Abstract
Background:
Detailed evaluations of hypoglycemia and associated indices based on continuous glucose monitoring (CGM) are limited in patients with diabetes of the exocrine pancreas. Our study sought to evaluate the frequency and pattern of hypoglycemic events and to investigate hypoglycemia-specific indices in this population.
Methods:
This was a cross-sectional study comprising 83 participants with diabetes of the exocrine pancreas. CGM and self-monitoring of blood glucose (SMBG) were performed on all participants for a minimum period of 72 hours. The frequency and pattern of hypoglycemic events, as well as hypoglycemia-related indices, were evaluated.
Results:
Hypoglycemia was detected in 90.4% of patients using CGM and 38.5% of patients using SMBG. Nocturnal hypoglycemic events were more frequent (1.9 episodes/patient) and prolonged (142 minutes) compared with day-time events (1.1 episodes/patient; 82.8 minutes, P < 0.05). The mean low blood glucose index was 2.1, and glycemic risk assessment diabetes equation hypoglycemia was 9.1%. The mean time spent below (TSB) <70 mg/dL was 9.2%, and TSB <54 mg/dL was 3.7%. The mean area under curve (AUC) <70 mg/dL was 1.7 ± 2.5 mg/dL/hour and AUC <54 mg/dL was 0.6 ± 1.3 mg/dL/hour. All of the CGM-derived hypoglycemic indices were significantly more deranged at night compared with during the day (P < 0.05).
Conclusion:
Patients with diabetes of the exocrine pancreas have a high frequency of hypoglycemic episodes that are predominantly nocturnal. CGM is superior to SMBG in the detection of nocturnal and asymptomatic hypoglycemic episodes. CGM-derived hypoglycemic indices are beneficial in estimating the risk of hypoglycemia.
Keywords: continuous glucose monitoring, hypoglycemia, hypoglycemic indices, diabetes of the exocrine pancreas
Introduction
Diabetes of the exocrine pancreas is associated with brittle glycemic control and an increased risk of hypoglycemia. The glycemic profile is typically characterized by hyperglycemic peaks interspersed with recurrent hypoglycemic episodes. 1 Pancreatic inflammation and damage is associated with the loss of β-cells, α-cells (glucagon), and δ-cells (somatostatin), as well as pancreatic-polypeptide (PP) cells of the pancreatic islets. This results in defective counter-regulation and brittle glycemic control, typically characterized by wide fluctuations in blood glucose levels.1,2 Malabsorption and variable nutrient absorption from the intestine further exacerbate the risk of hypoglycemia and contributes to extensive fluctuations in blood glucose levels. Repeated episodes of mild asymptomatic hypoglycemia are a risk factor for hypoglycemic unawareness, a state that is associated with significant morbidity.1,3-6 Furthermore, recurrent hypoglycemic episodes increase the challenges associated with attaining tight glycemic control.
A high rate of hypoglycemia was observed in patients with diabetes of the exocrine pancreas by self-monitoring of blood glucose (SMBG).7-11 Hypoglycemia is particularly challenging post-pancreatic resection, contributing to both morbidity and mortality in this patient population. 12 SMBG detects discrete capillary blood glucose levels and can be useful in detecting and managing symptomatic hypoglycemia; however, nocturnal hypoglycemia is frequently missed by SMBG. An additional shortcoming of SMBG is that it fails to provide meaningful information on different aspects of hypoglycemia.13,14 In contrast, continuous glucose monitoring (CGM) can provide integrated information on different aspects of daytime and nocturnal hypoglycemia, permitting a more detailed analysis. Such information will provide valuable insights for the planning of preventative strategies pertaining to hypoglycemia.13-15 Limited evidence suggests that hypoglycemic events identified by CGM were more frequent than those identified by SMBG in patients with diabetes of the exocrine pancreas and that use of CGM significantly reduced the incidence of hypoglycemia.16-18
Detailed evaluations of hypoglycemic events and other hypoglycemia-specific indices based on CGM are limited in patients with diabetes of the exocrine pancreas. These hypoglycemia-specific measures could be useful as an adjunct to glycated hemoglobin (HbA1c) for better overall assessment of glycemic status in patients with diabetes of the exocrine pancreas. They could also provide information regarding the burden of hypoglycemia in this patient population. Further, more in-depth knowledge of the various aspects of hypoglycemia and its indices will assist in the planning of preventative strategies. As well, such knowledge may provide novel insights into therapeutic regimens designed to reduce hypoglycemia. Prevention of recurrent hypoglycemic episodes will improve the overall quality of life and could contribute to a reduction in hypoglycemia-associated morbidity and mortality. Therefore, this study sought to use CGM to evaluate the frequency and pattern of hypoglycemic events and to estimate various hypoglycemia-specific indices in a large cohort with diabetes of the exocrine pancreas.
