Abstract
Polypeptide growth factors activate specific transmembrane receptors, leading to the induction of multiple intracellular signal transduction pathways which control cell function and fate. Recent studies have shown that growth factors promote cell survival by stimulating the serine-threonine protein kinase Akt, which appears to function primarily as an antiapoptotic agent by inactivating death-promoting molecules. We previously established C2 muscle cell lines lacking endogenous expression of insulin-like growth factor II (IGF-II). These cells underwent apoptotic death in low-serum differentiation medium but could be maintained as viable myoblasts by IGF analogues that activated the IGF-I receptor or by unrelated growth factors such as platelet-derived growth factor BB (PDGF-BB). Here we show that IGF-I promotes muscle cell survival through Akt-mediated induction of the cyclin-dependent kinase inhibitor p21. Treatment of myoblasts with IGF-I or transfection with an inducible Akt maintained muscle cell survival and enhanced production of p21, and ectopic expression of p21 was able to sustain viability in the absence of growth factors. Blocking of p21 protein accumulation through a specific p21 antisense cDNA prevented survival regulated by IGF-I or Akt but did not block muscle cell viability mediated by PDGF-BB. Our results define Akt as an intermediate and p21 as a critical effector of an IGF-controlled myoblast survival pathway that is active during early myogenic differentiation and show that growth factors are able to maintain cell viability by inducing expression of pro-survival molecules.
Polypeptide growth factors control diverse cellular processes by binding to and activating specific transmembrane receptors, leading to induction of distinct but overlapping intracellular signal transduction pathways (73). The insulin-like growth factors, IGF-I and -II, are structurally related circulating proteins of fundamental importance for normal somatic growth, and for the survival, proliferation, and terminal differentiation of different cell types (6, 32, 49, 68). The actions of both IGFs are mediated by the IGF-I receptor, a heterodimeric, ligand-activated tyrosine protein kinase that is structurally and functionally related to the insulin receptor (32, 46).
IGF action is critical for the formation and maintenance of skeletal muscle. Mice with a targeted disruption of the IGF-I receptor, or engineered to lack both IGF-I and IGF-II, exhibit marked muscle hypoplasia and die in the perinatal period because of inadequate muscle strength to inflate their lungs (3, 50). Conversely, mice with forced overexpression of IGF-I in muscle develop myofiber hypertrophy and increased muscle mass (5, 10). In cultured muscle cells IGF action enhances terminal differentiation through an autocrine pathway dependent on production of IGF-II (10, 18, 20, 21, 47, 51, 52, 59, 60, 63, 67). IGF-II also plays a central role in maintaining myoblast survival during the transition from proliferating to terminally differentiating muscle cells (45, 69). The signal transduction pathways and effectors of IGF-mediated muscle cell survival and differentiation have not been identified. It has been suggested that the intracellular signaling molecules, phosphatidylinositol 3-kinase (PI3-kinase) and mitogen-activated protein kinases, are involved in IGF-facilitated muscle differentiation (11, 33, 35, 56, 57, 64, 65), although the mechanisms by which these enzymes or other IGF-activated mediators may collaborate with myogenic regulatory factors to promote terminal differentiation have not been defined.
One of the earliest events in muscle differentiation is induction of the cyclin-dependent kinase inhibitor p21 (also known as Waf1 or Cip1 [4]) (16). This protein (24) and the related molecules p27 and p57 (4, 24) block progression through the cell cycle by forming ternary complexes with various cyclin–cyclin-dependent kinase complexes, including cyclin E/A–cdk-2 and D-type cyclin–cdk-4/6 (17, 30), thus inhibiting their enzymatic activity. Recent studies using cultured cells have shown that p21 is induced in differentiating myoblasts through the actions of MyoD (26–28) and have demonstrated that MyoD promotes cell cycle withdrawal through induction of p21 (27, 28). In addition, the combination of p21 and p57 has been shown to be essential for muscle differentiation during embryonic development in mice (76).
The serine-threonine protein kinase Akt is an important survival factor for a number of cell types (12, 40), and its mode of action has been investigated in great detail (2, 12, 14). Akt has been shown to prevent apoptotic cell death by phosphorylating and inhibiting several proapoptotic proteins, including Bad (13), caspase-9 (9), the forkhead transcription factor FKHR-L1 (8), and glycogen synthase kinase 3 (55). Akt also has been found to block the release of cytochrome c from mitochondria that is induced by several proapoptotic members of the Bcl-2 family (38). To date Akt has not been shown to promote cell viability by activating pro-survival molecules.
In this study we demonstrate that IGF-I promotes muscle cell survival during the initial phase of myogenic differentiation in cell culture through Akt-mediated induction of p21 expression. Forced expression of p21 was able to maintain complete myoblast viability in the absence of growth factors. Blocking of p21 protein accumulation could prevent both IGF-mediated and Akt-stimulated muscle cell viability but did not inhibit survival regulated by platelet-derived growth factor (PDGF). Our results define Akt as an intermediate and p21 as a critical effector of an IGF-controlled myoblast survival pathway and show that growth factors are able to sustain cell viability by inducing expression of pro-survival molecules.
MATERIALS AND METHODS
Materials.
Tissue culture supplies, fetal calf serum (FCS), newborn calf serum (NCS), horse serum, Dulbecco's modified Eagle's medium (DMEM), phosphate-buffered saline (PBS), G418, PDGF-BB, and TRIzol were purchased from Gibco BRL Life Technologies, Grand Island, N.Y. L6 myoblasts were obtained from the American Type Culture Collection, Manassas, Va. R3IGF-I was supplied by Gropep, Adelaide, Australia. Effectene was purchased from Qiagen, Chatsworth, Calif. Restriction enzymes, ligases, and polymerases were supplied by New England Biolabs, Beverly, Mass., and Promega Biotech, Madison, Wis. The pEGFP-N3 plasmid was purchased from Clontech, Palo Alto, Calif. Protease inhibitor tablets were obtained from Roche Molecular Biochemicals, Indianapolis, Ind. The bicinchoninic acid protein assay kit was from Pierce Chemical Co., Rockford, Ill. The kinase assay kit for Akt was purchased from New England Biolabs and was used according to the supplier's directions. Antibodies to p21 and CDK-4 used for immunoblotting were supplied by Santa Cruz Biotechnology. The p21 antibody used for immunocytochemistry was purchased from Pharmingin, San Diego, Calif.; the antibody to influenza hemagglutinin (HA) was from BabCO, Richmond, Calif., and the p65 (RelA) antibody was supplied by Rockland, Gilbertsville, Pa. The myogenin (clone F5D) and myosin heavy chain (clone MF 20) monoclonal antibodies were from the Developmental Studies Hybridoma Bank, University of Iowa, Iowa City. Horseradish peroxidase (HRP)-conjugated secondary antibodies were from Sigma Chemical Co., St. Louis, Mo. Slow Fade mounting reagent and secondary antibodies conjugated to fluorophores were purchased from Molecular Probes, Eugene, Oreg., and Southern Biotechnology Associates, Inc., Birmingham, Ala. Nitrocellulose was obtained from Schleicher and Schuell, Keene, N.H., and enhanced chemiluminescence (ECL) reagents were from Amersham Pharmacia Biotechnology, Piscataway, N.J. X-ray film was purchased from Kodak, Rochester, N.Y. All other chemicals were reagent grade and were obtained from commercial suppliers.
Cell culture.
C2 myoblasts stably transfected with the coding region of a mouse IGF-II cDNA in the antisense orientation (C2AS12 cells [69]) were grown on gelatin-coated tissue culture plates in DMEM supplemented with 10% heat-inactivated FCS, 10% heat-inactivated NCS, l-glutamine (2 mM), and G418 (400 μg/ml) (growth medium) until they were >95% confluent. C2 (74) and L6 myoblasts were grown on gelatin-coated plates in DMEM supplemented with 10% heat-inactivated FCS, 10% heat-inactivated NCS, and l-glutamine (2 mM). For all muscle cell lines, differentiation was initiated following washing with PBS by incubation in differentiation medium (DM), containing DMEM plus 2% horse serum, or in DM supplemented with PDGF-BB (0.4 nM) or the long lasting IGF-I analogue R3IGF-I (2 nM). At different intervals adherent cells were trypsinized and counted with a hemocytometer or Coulter particle counter.
