Abstract
Impaired consciousness during seizures severely affects quality of life for people with epilepsy but the mechanisms are just beginning to be understood. Consciousness is thought to involve large-scale brain networks, so it is puzzling that focal seizures often impair consciousness. Recent work investigating focal temporal lobe or limbic seizures in human patients and experimental animal models suggests that impaired consciousness is caused by active inhibition of subcortical arousal mechanisms. Focal limbic seizures exhibit decreased neuronal firing in brainstem, basal forebrain, and thalamic arousal networks, and cortical arousal can be restored when subcortical arousal circuits are stimulated during seizures. These findings open the possibility of restoring arousal and consciousness therapeutically during and following seizures by thalamic neurostimulation. When seizures cannot be stopped by existing treatments, targeted subcortical stimulation may improve arousal and consciousness, leading to improved safety and better psychosocial function for people with epilepsy.
Keywords: Epilepsy, consciousness, awareness, thalamus, deep brain stimulation, temporal lobe epilepsy
A Puzzle: Why Do Focal Seizures Cause Impaired Consciousness?
Conscious awareness depends on large-scale networks in the brain. Despite philosophical challenges in defining consciousness and awareness, normal conscious awareness clearly involves activity in widespread cortical and subcortical networks, and neurological disorders that disrupt these broad networks lead to impaired consciousness.1-3 It is therefore puzzling that focal seizures—which affect localized brain regions—often impair consciousness. It is not surprising that focal seizures may cause localized deficits. For example, it is understandable when temporal lobe limbic seizures alter emotions, olfaction, or memory. 4 But why do focal temporal lobe seizures so often cause people with epilepsy to lose their ability to meaningfully respond to the environment or to form experiences? Static lesions of the medial temporal lobes (trauma, infection/inflammation, degeneration, and so on) can cause amnesia and other cognitive or emotional changes without impaired consciousness. 5 Even with extensive bilateral medial temporal damage, people usually remain alert, interactive, and consciously aware of themselves and their surroundings. Focal temporal lobe or limbic seizures should similarly leave consciousness intact—unless additional mechanisms are involved beyond the medial temporal lobes. The same puzzle applies to other forms of focal epilepsy as well. Localized seizure activity in a frontal, parietal, or occipital lobe should cause selective deficits related to local circuit dysfunction. Impaired consciousness does not make logical sense unless more widespread network changes occur. What are the mechanisms of widespread network changes in focal seizures?
Network Inhibition Hypothesis and Other Mechanisms
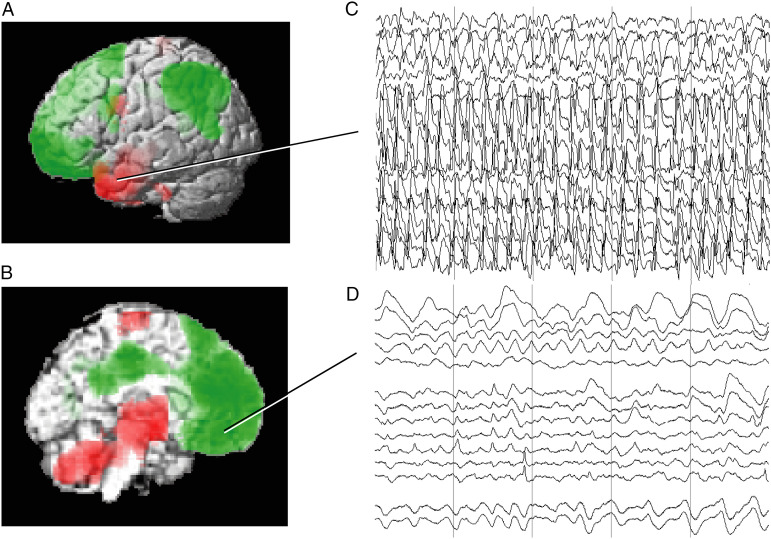
Temporal lobe seizures are the most common seizure type and have been most thoroughly investigated, although other focal seizures can also lead to impaired consciousness. In temporal lobe seizures, impaired consciousness is associated with abnormal activity in subcortical arousal systems and depressed cortical function resembling slow-wave sleep (Figure 1). Intracranial EEG during temporal lobe seizures with impaired consciousness shows polyspike discharges in one or both temporal lobes, but slow waves in the frontoparietal association cortex6-8 (Figures 1C and D). The frontoparietal slow waves are significantly reduced in temporal lobe seizures without impaired consciousness, which also on average show less bilateral medial temporal polyspike activity. 6 Ictal single-photon computed tomography (SPECT) in temporal lobe seizures with impaired consciousness shows increased cerebral blood flow (CBF) in the temporal lobe, but decreased CBF in the frontoparietal cortex; meanwhile, the upper brainstem and medial thalamus shows abnormal CBF increases9,10 (Figures 1A and B). The abnormal CBF increases in these subcortical structures may represent both abnormal increases and decreases in neuronal activity as described further below.