Methods
Subject Selection
Patients aged between 18 and 65 years who had been diagnosed with diabetes of the exocrine pancreas for at least 3 months and who were on self-adjusting insulin and under regular follow-up were eligible for this study. Diabetes of the exocrine pancreas includes the following: (1) post-pancreatic diabetes mellitus (PPDM), (2) pancreatic cancer-related diabetes (PCRD), and (3) cystic fibrosis-related diabetes (CFRD) as per American Diabetes Association (ADA). The diagnosis of PPDM is based on the presence of diabetes in the background of acute or chronic pancreatitis regardless of the timing of diabetes onset. 19 The diagnosis of fibrocalculous pancreatic diabetes (FCPD) was made on the basis of the previously defined criteria. 20 Alcohol-induced pancreatitis was defined as follows: (1) presence of alcohol consumption as described by the Alcohol Use Disorders Identification Test (AUDIT), whereby a score higher than eight was considered to be abnormal and (2) absence of other identifiable causes of pancreatitis. 21 The diagnosis of autoimmune pancreatitis requires the fulfillment of at least one of the following criteria: (1) pancreatic histopathological findings; (2) imaging; (3) serology (immunoglobulin [Ig]G4, IgG, antinuclear antibodies); (4) other organ involvement; and (5) response to steroid therapy. 22 Patients diagnosed with FCPD, diabetes secondary to alcohol-induced pancreatitis, and autoimmune pancreatitis were considered as patients with PPDM. Eligible patients were identified from the registry of our endocrine clinic and were invited to participate in this study over the phone. Patients were further screened for eligibility when they reported to the endocrine clinic at the Vydehi Institute of Medical Sciences and Research Centre, Bangalore. Exclusion criteria consisted of (1) patients on oral antidiabetic drugs; (2) those not on insulin; (3) those with a history of severe hypoglycemia in the preceding 3 months; (4) those on glucocorticoids; (5) use of beta-blockers; (6) HbA1c <6% or >12%; (7) those with undue fear of technical systems, alcohol, and/or drug misuse; and (8) patients with major psychological/psychiatric problems. After screening, a total of 92 patients were included in the final cohort. The study protocol was approved by the ethical committee of the Vydehi Institute of Medical Sciences and Research Centre, and the study conforms with the Good Clinical Practice provisions of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Clinical and Laboratory Evaluation
We collected information on the duration of diabetes, steatorrhea, and abdominal pain; history of hypoglycemic episodes; enzyme replacement therapy; pancreatic surgeries; initial and current treatment; details of insulin therapy, and the most recent HbA1c measurement. All patients underwent anthropometric assessment of height, weight, and body mass index (BMI).
Following a ten-hour overnight fast, fasting blood samples were drawn from patients with FCPD for estimation of the levels of fasting plasma glucose (FPG), HbA1c, the fasting lipid profile, and serum creatinine. Plasma glucose concentration was estimated using the hexokinase enzymatic reference method. HbA1c level was estimated by the high-performance liquid chromatography method using a BIORAD D10 analyzer (Bio-Rad, NY, USA). Enzymatic methods were used to determine serum total cholesterol and triglyceride concentrations. High-density lipoprotein-cholesterol (HDL-C) and low-density lipoprotein (LDL) levels were estimated using the direct measure polymer poly-anion method. The remaining tests were performed using a fully automated Beckman Coulter DXC 860i autoanalyzer (Beckman Coulter Inc., CA, USA).
