Abstract
Aim:
Sensor-augmented pumps with predictive low glucose suspend function (PLGS-SAP) help patients avoid hypoglycemia and improve quality of life: in this retrospective study, we investigated long-term effects of PLGS-SAP on metabolic outcomes, acute and chronic diabetic complications, in particular cardiovascular events.
Materials and Methods:
One hundred thirty-nine adults with type 1 diabetes (T1D) treated for more than 10 years with continuous subcutaneous insulin infusion (CSII) were followed for 5 years; 71 (Group 1) started to use PLGS-SAP, and 68 (Group 2) maintained on their non-PLGM insulin pump. Glucose control measures (hemoglobin A1c [HbA1c], acute diabetic complications), clinical outcomes (body mass index [BMI], arterial hypertension, dyslipidemia), chronic diabetes-related complications, and device utilization (continuous glucose monitoring utilization, use of temporary basal rates or special boluses, carbohydrate counting usage) were assessed.
Results:
The reduction of HbA1c was significant in Group 1 (from 7.5% ± 1.1% to 7.0% ± 1.0%, P = .02), while in Group 2 it did not reach statistical significance (from 7.5% ± 1.1% to 7.4% ± 0.9%, P = .853). BMI increased significantly in Group 2 (from 25.3 ± 2.8 to 25.7 ± 3.4, P < .001), but not in Group 1 (from 25.2 ± 3.5 to 25.2 ± 2.8, P = .887). There were no statistically significant differences in occurrence of acute diabetes complications, other clinical outcomes, prevalence of diabetes-related complications, or device utilization between the groups.
Conclusions:
In our five-year follow-up experience with T1D CSII users, PLGS-SAP has resulted efficient in improving metabolic control and maintaining the body weight.
Keywords: sensor-augmented pump, predictive low glucose suspend, continuous subcutaneous insulin infusion, type 1 diabetes mellitus, body mass index
Introduction
Continuous subcutaneous insulin infusion (CSII) has been demonstrated, in both type 1 and type 2 diabetes, of all age groups to provide patients improved glycemic control without increasing risk of severe hypoglycemic episodes.1-3 The Diabetes Control and Complications Trial study demonstrated that many long-term complications such as cardiovascular disease, 4 nephropathy, neuropathy, and retinopathy 5 are reduced by an intensive therapeutic approach. Forty percent of the intensive therapy group were treated by CSII. Our experience with long-term CSII users has provided evidence that the fractional use (percentage of diabetes duration treated with CSII) is correlated with the reduced incidence of microvascular and macrovascular diabetes-related complications. 6
CSII therapy has undergone several innovative steps, with integration of continuous glucose monitoring (CGM) and increasing automation in the insulin delivery. Generally these innovations were associated with improved diabetes management. 7 The integrated systems of CSII with CGM, sensor-augmented pump (SAP), allow to suspend automatically insulin infusion in case of low sensor glucose values (low glucose suspend [LGS]) or in case low sensor glucose values are predicted 30 minutes in advance (predictive low glucose suspend [PLGS]) and automatically resumes basal insulin delivery as hypoglycemia is averted.8,9 This system helps patients avoid hypoglycemia10,11 while maintaining overall glycemic control and improve the quality of life (QoL). 12 In Italy, the algorithm SmartGuard was introduced in 2015 within the Medtronic MiniMed 640G insulin pump. Currently, more PLGS algorithms using various sensor and pump models that communicate with each other are entering clinical practice.
Real-life studies have confirmed the safety of this system. 13 Short-term studies provided efficacy data, but no long-term efficacy studies are available yet.
The aim of this retrospective study was to investigate the long-term effect of SAP with PLGS in terms of acute complications, metabolic outcomes, and chronic macrovascular and microvascular complications, in particular cardiovascular events in relationship to the use of PLGS in real life.
Methods
Study Design and Population
This is a single-center retrospective analysis that includes 139 adults with type 1 diabetes (T1D) treated for more than 10 years with CSII and followed for an average of 5 years until 31 December 2019.
