Abstract
Introduction:
We recently published the results of a pilot study measuring glucose in tear fluid. We now show the results of an additional 24 patients.
Methods:
Twenty-four subjects were recruited from Haaglanden Diabetes Centre. The patients reported in a fasting state and were given a meal with half the usual dose of insulin during the test. The device was applied under the lower eyelid. Glucose levels from capillary blood and interstitial fluid with a flash glucose measurement device were recorded every 15 minutes; the current from the tear glucose sensor was recorded continuously. The eye surface and tolerability were regularly checked. A calibration algorithm to convert tear glucose to blood values was built using a neural network regression model and validated.
Results:
No adverse events were attributed to the sensor coil placed under the lower eyelid. The mean absolute relative difference for the 24-patient subset was 16.7 (after 6 hours total time in the eye). The median absolute relative difference was 13.3. Compared to published data from Abbott (15.7 on day 1), the present device is comparable to Libre, considering that the device was allowed only one hour of equilibration time before the measurements were made.
Conclusion:
The NovioSense Tear glucose sensor measures blood glucose values with an acceptable accuracy and may become a good alternative to invasive devices.
Keywords: glucose monitoring, glucose sensor, tear glucose, type 1 diabetes
Introduction
Good glycemic control is extremely important in type 1 diabetes mellitus to reduce the risk of both microvascular and macrovascular complications.1 -3 Despite the availability of insulins, pump systems, and self-management techniques, obtaining good control is difficult and not obtained by more than half of the patients. Frequency of self-monitoring of blood glucose is inversely related to glycated hemoglobin, but the mean use of finger strips in type 1 has been shown to be no more than 2.5 per day.4 -6 The use of continuous glucose measurement (CGM) systems leads to better control and more time in range with less hypoglycemia and notably a better quality of life. 7 Current CGM systems are invasive, costly, and can give skin problems. Also, the visibility of the external device is an obstacle for some patients. Therefore, there is a need for noninvasive, nonvisible devices. Noninvasive glucose measurement via the skin (BioMKR, GlucoWise, and Glucotrack) is under development or clinical testing. 8 Until our first report in 2018, 9 attempts to measure glucose in tears have not resulted in the creation of a reliable device. This was reviewed by Baca et al in 2007 and since then little has changed. 10 The largest challenge in measuring in the eye has been identifying a method to measure in the eye without causing abrasion of the sclera resulting in artificially high glucose values while also being able to measure sufficient volumes of consistent tears in the eye. This report demonstrated the clinical evidence that these challenges have now been largely mitigated with the use of this sensor. After a successful pilot study 9 we now show the results of the next 24 study participants.
Study Design (Statistical Method)
A power and sample size calculation was carried out using the historical data from our first phase II six-patient clinical trial. From this we determined that a sample size of 24 patients was required to evaluate the data with statistical confidence and standard power of no less than 0.9 at a confidence of 95% (α = 0.05).
Patients and Methods
The device assembly, coating specifications, electrochemical measurement technique, calibration of the device, as well as functionality and use of the sensor are described in a previous report. 9 In short, the device consists of a flexible, spring-shaped coil coated with a proprietary hydrogel. The sensor has a classical first-generation enzymatic detection design. The devices are factory calibrated, which involve extensive calibration on a per-lot basis to determine a suitable set of calibration parameters for the device along with comparison to a proprietary model and data set on the performance of the sensor over time. The sensor can be easily placed into the fornix of the lower eyelid. In the moist surroundings of the lower eyelid, the gel coating will swell, creating a contact between the coil and fluid in the eye. The device contains no power source, and in the final embodiment, the readings are triggered by an external telemetry device, for example, a phone or stand-alone reader. For this study, we used the same prototype devices as employed in the pilot trial.
Patients with type 1 diabetes, already wearing a Freestyle Libre device for at least 24 hours, were recruited from the Diabetes Centre of Haaglanden Medical Centre. All patients were ≥18 years old on the date they signed the informed consent. Exclusion criteria were prior eye surgery, any history of disease of the eye like conjunctivitis, keratitis, dry eye, and wearing contact lenses, not willing or able to comply to the protocol, or any signs or symptoms of an additional disease except diabetes.
Before positioning the device, the ophthalmologist examined each subject to exclude eye disease, trauma, or infection. An extensive ophthalmologic exam was performed to exclude any pre-existent anterior surface a disease test for lacrimal secretion, tear breakup time, Schirmer tear test, corneal punctate staining, the appearance of the anterior chamber, and photography of the ocular surface. Before insertion, the device was tested in sterile water to exclude the risk of a short circuit. After the insertion, the sensor was left in the eye for one hour to stabilize before the blood (Roche Accu-Chek Inform II) and interstitial glucose samples were taken and then recorded every 15 minutes for 5 hours; the current measured with tear glucose sensor was recorded continuously. The eye surface and lower eyelid and tolerability were regularly monitored by the ophthalmologist and through questionnaires. One hour after start of the measurements, the patients took their normal meal with half the dose of insulin in order to let glucose values rise as high as about 15 mmol/L.