CGM Monitoring
Following determination of eligibility, the CGM system Medtronic iPro2 (Medtronic, USA), was inserted in all eligible patients by a trained professional. Patients were advised to remain hospitalized for a minimum period of three days. Bedside glucose monitoring (SMBG) was performed at frequent intervals (prebreakfast, prelunch, predinner, two hours after dinner, midnight 3a.m and whenever the patient develops symptoms of hypoglycemia) using an Accuchek Instant blood glucose meter (Abbott Diabetes Care, Alameda, CA, USA). Patients and nursing staff were instructed on recording events of hypoglycemia (symptomatic and asymptomatic), insulin doses, and physical activity, as well as maintenance of a food diary. The plasma glucose concentrations were maintained within the preprandial target range of 90-130 mg/dL and with two-hour postprandial values below 180 mg/dL. At the follow-up visit, adverse events including severe hypoglycemia, device-related issues, and any other notable adverse events, regardless of cause, were identified and reported. The final analysis included only those patients with a minimum of 72 hours of CGM data.
Measures of Glycemic Variability and Hypoglycemia
CGM-derived metrics of glycemic variability and hypoglycemia were estimated using the GlyCulator 2 (https://apps.konsta.com.pl/app/glyculator). The following parameters of glycemic variability were estimated: (1) SD of the mean of the sensor values; (2) mean amplitude of glycemic excursion (MAGE); (3) continuous overall net glycemic action (CONGA); (4) M value, and (5) coefficient of variance (%CV). Hypoglycemic indices derived from CGM included (1) low blood glucose index (LBGI), (2) area under curve the (AUC) <70 mg/dL, (3) AUC <54 mg/dL, (4) time spent below (TSB) <70 mg/dL, (5) glycemic risk assessment diabetes equation (GRADE) hypoglycemia, (6) number of hypoglycemic events, (7) number of prolonged hypoglycemic events (>120 minutes), and (8) mean duration of hypoglycemic event.
Sample Size
Sample size was estimated considering the low blood glucose index as an outcome. Assuming the expected SD of 0.82, to estimate a mean with 95% confidence and a precision of 0.2, the required sample size was 68.
Statistical Analysis
Assumption of normality was assessed. Descriptive statistics were reported as mean with SD for normally distributed continuous variables, variables that were non-normal were reported with mean and SD along with median with 25th and 75th percentiles to understand the distribution of each variable. Categorical variables were reported as numbers and percentages. Comparison of hypoglycemic outcomes between night and day was performed using the Wilcoxon Signed-Rank test. P value of less than 5% was considered statistically significant. All the analyses were performed using SPSS version 25.0.
Results
Baseline Characteristics
Demographic information and biochemical measurements of the study group are presented in Table 1. A total of 92 patients met the eligibility criteria for enrolment in the study and completed the CGM procedure. Nine patients (six females and three males) were withdrawn from the study due to sensor displacement and technical issues. The final cohort was composed of 83 patients with diabetes of the exocrine pancreas. The distribution was as follows: 51.81% of patients (n = 43) were diagnosed with FCPD, 33.73% (n = 28) of patients had alcoholic pancreatitis, 8.43% (n = 7) had post-pancreatic resection (n = 5 patients underwent resection secondary to pancreatic malignancy, while n = 2 patients underwent resection owing to chronic intractable pain secondary to chronic pancreatitis), 4.82% (n = 4) had pancreatic cancer, and 1.21% (n=1) had pancreatitis attributed to an autoimmune etiology. Overall, 74 patients were diagnosed with PPDM, nine patients had PCRD, while none had CFRD. Among the study population, 56.6% of the patients were males. The mean age of the participants was 35 ± 8.2 years, and the duration of diabetes was 5.1 years. The mean BMI was 18.9 ± 2.8 kg/m2. The mean HbA1c value of the participants was 9.5 ± 1.6%, and the patients required approximately 0.6 U of insulin per kg of body weight administered daily by multiple subcutaneous injections. No ketoacidosis was reported during the hospital stay.
Table 1.
Baseline Characteristics of the Study Group.
| Parameters | Mean ± SD |
|---|---|
| Age (years) | 35.0 ± 8.2 |
| Gender | |
| Male (n) | 47 |
| Female (n) | 36 |
| BMI (kg/m2) | 18.9 ± 2.8 |
| Duration of diabetes (years) | 5 (1, 8) |
| FPG (mg/dL) | 191.8 ± 79.3 |
| HbA1c % | 9.5 ± 1.6 |
| Serum creatinine (mg/dL) | 0.7 ± 0.3 |
| Total cholesterol (mg/dL) | 166.1 ± 41.3 |
| TG (mg/dL) | 154.2 ± 85.4 |
| HDL (mg/dL) | 41.7 ± 9.4 |
| LDL (mg/dL) | 97.4 ± 35.4 |
Data are represented by mean ± SD for normally distributed continuous variables, while variables that were non-normal were reported as mean and SD along with median with interquartile range to understand the distribution of each variables.