The patients started CSII between 1997 and 2015 and were followed for a median (interquartile range [IQR]) of 17 (15-22) years (mean 18 ± 4 years).
The most common clinical indications for CSII were poor metabolic control with high HbA1c levels despite MDI intensive therapy, recurrent hypoglycemia, and excessive glucose variability. A secondary indication was a need for more flexible therapy, which improves QoL.
We compared the outcomes of two groups. Group 1 were 71 of these patients who started to use Medtronic 640G between 2015 and 2018. Group 2 were 68 patients maintained on their non-SAP PLGM insulin pump (MiniMed Paradigm Veo 554/754, My Life Omnipod, Roche Accu-Check Spirit Combo, Animas Vibe, Dana Diabecare in association with Enlite, Dexcom G4/G5 sensors). The only indication for starting Medtronic 640G was previous device warranty expiration. When patients switched to Medtronic 640G they had a specific training on the use of the PLGS function and the device management while the follow-up visits were similar in both groups.
Descriptive Outcome Measures
Glucose control measures: HbA1c values were calculated as a mean value of at least three measurements per year of follow-up. Severe hypoglycemic events were defined as having low blood glucose levels that required external assistance with subsequent hospitalization; severe hyperglycemia was considered when metabolic decompensation called for medical intervention and hospitalization was required. The occurrence of both acute complications was averaged per year of follow-up.
Clinical outcomes: Body mass index (BMI) (the mean value was obtained from at least three measurements/year), incidence of arterial hypertension (defined as systolic blood pressure equal to or above 140 mm Hg, or diastolic blood pressure equal to or above 90 mm Hg, or both) requiring antihypertensive agents, and incidence of dyslipidemia (defined as total cholesterol level equal to or above 220 mg/dL, or low-density lipoprotein levels equal to or above 100 mg/dL, or triglyceride levels equal to or above 150 mg/dL, or their combination) requiring lipid-lowering drug therapy.
Diabetes-related complications: Presence of peripheral artery disease requiring aspirin or clopidogrel therapy, incidence of any cardiovascular event (acute myocardial infarction, ischemic cardiac event following percutaneous transluminal coronary angioplasty [PTCA] with stent implantation, any cardiac event requiring pacemaker implantation, stroke, transient ischemic attack, foot ulcer, lower-extremity endovascular or surgical revascularization, major or minor lower limb amputation), laser treatment of retinopathy, vitrectomy, haemodialysis start, and renal transplantation.
Device utilization: Frequency of CGM utilization, use of temporary basal rates or special boluses (dual or square wave), and carbohydrate counting usage.
The study was approved by the local Institutional Review Board, and written informed consent was obtained from each participant.
Statistical Methods
Descriptive statistics were used to summarize patient characteristics. This includes mean and standard deviation, minimum, maximum and median with the IQR for continuous variables, and counts and percentages for categorical variables. For the latter, denominators only include non-missing values (ie, the difference between total number of patients and denominators represent the number of missing values). Continuous variables were compared using the Wilcoxon test and comparisons of categorical variables have been performed by means of the chi-square test or Fisher’s exact test for extreme proportions, as appropriate. For the pre–post comparisons, the generalized estimating equation (GEE) model was used, to adjust for multiple measures per patient. Statistical tests are based on a two-sided significance level of .05. The SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) was used to perform statistical analyses. In order to minimize missing data in the endpoint evaluation, the following parameters were imputed during follow-up, considering blank as “absence of condition” (best case): LGS, severe hypoglycemia, and hyperglycemia. Other variables with missing data were not imputed. Adjustments for multiple comparisons (for pre–post comparisons) were performed by the GEE model.
Results
Baseline Characteristics
Baseline characteristics of the study population are shown in Table 1. No significant baseline demographic or clinical differences were observed between the study groups.
Table 1.