RNA isolation and RNase protection assays.
Total RNA was isolated from cells using TRIzol and quantitated by spectrophotometry (Beckman DU 640 instrument). RNA integrity was assessed by electrophoresis through 1% agarose–formaldehyde gels after staining with ethidium bromide. Solution hybridization RNase protection assays were performed as previously described (67) using single stranded [α-32P]CTP-labeled antisense riboprobes synthesized in vitro from linearized plasmid templates. Results were quantitated with a phosphorimager (Bio-Rad GS 525 Molecular Imaging system).
Protein isolation and immunoblotting.
Protein extracts were isolated after washing cells twice with cold PBS by incubation for 30 min at 4°C in radioimmunoprecipitation assay buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1.0% NP-40, 1.0% deoxycholate) containing protease inhibitors, 1 μM okadaic acid, and 1 μM sodium orthovanadate. After removal of insoluble material by centrifugation (Eppendorf 5415 C bench centrifuge) at 14,000 rpm for 10 min at 4°C, the protein concentration was determined by bicinchoninic acid assay. Protein extracts (60 μg) were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing and reducing conditions before transfer onto 0.2 μm-pore-size nitrocellulose membranes at 18 V for 45 min using a semidry blotter. The membranes were blocked for 2 h at 25°C using blocking buffer (Tris-buffered saline plus 0.1% Tween 20 containing 5% nonfat dry milk) before being incubated with primary antibody (anti-p21 [1:75] and anti-CDK4 [1:500] in blocking buffer). Following incubation with HRP-conjugated secondary antibodies (1:2,000 in blocking buffer), proteins were detected by ECL, followed by exposure to X-ray film. Results were quantitated by densitometry (Bio-Rad GS 700 densitometer).
Construction of bicistronic expression plasmids for p21 and iAkt.
A 700-bp SalI/BglII fragment containing the internal ribosome entry site (IRES) from mouse encephalomyocarditis virus (25) was subcloned into the polylinker of pEGFP-N3 to generate pIRES-EGFP. BamHI/SalI DNA fragments containing the coding region of murine p21 cDNA in the sense or antisense orientation were excised from pBluescript and subcloned 5′ to the IRES to generate p21-IRES-EGFP or p21AS-IRES-EGFP. A modified human Akt-1 cDNA (iAkt) was added 5′ to the IRES as a 2.1-kb BamHI-SalI fragment. iAkt has been described previously (41) and contains (i) a truncated amino terminus lacking the pleckstrin homology region and encoding a myristoylation sequence and (ii) a carboxyl terminus fused in frame to the modified ligand binding domain of mouse estrogen receptor α. Akt enzymatic activity is induced by incubation with 4-hydroxytamoxifen (HT) (41).
Transfections.
Myoblasts were plated at 13,000 cells/cm2 onto 12-well tissue culture dishes or 4-well slide chambers and incubated for 24 h in growth medium. DNA-mediated gene transfer was performed using Effectene, following the protocol specified by the manufacturer. For transfections of 12-well dishes or slide chambers 1 μg of p21-IRES-EGFP DNA or 2 μg of p21AS-IRES-EGFP DNA was used, and for cotransfections a total of 2 μg of DNA was added to cells. After incubation with DNA for 16 to 18 h, fresh growth medium was added to the cells. When cells reached confluent density at ∼48 h after transfection, they were incubated in DM without or with growth factors for an additional 24 or 48 h. Transfection efficiencies ranged from 12 to 20% for C2AS12 cells, from 17 to 20% for L6 cells, and from 20 to 25% for C2 myoblasts.
Survival assays of transfected cells.
Twelve-well dishes of proliferating myoblasts were transfected in parallel as described above. When cells reached a confluent density at ∼48 h after transfection, two wells were harvested and both total cell number [T0 (total)] and transfection efficiency were determined. The latter was assessed by averaging the fraction of cells expressing EGFP in 20 hemocytometer fields at a magnification of ×200 [T0 (transfected)]. The remaining wells were incubated in DM without or with growth factors for 24 or 48 h, and cells were then harvested and total and transfected cells were counted. Survival of transfected cells at 24 h was determined by the following formula: percent survival = {[T24 (transfected)/T0 (transfected)] × [T24 (total)/T0 (total)]} × 100. Survival at 48 h was assessed similarly.
Immunocytochemistry.
Cells cultured on slide chambers or 12-well dishes were washed twice with PBS before fixation in 1% paraformaldehyde for 10 min. Cells were then solubilized for 5 min in a solution of PBS containing 0.2% Triton X-100 (PBST), washed twice with PBST, and incubated with primary antibodies polyclonal rabbit anti-p21 immunoglobulin G (IgG) (1:2,000), monoclonal anti-HA IgG (1:100), polyclonal rabbit anti-p65 (Rel A) (1:2,000), monoclonal antimyogenin (1:200), or monoclonal anti-myosin heavy chain (anti-MHC) (1:200) in PBST plus 3% bovine serum albumin for 3 h overnight. The cells were next washed three times with PBST before incubation with polyclonal secondary antibodies, either Alexa 594-tagged goat anti-rabbit IgG, fluorescein-conjugated goat anti-mouse IgG, or Texas red-labeled goat anti-mouse IgG (1:2,000), for 45 min in the dark. Slides were mounted using Slow Fade. Fluorescent images were captured with a Nikon Eclipse TE 300 fluorescent microscope using Scion Image 1.62 software.
Akt kinase assay.
Immune complex-kinase assays were performed using cells transfected with iAkt by a protocol modified from an assay kit purchased from New England Biolabs. Cell lysates (60 μg) were incubated with anti-HA antibody (5 μg/reaction) overnight at 4°C. Immune complexes were then incubated for 3 h at 4°C with protein A-conjugated agarose beads (20 μl of 50% slurry/reaction) before being washed twice in cell lysis buffer and twice in kinase buffer. After resuspension in kinase assay buffer containing the Akt substrate GSK-3α, the reaction was allowed to proceed at 30°C for 30 min. After the reaction was stopped by addition of concentrated SDS-PAGE loading buffer, samples were separated by SDS-PAGE and transferred to nitrocellulose membranes, as described above. Immunoblotting was performed using primary antibodies to phospho-GSK-3α, followed by addition of HRP-conjugated secondary antibodies, detection by ECL, and exposure to X-ray film. Results were quantitated by densitometry.
Statistical analysis.
Results are presented as the mean ± standard error of the mean (SEM). Statistical significance was determined using the independent Student's t test for paired samples. Results were considered statistically significant when the P value was <0.05.
RESULTS
Prevention of myoblast death by IGF-I or PDGF.
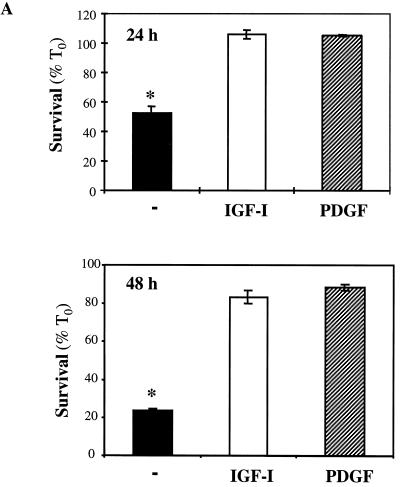
In previous studies we showed that C2 myoblasts engineered to lack IGF-II by stable transfection with a mouse IGF-II cDNA in the antisense orientation (C2AS12 cells) underwent rapid apoptotic death when incubated in DM and that cell death could be prevented by IGF-II, IGF-I, or several other growth factors (45, 69). As seen in Fig. 1A, 50% of myoblasts remained viable after a 24-h incubation in DM, and only 23% were viable after 48 h. A further decrease in cell survival was observed after longer incubation in DM (45, 69). Addition of the IGF-I analogue R3IGF-I maintained nearly complete cell survival, as did treatment with PDGF-BB.
FIG. 1.