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides.
Hypoglycemic Outcomes
Data from CGM revealed that 75 patients had at least one hypoglycemic event. Among these, 29.3% of patients (n = 22) developed symptoms, while 70.7% (n = 53) were asymptomatic. Level 1 hypoglycemia (blood glucose <70 mg/dL and >54 mg/dL) was observed in 40.9% of patients, while level 2 hypoglycemia (blood glucose <54 mg/dL; did not require external assistance for recovery) was recorded in 42.1% of patients. Data from SMBG revealed that 32 (38.5%) patients had at least one hypoglycemic episode. Of these, 68.7% developed symptoms (n= 22), while the remainder (31.3%, n = 10) were asymptomatic. Severe hypoglycemia was reported by six patients, one of whom had two episodes of severe hypoglycemia.
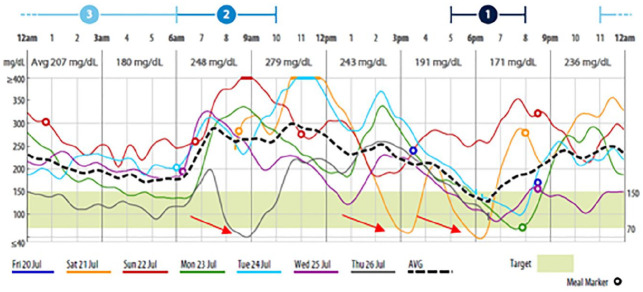
CGM recorded a mean number of 2.9 hypoglycemic episodes per patient (Table 2). Approximately 62 patients (74.7%) had at least one nocturnal hypoglycemic episode (12 am – 6 am) detected by CGM, 80.6% (n = 50) of whom did not develop any symptoms. SMBG recorded nocturnal hypoglycemic episodes in 22 patients (26.5%), 45.4% of whom (n = 10) were asymptomatic. The mean number of recorded hypoglycemic episodes was significantly higher during the night than during the day (1.9 vs 1.1, P < 0.001). Similarly, the mean duration of hypoglycemic episodes recorded by CGM was significantly higher at night than during the day (142 minutes vs 82.8 minutes, P < 0.001). The period between 3 and 6 AM was the most common time for nocturnal hypoglycemia. A typical CGM graph depicting hypoglycemia in patients with diabetes of the exocrine pancreas is shown in Figure 1.
Table 2.
Hypoglycemic Outcomes.
| Parameters | WHOLE (n = 83) | NIGHT | DAY | P value* |
|---|---|---|---|---|
| LBGI | 2.1 ± 2.7 | 2.3 ± 3.8 | 1.3 ± 2.0* | 0.011 |
| 1 (0.4-2.4) | 0.9 (0.2-2) | 0.7 (0.1-1.5) | ||
| AUC <70 mg/dL | 1.70 ± 2.5 | 1.9 ± 3.2 | 1.0 ± 1.7* | 0.031 |
| 0.7 (0.3-1.7) | 0.6 (0-1.7) | 0.5 (0-1.5) | ||
| AUC <54 mg/dL | 0.6 ± 1.3 | 0.7 ± 1.6 | 0.2 ± 0.50* | 0.003 |
| 0 (0-0.7) | 0 (0-0.1) | 0 (0-0) | ||
| Time spent below 70 mg/dL % | 9.2 ± 10.4 | 10.4 ± 13.6 | 5.8 ± 7.6* | 0.003 |
| 4.4 (2-13.7) | 5.5 (0-13.3) | 2.5 (0-10) | ||
| Time spent below 54 mg/dL % | 3.7 ± 6.5 | 3.6 ± 9.4 | 1.7 ± 3.4 | 0.077 |
| 0 (0-5.2) | 0 (0-1.3) | 0 (0-1.1) | ||
| GRADE _ hypoglycemia | 9.1 ± 11.0 | 13.1 ± 19.4 | 5.9 ± 8.6* | <0.001 |
| 5.1 (1.4-12.8) | 4.4 (0.6-14.2) | 1.8 (0.1-8.4) | ||
| No. of hypoglycemic events | 2.9 ± 2.7 | 1.9 ± 2.4 | 1.1 ± 1.2* | <0.001 |
| 2.0 (1.0-4.5) | 1 (0-3) | 1 (0-1) | ||
| Mean duration of hypoglycemic event (min) | 177.3 ± 143.5 | 142 ± 157.8 | 82.8 ± 83.4* | <0.001 |
| 157.8 (63.9-227.3) | 135.5 (0-164.5) | 54.8 (0-148.3) |
Data are represented by mean ± SD for normally distributed continuous variables, while variables that were non-normal were reported as mean and SD along with median with interquartile range to understand the distribution of each variables. P value < 0.05 was considered significant.