Baseline Characteristics.
| Characteristics | Total (n = 139) |
Group 1 (n = 71) |
Group 2 (n = 68) |
P value |
|---|---|---|---|---|
| Age (years) | 50 ± 15 | 50 ± 14 | 50 ± 17 | .993 |
| Age at diabetes onset (years) | 23 ± 13 | 23 ± 13 | 24 ± 14 | .905 |
| Age at CSII first usage (years) | 31 ± 12 | 31 ± 12 | 31 ± 13 | .988 |
| Time between CSII start and last follow-up (years) | 13 ± 6 | 14 ± 6 | 11 ± 6 | .019 |
Data are means ± standard deviation.
CSII, continuous subcutaneous insulin infusion.
Some data (number and percentage of non-missing values, minimum, maximum, and median with the IQR) have been reported in supplemental Table 1.
Descriptive Outcomes
The mean follow-up was 5 ± 1 years (range 2-9).
HbA1c levels, analyzed annually from PLGM initiation, over a five-year period, show significant reduction only in Group 1 (from 7.5% ± 1.1% to 7.0% ± 1.0%, P = .02), while in Group 2, the reduction of HbA1c levels did not reach statistical significance (from 7.5% ± 1.1% to 7.4% ± 0.9%, P = .853), as there were no significant differences between groups during the follow-up (Table 2). Time courses in HbA1c levels of both groups are illustrated in Figure 1.
Table 2.
Follow-up Characteristics of HbA1c and BMI Between Groups.
| Characteristics | Total (n = 139) |
Group 1 (n = 71) |
Group 2 (n = 68) |
P value | |
|---|---|---|---|---|---|
| FU#1 | HbA1c (%) | 7.5 ± 1.1 | 7.5 ± 1.1 | 7.5 ± 1.1 | .863 |
| BMI (kg/m2) | 25.3 ± 3.1 | 25.2 ± 3.5 | 25.3 ± 2.8 | .976 | |
| FU#2 | HbA1c (%) | 7.4 ± 1.0 | 7.3 ± 0.9 | 7.5 ± 1.1 | .121 |
| BMI (kg/m2) | 25.3 ± 3.4 | 25.2 ± 3.7 | 25.5 ± 2.9 | .634 | |
| FU#3 | HbA1c (%) | 7.3 ± 1.0 | 7.3 ± 0.8 | 7.3 ± 1.2 | .630 |
| BMI (kg/m2) | 25.7 ± 3.3 | 25.6 ± 3.5 | 25.9 ± 3.1 | .419 | |
| FU#4 | HbA1c (%) | 7.2 ± 0.9 | 7.1 ± 0.9 | 7.4 ± 1.0 | .050 |
| BMI (kg/m2) | 25.3 ± 3.6 | 25.2 ± 3.7 | 25.5 ± 3.5 | .623 | |
| FU#5 | HbA1c (%) | 7.1 ± 1.0 | 7.0 ± 1.0 | 7.4 ± 0.9 | .146 |
| BMI (kg/m2) | 25.6 ± 3.0 | 25.2 ± 2.8 | 25.7 ± 3.4 | .762 | |
| Changes from baseline to final values (FU#1-FU#5) | HbA1c (%) | −0.2 ± 0.8 | −0.3 ± 0.7 | 0.1 ± 0.9 | .062* |
| BMI (kg/m2) | 0.2 ± 1.3 | −0.1 ± 1.4 | 0.7 ± 1.0 | .083* | |
Data are means ± standard deviation, *refers to repeated-measures mixed model for between-group comparison in patients with both FU#1 and FU#5 HbA1c and BMI values.
BMI, body mass index; HbA1c, hemoglobin A1c.
Figure 1.
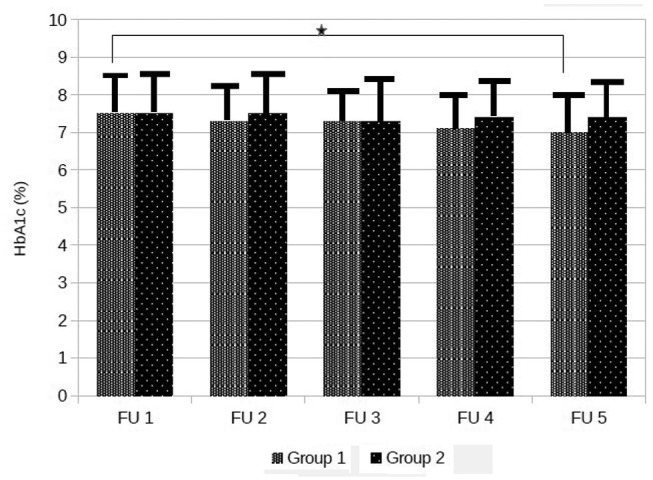
Time courses in HbA1c levels.