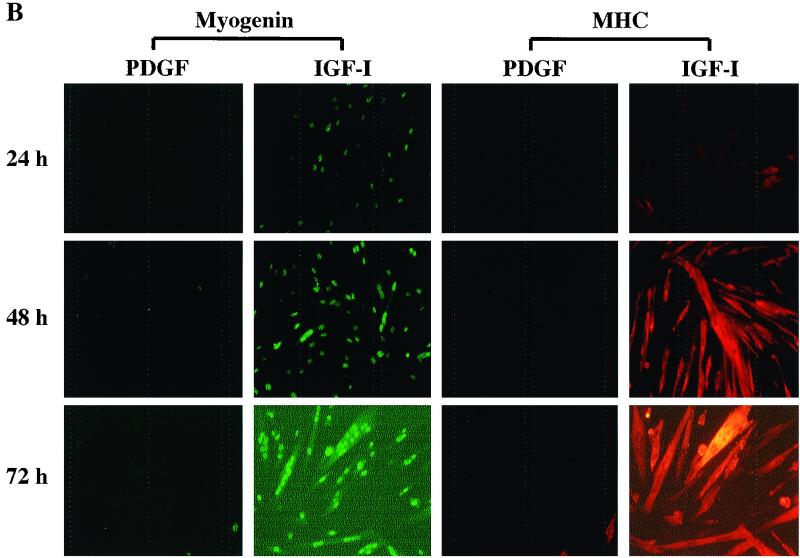
IGF-I and PDGF maintain myoblast survival, but only IGF-I promotes differentiation. (A) Cell counts (expressed as the percentage at time zero [T0]) of C2AS12 myoblasts after a 24- or 48-h incubation in DM with or without R3IGF-I (2 nM) or PDGF-BB (0.4 nM). The asterisk indicates that significantly fewer cells survived (P < 0.0006) in untreated than in growth factor-treated cells. Results are means ± SEMs from three experiments, each performed in duplicate. (B) IGF-I induces muscle differentiation. Results of immunocytochemical staining for myogenin and MHC after incubation of C2AS12 myoblasts in DM with PDGF-BB or R3IGF-I for 24 to 72 h are shown.
Stimulation of differentiation by IGF-I.
To demonstrate that C2AS12 myoblasts were capable of undergoing normal biochemical and morphological differentiation, cells were incubated in DM with growth factors for 24 to 72 h, followed by fixation and immunostaining with antibodies for myogenin or MHC. As shown in Fig. 1B, incubation with PDGF-BB did not induce either protein and did not trigger formation of myotubes. By contrast, treatment with IGF-I led to the progressively increased expression of both myogenin and MHC and to the appearance of multinucleated myofibers by 48 and 72 h. Thus addition of IGF-I maintains survival and promotes differentiation of C2AS12 myoblasts, but PDGF only sustains survival.
Induction of p21 expression after treatment with IGF-I.
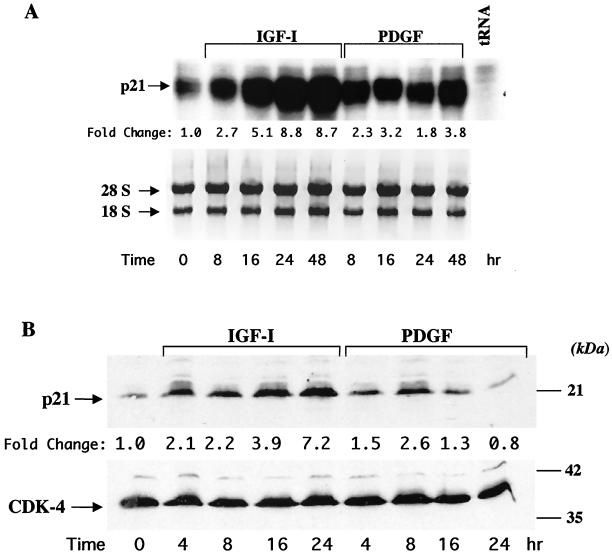
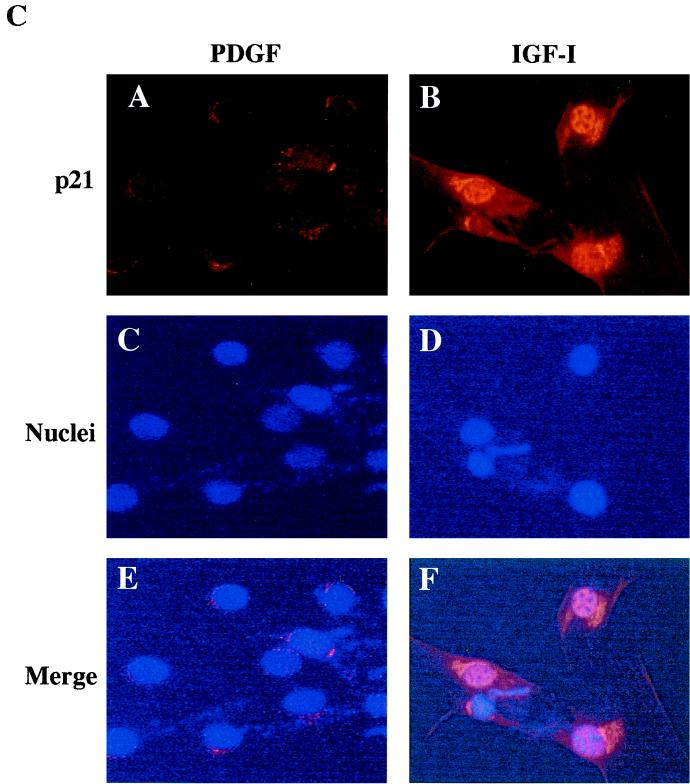
The results in Fig. 1 prompted examination of mechanisms of growth factor-mediated myoblast survival. The cyclin-dependent kinase inhibitor p21 has been implicated as a survival factor for several cell types, including differentiating myoblasts (48, 58, 72). We first asked if either IGF-I or PDGF stimulated expression of p21. Figure 2 shows results of time course studies. As seen in the immunoblot in Fig. 2B, incubation of confluent myoblasts in DM with IGF-I led to a progressive increase in the abundance of p21, while PDGF treatment caused at best a transient rise in protein accumulation. Neither growth factor altered the expression of CDK-4. In addition, as shown by immunocytochemistry, after a 24-h incubation with IGF-I, p21 could be detected in nearly every myoblast, while few cells expressed p21 after a similar treatment with PDGF (Fig. 2C). Analogous results were observed when steady-state levels of p21 mRNA were measured (Fig. 2A). IGF-I caused a progressive increase in p21 transcript abundance, while PDGF had little effect. Thus, IGF-I caused a sustained induction of p21 mRNA and protein expression.
FIG. 2.
Treatment with IGF-I stimulates expression of p21. (A) The autoradiograph shows results of a representative RNase protection assay performed using total RNA isolated from C2AS12 cells incubated with either R3IGF-I (2 nM) or PDGF-BB (0.4 nM) for the indicated times. The numbers below each panel correspond to the change in mRNA abundance relative to that at time zero. The bottom panel shows a photograph of an ethidium bromide-stained gel of the RNA used in these studies. Similar results were seen in three independent experiments. (B) The upper panel shows a representative immunoblot using an antibody to p21 and whole-cell protein extracts from C2AS12 cells incubated with either R3IGF-I (2 nM) or PDGF-BB (0.4 nM) for the indicated times. The numbers below each panel correspond to the change in protein abundance relative to that at time zero. Similar results were seen in seven independent experiments. The lower panel shows the same blot after being stripped and incubated with an antibody to CDK-4. (C) Representative fluorescence micrographs of C2AS12 myoblasts treated with either R3IGF-I (2 nM) or PDGF (0.4 nM) for 24 h as described in Materials and Methods and stained with anti-p21 antibody (A and B) or Hoechst dye (C and D). Panels E and F are merged images of panels A and C and panels B and D, respectively.
Forced expression of p21 promotes myoblast survival.