P value between day and night parameters.
AUC, area under curve; GRADE, glycemic risk assessment diabetes equation score; LBGI, low blood glucose index.
Figure 1.
A typical CGM graph of patient with diabetes of the exocrine pancreas. Dotted black lines represent integrated CGM curve; red arrows represent hypoglycemia. CGM, continuous glucose monitoring.
Measures of Glycemic Variability and Hypoglycemic Indices as Determined by CGM
The baseline CGM-derived measures of glycemic variability are provided in Table 3. The mean cohort values of MAGE, SD, and %CV were 138.9 ± 57.7 mg/dL, 57.3 ± 24.3 mg/dL, and 30 ± 11.4%, respectively. The mean CGM-derived 24-hour glucose concentration was 193.3 ± 56.9 mg/dL. In general, significant differences were observed between daytime and nocturnal CGM-derived hypoglycemic events and indices. The mean TSB <70 mg/dL was 9.2%, and <54 mg/dL was 3.7% (Table 2). The mean TSB <70 mg/dL was significantly higher during the night in comparison with during the day (10.4% vs 5.8%, P <0.05). Although TSB <54 mg/dL was numerically higher during the night (3.6%) than during the day (1.7%), the result was not statistically significant (P = 0.07). The mean AUC <70 mg/dL at night (1.9 ± 3.2) was significantly higher than during the day (1.0 ± 1.7, P = 0.03). Similarly, the mean AUC <54 mg/dL was significantly higher during night (0.7 ± 1.6) than during the day time (0.2 ± 0.5, P <0.05). The mean LBGI and GRADE hypoglycemia were also significantly higher at night (2.3 ± 3.8 and 13.1 ± 19.4, respectively) than during the day (1.3 ± 2.0 and 5.9 ± 8.6, respectively; P <0.05).
Table 3.
Baseline CGM-Derived Glycemic Parameters.
| Parameters | Whole (n = 83) |
|---|---|
| Mean | 193.3 ± 56.9; 184.3 (159.3-222.5) |
| Median | 188.4 ± 60.1; 180 (147.5-220.0) |
| SD | 57.3 ± 24.3; 55.3 (37-79.7) |
| CV % | 30 ± 11.4; 27.2 (20.5-38.2) |
| LBGI | 2.1 ± 2.7; 1 (0.4-2.4) |
| HBGI | 13.5 ± 10.3; 11.2 (5.6-18.1) |
| M100 | 268.1 ± 108.1; 252.9 (202.1-330) |
| J Index | 68 ± 36.8; 63.5 (39.1-93.3) |
| GRADE | 12.9 ± 6.6; 11.6 (8.6-16.4) |
| MAGE | 138.9 ± 57.7; 132.3 (89.6-186.2) |
| MODD | 60.6 ± 28.5; 53.4 (40.3-82.5) |
| CONGA_6hrs | 45.8 ± 20.5; 42.7 (30.3-59.5) |
Data are represented by mean ± SD for normally distributed continuous variables, while variables that were non-normal were reported as mean and SD along with median with interquartile range to understand the distribution of each variables.
CGM, continuous glucose monitoring; CONGA_6 hrs, continuous overall net glycemic action at four hours; CV, coefficient of variation; eA1c, estimated glycated haemoglobin; GRADE, glycemic risk assessment diabetes equation score; HBGI, high blood glucose index; J index, measure of quality of glycemic control; LBGI, low blood glucose index; M100 (mg/dL), weighted average of glucose values; MAGE, mean amplitude of glycemic excursions; MODD, absolute means of daily differences.