Figure 1.
Local and long-range network effects in temporal lobe seizures consistent with decreased cortical physiological arousal. (A and B) Group analysis of SPECT ictal–interictal difference imaging during temporal lobe seizures. CBF increases (red) are present in the temporal lobe (A) and in the medial thalamus and upper brainstem (B). Decreases (green) are seen in the lateral frontoparietal association cortex (A) and in the interhemispheric frontoparietal regions (B). (C, D) Intracranial EEG recordings from a patient during a temporal lobe seizure. High-frequency polyspike-and-wave seizure activity is seen in the temporal lobe (C). The orbital and medial frontal cortex (and other regions, EEG not shown) do not show polyspike activity, but instead large-amplitude, irregular slow rhythms resembling coma or sleep (D). Vertical lines in (C) and (D) denote 1-s intervals. Note that the EEG and SPECT data were from similar patients, but were not simultaneous, and are shown together here for illustrative purposes only ((A, B) Modified from Blumenfeld et al. 9 with permission. (C, D) Modified from Englot et al. 6 with permission).
These initial findings in human patients led to the network inhibition hypothesis for impaired consciousness in focal temporal lobe seizures.11,12 This hypothesis proposes that seizure activity in limbic circuits spreads to subcortical inhibitory areas, causing decreased subcortical arousal. This leads to decreased arousal in the cortex and impaired consciousness. Further support for the network inhibition hypothesis has come from experimental animal models, which also provide mechanistic insights. Focal hippocampal seizures in rats in a post-pilocarpine spontaneous seizure model produced behavioral arrest and cortical slow waves resembling those seen in human patients with temporal lobe epilepsy. 13 Focal hippocampal seizures in rats and mice induced by brief 2s, 60 Hz hippocampal stimulation also exhibit behavioral arrest and cortical slow waves resembling human temporal lobe seizures.13-18 These findings established the validity of the rodent models for investigating impaired behavior and physiology in focal limbic seizures.
Human CBF and EEG data (Figure 1) suggested that the state of the cortex is depressed rather than activated during focal limbic seizures. Rodent model studies have verified that cortical physiology during focal limbic seizures closely resembles states of decreased arousal such as deep anesthesia or sleep. Like in human limbic seizures, the cortex in rodent models shows slow waves, decreased CBF, and hypometabolism13-19 (Figure 2). Cortical neuronal recordings showed Up and Down states of action potential firing and membrane potential oscillations mimicking the physiology of deep sleep and anesthesia.13-16,19,20
Figure 2.