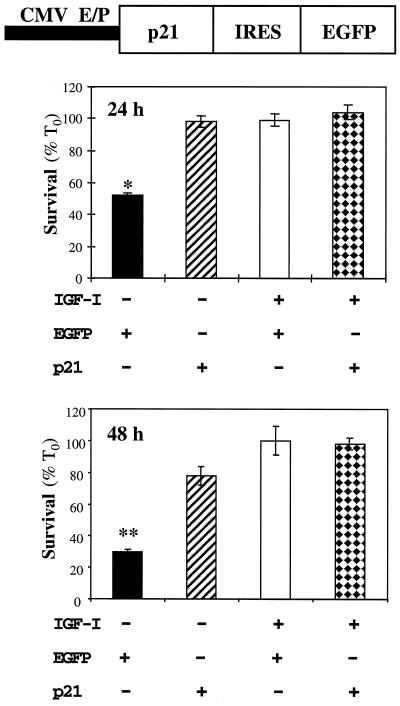
We next asked if p21 could function as a survival factor in the absence of growth factors. We generated a bicistronic expression plasmid using a mouse p21 cDNA, followed by a cassette containing an IRES derived from murine encephalomyocarditis virus (25) and the marker protein EGFP (Fig. 3). C2AS12 myoblasts were transiently transfected with this plasmid or with a control encoding EGFP, and survival was assessed by cell counting after 24 or 48 h in DM. As seen in Fig. 3, nearly 100% of myoblasts transfected with p21 remained alive after 24 h in the absence of IGF-I and 78% ± 6% remained alive at 48 h, while 53% ± 1% of cells transfected with EGFP survived at 24 h and only 30% ± 1% survived at 48 hr (P < 0.001 and P < 0.003, respectively). Complete survival was seen after incubation with IGF-I or with PDGF (data not shown), indicating that neither plasmid was toxic to the cells. As observed with endogenous p21, the transfected protein was located in the nucleus (data not shown). Thus, forced expression of p21 promotes muscle cell survival in the absence of exogenous growth factors.
FIG. 3.
Forced expression of p21 stimulates myoblast survival. C2AS12 cells were transfected with the p21-IRES-EGFP expression plasmid shown above the graph, or with EGFP alone, as described in Materials and Methods. Cell counts of transfected myoblasts were performed after a 24- or 48-h incubation in DM or in DM supplemented with R3IGF-I (2 nM). Results are presented as means ± SEMs from four experiments, each performed in duplicate. The asterisks indicate that survival was significantly less in myoblasts transfected with EGFP than in p21-transfected or IGF-I-treated cells (∗, P < 0.001, ∗∗, P < 0.003). CMV/EP, enhancer-promoter from cytomegalovirus.
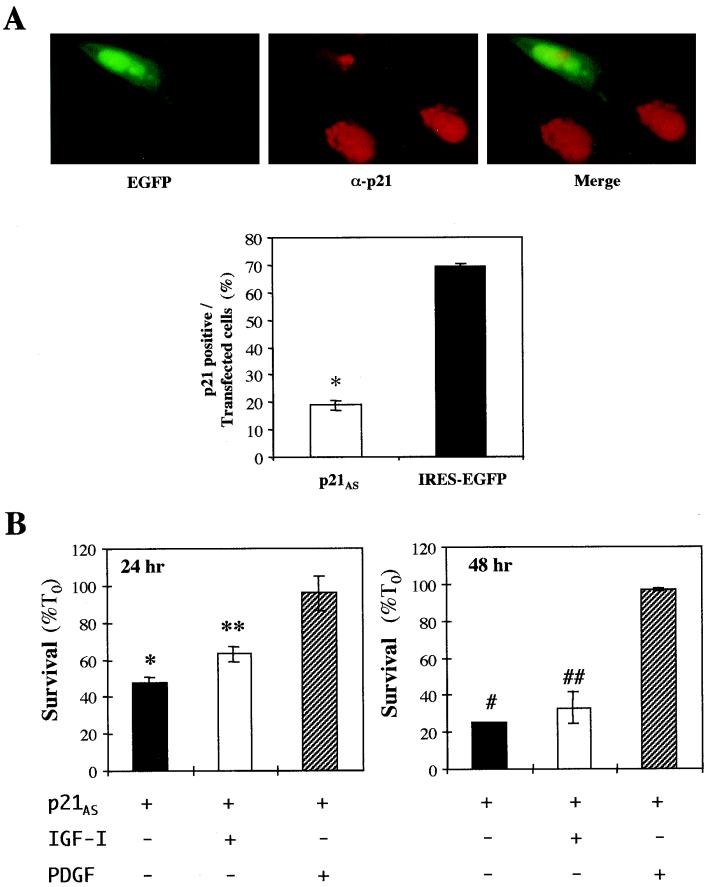
A p21 antisense cDNA blocks IGF-mediated myoblast survival.
We next asked if inhibition of p21 expression could prevent IGF-regulated muscle cell survival. We first tested the effectiveness of a p21 antisense cDNA (p21AS) in blocking IGF-stimulated p21 protein expression. C2AS12 myoblasts were transiently transfected with an expression plasmid containing a mouse p21 cDNA in the antisense orientation (p21AS-IRES-EGFP). Cells were immunostained for p21 after a 24-h incubation in DM supplemented with IGF-I. As shown in Fig. 4A, IGF-stimulated expression of p21 was diminished in myoblasts transfected with the p21AS cDNA. In only 19% ± 1.8% of cells could p21 protein be detected by immunocytochemistry, compared with 70% ± 1.2% of myoblasts transfected with EGFP (P < 0.001). Thus, the p21AS plasmid blocked IGF-induced p21 protein expression. The p21AS cDNA also inhibited IGF-promoted myoblast viability. As seen in Fig. 4B, IGF-I had little effect on survival after 24 hr (63% ± 2% with IGF-I versus 48% ± 5% without IGF-I; P = 0.06 [not significant]) and did not prevent enhanced death at 48 h (33% ± 6% with IGF-I versus 28% ± 3% without IGF-I; P = 0.65, [not significant]). By contrast, nearly 100% of transfected cells remained alive at 24 and 48 h after incubation with PDGF-BB. Therefore, expression of p21 appears to be required for IGF-regulated muscle cell survival.
FIG. 4.
Inhibition of p21 expression prevents IGF-stimulated myoblast survival. (A) Expression of a p21AS cDNA blocks IGF-induced accumulation of p21 protein. C2AS12 cells were transiently cotransfected with the p21AS-IRES-EGFP and EGFP expression plasmids, as described in Materials and Methods. Cells were then incubated in DM plus R3IGF-I (2 nM) for 24 h. The top panels show photomicrographs of a group of cells (magnification, ×400). The left panel shows expression of EGFP, and the center panel shows results of immunostaining for p21. In the right panel merged EGFP and p21 images are displayed. Below the micrographs results are presented as the percentage of cells transfected with either the p21AS-IRES-EGFP or IRES-EGFP expression plasmid that also express p21 protein (means ± SEMs from three experiments, counting 100 cells per experiment; ∗, P < 0.0001). (B) Inhibition of p21 blocks IGF-mediated myoblast survival. C2AS12 cells were transiently transfected with the p21AS-IRES-EGFP expression plasmid. Cell counts of transfected myoblasts were performed at 24 and 48 h after incubation in DM or in DM supplemented with R3IGF-I (2 nM) or PDGF-BB (0.4 nM). Results are presented as the means ± SEMs from three independent experiments, each performed in duplicate. The symbols indicate that survival was significantly less in myoblasts transfected with p21AS and treated with DM or R3IGF-I than in cells transfected with p21AS and incubated with PDGF-BB at 24 h (∗, P < 0.01; ∗∗, P < 0.03) or at 48 h (#, P < 0.02; ##, P < 0.001).