Discussion
The present study represents an important expansion of our prior work on CGM-derived measures of glycemic variability in patients with T2D and FCPD. 23 In this study, we used CGM to assess hypoglycemic events and hypoglycemia-associated indices in patients with diabetes of the exocrine pancreas. We found that the majority of patients experienced hypoglycemic events with significant alterations in hypoglycemic indices. As well, nocturnal hypoglycemic events and indices were more prevalent and unstable than those observed during the day. To our knowledge, this is the first study to evaluate various hypoglycemic indices in a large cohort with diabetes of the exocrine pancreas.
Diabetes of the exocrine pancreas is characterized by glycemic variability and instability with broad fluctuations in plasma glucose. 1 In tropical countries, the forms of diabetes of the exocrine pancreas that are most commonly observed include chronic alcoholic pancreatitis and FCPD. A high frequency of hypoglycemia has been demonstrated in patients with diabetes of the exocrine pancreas using SMBG. Studies in patients that had developed diabetes secondary to pancreatic resection reported incidences of hypoglycemia detected by SMBG in the majority of their patients.8-11 In a study involving patients who underwent pancreatectomy, severe hypoglycemia was reported in 41% of patients, and nonsevere hypoglycemia was reported in 79% of patients, 8 while a separate study reported symptomatic hypoglycemia in 50% of their patients. 10 In a retrospective analysis of patients with PPDM (secondary to alcoholism, biliary tract disease, and idiopathic etiology), hypoglycemia was detected by SMBG in 78% of patients treated with insulin. 7 A similar observation was reported in patients with diabetes secondary to hereditary pancreatitis. 24
Detailed evaluations of hypoglycemia using CGM in patients with diabetes of the exocrine pancreas are limited thus far. In our study, CGM revealed that the majority of the patients (90.4%) with diabetes of the exocrine pancreas experienced at least one hypoglycemic event. These findings are consistent with those reported by a previous study involving 60 patients with diabetes of the exocrine pancreas, in which hypoglycemic events detected with CGM were five times higher than those detected with SMBG. 17 As well, it has been shown that CGM contributes to a reduction in the number of hypoglycemic episodes through improved glycemic control.17,25-27 In a prior study, usage of CGM was associated with a 44% reduction in hypoglycemic events in patients with T1D. 25 Similarly, the use of CGM led to a reduction in hypoglycemic events in patients with diabetes post-pancreatic resection. 18
Hypoglycemic indices are useful in estimating the risk of hypoglycemia. They also assist in assessing the overall quality of glycemic control. LBGI is an indicator of risk for severe hypoglycemia in patients with diabetes, and the highest risk was seen in patients with LBGI >5. 28 In our study, the mean LBGI was 2.1, while a subset of patients with HbA1c <8.5% had a mean LBGI of 5.2. In a study involving 50 patients with T1D on continuous subcutaneous insulin infusion therapy, LBGI was 3.49 with an HbA1c of 7.5%. 29 In another study composed of patients with T1D, LBGI was 5.7 in patients with an HbA1c of <7.2, while in patients with HbA1c >9.2%, LBGI was 2.2. 30 GRADE hypoglycemia is an indicator of the degree of hypoglycemic risk, and it has been suggested that values >5 are associated with a higher risk of hypoglycemia. 31 The GRADE hypoglycemia value of 9.1 observed in this study is consistent with a value of 8.4 reported by a study composed of patients with T1D. 32 TSB represents the duration of hypoglycemia, while AUC <70 mg/dL represents hypoglycemic exposure; in normal individuals, both are close to zero. Although there are no recommended cut-off values for TSB and AUC <70 mg/dL, our study results are similar to those reported in a study composed of patients with T1D. 33 Our findings suggest that patients with diabetes of the exocrine pancreas have greater instability in hypoglycemic indices than has been previously reported in patients with T1D. This instability in hypoglycemic indices could be attributed to higher glycemic variability, exocrine insufficiency, malabsorption, and deficient counter-regulatory glucagon response in this population.34,35
Information derived from CGM is of vital importance in improving glycemic control and planning preventative strategies which include education of the patient (regarding diet, exercise, dose adjustments, self-monitoring of glucose), use of long-acting insulins (degludec, glargine) and flexible dosing, and individualized glycemic goals, help in reducing the incidence of hypoglycemia and its associated morbidity. Furthermore, CGM-derived hypoglycemic indices will guide the clinician in assessing the patient-specific risk of hypoglycemia and could help in monitoring the response to therapy.