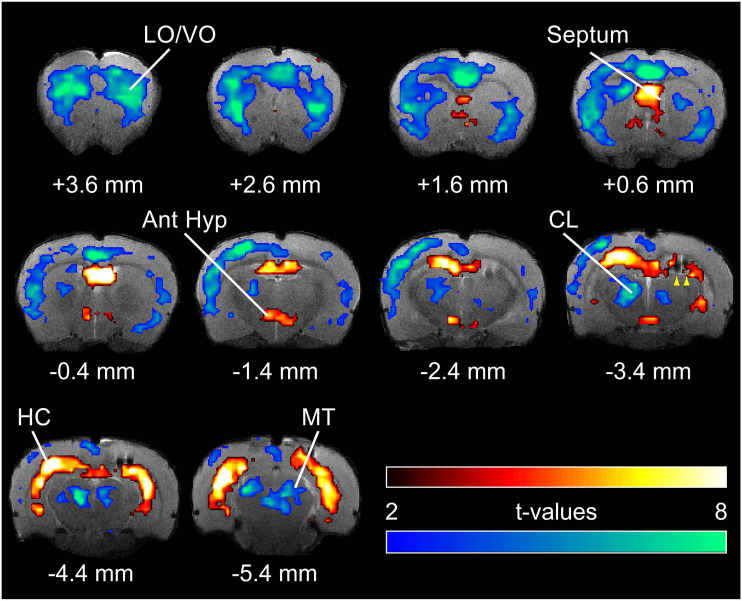
Hippocampal, cortical, and subcortical blood oxygen-level–dependent (BOLD) fMRI changes during focal limbic seizures in a rat model. T-map of ictal changes during focal seizures (vs 30 s pre-seizure baseline) reveals a complicated network of changes. Widespread cortical decreases are accompanied by mixed subcortical increases and decreases. Increases are seen in known areas of seizure propagation such as the hippocampus (HC) and lateral septum as well as in sleep-promoting regions such as the anterior hypothalamus (Ant Hyp). Decreases are seen in the cortex, most prominently in lateral and ventral orbital frontal cortex (LO/VO) and in medial regions including cingulate and retrosplenial cortex. Decreases are also seen in arousal-promoting regions such as the thalamic intralaminar nuclei including centrolateral nucleus (CL), as well as in the midbrain tegmentum (MT). The arrowheads at AP −3.4 mm signify the hippocampal electrode artifact. Warm colors represent fMRI increases, and cool colors, decreases, superimposed on coronal anatomical images from the template animal. AP coordinates in millimeters are relative to bregma. 10 animals, with FDR corrected threshold P < .05. Reproduced with permission from Motelow et al. 19
Why is cortical arousal depressed during focal limbic seizures? Human data showed altered activity in subcortical arousal areas such as the upper brainstem and thalamus (Figure 1B), leading to the network inhibition hypothesis. Abnormal CBF in subcortical structures during seizures can be associated with either abnormal increases or decreases in neuronal activity, which should therefore be measured directly. 21 The mechanisms have been elaborated further through a series of rodent model studies (Figure 3). Depressed cortical arousal is linked to decreased subcortical arousal in cholinergic and other neurotransmitter systems. Cortical and thalamic cholinergic transmission is reduced during focal limbic seizures, based on measurements with electrochemical biosensors and genetically encoded fluorescent neurotransmitter sensors.19,22 Functional magnetic resonance imaging (fMRI) showed decreased activity in the intralaminar thalamus and upper brainstem tegmentum during focal limbic seizures 19 (Figure 2). Direct neuronal recordings showed reduced firing of cholinergic neurons in the nucleus basalis, cholinergic neurons in the pedunculopontine tegmental nucleus, putative glutamatergic neurons in the intralaminar thalamic central lateral nucleus, and serotonergic neurons in the brainstem raphe nuclei.19,23-25 Interestingly, neurons in different parts of the thalamus show different activity patterns during focal limbic seizures, depending on their well-known cortical network connections. For example, the limbic anterior nucleus shows high-frequency seizure discharges, whereas at the same time, the ventral posterior medial relay nucleus shows sleep spindle-like activity, and the arousal-related paratenial and intralaminar central lateral nuclei show intermittent burst firing with an overall decrease in mean neuronal firing rate. 23
Figure 3.
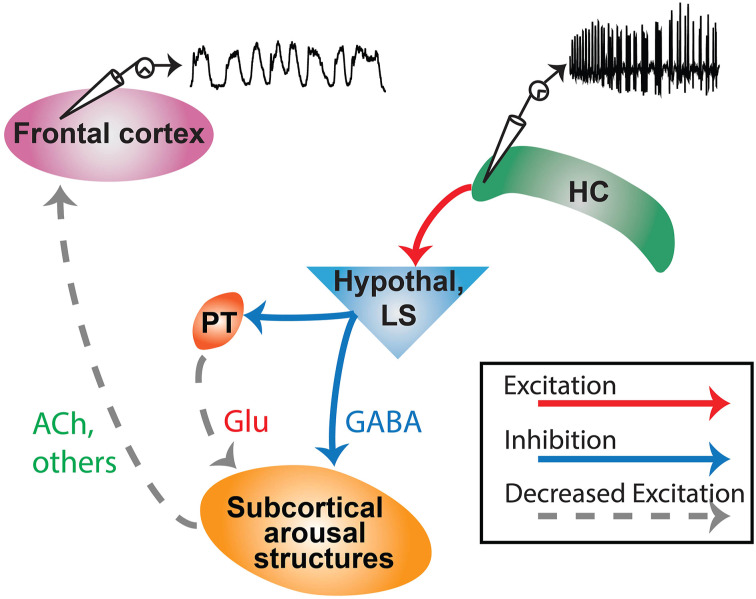
Network inhibition hypothesis. A simplified model demonstrating propagation of excitatory seizure activity from hippocampus (HC) to subcortical inhibitory areas such as anterior hypothalamus (Hypothal) or lateral septum (LS). From there, parallel pathways of direct inhibition (GABA) and indirect de-excitation (e.g., reduced glutamate, Glu from paratenial nucleus, PT) cause subcortical arousal structures to have reduced activity. This leads to reduced subcortical arousal projections to the cortex, including reduced acetylcholine (Ach) and reduction of other neurotransmitter systems, leading to cortical slow-wave activity and impaired consciousness. Modified with permission from Motelow et al. 19