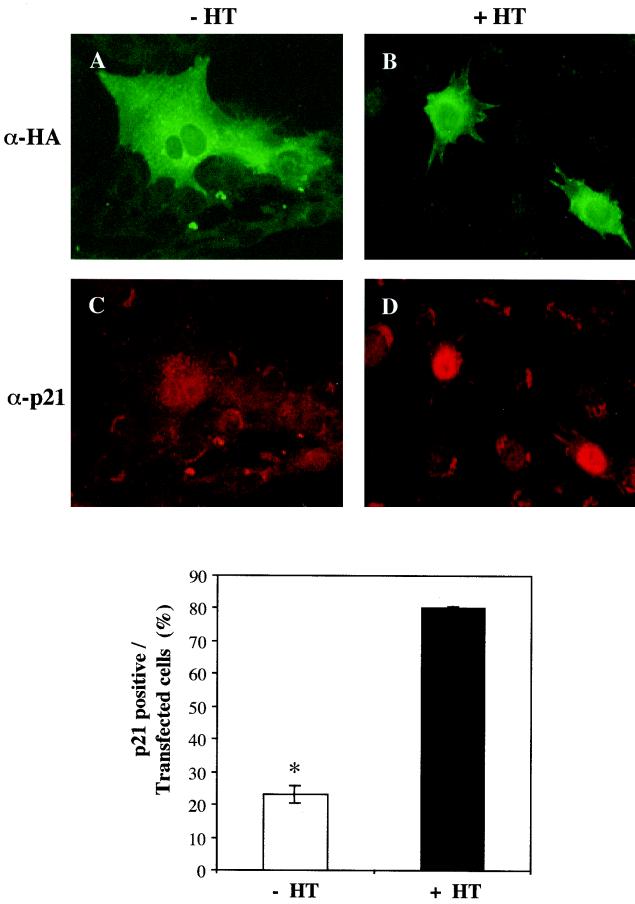
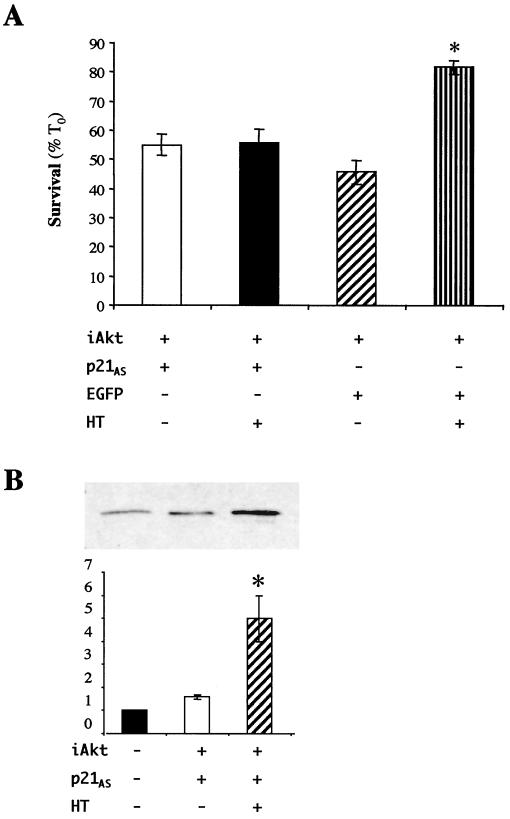
Akt induces p21 protein expression.
In recent studies we showed that IGF-I treatment resulted in the sustained stimulation of Akt kinase activity in muscle cells and that Akt could maintain myoblast survival in the absence of growth factors (45). Based on these observations and on the results in Fig. 3 and 4, we next asked if Akt was involved in IGF-mediated stimulation of p21. To test this hypothesis, myoblasts were transiently transfected with an inducible Akt expression plasmid, iAkt, and cells were treated with the inducer HT or with ethanol vehicle. As shown in Fig. 5, treatment with HT caused the accumulation of p21 in the majority of transfected myoblasts. Only 23% ± 2.7% of transfected cells expressed p21 in the absence of HT, compared with 80% ± 1.0% after treatment with HT (P < 0.0004). Thus, forced expression of Akt induces p21 in our muscle cell line.
FIG. 5.
Akt induces p21 expression. (Top panels) Representative fluorescence micrographs of C2AS12 muscle cells transiently transfected with the iAkt-IRES-EGFP expression plasmid, as described in Materials and Methods. Following a 24-h incubation in DM alone (A and C) or in DM containing HT (1 μM) (B and D), expression of iAkt was assessed by immunostaining using an anti-HA (α-HA) primary antibody and a fluorescein-labeled secondary antibody (A and B). Expression of p21 (C and D) also was determined by immunofluorescence. (Bottom panel) The graph shows quantitation of p21 expression in cells transfected with the iAkt-IRES-EGFP plasmid following treatment with DM alone or containing HT (means ± SEMs from three experiments, counting 100 cells per experiment; ∗, P < 0.003).
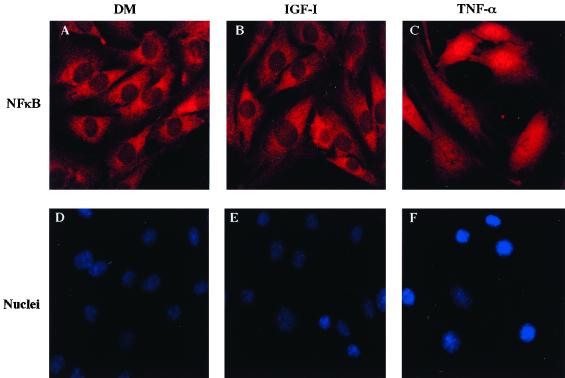
IGF-I treatment does not lead to activation of NF-κB.
Recent studies have indicated that activation of the transcription factor NF-κB was required for the antiapoptotic effects of PDGF through a mechanism that involved Akt (61). To determine if NF-κB also functioned as a component of an IGF-mediated survival pathway, we first determined if treatment of myoblasts with IGF-I caused translocation of the NF-κB subunit p65 Rel A from the cytoplasm to the nucleus. As shown in Fig. 6, p65 is readily detected in C2AS12 muscle cells, as assessed by immunocytochemistry. In untreated myoblasts the protein is found predominantly in the cytoplasm (Fig. 6A). Incubation with IGF-I for up to 2 h did not result in the appearance of p65 in the nucleus (Fig. 6B), although treatment with tumor necrosis factor alpha caused translocation in the majority of cells (Fig. 6C) (73% ± 5% of 250 cells counted). Similar results were observed after shorter incubation times (data not shown). Thus, we tentatively conclude that NF-κB does not play a role in IGF-mediated muscle cell survival, as IGF treatment is unable to stimulate its translocation into the nucleus.
FIG. 6.
IGF-I treatment does not cause nuclear translocation of the NF-κB subunit p65 Rel A. (A to C) Representative fluorescence micrographs of C2AS12 myoblasts incubated in DM without or with either R3IGF-I (2 nM) or tumor necrosis factor alpha (1.2 nM) for 2 h and immunostained with an antibody to p65 Rel A. (D to F) Same cells as in panels A to C stained with Hoechst dye.
A p21 antisense cDNA blocks Akt-stimulated myoblast survival.
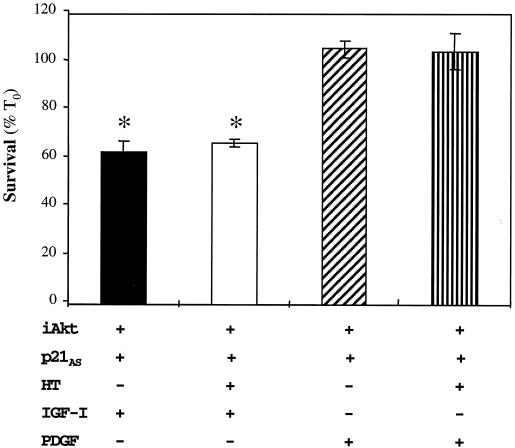
To determine if p21 is downstream of Akt in an IGF-stimulated pathway of muscle cell survival, C2AS12 myoblasts were transiently cotransfected with iAkt and either the p21AS cDNA or an EGFP control plasmid. As shown in Fig. 7A, Akt promoted muscle cell viability in the presence of EGFP but not when cotransfected with the p21AS cDNA. Coexpression of iAkt and the p21AS plasmid did not block induction of Akt kinase activity by HT (Fig. 7B), indicating that the inhibitory effects of p21AS on Akt were not direct. To provide additional evidence in support of a survival pathway involving the activated IGF-I receptor, Akt, and p21, myoblasts were incubated with HT and IGF-I after cotransfection with iAkt and p21AS. As seen in Fig. 8, IGF-I did not enhance viability, while under similar conditions PDGF promoted nearly complete survival. These results show that induction of p21 is a central component of an IGF- and Akt-stimulated muscle cell survival pathway and demonstrate that PDGF maintains muscle cell viability by a mechanism that does not require p21.