It was the intention of this study to determine the correct algorithm for conversion from tear glucose to blood glucose values; thus, after data collection, each sensor was postcalibrated following removal from the eye. The conversion algorithm for tears to blood used a combined calibration model from the factory calibration factors and the postcalibration; however, in practice, the results of using either/or method did not affect the mean absolute relative difference (MARD) or Clarke error grid data. All amperometry-based glucose-sensing techniques require an algorithm that contains multiple steps, especially in the case of continuous monitoring: filtering, smoothing, and application of a calibration factor. Intuitively this process requires additional steps when measuring blood glucose indirectly, that is, in a non-blood matrix in order to convert to blood equivalent values. It was the purpose of this study to provide data to first examine the feasibility for measuring glucose in the tears in a clinical setting and secondly allow for the generation of an appropriate calibration algorithm for the determination of the conversion of blood to tear glucose. Due to the lack of information in the literature this study and our previous report 9 stand as the seminal works in the determination of blood glucose values from tears. In the course of this we have used a neural network model to carry out the conversion from tear to blood values due to its ability when properly cross-validated (as was done here) to account for the as-yet unknown parameters for conversion. This is discussed in detail in the supplemental material.
For statistical analysis, the MARD and median absolute relative difference (MedARD) were calculated.
The study was approved by METC Zuidwest Holland, ID: NL66681.098.18 and Dutch Health Care Inspectorate, ID: VGR2009189 conducted between October 26, 2018 and January 18, 2019.
Results
Thirteen males, 11 females, aged 19-73 years, mean diabetes duration 25 years, were tested. Measured glucose values during the test ranged from 3.6 to 19.9 mmol/L. All patients completed the study. During the study, data from three patients were deemed unusable as the probe wires became irreversibly detached from their mounting and thereby the device became inoperable. The power was recalculated and found to be above 0.8. The study produced a total of 368 data points, which are further analyzed below.
Glucose in tear fluid was found to be significantly lower compared to blood glucose (Libre). An example of the tear glucose levels versus time is shown in Figure 1 (black line). Several parameters (noise, patient-specific factors, per-batch variability) were identified that affect the tear-to-equivalent blood value. The model for tear-to-blood glucose conversion published previously was only able to account for around 70% of the variation in the data. 9
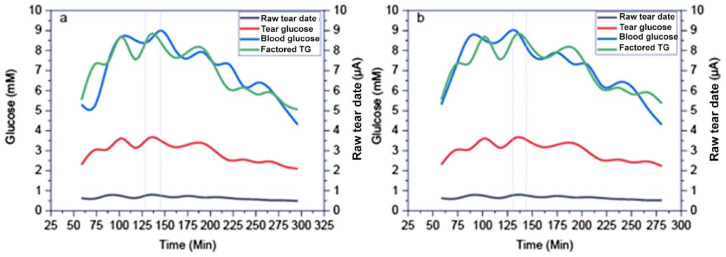
Figure 1.
Tear glucose versus time for patient 016. (a) Non-time-corrected data. (b) The time-corrected data (15 minutes). Timeshift is indicated by the gray dotted line.
The clark error grid arising from analysis according to our previous report (Kownacka et al.) 9 is shown in Figure 2. The corrected tear glucose values according to this model are shown in Figure 1 (red line).
Figure 2.
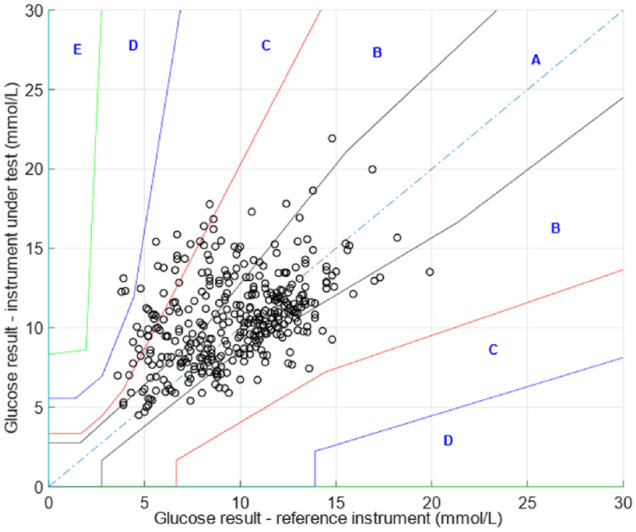
Error grid using previously published algorithm for the tear-to-blood glucose concentration.