Data are means ± standard deviation, *P = .020.
HbA1c, hemoglobin A1c.
There were no statistically significant differences in occurrence of acute diabetes complications in both study groups as shown in Tables 3 and 4. Additional data of missing values, minimum, maximum, and median with the IQR are provided in supplemental Tables 2 and 3.
Table 3.
Annual Glucose Control Measures Group 1.
| Parameter | Summary statistics | FU#1 | FU#2 | FU#3 | FU#4 | FU#5 | P value |
|---|---|---|---|---|---|---|---|
| HbA1c (%) | Mean ± SD | 7.5 ± 1.1 | 7.3 ± 0.9 | 7.3 ± 0.8 | 7.1 ± 0.9 | 7.0 ± 1.0 | .020 |
| Severe hypoglycemia | %, n/Patients | 0.0% (0/70) | 3.0% (2/66) | 1.9% (1/52) | 0.0% (0/55) | 0.0% (0/38) | .589 |
| Severe hyperglycemia | %, n/Patients | 0.0% (0/70) | 1.5% (1/66) | 1.9% (1/52) | 0.0% (0/55) | 0.0% (0/38) | .589 |
HbA1c, hemoglobin A1c; SD, standard deviation.
Table 4.
Annual Glucose Control Measures Group 2.
| Parameter | Summary statistics | FU#1 | FU#2 | FU#3 | FU#4 | FU#5 | P value |
|---|---|---|---|---|---|---|---|
| HbA1c (%) | Mean ± SD | 7.5 ± 1.1 | 7.5 ± 1.1 | 7.3 ± 1.2 | 7.4 ± 1.0 | 7.4 ± 0.9 | .853 |
| Severe hypoglycemia | %, n/Patients | 0.0% (0/67) | 8.6% (5/58) | 2.4% (1/42) | 2.6% (1/39) | 0.0% (0/20) | .422 |
| Severe hyperglycemia | %, n/Patients | 0.0% (0/67) | 0.0% (0/58) | 2.4% (1/42) | 0.0% (0/39) | 0.0% (0/20) |
HbA1c, hemoglobin A1c; SD, standard deviation.
BMI increased significantly in Group 2 (from 25.3 ± 2.8 to 25.7 ± 3.4, P < .001), but not in Group 1 (from 25.2 ± 3.5 to 25.2 ± 2.8, P = .887) as shown in Figure 2, but there were no significant differences between groups during the follow-up (Table 2).
Figure 2.
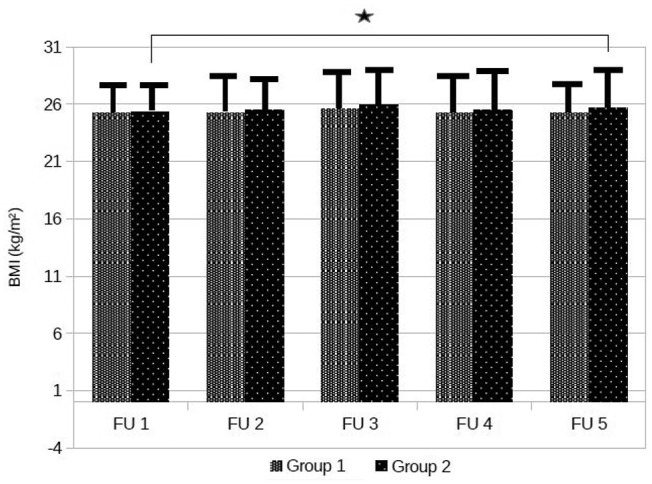
Time courses in BMI values.
Data are means ± standard deviation, *P = .001.