FIG. 7.
Inhibition of p21 expression blocks Akt-mediated myoblast survival. (A) C2AS12 cells were transiently cotransfected with iAkt-IRES-EGFP and either the p21AS-IRES-EGFP or EGFP expression plasmid, as described in Materials and Methods. Cell counts of transfected myoblasts were performed 24 h after incubation in DM or in DM plus HT (1 μM). Results are presented as the means ± SEMs from three independent experiments, each performed in duplicate. Survival was significantly greater in HT-treated cells cotransfected with EGFP than with p21AS (∗, P < 0.003). (B) Inhibition of p21 expression does not block induction of Akt kinase activity. Results are of in vitro kinase assays for Akt using immunoprecipitates from cells cotransfected with the iAkt-IRES-EGFP and p21AS-IRES-EGFP expression plasmids and incubated without or with HT for 4 h are shown. Results from a representative experiment are pictured in the top panel. Results of three independent experiments (means ± SEMs) are plotted in the bottom panel. Values on the y axis represent arbitrary densitometric units. Significantly more Akt enzymatic activity could be measured after treatment with HT (∗, P < 0.003).
FIG. 8.
IGF-I cannot maintain survival in the absence of p21 expression. C2AS12 cells were transiently cotransfected with p21AS-IRES-EGFP and iAkt-IRES-EGFP expression plasmids, as described in Materials and Methods. Cell counts of transfected myoblasts were performed 24 h after incubation in DM or in DM supplemented with R3IGF-I (2 nM) or PDGF-BB (0.4 nM). Results are presented as the means ± SEMs from three independent experiments, each performed in duplicate. The asterisks indicate that survival was significantly less in transfected myoblasts treated with IGF-I than in cells incubated with PDGF (P < 0.01).
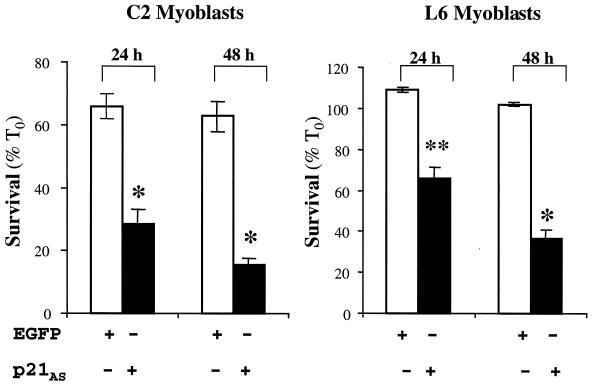
A p21 antisense cDNA inhibits survival of several muscle cell lines.
A stringent test of the hypothesis that IGF-induced expression of p21 is required for muscle cell survival during the transition from proliferating to terminally differentiating myoblasts would be to disrupt this circuit in parental C2 cells and in other muscle cell lines that normally produce IGF-II (62, 71, 74). As shown previously, C2 myoblasts undergo limited apoptotic death during the first 24 h after incubation in DM (45). As seen in Fig. 9, 65 to 70% of C2 myoblasts transfected with EGFP remained viable after 24 or 48 h. Similar results were seen in nontransfected cells (reference 45 and data not shown). By contrast, only 29% ± 5% of cells transfected with the p21 antisense cDNA were alive after 24 h in DM, and only 15% ± 2% survived after 48 h. Equivalently dramatic results were seen with L6 myoblasts. Nearly 100% of nontransfected cells or myoblasts transfected with EGFP were alive after 24 or 48 h in DM, compared with 66% ± 6% of cells transfected with the p21 antisense plasmid at 24 h and 37% ± 5% at 48 h. In aggregate, these observations show that p21 is a central component of an IGF-regulated muscle cell survival pathway that operates during the early phases of muscle differentiation.
FIG. 9.
Inhibition of p21 expression blocks muscle cell survival. C2 or L6 myoblasts were transiently transfected with the p21AS-IRES-EGFP or EGFP expression plasmid. Cell counts of transfected myoblasts were performed at 24 and 48 h after incubation in DM. Results are presented as the means ± SEMs from three independent experiments, each performed in duplicate. The asterisks indicate that survival was significantly less in myoblasts transfected with p21AS-IRES-EGFP than in cells transfected with EGFP at either time point (∗, P < 0.01; ∗∗, P < 0.03).
DISCUSSION
In this paper we establish that IGF-I promotes muscle cell survival as an early event in differentiation through a pathway that involves activation of Akt and the subsequent induction of p21. We have demonstrated previously that cultured myoblasts engineered to lack IGF-II underwent apoptotic death when incubated in low-serum DM, with nearly 85% of cells becoming nonviable within 72 h, and we found that survival could be maintained by IGF-I, IGF-II, or other IGF analogues (69). These prior observations indicated that IGF-II functioned as an autocrine or paracrine survival factor for differentiating muscle cells by activating the IGF-I receptor (69). We subsequently showed that IGF action sustained myoblast viability through induction of PI3-kinase and Akt (45). By contrast, the unrelated growth factor PDGF-BB also promoted survival of IGF-II-deficient myoblasts but did so through a signaling pathway that involved activation of Mek and Erks (45). We now find that IGF-I induces p21 through Akt-dependent mechanisms and that p21 is the critical effector of IGF-mediated muscle cell survival. Treatment with IGF-I led to accumulation of p21 mRNA and protein, and forced expression of p21 was sufficient to maintain myoblast survival in the absence of growth factors. In addition, interference with p21 protein production by a specific antisense cDNA blocked the ability of IGF-I or Akt to maintain myoblast viability but did not perturb PDGF-stimulated survival. Induction of p21 by IGF action also appears to precede the initiation of myogenic differentiation, at least as measured by accumulation of myogenin and MHC, and myofiber formation. In aggregate, these results define p21 as a key agent of viability in differentiating muscle cells and demonstrate that IGF-I and Akt stimulate expression of at least one critical prosurvival molecule in myoblasts.
A variety of studies have documented the central role of the PI3-kinase–Akt pathway in preventing apoptotic cell death (reviewed in reference 12). Forced expression of active PI3-kinase or Akt is able to maintain survival under conditions that otherwise induce death, including withdrawal of growth factors (22, 37, 39, 43, 75), while inhibition of PI3-kinase interferes with growth factor-mediated survival (15, 19, 37, 39, 43, 44, 75). At least four proapoptotic substrates have been shown to be inactivated by Akt (8, 9, 12, 13, 55). Phosphorylation of pro-caspase 9 inhibits its proteolytic processing and activation, thus preventing stimulation of the caspase enzymatic cascade, the executioners of cell death (9). Phosphorylation by Akt on serine 136 of Bad, a proapoptotic member of the Bcl-2 family (1, 53), causes its sequestration by 14-3-3 proteins, thus impairing its ability to bind and inactivate antiapoptotic members of the Bcl-2 family (13). Phosphorylation and inactivation of glycogen synthase kinase-3 by Akt maintains cell survival by unknown mechanisms that may be linked to changes in intermediary metabolism (55). Inhibition of the forkhead transcription factor FKHR-L1 and other related proteins (7, 42, 70) through phosphorylation by Akt causes its accumulation in the cytoplasm rather than in the nucleus and prevents activation of genes involved in promoting cell death (8). It is not yet known if any of these substrates of Akt are involved in muscle cell death, although in preliminary studies we can detect expression of Bad and Gsk3α and -β in our cells (data not shown).
Relatively little is known about the mechanisms by which growth factors induce survival molecules, in contrast to the extensive information about inhibition of death-promoting pathways. Our present results identify p21 as a survival molecule for cultured muscle cells and place it downstream of Akt in an IGF-regulated survival pathway. Treatment of myoblasts with IGF-I or forced expression of an inducible Akt resulted in accumulation of p21 protein, and p21 could maintain muscle cell viability in the absence of growth factors. Inhibition of p21 production by an antisense cDNA prevented both Akt- and IGF-stimulated survival. Since the p21 antisense plasmid also reduced the viability of differentiating myoblasts expressing IGFs, the significance of our results appears not to be limited to muscle cells engineered to lack IGF-II. Further study will be required to determine if similar mechanisms operate in primary muscle cells or in vivo. Taken together, our observations also extend data of Fujio et al., who found that in growth-arrested myoblasts expression of both p21 and Akt increased with approximately similar kinetics (23), and confirm and extend other observations showing that forced expression of p21 could enhance muscle cell viability in culture (72).
