Abstract
Objectives
In patients with systemic autoimmune rheumatic disorders (SARDs), vaccination with SARS-CoV-2 mRNA vaccines has been proposed. The aim of this study is to evaluate the immune response elicited by vaccination with mRNA vaccine, testing IgM, IgA and IgG antibodies to SARS-CoV-2 receptor-binding domain (RBD) and measuring neutralising antibodies.
Methods
IgG, IgM and IgA anti-RBD antibodies were measured in 101 patients with SARDs. Antibodies inhibiting the interaction between RBD and ACE2 were evaluated. Antibody avidity was tested in a chaotropic ELISA using urea. Twenty-one healthcare workers vaccinated with mRNA vaccine served as control group.
Results
Anti-RBD IgG and IgA were produced after the first dose (69% and 64% of the patients) and after the boost (93% and 83%). Antibodies inhibiting the interaction of RBD with ACE2 were detectable in 40% of the patients after the first dose and 87% after boost, compared with 100% in healthy controls (p<0.01). Abatacept and mycophenolate had an impact on the titre of IgG anti-RBD antibodies (p<0.05 and p<0.005, respectively) and on the amount of neutralising antibodies. No effect of other therapies was observed. Vaccinated patients produce high avidity antibodies, as healthy controls.
Conclusions
These data show that double-dose vaccination induced in patients with SARDs anti-RBD IgG and IgA antibodies in amounts not significantly different from controls, and, most interestingly, characterised by high avidity and endowed with neutralising activity.
Keywords: COVID-19, vaccination, autoimmune diseases
Key messages.
Previous studies showed that mRNA vaccines induce anti-SARS-CoV-2 antibodies in patients with systemic autoimmune rheumatic disorders (SARDs).
Few data are available on the functional ability of induced anti-SARS-CoV-2 antibodies.
In a monocentric cohort of patients with SARDs, vaccination with two doses of mRNA vaccine induces IgG and IgA anti-receptor-binding domain (RBD) antibodies with neutralising ability; the characterisation of antibody quality by means of avidity analysis has shown that vaccinated patients with SARDs produce anti-RBD antibodies of high avidity.
This study supports the current indications on the vaccination of patients with SARDs and further stresses the need to pay particular attention to immune suppressive treatment with mycophenolate or abatacept.
Introduction
Patients affected by systemic autoimmune rheumatic disorders (SARDs) represent a high-risk group for severe COVID-19. In those patients, in addition to known risk factors for the general population, glucocorticoids (GCs) use, immunosuppressive treatments and disease activity have been associated with an increased risk of hospitalisation and COVID-19-related mortality.1 2
Thus, considering the possible adverse course of COVID-19 in patients with SARDs and the favourable safety profile of the mRNA vaccines in the general population, scientific societies agree on the recommendation of COVID-19 vaccination in patients with SARDs.3 4
Previous studies on pneumococcal and influenza vaccination showed a marked reduction of the humoral response under treatment with anti-CD20, while scarce and controversial data are available on abatacept. Moreover, some studies showed a reduced immunogenicity of anti-pneumococcal vaccination during high doses of GCs and tofacitinib.5
Recently, several studies on patients with SARDs showed that different immunosuppressive therapies impair the immune response to SARS-CoV-2 vaccines.6–14 However, the reduced antibody response can be the result of the disease itself and not only the effect of therapy. Moreover, only limited information is available on the quality of the antibodies elicited by vaccination (eg, neutralising ability, avidity).
The aim of the present work is to evaluate the immune response elicited by vaccination with mRNA vaccine, Moderna mRNA-1273 or Pfizer BNT126b2, testing IgM, IgA and IgG antibodies to SARS-CoV-2 receptor-binding domain (RBD) and measuring the amounts of neutralising antibodies.
Patients and methods
One hundred one adult patients with an established diagnosis of SARDs eligible for SARS-CoV-2 vaccination and regularly followed at the Rheumatology Unit, Pisa University Hospital were recruited for the study.
For each patient, the following clinical data were collected at the time of enrolment in the study: age, diagnosis, disease duration, ongoing therapies (GCs, conventional disease-modifying antirheumatic drugs (cDMARDs), biological DMARDs (bDMARDs), antimalarials, intravenous immunoglobulin (IVIg)), presence of hypogammaglobulinaemia.