The next important question is why subcortical arousal systems are depressed during focal limbic seizures. fMRI revealed that subcortical nuclei containing GABAergic neurons, such as the lateral septal nuclei and anterior hypothalamus, show increased activity during seizures13,14,19 (Figure 2). Increased neuronal firing in these regions was also confirmed by direct neuronal recordings.13,19 The network inhibition hypothesis posits that these subcortical GABAergic systems are activated by limbic seizures and in turn inhibit subcortical arousal (Figure 3). Support for this mechanism comes from experiments showing that (1) cutting the fornix prevents seizure activity from spreading to the lateral septum, and also prevents cortical slow wave activity and behavioral arrest 14 ; and (2) direct stimulation of the lateral septum without seizure activity is capable of producing cortical slow-wave activity, decreased cortical cholinergic neurotransmission, and behavioral arrest.14,26
Recent work suggests that there may be both direct and indirect pathways leading to suppressed subcortical arousal. For example, anatomical tracer studies showed direct projections of lateral septal neurons to the nucleus basalis (direct inhibition), as well as indirect projections of lateral septal neurons to nucleus basalis via the thalamic paratenial nucleus (indirect de-excitation) 27 (Figure 3). Additional evidence for decreased excitatory input as one mechanism of reduced subcortical arousal comes from patch-clamp recordings showing increased input impedance, fewer excitatory post synaptic potential-like events, and reduced membrane potential fluctuations (synaptic noise) in pedunculopontine tegmental nucleus neurons during seizures. 25 In summary, inhibitory activity (e.g., in the lateral septum) during limbic seizures may suppress subcortical arousal systems through direct inhibition as well through indirect de-excitation (Figure 3). Either way, subcortical arousal systems are suppressed, raising the question of whether therapeutic interventions might improve arousal by stimulating subcortical arousal systems during seizures (see next section).
Aside from decreased arousal as proposed by the network inhibition hypothesis, other mechanisms may contribute to impaired consciousness in focal seizures. In addition to cortical slow-wave activity, other abnormal cortical rhythms could also disrupt function. For example, focal temporal lobe seizures sometimes exhibit some propagation of ictal fast rhythms to the neocortex even without evolution to full bilateral tonic–clonic seizure activity. 8 In addition, it has been demonstrated that increased cortical–cortical and cortical–thalamic synchrony during focal seizures can impair the global neuronal workspace necessary for normal consciousness. 28 Increased synchrony is associated with loss of consciousness during focal temporal lobe, parietal lobe, as well as frontal lobe seizures.29-32 Recent work has also shown that focal frontal lobe seizures with impaired consciousness have an overall increase in EEG power across a broad range of frequencies, not just slow waves. 33 These findings suggest other mechanisms aside from depressed arousal may participate in loss of consciousness during focal seizures, especially for seizures arising outside the medial temporal lobe.
Impaired consciousness during the postictal period may have consequences for patients that are as severe as ictal impairment. 34 In addition to behavioral unresponsiveness, postictal impaired consciousness may increase risk of sudden unexpected death in epilepsy (SUDEP) due to lack of airway protection.24,35,36 The mechanisms of impairment in the postictal period are still under investigation, but both human and animal model studies of focal limbic seizures show persistent cortical slow-wave activity and decreased cortical CBF and metabolism in the postictal period.6,7,9,13,19,37 In addition, during the postictal period, cholinergic neurotransmission remains depressed, fMRI signals in the central thalamus and upper brainstem tegmentum are reduced, firing of thalamic intralaminar neurons and of subcortical cholinergic and serotonergic neurons is reduced, and behavioral responsiveness is impaired in association with cortical slow-wave activity.19,22-24,37 These findings suggest that persistently depressed arousal in subcortical and cortical networks may play an important role in postictal impaired consciousness following focal limbic seizures.