Due to the number of parameters involved in the conversion and complexity of the interactions, a neural network regression algorithm was developed and cross-validated to convert tear glucose values-to-blood glucose equivalent values. The neural network can calibrate not only for sensor response but also for the unknown physiological conversion parameters.
The development of the regression method of the neural network is discussed in detail in the supplemental information. The time versus blood glucose equivalent tear glucose values are shown in Figure 1 (green line) as well as the corresponding blood glucose values obtained from the Libre device (blue line).
The error grid analysis shown below (Figure 3) was derived from the trials using the neural network regression model. The error grid analysis of the 24 patient data set exhibited an excellent correlation between blood and tear glucose; 99.7% of data was found in the A+B region (Figure 3).
Figure 3.
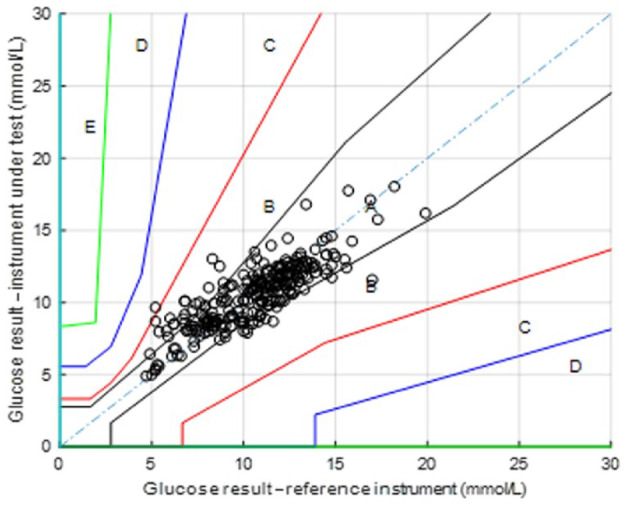
Error grids produced from the neural network regression model.
The calculated MARD for the 24-patient subset was determined to be 16.7 and MedARD was found to be 13.3. A significant number of outlying data points (10% of the data) skewed the MARD considerably.
Although the study was not designed to test compliance to ISO 15197:2013 in terms of protocol, study, sample size, and concentration range, it is a valuable measure for NovioSense device’s potential. The NovioSense tear glucose sensor could measure 80% of the data points within ±15 mg/dL (±15%) accuracy criteria (Figure 4).
Figure 4.
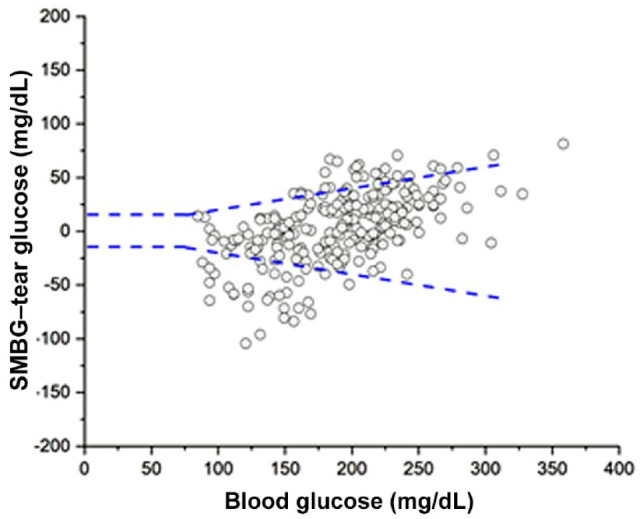
Analysis of NovioSense data versus blood glucose data following ISO 15197:2013 standard.
Tolerability
Detailed microscopic examination of the eye and inferior conjunctival fornix carried out at various points of the trajectory of the study did not show significant adverse events. The pictures of patients wearing the tear glucose sensor in the eye taken at different points of the clinical trial phase II can be found in our previously published paper. 9 Results from ophthalmological measurements have shown good tolerability of wearing NovioSense tear glucose sensor. However, some of the subjects developed a slight conjunctival redness (mild irritation) due to the probe wires exiting the eye, not as a result of the sensor itself. All patients could finish the five-hour test. The results of the questionnaires on tolerability are presented in Table 1. Most of the patients confirmed good tolerance to the device being present in the eye. Only two subjects had marginally less subjective tolerance to the ocular insert.
Table 1.