Diagnosis was categorised according to the following three main categories: inflammatory arthritis (IA), connective tissue diseases (CTDs), systemic vasculitis (SV).
Twenty-one healthcare workers (normal healthy subjects–NHS), vaccinated with mRNA BNT126b2, served as control group (mean age±SD=46.8±12.9; male/female=5/16).
Whole blood was collected 12–20 days after the first dose (T1) and 21 days after the second (T2). Sera were collected and kept frozen at −60°C until use.
All the patients and controls had not contracted SARS-CoV-2 infection before recruitment for the study.
Anti-RBD antibody titres
Antibodies were detected by solid phase assay, on plates coated with recombinant RBD (SARS-CoV-2 spike protein aa319–541), as previously described.15 IgG, IgM and IgA anti-RBD antibodies were measured.
Analysis of neutralising antibodies
To detect neutralising antibodies, the kit SPIA (Spike Protein Inhibition Assay, DiaMetra, Perugia, Italy) was employed according to manifacturer’s instructions. This assay is based on the competition between patient’s antibodies and the peroxidase-conjugated ACE2 for the binding to viral RBD coated on the solid phase.
Inhibition value was calculated using this formula:
% inhibition=[1−(Absorbance Sample)/(Absorbance Calibrator)]×100
Evaluation of antibody avidity
Antibody avidity was evaluated in a subgroup of 25 patients, by means of an Avidity ELISA, employing different concentrations of urea as chaotropic reagent. The Avidity Index (AI) was calculated as the extrapolated urea concentration that displaces 50% of serum binding with respect to the control wells using the approach described by Polanec et al.16
Statistical analysis
Statistical analysis was performed using IBM-SPSS 20 Statistics and GraphPad Prism statistical packages.
To evaluate the impact of therapies (GCs, cDMARDs, abatacept or anti-tumor necrosis factors agents) on anti-RBD antibody titres and neutralising antibodies after the first and the second dose, we conducted a linear mixed-effects model on the whole sample. Therapies were included as fixed effects in the model (independent variables) and anti-RBD or neutralising antibodies as dependent variables. We also evaluated interaction between therapies.
Antibody levels at different time points were compared by Kruskall-Wallis. Results of anti-RBD Ig were expressed as odds ratio (OR) of a positive internal control set at 1.0. Cut-off values have been set at the 97.5th percentile of the NHS evaluated before vaccination.
P values of <0.05 were considered significant.
Results
One hundred one patients with SARDs were enrolled in the study. Diagnosis were grouped as following: 54 CTDs, 32 IA, 15 SV. Comorbid hypogammaglobulinaemia was present in 10 patients, of these 7 were on IVIg. Demographic data, diagnosis and ongoing therapies are summarised in table 1.