The mechanisms by which IGF signaling through Akt induces p21 mRNA and protein expression are unknown. The myogenic transcription factor MyoD has been shown to stimulate p21 gene expression (27, 28). MyoD is induced in our cells but not by Akt (data not shown), thus indicating that MyoD does not contribute to this pathway of muscle cell survival. Recent reports have demonstrated that in some cell types Akt can activate the heterodimeric transcription factor NF-κB through steps involving phosphorylation and induction of the kinases that phosphorylate the NF-κB inhibitor IκB and target it for degradation (36, 40, 54, 61, 66). The net effect is translocation of NF-κB from the cytoplasm to the nucleus, where it can stimulate target gene transcription (36, 40, 61, 66). Although treatment with IGF-I or IGF-II has been shown to activate NF-κB family members p65 and p50 in primary cerebellar neurons (29) and to transiently induce NF-κB DNA binding activity in L6E9 muscle cells (34), we find no evidence for IGF-induced nuclear translocation of the p65 subunit of NF-κB in our cell model. Therefore, even though in some cell types activation of NF-κB can lead to stimulation of p21 expression (31), it is unlikely that this transcription factor plays a role in IGF-regulated myoblast survival.
Previous studies have established that mice with targeted disruptions of the genes encoding both p21 and the related cyclin-dependent kinase inhibitor p57 had defective muscle differentiation during development and exhibited increased muscle cell apoptosis (76). Marked muscle hypoplasia also has been observed during embryonic development in mice lacking the IGF-I receptor (50). It is not known whether diminished muscle mass in IGF-I receptor-deficient mice is a consequence of decreased differentiation or enhanced myocyte death or whether defective p21 expression is implicated. Similarly, the physiological relevance of IGF-mediated inhibition of cell death in skeletal muscle remains to be established. Such regulation could potentially provide a mechanism for modulating muscle mass during embryonic or adult life. In support of this hypothesis, it has been shown that mice overexpressing IGF-I in muscle do not exhibit the normal decline in muscle mass that occurs during aging (5). Selective stimulation of IGF action in muscle may provide a therapeutic means to diminish the rate of myofiber loss seen in other disorders.
ACKNOWLEDGMENTS
We thank Richard Roth, Stanford University, for iAkt and Bert Vogelstein, Johns Hopkins University School of Medicine, for the mouse p21 cDNA. We appreciate the technical assistance of Barb Rainish and Daniel Everding.
This study was supported by research grant 5RO1-DK42748 from the National Institutes of Health to P.R.
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Alessi D R, Downes C P. The role of PI 3-kinase in insulin action. Biochim Biophys Acta. 1998;1436:151–164. doi: 10.1016/s0005-2760(98)00133-7. [DOI] [PubMed] [Google Scholar]
- 3.Baker J, Liu J P, Robertson E J, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 4.Ball K L. p21: structure and functions associated with cyclin-CDK binding. Prog Cell Cycle Res. 1997;3:125–134. doi: 10.1007/978-1-4615-5371-7_10. [DOI] [PubMed] [Google Scholar]
- 5.Barton-Davis E R, Shoturma D I, Musaro A, Rosenthal N, Sweeney H L, Kurabayashi M, Dutta S, Kedes L. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baserga R, Resnicoff M, D'Ambrosio C, Valentinis B. The role of the IGF-I receptor in apoptosis. Vitam Horm. 1997;53:65–98. doi: 10.1016/s0083-6729(08)60704-9. [DOI] [PubMed] [Google Scholar]
- 7.Biggs W H, 3rd, Meisenhelder J, Hunter T, Cavenee W K, Arden K C. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 9.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 10.Coleman M E, DeMayo F, Yin K C, Lee H M, Geske R, Montgomery C, Schwartz R J. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- 11.Coolican S A, Samuel D S, Ewton D Z, McWade F J, Florini J R. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- 12.Datta S R, Brunet A, Greenberg M E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 13.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 14.Downward J. Role of phosphoinositide-3-OH kinase in Ras signaling. Adv Second Messenger Phosphoprotein Res. 1997;31:1–10. doi: 10.1016/s1040-7952(97)80004-3. [DOI] [PubMed] [Google Scholar]
- 15.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 16.El-Deiry W S. p21/p53, cellular growth control and genomic integrity. Curr Top Microbiol Immunol. 1998;227:121–137. doi: 10.1007/978-3-642-71941-7_6. [DOI] [PubMed] [Google Scholar]
- 17.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 18.Engert J C, Berglund E R, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol. 1996;135:431–440. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eves E M, Xiong W, Bellacosa A, Kennedy S G, Tsichlis P N, Rosner M R, Hay N. Akt, a target of phosphatidylinositol 3-kinase, inhibits apoptosis in a differentiating neuronal cell line. Mol Cell Biol. 1998;18:2143–2152. doi: 10.1128/mcb.18.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewton D A, Roof S L, Magri K A, McWade F J, Florini J R. IGF-II is more active than IGF-I in stimulating L6A1 myogenesis: greater mitogenic actions of IGF-I delay differentiation. J Cell Physiol. 1994;16:277–284. doi: 10.1002/jcp.1041610212. [DOI] [PubMed] [Google Scholar]
- 21.Florini J R, Magri K A, Ewton D Z, James P L, Grindstaff K, Rotwein P S. “Spontaneous” differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. J Biol Chem. 1991;266:15917–15923. [PubMed] [Google Scholar]
- 22.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 23.Fujio Y, Guo K, Mano T, Mitsuuchi Y, Testa J R, Walsh K. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol Cell Biol. 1999;19:5073–5082. doi: 10.1128/mcb.19.7.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funk J O, Galloway D A. Inhibiting CDK inhibitors: new lessons from DNA tumor viruses. Trends Biochem Sci. 1998;23:337–341. doi: 10.1016/s0968-0004(98)01242-0. [DOI] [PubMed] [Google Scholar]
- 25.Ghattas I R, Sanes J R, Majors J E. The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol. 1991;11:5848–5859. doi: 10.1128/mcb.11.12.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo K, Walsh K. Inhibition of myogenesis by multiple cyclin-Cdk complexes. J Biol Chem. 1997;272:791–797. doi: 10.1074/jbc.272.2.791. [DOI] [PubMed] [Google Scholar]
- 27.Guo K, Wang J, Andres V, Smith R C, Walsh K. MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol Cell Biol. 1995;15:3823–3829. doi: 10.1128/mcb.15.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 29.Heck S, Lezoualc'h F, Engert S, Behl C. Insulin-like growth factor-1-mediated neuroprotection against oxidative stress is associated with activation of nuclear factor kappaB. J Biol Chem. 1999;274:9828–9835. doi: 10.1074/jbc.274.14.9828. [DOI] [PubMed] [Google Scholar]
- 30.Jacks T, Weinberg R A. The expanding role of cell cycle regulators. Science. 1998;280:1035–1036. doi: 10.1126/science.280.5366.1035. [DOI] [PubMed] [Google Scholar]
- 31.Javelaud D, Wietzerbin J, Delattre O, Besancon F. Induction of p21Waf1/Cip1 by TNFalpha requires NF-kappaB activity and antagonizes apoptosis in Ewing tumor cells. Oncogene. 2000;19:61–68. doi: 10.1038/sj.onc.1203246. [DOI] [PubMed] [Google Scholar]
- 32.Jones J I, Clemmons D R. Insulin-like growth factors and their binding proteins: biological actions. Endocrinol Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 33.Kaliman P, Canicio J, Shepherd P R, Beeton C A, Testar X, Palacin M, Zorzano A. Insulin-like growth factors require phosphatidylinositol 3-kinase to signal myogenesis: dominant negative p85 expression blocks differentiation of L6E9 muscle cells. Mol Endocrinol. 1998;12:66–77. doi: 10.1210/mend.12.1.0047. [DOI] [PubMed] [Google Scholar]
- 34.Kaliman P, Canicio J, Testar X, Palacin M, Zorzano A. Insulin-like growth factor-II, phosphatidylinositol 3-kinase, nuclear factor-kappaB and inducible nitric-oxide synthase define a common myogenic signaling pathway. J Biol Chem. 1999;274:17437–17444. doi: 10.1074/jbc.274.25.17437. [DOI] [PubMed] [Google Scholar]