BMI, body mass index.
There were no statistically significant changes for clinical outcomes, beyond BMI, as well as prevalence of diabetes-related complications or device utilization between the groups (Supplemental Tables 4–6).
Discussion
This retrospective analysis follows 139 adult patients with over 10 years of experience with CSII (initiation between 1997 and 2015), as 71 of them transition into PLGM system that combines CSII, CGM, and predictive algorithms (Group 1), and is a single-center study for a mean of 5 years. The first Italian guidelines on the indications for CSII were published in 2009. 14 As mentioned previously, the indications for CSII were poor metabolic control, recurrent hypoglycemia, excessive glucose variability, and a need for more flexible insulin therapy. In the course of the years, the technologies were frequently updated depending on the clinical indications (eg, hypoglycemia unawareness), patient motivation, ability, and preference (eg, patch pumps vs conventional pumps). When comparing the above group to those who maintained their current system (Group 2), a significant statistical and clinical reduction of HbA1c (by 0.5 percentage point) was observed in the group that used the PLGS function. The reduction in HbA1c is not usually seen with PLGS as its main function is to reduce hypoglycemia as described by Beato-Víbora et al. 12 In their study, Beato-Víbora et al have evaluated a population of 162 T1D subjects (mean age 32 ± 17 years, 46 were children), with a median follow-up of 12 months (6-18), but the reduction of HbA1c was not significant (by 0.1 percentage point). On the other hand, similar decrease in HbA1c levels (from 7.61% to 6.88%, P < .05) was observed in a study of Gaweł et al, 15 conducted in a pediatric population with a shorter follow-up period (10.8 months). In our study the follow-up period was long enough to exclude the greater therapeutic adherence observed in the first few months of using a new device, because of higher interest for the device and due to more intensive educational therapy. 7 The decrease in HbA1c might be due to our clinical practice, to enhance basal rate with PLGM taking advantage of the security from hypoglycemia the system provides.
We observed a significant increase in BMI in Group 2 while the BMI was maintained with the use of PLGS. We hypothesize that this might be secondary to reduction in the nonsevere hypoglycemia events, which are usually mitigated by the patients by “defensive eating.” To our knowledge, there are no studies that have evaluated long-term BMI courses in patients using a SAP with PLGS algorithm.
Both groups showed no statistically significant increase or decrease in incidence of acute complications during the follow-up period, although the absolute number of severe hypoglycemia episodes were 3 (4.22%) in Group 1 vs 7 (10.29%) in Group 2. The lack of effect of PLGS in reduction of severe hypoglycemia, seen in this study, is probably due to the fact that patients were not selected for hypoglycemia unawareness and, therefore, did not have many severe hypoglycemic episodes at baseline.
No significant differences were noted in the presence or progression of microvascular and macrovascular diabetes-related complications, in either of the groups during the follow-up. It is likely that a longer follow-up period is necessary in order to fully evaluate the possible benefits of the PLGS in this compound. A nonsignificant trend in the incidence of ischemic cardiac event following PTCA with stent implantation (2 [2.81%] vs 3 [4.41%]) and laser therapy of retinopathy (2 [2.81%] vs 3 [4.41%]) in Group 1 vs Group 12, respectively, was noted.
The limitation of a retrospective analysis was addressed partly by the fact that all patients underwent the same number of visits and received a standardized education in medical nutritional counseling and low glucose management, except of a specific training to use the PLGS function in patients who had their device switched on. In addition, the indication for initiating PLGM was due to warranty expiration; thus, the groups were very similar at baseline. Another major limitation is that we lack data on mild and moderate hypoglycemia and glucose variability: as the usage frequency of sensors in both groups varied from 50% to 75% (data not shown) of the time and the glucose sensor models used by patients in Group 2 were different, it was not possible to compare CGM metrics data. 16
Conclusion
SAP with the PLGS algorithm is the first step toward the hybrid closed-loop systems and is used in many countries. In our five-year follow-up real-life experience with long-term T1D CSII users, it has resulted efficient in improving metabolic control and maintaining the body weight. Longer follow-up is needed to demonstrate the effect on chronic diabetes-related complications.