Restoring Conscious Arousal by Neurostimulation
Understanding the network mechanisms for impaired arousal and consciousness during and following focal limbic seizures suggests new potential avenues for therapeutic intervention. Neurostimulation targeted at subcortical arousal structures could be used to restore arousal and consciousness in the ictal and postictal periods.38,39 Although the primary goal of epilepsy treatment is to stop seizures, current treatments do not work in all patients. Medications, surgical resection, or neurostimulation are often effective; however, given the large number of people living with epilepsy, this leaves a substantial population who have continued focal seizures with impaired consciousness. For example, people with medically refractory temporal lobe epilepsy who cannot have surgical resection are currently offered deep brain stimulation of the anterior thalamic nuclei or responsive medial temporal lobe neurostimulation.40,41 However, these treatments only reduce seizures by about 70%, which leaves a substantial number of patients with seizures causing impaired consciousness; these patients currently have no further treatment options.
Studies in the rodent model have shown that optogenetic stimulation of upper brainstem cholinergic pedunculopontine tegmental nucleus neurons converts cortical slow-wave activity to an awake physiological rhythm during focal limbic seizures. 42 Although optogenetic stimulation is not available for human use, electrical stimulation is available with existing neurostimulation devices. Stimulation of the thalamic intralaminar central lateral nucleus improves arousal in rodent and non-human primates, as well as in patients with chronically impaired consciousness.43-47 During focal limbic seizures in rats, stimulation of the intralaminar thalamic central lateral nucleus and of the pontine nucleus oralis converts cortical slow waves into an awake rhythm and converts behavioral arrest into normal exploratory behavior. 15 In addition, stimulation of the thalamic intralaminar central lateral nucleus restores normal exploratory behavior, sucrose reward-seeking behavior, and conditioned shock avoidance in the postictal period following focal limbic seizures with secondary generalization. 48
These encouraging findings have led to two human clinical trials to stimulate the thalamus in an effort to restore consciousness during focal temporal lobe seizures which cannot be stopped by other treatments. Both begin with an effort to stop seizures by traditional means of neurostimulation and then augment this with a palliative effort to reduce the behavioral severity of seizures that escape traditional treatment. In one approach, stimulation of the thalamic pulvinar has been shown to reduce impaired consciousness and speed behavioral recovery in temporal lobe seizures. 49 In another trial beginning this year, stimulation of the thalamic intralaminar central lateral nucleus will be provided for temporal lobe seizures that are not interrupted by responsive medial temporal stimulation, and behavior will be assessed by automatic behavioral testing technology (see https://braininitiative.nih.gov/funded-awards/thalamic-stimulation-prevent-impaired-consciousness-epilepsy).
Conclusions and Future Directions
Converging evidence from human intracranial EEG and SPECT studies along with experimental animal models supports an important role for depressed subcortical arousal in producing impaired consciousness during and following focal seizures arising from the medial temporal lobe. Initial promising results demonstrate potential therapeutic benefit of thalamic stimulation to restore consciousness in otherwise refractory temporal lobe seizures, and further studies are under way. Additional work is needed to better understand mechanisms of impaired consciousness in the postictal period, which may differ from ictal impairments. In addition, the relative contributions of different mechanisms including impaired arousal, abnormally enhanced long-range synchrony, and possibly other mechanisms in producing impaired consciousness require further investigation, especially for seizures arising outside the temporal lobe. Mechanisms of impaired consciousness in focal frontal, parietal, and occipital lobe seizures have been relatively less studied than in temporal lobe seizures and should be investigated further. In addition, although some work has been done to understand impaired consciousness in generalized seizures such as absence and tonic–clonic seizures,50,51 further investigation is needed for these seizure types as well. Hopefully, with ongoing mechanistic studies in human patients and experimental animal models, novel treatment approaches will continue to emerge to improve consciousness and to better quality of life for people with epilepsy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH/NINDS R01 NS066974, R01 NS096088, R01 NS055829, R37 NS10090, UG3/UH3 NS112826, the Mark Loughridge and Michele Williams Foundation, and the Betsy and Jonathan Blattmachr family.