Our study has a few limitations. First, the majority of the patients in our study belonged to the PPDM (chronic) group, and we excluded patients diagnosed with postacute pancreatitis diabetes mellitus which is the most common subtype of diabetes of the exocrine pancreas. 36 In addition, there were limited patients belonging to the PCRD group and none in the CFRD group. Further CGM-based studies might be required to assess hypoglycemic indices in PCRD and CFRD categories. Second, it is possible that the interstitial glucose readings obtained by CGM were lower compared with capillary blood glucose levels, which could have resulted in overreporting of hypoglycemic events.37,38 Lastly, hypoglycemic indices were assessed during hospital admission, and it is therefore possible that they do not reflect hypoglycemia that occurs at home. Further prospective studies are required to validate our findings in nonhospitalized patients on a stable treatment regimen.
Conclusion
Patients with diabetes of the exocrine pancreas have a high frequency of hypoglycemic episodes that predominantly occur at night. CGM is superior to SMBG in the detection of nocturnal and asymptomatic hypoglycemic episodes. CGM-derived hypoglycemic indices are beneficial in estimating the risk of hypoglycemia.
Acknowledgments
The authors wish to thank Sumitra Selvan for her help with data analysis. We would also like to thank all the participants in the study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Channabasappa Shivaprasad  https://orcid.org/0000-0003-2847-1747
https://orcid.org/0000-0003-2847-1747
References
- 1. Ewald N, Hardt PD. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J Gastroenterol. 2013;19:7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larsen S, Hilsted J, Tronier B, Worning H. Pancreatic hormone secretion in chronic pancreatitis without residual beta-cell function. Acta Endocrinol (Copenh). 1988;118:357-364. [DOI] [PubMed] [Google Scholar]
- 3. Shafiee G, Mohajeri-Tehrani M, Pajouhi M, et al. The importance of hypoglycemia in diabetic patients. J Diabetes Metab Disord. 2012;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab. 2013;17:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Snell-Bergeon JK, Wadwa RP. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol Ther. 2012;14(suppl 1): s51-s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martín-Timón I, del Cañizo-Gómez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetic patients. World J Diabetes. 2015;6:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Linde J, Nilsson LH, Barany FR. Diabetes and hypoglycemia in chronic pancreatitis. Scand J Gastroenterol. 1977; 12:369-373. [DOI] [PubMed] [Google Scholar]
- 8. Parsaik AK, Murad MH, Sathananthan A, et al. Metabolic and target organ outcomes after total pancreatectomy: Mayo Clinic experience and meta-analysis of the literature. Clin Endocrinol (Oxf). 2010;73:723-731. [DOI] [PubMed] [Google Scholar]
- 9. Maeda H, Hanazaki K. Pancreatogenic diabetes after pancreatic resection. Pancreatology. 2011;11:268-276. [DOI] [PubMed] [Google Scholar]
- 10. Jamil LH, Chindris AM, Gill KR, et al. Glycemic control after total pancreatectomy for intraductal papillary mucinous neoplasm: an exploratory study. HPB Surgery. 2012; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barbier L, Jamal W, Dokmak S, et al. Impact of total pancreatectomy: short-and long-term assessment. HPB (Oxford). 2013;15:882-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scott ES, Fulcher GR, Clifton-Bligh RJ. Sensor-augmented CSII therapy with predictive low-glucose suspend following total pancreatectomy. Endocrinol Diabetes Metab Case Rep. 2017;2017(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki S, Kajiyama H, Takemura A, et al. The clinical outcomes after total pancreatectomy. Dig Surg. 2017;34(2):142-150. [DOI] [PubMed] [Google Scholar]
- 14. Boland E, Monsod T, Delucia M, et al. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24:1858-1862. [DOI] [PubMed] [Google Scholar]
- 15. Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr. 2013;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weber KK, Lohmann T, Busch K, et al. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes. 2007;115:491-494. [DOI] [PubMed] [Google Scholar]
