J., Qu L.Z., Wang M. Protocatechuic acid ameliorated palmitic acid-induced oxidative damage in endothelial cells through activating endogenous antioxidant enzymes via an AMPK-dependent pathway. J Agric Food Chem. 2018;66:10400–10409. doi: 10.1021/acs.jafc.8b03414. [DOI] [PubMed] [Google Scholar]
- 162.Guo F., Wu R., Xu J. Salicin prevents TNF-α-induced cellular senescence in human umbilical vein endothelial cells (HUVECs) Artif Cells Nanomed Biotechnol. 2019;47:2618–2623. doi: 10.1080/21691401.2019.1629949. [DOI] [PubMed] [Google Scholar]
- 163.Zhang M., Pan H.C., Xu Y.J., Wang X.T., Qiu Z.H., Jiang L. Allicin decreases lipopolysaccharide induced oxidative stress and inflammation in human umbilical vein endothelial cells through suppression of mitochondrial dysfunction and activation of Nrf2. Cell Physiol Biochem. 2017;41:2255–2267. doi: 10.1159/000475640. [DOI] [PubMed] [Google Scholar]
- 164.Li C.L., Liu X.H. Allicin alleviates inflammation of diabetic macroangiopathy via the Nrf2 and NF-kB pathway. Eur J Pharmacol. 2020;876:173052. doi: 10.1016/j.ejphar.2020.173052. [DOI] [PubMed] [Google Scholar]