Results of the Tolerability Questionnaire.
| Question | Mean | Median | Mode |
|---|---|---|---|
| First screening session | |||
| 1 = Not at all 5 = Yes definitely | |||
| 1. Was the positioning of the sensor uncomfortable? (1-5) | 1.8 | 2.0 | 1 |
| 2. Can you feel the sensor? (1-5) | 1.7 | 1.5 | 1 |
| 3. Did you tend to rub your eye more than usual? (1-5) | 1.4 | 1.0 | 1 |
| 0 = Completely tolerable 10 = Not tolerable at all | |||
| 4. Overall, how unpleasant, up to now, is it to wear the insert? (0-10) | 2.1 | 2.0 | 2 |
| Final screening session | |||
| 1 = Not at all 5 = Yes definitely | |||
| 5. Did you find the removal procedure unpleasant? (1-5) | 1.0 | 1.0 | 1 |
| 6. Did you feel the sensor in the eye? (1-5) | 1.7 | 2.0 | 2 |
| 7. Was it unpleasant to wear the sensor? (1-5) | 1.5 | 1.0 | 1 |
| 8. Did you tend to rub your eye more than usual? (1-5) | 1.5 | 1.5 | 1 |
| 9. Did you experience more tear production than usual while wearing the sensor? (1-5) | 1.1 | 1.0 | 1 |
| 10. Did you feel any itching sensation when wearing the device in the eye? (1-5) | 1.2 | 1.0 | 1 |
| 11. Did you feel any burning sensation while wearing the sensor? (1-5) | 1.4 | 1.0 | 1 |
| 12. Is your eye redder? (1-5) | 1.6 | 2.0 | 2 |
| 0 = Not tolerable at all 10 = Completely tolerable | |||
| 13. Overall how comfortable was the sensor to wear in your eye? (0-10) | 8.8 | 9.0 | 9 |
Discussion
Tear glucose was found to be much lower in tears than in blood glucose, which was consistent with reported data in our previous article and is also well known from literature.9 -14 The most recent publication on this topic is that of the group of Wang showing similar results for an eye glasses–based device. 13 Our results suggest that the lag time between tear glucose and blood glucose appears to be around 15 minutes (Figure 3), which is similar to prior reports of interstitial fluid used, for example, in the Libre device. 15 Evidence of the tear and blood glucose correlation with a lag time of 15 minutes was already published in 2006 by Dr Wayne March et al. 16 Also, several other studies have indicated that tear and blood sugar delay is around 10 minutes.8,16,17 However, according to Zhang et al, the delay between blood and tear glucose concentration was around five minutes. 18 The clinical relevance of a delay is especially important with rapid rising or lowering of blood glucose values.
It is expected that tear glucose may not perfectly track with blood glucose; however, we showed that there is undoubtedly a significant correlation between blood and tear glucose.
Many factors affect the ability of the sensor to accurately predict glucose levels in tears of patients with diabetes. Therefore, in this study, a robust calibration with a newly derived algorithm utilizing a neural network for the conversion of tear glucose to blood glucose was employed. The results suggest that the utilized neural network can enhance the accuracy of the noninvasive ocular insert. A neural network approach was taken using regression analysis to determine blood equivalent values given the tear glucose and sensor parameters.
Accuracy metrics showed that the noninvasive nature of the NovioSense device could measure the blood glucose values from tears that are comparable with CGM devices available in the market, allowing patients to measure their glucose on demand, at least for the glucose values measured during this study. The Abbott Freestyle Libre device generally shows an MARD equal to 15.7 on the first day. Comparing the NovioSense device to this, it can be seen that the values are similar, considering that the device was allowed only five hours of performance time before the MARD was measured, and this is rather promising.
It should be noted that the ocular insert utilizes a factory calibration system, meaning no user calibrations are required during sensor wear duration. The sensor utilizes a cross-validated artificial intelligence technique to transform tear glucose to blood glucose. However, further research is needed to assess the effectiveness and performance for a longer period and at more glucose variation, for example hypoglycemia and glucose values above 15 mmol/L. Since this a pilot clinical study we did not want to expose patients to extreme glucose values. As published previously in both ex vivo and in vitro data the NovioSense sensor performs excellently in the lower glucose concentration regime (<4 mmol/L). 9
Also, tolerability for more than five hours has to be studied, which is only possible with a wireless device.
Conclusion
We have conducted the first clinical trial, using a statistically significant sample size at a power of 0.8, showing that the NovioSense device measures glucose values from tears with an acceptable accuraccy, which is similar to that shown by commercial CGM devices on the first day. Further studies with more variation in glucose values and longer duration are warranted after building in a microchip for interface with, for example, a mobile phone.