Table 1.
Demographic data, diagnosis and ongoing therapies at the time of enrolment
| Whole cohort (101) |
CTDs (54) |
IA (32) |
SV (15) |
P value | |
| Female, N (%) | 78 (77) | ||||
| Age at enrolment (mean±SD) | 57.4±14.3 | 55.6±14.1 | 63.2±10.2 | 51.1±18.7 | 0.02 |
| Disease duration (mean±SD) | 12.8±10.4 | 12.7±10.6 | 16.5±11.5 | 6±5.3 | 0.04 |
| Hypogammaglobulinaemia, N (%) | 10 (9.9) | 4 (7.4) | 5 (14.2) | 1 (6.6) | ns |
| Ongoing therapies | |||||
| GCs | 36 (35.5%) | 22 (40.7%) | 6 (18.7%) | 8 (53.3%) | 0.03 |
| Daily dose prednisone equivalent (mean±SD) | 4.6±4 | 4.8±4.4 | 3.5±0.8 | 5±4.5 | ns |
| Antimalarials | 42 (41.5%) | 37 (68.5%) | 5 (15.6%) | 0 | <0.01 |
| cDMARDs | 47 (46.5%) | 24 (44%) | 19 (59.3) | 4 (26.6) | ns |
| Methotrexate | 24 (23.7%) | 5 (9.2%) | 16 (50%) | 3 (20%) | <0.01 |
| Azatioprine | 5 (4.9%) | 4 (7.4%) | 0 | 1 (6.6%) | ns |
| Mycophenolate mofetil | 9 (8.9%) | 9 (16.6%) | 0 | 0 | 0.01 |
| Cyclosporine | 4 (3.9%) | 4 (7.4%) | 0 | 0 | ns |
| Cyclophosphamide | 2 (1.9%) | 2 (3.7%) | 0 | 0 | ns |
| Tacrolimus | 1 (0.9%) | 1 (1.8%) | 0 | 0 | ns |
| Leflunomide | 2 (1.9%) | 0 | 2 (6.25%) | 0 | ns |
| cDMARDs+GC | 14 (13.8%) | 9 (16.6%) | 4 (12.5%) | 1 (6.6%) | ns |
| bDMARDs | 35 (34.6%) | 2 (3.7%) | 23 (42.6%) | 10 (66.6%) | <0.01 |
| TNF-alpha inhibitors | 20 (19.8%) | 0 | 14 (43.7%) | 6 (40%) | <0.01 |
| Tocilizumab | 3 (2.9%) | 0 | 1 (3.1%) | 2 (13.3%) | 0.02 |
| Abatacept | 8 (7.9%) | 0 | 8 (25%) | 0 | <0.01 |
| Belimumab | 2 (1.9%) | 2 (3.7%) | 0 | 0 | ns |
| Mepolizumab | 2 (1.9%) | 0 | 0 | 2 (13.3%) | 0.03 |
| IVIg | 7 (6.9%) | 6 (11.1%) | 1 (3.1%) | 0 | ns |
| Rituximab within the last 2 years* | 4 (3.9%) | 2 (3.7%) | 1(3.1%) | 1(6.6%) | ns |
| No treatment, N (%) | 3 (2.9) | ||||
*Patients received last dose of rituximab at least 5 months before vaccination.
bDMARDs, biological disease-modifying antirheumatic drugs; cDMARDs, conventional disease-modifying antirheumatic drugs; CTDs, connective tissue diseases; GCs, glucocorticoids; IA, inflammatory arthritis; IVIg, intravenous immunoglobulin; ns, not significant; SV, systemic vasculitis.
Anti-RBD antibodies in vaccinated patients
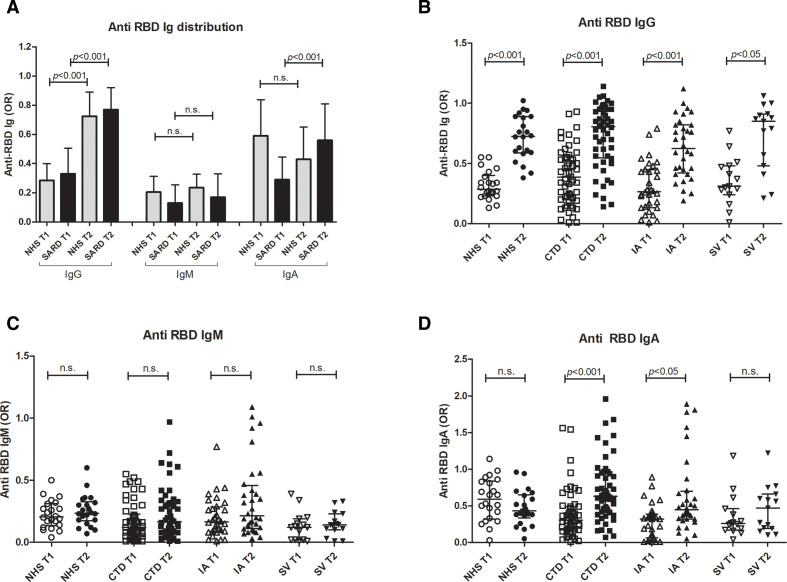
Anti-RBD IgG was produced in 69% patients after the first dose and in 93% after the second one. IgM and IgA anti-RBD were also induced by the first dose (in 25% and 64% of the patients, respectively) and the second (in 36% and 83% of the patients, respectively). Levels of anti-RBD IgG and IgA antibodies increased after the second dose, as reported in figure 1A; data obtained in vaccinated healthcare workers are also shown as control. Analysing subgroups of patients, those affected by CTD or IA or SV produced similar levels of anti-RBD antibodies, with a similar increase after boost (figure 1B–D).
Figure 1.