ORCID iD
Hal Blumenfeld https://orcid.org/0000-0003-0812-8132
References
- 1.Koch C, Massimini M, Boly M, Tononi G. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci. 2016;17(5):307-321. [DOI] [PubMed] [Google Scholar]
- 2.Dehaene S, Lau H, Kouider S. What is consciousness, and could machines have it?. Science. 2017;358(6362):486-492. [DOI] [PubMed] [Google Scholar]
- 3.Laureys S, Gosseries O, Tononi G. The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology. 2nd Edition. Cambridge, MA: Academic Press; 2015. [Google Scholar]
- 4.Catani M, Dell’Acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37(8):1724-1737. [DOI] [PubMed] [Google Scholar]
- 5.Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Englot DJ, Yang L, Hamid H, et al. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain: J Neurol. 2010;133(Pt 12):3764-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenfeld H, Rivera M, McNally KA, Davis K, Spencer DD, Spencer SS. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004;63:1015-1021. [DOI] [PubMed] [Google Scholar]
- 8.Lieb JP, Dasheiff RM, Engel J, Jr, Genton P, Genton P. Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia. 1991;32(6):822-837. [DOI] [PubMed] [Google Scholar]
- 9.Blumenfeld H, McNally KA, Vanderhill SD, et al. Positive and negative network correlations in temporal lobe epilepsy. Cerebr Cortex. 2004;14(8):892-902. [DOI] [PubMed] [Google Scholar]
- 10.Lee KH, Meador KJ, Park YD, et al. Pathophysiology of altered consciousness during seizures: Subtraction SPECT study. Neurology. 2002;59(6):841-846. [DOI] [PubMed] [Google Scholar]
- 11.Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3(3):219-231. [DOI] [PubMed] [Google Scholar]
- 12.Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurol. 2012;11(814–8269):814-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28(36):9066-9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;29(41):13006-13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kundishora AJ, Gummadavelli A, Ma C, et al. Restoring conscious arousal during focal limbic seizures with deep brain stimulation. Cerebr Cortex. 2017;27(3):1964-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieu L-A, Singla S, Sharafeldin A, et al. A novel mouse model of focal limbic seizures that reproduces behavioral impairment associated with cortical slow wave activity. BioRxiv 2021. doi: 10.1101/2021.05.05.442811. [DOI] [Google Scholar]
- 17.Sieu L-A, Singla S, McCafferty C, et al. Mouse model of electrically inducible focal seizures with impaired consciousness. 2018;Soc Neurosci Abstr. Abstract No 56102 Available at: http://wwwsfnorg/Meetings/Past-and-Future-Annual-Meetings 2018 [Google Scholar]
- 18.Gummadavelli A, Martin R, Goshay D, et al. Cortical low-frequency power correlates with behavioral impairment in animal model of focal limbic seizures. In review; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motelow JE, Li W, Zhan Q, et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 2015;85(3):561-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue Z, Freedman IG, Vincent P, et al. Up and down states of cortical neurons in focal limbic seizures. Cerebr Cortex. 2020;30(5):3074-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra AM, Ellens DJ, Schridde U, et al. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. J Neurosci. 2011;31(42):15053-15064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sieu L-A, Singla S, Sharafeldin A, et al. Mouse model of focal limbic seizures with impaired behavior associated with cortical slow waves and reduced cholinergic arousal. AES Abstracts. 2020. Available at: http://wwwaesnetorg/2021. [Google Scholar]
- 23.Feng L, Motelow JE, Ma C, et al. Seizures and sleep in the thalamus: focal limbic seizures show divergent activity patterns in different thalamic nuclei. J Neurosci. 2017;37(47):11441-11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan Q, Buchanan GF, Motelow JE, et al. Impaired serotonergic brainstem function during and after seizures. J Neurosci. 2016;36(9):2711-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews JP, Yue Z, Ryu JH, Neske G, McCormick DA, Blumenfeld H. Mechanisms of decreased cholinergic arousal in focal seizures: in vivo whole-cell recordings from the pedunculopontine tegmental nucleus. Exp Neurol. 2019;314:74-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Motelow JE, Zhan Q, et al. Cortical network switching: possible role of the lateral septum and cholinergic arousal. Brain Stimulation. 2015;8(1):36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao CW, Feng L, Sieu LA, Pok B, Gummadavelli A, Blumenfeld H. Parallel pathways to decreased subcortical arousal in focal limbic seizures. Epilepsia. 2020;61(12):e186-e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartolomei F. Coherent neural activity and brain synchronization during seizure-induced loss of consciousness. Arch Ital Biol. 2012;150(2-3):164-171. [DOI] [PubMed] [Google Scholar]