Supplemental Material
Supplemental material, GEELHOED_Supplementary_Information_to_Manuscript_1 for Performance of the Prototype NovioSense Noninvasive Biosensor for Tear Glucose in Type 1 Diabetes by Petronella Geelhoed-Duijvestijn, Dovile Vegelyte, Alicja Kownacka, Nicoleta Anton, Maurits Joosse and Christopher Wilson in Journal of Diabetes Science and Technology
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dovile Vegelyte and Alicja Kownaca are employees from NovioSense B.V. Christopher Wilson is the CEO of NovioSense B.V. The costs of the clinical study were payed for to the Haaglanden Medical Centre by NovioSense B.V. No individual payments were made. Petronella Geelhoed-Duijvestijn, Maurits Joosse, and Nicoleta Anton have no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by NovioSense B.V., Nijmegen, The Netherlands.
ORCID iD: Petronella Geelhoed-Duijvestijn  https://orcid.org/0000-0003-1254-2635
https://orcid.org/0000-0003-1254-2635
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose system. Diabetes Technol Ther. 2015;17(11):787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 3. Ceriello A, Ihnat MA, Thorpe JE. The “metabolic memory”: is more than just tight glucose control necessary to prevent diabetic complications? J Clin Endocrinol Metab. 2009;94(2):410-415. [DOI] [PubMed] [Google Scholar]
- 4. Lee WC, Smith E, Chubb B, Wolden ML. Frequency of blood glucose testing among insulin-treated diabetes mellitus patients in the United Kingdom. J Med Econ. 2014;17(3):167-175. [DOI] [PubMed] [Google Scholar]
- 5. Moström P, Ahlén E, Imberg H, Hansson PO, Lind M. Adherence of self-monitoring of blood glucose in persons with type 1 diabetes in Sweden. BMJ Open Diabetes Res Care. 2017;5(1):e000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller KM, Beck RW, Bergenstal RM, et al. T1D Exchange Clinic Network. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slattery D, Choudhary P. Clinical use of continuous glucose monitoring in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(suppl 2):S55-S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim J, Campbell AS, de Ávila BE, Wang J. Wearable biosensors for healthcare monitoring. Nat Biotechnol. 2019;37(4):389-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kownacka AE, Vegelyte D, Joosse M, et al. Clinical evidence for use of a noninvasive biosensor for tear glucose as an alternative to painful finger-prick for diabetes management utilizing biopolymer coating. Biomacromolecules. 2018;19(11):4504-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baca JT, Finegold DN, Asher SA. Tear glucose analysis for the noninvasive detection and monitoring of diabetes mellitus. Ocul Surf. 2007;5(4):280-293. [DOI] [PubMed] [Google Scholar]
- 11. Lee SH, Cho YC, Choy YB. Noninvasive self-diagnostic device for tear collection and glucose measurement. Sci Rep. 2019;9(1):4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu MX, Miyajima K, Takahashi D, et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat Commun. 2017;8(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sempionatto JR, Brazaca LC, Garcia-Carmona L, Bolat G, Campbell AS, Martin A. Eyeglasses-based tear biosensing system: non-invasive detection of alcohol, vitamins and glucose. Biosens Bioelectron. 2019. Jul 15;137:161-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cha KH, Jensen GC, Balijepalli AS, Cohan BE, Meyerhoff ME. Evaluation of commercial glucometer test strips for potential measurement of glucose in tears. Anal Chem. 2014;86(3):1902-1908. [DOI] [PubMed] [Google Scholar]
- 15. Basu A, Dube S, Slama M, et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes. 2013;62(12):4083-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. March W, Lazzaro D, Rastogi S. Fluorescent measurement in the non-invasive contact lens glucose sensor. Diabetes Technol Ther. 2006;8(3):312-317. [DOI] [PubMed] [Google Scholar]
- 17. Iguchi S, Kudo H, Saito T, et al. A flexible and wearable biosensor for tear glucose measurement. Biomed Microdevices. 2007;9(4):603-609. [DOI] [PubMed] [Google Scholar]
- 18. Zhang J, Hodge W, Hutnick C, Wang X. Noninvasive diagnostic devices for diabetes through measuring tear glucose. J Diabetes Sci Technol. 2011;5(1):166-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, GEELHOED_Supplementary_Information_to_Manuscript_1 for Performance of the Prototype NovioSense Noninvasive Biosensor for Tear Glucose in Type 1 Diabetes by Petronella Geelhoed-Duijvestijn, Dovile Vegelyte, Alicja Kownacka, Nicoleta Anton, Maurits Joosse and Christopher Wilson in Journal of Diabetes Science and Technology