- 17. Ruxer J, Mozdzan M, Loba J, et al. Usefulness of continuous glucose monitoring system in detection of hypoglycaemic episodes in patients with diabetes in course of chronic pancreatitis. Pol Arch Med Wewn. 2005;114(4):953-957. [PubMed] [Google Scholar]
- 18. Okabayashi T, Nishimori I, Yamashita K, et al. Continuous postoperative blood glucose monitoring and control by artificial pancreas in patients having pancreatic resection: a prospective randomized clinical trial. Arch Surg. 2009;144:933-937. [DOI] [PubMed] [Google Scholar]
- 19. Petrov MS. Diabetes of the exocrine pancreas: American Diabetes Association-compliant lexicon. Pancreatology. 2017;17:523-526. [DOI] [PubMed] [Google Scholar]
- 20. Mohan V, Premalatha G, Pitchumoni CS. Tropical chronic pancreatitis: an update. J Clin Gastroenterol. 2003;36:337-346. [DOI] [PubMed] [Google Scholar]
- 21. Nordback I, Sand J, Andrén-Sandberg Å. Criteria for alcoholic pancreatitis. Pancreatology. 2007;7:100-104. [DOI] [PubMed] [Google Scholar]
- 22. Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [DOI] [PubMed] [Google Scholar]
- 23. Shivaprasad C, Aiswarya Y, Kejal S, et al. Comparison of CGM-derived measures of glycemic variability between pancreatogenic diabetes and type 2 diabetes mellitus. J Diabetes Sci Technol. 2019:1932296819860133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dytz MG, Marcelino PA, de Castro Santos O, et al. Clinical aspects of pancreatogenic diabetes secondary to hereditary pancreatitis. Diabetol Metab Syndr. 2017;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davey RJ, Jones TW, Fournier PA. Effect of short-term use of a continuous glucose monitoring system with a real-time glucose display and a low glucose alarm on incidence and duration of hypoglycemia in a home setting in type 1 diabetes mellitus. J Diabetes Sci Technol. 2010;4:1457-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leinung M, Thompson S, Nardacci E. Benefits of continuous glucose monitor use in clinical practice. Endocr Pract. 2010;16:371-375. [DOI] [PubMed] [Google Scholar]
- 27. Battelino T, Phillip M, Bratina N, et al. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kovatchev BP, Cox DJ, Gonder-Frederick LA, et al. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21:1870-1875. [DOI] [PubMed] [Google Scholar]
- 29. Crenier L, Abou-Elias C, Corvilain B. Glucose variability assessed by low blood glucose index is predictive of hypoglycemic events in patients with type 1 diabetes switched to pump therapy. Diabetes Care. 2013;36:2148-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsujino D, Nishimura R, Onda Y, et al. The relationship between HbA1c values and the occurrence of hypoglycemia as assessed by continuous glucose monitoring in patients with type 1 diabetes. Diabetol Metab Syndr. 2016;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hill NR, Hindmarsh PC, Stevens RJ, et al. A method for assessing quality of control from glucose profiles. Diabet Med. 2007;24:753-758. [DOI] [PubMed] [Google Scholar]
- 32. El-Laboudi AH, Godsland IF, Johnston DG, et al. Measures of glycemic variability in type 1 diabetes and the effect of real-time continuous glucose monitoring. Diabetes Technol Ther. 2016;18:806-812. [DOI] [PubMed] [Google Scholar]
- 33. Gómez AM, Henao DC, Imitola Madero A, et al. Defining high glycemic variability in type 1 diabetes: comparison of multiple indexes to identify patients at risk of hypoglycemia. Diabetes Technol Ther. 2019;21:430-439. [DOI] [PubMed] [Google Scholar]
- 34. Raue G, Keim V. Secondary diabetes in chronic pancreatitis. Z Gastroenterol. 1999;suppl 1:4-9. [PubMed] [Google Scholar]
- 35. Scavini M, Dugnani E, Pasquale V, et al. Diabetes after pancreatic surgery: novel issues. Curr Diab Rep. 2015;15:16. [DOI] [PubMed] [Google Scholar]
- 36. Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rossetti P, Bondia J, Vehí J, et al. Estimating plasma glucose from interstitial glucose: the issue of calibration algorithms in commercial continuous glucose monitoring devices. Sensors (Basel). 2010;10:10936-10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Basu A, Dube S, Slama M, et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes. 2013;62:4083-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]