- 29.Bonini F, Lambert I, Wendling F, McGonigal A, Bartolomei F. Altered synchrony and loss of consciousness during frontal lobe seizures. Clin Neurophysiol. 2016;127(2):1170-1175. [DOI] [PubMed] [Google Scholar]
- 30.Lambert I, Arthuis M, McGonigal A, Wendling F, Bartolomei F. Alteration of global workspace during loss of consciousness: A study of parietal seizures. Epilepsia. 2012;53(12):2104-2110. [DOI] [PubMed] [Google Scholar]
- 31.Arthuis M, Valton L, Régis J, et al. Impaired consciousness during temporal lobe seizures is related to increased long-distance cortical-subcortical synchronization. Brain : J Neurol. 2009;132(Pt 8):2091-2101. [DOI] [PubMed] [Google Scholar]
- 32.Guye M, Regis J, Tamura M, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129(7):1917-1928. [DOI] [PubMed] [Google Scholar]
- 33.Salardini E, Vaddiparti A, Kumar A, et al. Mechanism of impaired consciousness in frontal lobe seizures investigated with intracranial EEG. AES Abstracts 2020 Abstract No 42 Available at: http://wwwaesnetorg/2020.
- 34.Schmidt D, Noachtar S. Outlook: the postictal state-future directions for research. Epilepsy Behav. 2010;19(2):191-192. [DOI] [PubMed] [Google Scholar]
- 35.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966-977. [DOI] [PubMed] [Google Scholar]
- 36.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci Unit States Am. 2010;107(37):16354-16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gummadavelli A, Martin R, Goshay D, et al. Cortical low-frequency power correlates with behavioral impairment in animal model of focal limbic seizures. Epilepsia. 2021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gummadavelli A, Kundishora AJ, Willie JT, et al. Neurostimulation to improve level of consciousness in patients with epilepsy. Neurosurg Focus. 2015;38(6):E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambert I, Bartolomei F. Why do seizures impair consciousness and how can we reverse this?. Curr Opin Neurol. 2020;33(2):173-178. [DOI] [PubMed] [Google Scholar]
- 40.Geller EB, Skarpaas TL, Gross RE, et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58(6):994-1004. [DOI] [PubMed] [Google Scholar]
- 41.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908. [DOI] [PubMed] [Google Scholar]
- 42.Furman M, Zhan Q, McCafferty C, et al. Optogenetic stimulation of cholinergic brainstem neurons during focal limbic seizures: effects on cortical physiology. Epilepsia. 2015;56(12):e198-e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiff ND. Moving toward a generalizable application of central thalamic deep brain stimulation for support of forebrain arousal regulation in the severely injured brain. Ann N Y Acad Sci. 2012;1265:56-68. [DOI] [PubMed] [Google Scholar]
- 44.Schiff ND, Giacino JT, Kalmar K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448(7153):600-603. [DOI] [PubMed] [Google Scholar]
- 45.Redinbaugh MJ, Phillips JM, Kambi NA, et al. Thalamus modulates consciousness via layer-specific control of cortex. Neuron. 2020;106(1):66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker JL, Ryou J-W, Wei XF, Butson CR, Schiff ND, Purpura KP. Robust modulation of arousal regulation, performance, and frontostriatal activity through central thalamic deep brain stimulation in healthy nonhuman primates. J Neurophysiol. 2016;116(5):2383-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Lee HJ, Weitz AJ, et al. Frequency-selective control of cortical and subcortical networks by central thalamus. Elife. 2015;4:e09215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, Galardi MM, Pok B, et al. Thalamic stimulation improves postictal cortical arousal and behavior. J Neurosci. 2020;40(38):7343-7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Filipescu C, Lagarde S, Lambert I, et al. The effect of medial pulvinar stimulation on temporal lobe seizures. Epilepsia. 2019;60(4):e25-e30. [DOI] [PubMed] [Google Scholar]
- 50.Guo JN, Kim R, Chen Y, et al. Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurol. 2016;15(13):1336-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blumenfeld H, Varghese GI, Purcaro MJ, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain: J Neurol. 2009;132(Pt 4):999-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]