Supplemental Material
Supplemental material, SUPPLEMENTARY_FILES for Predictive Low Glucose Suspend Algorithm in Real Life: A Five-Year Follow-Up Retrospective Analysis by Claudio Tubili, Daniela Pollakova, Maria Rosaria Nardone and Ugo Di Folco in Journal of Diabetes Science and Technology
Acknowledgments
The authors would like to express their sincere gratitude to Professor Ohad Cohen, Medical Director Medtronic, who helped to write and edit the manuscript.
Footnotes
Author Contributions: UDF and CT designed the research. UDF, CT, DP, and MRN acquired the data and conducted the research. CT and UDF analyzed the data. UDF and CT had primary responsibility for final content. DP and MRN critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. UDF and CT are the guarantors of this work and, as such, have full access to all the data in the study and take full responsibility for the integrity and accuracy of the data.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ugo Di Folco  https://orcid.org/0000-0003-0531-7691
https://orcid.org/0000-0003-0531-7691
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bode BW, Steed RD, Davidson PC. Reduction in severe hypoglycemia with long-term continuous subcutaneous insulin infusion in type 1 diabetes. Diabetes Care. 1996;19:324-327. [DOI] [PubMed] [Google Scholar]
- 2. Blackman SM, Raghinaru D, Adi S, et al. Insulin pump use in young children in the T1D exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatr Diabetes. 2014;15:564-572. [DOI] [PubMed] [Google Scholar]
- 3. Yeh H-C, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and metaanalysis. Ann Intern Med. 2012;157:336-347. [DOI] [PubMed] [Google Scholar]
- 4. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 6. Tubili C, Di Folco U, Nardone MR, Clementi A. A single center long-term continuous subcutaneous insulin infusion (CSII) experience: higher fractional use is associated with less diabetes complications. J Diabetes Sci Technol. 2017;11(5):1057-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ziegler R, Tubili C, Chico A, et al. ProAct study: new features of insulin pumps improve diabetes management and glycemic control in patients after transition of continuous subcutaneous insulin infusion systems. Diabetes Technol Ther. 2013;15(9):738-743. [DOI] [PubMed] [Google Scholar]
- 8. O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensorguided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52:1250-1257. [DOI] [PubMed] [Google Scholar]
- 9. Batellino T, Nimri R, Dovc K, Phillip M, Bratina N. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: a randomized controlled trial. Diabetes Care. 2017;40(6):764-770. [DOI] [PubMed] [Google Scholar]
- 10. Forlenza GP, Li Z, Buckingham BA, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care. 2018;41:2155-2161. [DOI] [PubMed] [Google Scholar]
- 11. Bosi E, Choudhary P, de Valk HW, et al. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:462-472. [DOI] [PubMed] [Google Scholar]
- 12. Beato-Víbora PI, Quirós-López C, Lazaro-Martín L, et al. Impact of sensor-augmented pump therapy with predictive low-glucose suspend function on glycemic control and patient satisfaction in adults and children with type 1 diabetes. Diabetes Technol Ther. 2018;20:738-743. [DOI] [PubMed] [Google Scholar]
- 13. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316:1407-1408. [DOI] [PubMed] [Google Scholar]
- 14. AMD, SID. Standard italiani per la cura del diabete mellito 2009-2010. Edizioni Infomedica. [Google Scholar]
- 15. Gaweł WB, Deja G, Kamińska H, Tabor A, Skała-Zamorowska E, Jarosz-Chobot P. How does a predictive low glucose suspend (PLGS) system tackle pediatric lifespan challenges in diabetes treatment? Real world data analysis. Pediatr Diabetes. 2020;21(2):280-287. [DOI] [PubMed] [Google Scholar]
- 16. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, SUPPLEMENTARY_FILES for Predictive Low Glucose Suspend Algorithm in Real Life: A Five-Year Follow-Up Retrospective Analysis by Claudio Tubili, Daniela Pollakova, Maria Rosaria Nardone and Ugo Di Folco in Journal of Diabetes Science and Technology