- 35.Kaliman P, Vinals F, Testar X, Palacin M, Zorzano A. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J Biol Chem. 1996;271:19146–19151. doi: 10.1074/jbc.271.32.19146. [DOI] [PubMed] [Google Scholar]
- 36.Kane L P, Shapiro V S, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 37.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy S G, Kandel E S, Cross T K, Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 40.Khwaja A. Akt is more than just a Bad kinase. Nature. 1999;401:33–34. doi: 10.1038/43354. [DOI] [PubMed] [Google Scholar]
- 41.Kohn A D, Barthel A, Kovacina K S, Boge A, Wallach B, Summers S A, Birnbaum M J, Scott P H, Lawrence Jr J C, Roth R A. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 42.Kops G J, de Ruiter N D, De Vries-Smits A M, Powell D R, Bos J L, Burgering B M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 43.Kulik G, Klippel A, Weber M J. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulik G, Weber M J. Akt-dependent and -independent survival signaling pathways utilized by insulin-like growth factor I. Mol Cell Biol. 1998;18:6711–6718. doi: 10.1128/mcb.18.11.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawlor M A, Feng X, Everding D R, Sieger K, Stewart C E H, Rotwein P. Dual control of muscle cell survival by distinct growth factor regulated signaling pathways. Mol Cell Biol. 2000;20:3256–3265. doi: 10.1128/mcb.20.9.3256-3265.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LeRoith D, Werner H, Beitner-Johnson D, Roberts C T Jr. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocrinol Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 47.Levinovitz A, Jennische E, Oldfors A, Edwall D, Norstedt G. Activation of insulin-like growth factor II expression during skeletal muscle regeneration in the rat: correlation with myotube formation. Mol Endocrinol. 1992;6:1227–1234. doi: 10.1210/mend.6.8.1406701. [DOI] [PubMed] [Google Scholar]
- 48.Levkau B, Koyama H, Raines E W, Clurman B E, Herren B, Orth K, Roberts J M, Ross R. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol Cell. 1998;1:553–563. doi: 10.1016/s1097-2765(00)80055-6. [DOI] [PubMed] [Google Scholar]
- 49.Lindquist J M, Rehnmark S. Ambient temperature regulation of apoptosis in brown adipose tissue. Erk1/2 promotes norepinephrine-dependent cell survival. J Biol Chem. 1998;273:30147–30156. doi: 10.1074/jbc.273.46.30147. [DOI] [PubMed] [Google Scholar]
- 50.Liu J P, Baker J, Perkins A S, Robertson E J, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igflr) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 51.Montarras D, Aurade F, Johnson T, Ilan J, Gros F, Pinset C. Autonomous differentiation in the mouse myogenic cell line, C2, involves a mutal positive control between insulin-like growth factor II and MyoD, operating as early as at the myoblast stage. J Cell Sci. 1996;109:551–560. doi: 10.1242/jcs.109.3.551. [DOI] [PubMed] [Google Scholar]
- 52.Musaro A, Rosenthal N. Maturation of the myogenic program is induced by postmitotic expression of insulin-like growth factor I. Mol Cell Biol. 1999;19:3115–3124. doi: 10.1128/mcb.19.4.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newton K, Strasser A. The Bcl-2 family and cell death regulation. Curr Opin Genet Dev. 1998;8:68–75. doi: 10.1016/s0959-437x(98)80064-6. [DOI] [PubMed] [Google Scholar]
- 54.Ozes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 55.Pap M, Cooper G M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 56.Pinset C, Garcia A, Rousse S, Dubois C, Montarras D. Wortmannin inhibits IGF-dependent differentiation in the mouse myogenic cell line C2. C R Acad Sci Ser III. 1997;320:367–374. doi: 10.1016/s0764-4469(97)85024-x. [DOI] [PubMed] [Google Scholar]
- 57.Pinset C, Montarras D, Chenevert J, Minty A, Barton P, Laurent C, Gros F. Control of myogenesis in the mouse myogenic C2 cell line by medium composition and by insulin: characterization of permissive and inducible C2 myoblasts. Differentiation. 1988;38:28–34. doi: 10.1111/j.1432-0436.1988.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 58.Poluha W, Poluha D K, Chang B, Crosbie N E, Schonhoff C M, Kilpatrick D L, Ross A H. The cyclin-dependent kinase inhibitor p21/WAF1 is required for survival of differentiating neuroblastoma cells. Mol Cell Biol. 1996;16:1335–1341. doi: 10.1128/mcb.16.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quinn L S, Haugk K L. Overexpression of the type-1 insulin-like growth factor receptor increases ligand-dependent proliferation and differentiation in bovine skeletal myogenic cultures. J Cell Physiol. 1996;168:34–41. doi: 10.1002/(SICI)1097-4652(199607)168:1<34::AID-JCP5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 60.Quinn L S, Steinmetz B, Maas A, Ong L, Kaleko M. Type-1 insulin-like growth factor receptor overexpression produced dual effects on myoblast proliferation and differentiation. J Cell Physiol. 1994;159:387–398. doi: 10.1002/jcp.1041590302. [DOI] [PubMed] [Google Scholar]
- 61.Romashkova J A, Makarov S S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 62.Rosen K M, Wentworth B M, Rosenthal N, Villa-Komaroff L. Specific, temporally regulated expression of the insulin-like growth factor II gene during muscle cell differentiation. Endocrinology. 1993;133:474–481. doi: 10.1210/endo.133.2.8393762. [DOI] [PubMed] [Google Scholar]
- 63.Rosenthal S M, Brunetti A, Brown E J, Mamula P W, Goldfine I D. Regulation of insulin-like growth factor (IGF) I receptor expression during muscle cell differentiation. Potential autocrine role of IGF-II. J Clin Investig. 1991;87:1212–1219. doi: 10.1172/JCI115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarbassov D D, Jones L G, Peterson C A. Extracellular signal-regulated kinase-1 and -2 respond differently to mitogenic and differentiative signaling pathways in myoblasts. Mol Endocrinol. 1997;11:2038–2047. doi: 10.1210/mend.11.13.0036. [DOI] [PubMed] [Google Scholar]
- 65.Sarbassov D D, Peterson C A. Insulin receptor substrate-1 and phosphatidylinositol 3-kinase regulate extracellular signal-regulated kinase-dependent and -independent signaling pathways during myogenic differentiation. Mol Endocrinol. 1998;12:1870–1878. doi: 10.1210/mend.12.12.0205. [DOI] [PubMed] [Google Scholar]
- 66.Sizemore N, Leung S, Stark G R. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stewart C E H, James P L, Fant M E, Rotwein P. Overexpression of insulin-like growth factor-II induces accelerated myoblast differentiation. J Cell Physiol. 1996;169:23–32. doi: 10.1002/(SICI)1097-4652(199610)169:1<23::AID-JCP3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 68.Stewart C E H, Rotwein P. Growth, differentiation and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 69.Stewart C E H, Rotwein P. Insulin-like growth factor-II is an autocrine survival factor for differentiating myoblasts. J Biol Chem. 1996;271:11330–11338. doi: 10.1074/jbc.271.19.11330. [DOI] [PubMed] [Google Scholar]
- 70.Tang E D, Nunez G, Barr F G, Guan K L. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 71.Tollefsen S E, Sadow J L, Rotwein P. Coordinate expression of insulin-like growth factor II and its receptor during muscle differentiation. Proc Natl Acad Sci USA. 1989;86:1543–1547. doi: 10.1073/pnas.86.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Walsh K. Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science. 1996;273:359–361. doi: 10.1126/science.273.5273.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weiss A, Schlessinger J. Switching signals on or off by receptor dimerization. Cell. 1998;94:277–280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- 74.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 75.Yao R, Cooper G M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 76.Zhang P, Wong C, Liu D, Finegold M, Harper J W, Elledge S J. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 1999;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]