Distribution of anti-RBD immunoglobulins. Distribution of IgG, IgM and IgA anti-RBD in patients with SARD as compared with NHS after first dose (T1) and after the boost (T2) (A); distribution of IgG (B), IgM (C) and IgA (D) after the first dose (T1) and after the boost (T2) in patients with SARD subdivided into disease groups (CTD, IA, SV). Results are represented as OR of a positive internal control; p<0.05 was considered as significant. CTD, connective tissue disorder; IA, inflammatory arthritis; NHS, normal healthy subjects; RBD, receptor-binding domain; SARD, systemic autoimmune rheumatic disorder; SV, systemic vasculitis.
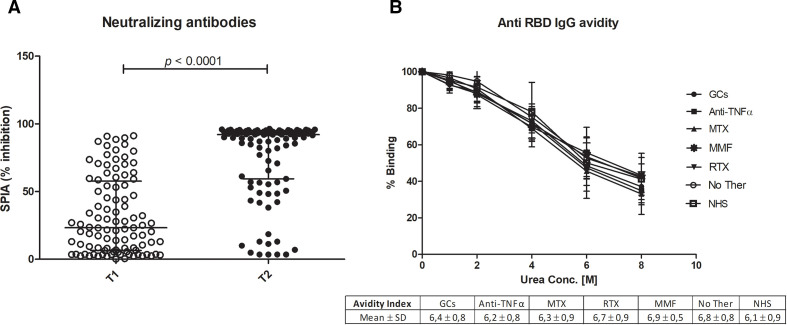
As far as the production of neutralising antibodies is concerned, antibodies inhibiting the interaction of RBD with ACE2 were detectable in 40% of the patients after the first dose and 87% after boost, compared with 100% in healthy controls (p<0.01). The level of neutralising antibodies is lower than the one observed in controls (p<0.05) and not related to diagnosis or ongoing treatments (figure 2A).
Figure 2.
Neutralising ability and avidity of anti-RBD antibodies. (A) The distribution of immunoglobulin inhibitory activity measured by SPIA kit after the first dose of vaccine (T1) and after the boost (T2). Results are expressed as the percentage of inhibition of the binding of labelled ACE2 receptor to RBD coated plates. P<0.05 was considered as significant. (B) Avidity of anti-RBD IgG from vaccinated patients grouped according to treatment (GCs, anti-TNF, MTX, MMF, RTX or No Ther) and from healthy controls (NHS). For the different urea concentrations, mean binding values and SD obtained in each patient group are represented. Avidity Index is indicated in the table below (B). GCs, glucocorticoids; MMF, mycophenolate; MTX, methotrexate; NHS, normal healthy subjects; No Ther, untreated; RBD, receptor-binding domain; RTX, rituximab; SPIA, Spike Protein Inhibition Assay.
Influence of therapies
We then analysed the effect of therapy by means of linear mixed models, comparing patients treated or not with GCs or cDMARDs or abatacept or anti-TNF agents. Patients under treatment with abatacept develop lower titres of IgG anti-RBD antibodies (p=0.02). Other therapies do not significantly affect the titre of anti-RBD or neutralising antibodies. However, when we analysed separately the 24 patients treated with methotrexate (MTX) and the 8 treated with mycophenolate (MMF) included in the cDMARD group, no effect of MTX was shown, while patients on MMF developed lower amounts of anti-RBD and neutralising antibodies (p<0.005), in a dose-dependent manner (p<0.05).
In patients receiving combination therapies, a significant decrease of antibody response is observed when anti-TNF is associated with GCs (p<0.05) or with cDMARDs (p<0.02).
Antibody avidity
Antibody avidity was evaluated by means of a chaotropic ELISA in 33 patients (3 untreated, 8 in monotherapy with GCs, 10 with anti-TNFα, 4 with MTX, 4 with MMF and 14 controls; 4 patients who have received rituximab >5 months before vaccination were also included). Results are expressed as AI, corresponding to the urea concentration that removes 50% of IgG antibodies bound to solid phase RBD. As shown in figure 2B, vaccinated patients produce high avidity antibodies after the second dose, similarly to healthy controls. Moreover, no significant differences in avidity could be ascribed to different treatments.
Discussion
In the present study, we have evaluated the response to anti-SARS-CoV-2 mRNA vaccines in a monocentric population of patients with SARDs compared with healthy controls, assessing its efficacy by measuring anti-RBD antibody titres, antibody avidity and neutralising ability.
In patients with SARDs, two-dose vaccination induced anti-RBD IgG and IgA antibodies in amounts not significantly different from controls, in the whole group of patients or in the single diseases and, most interestingly, characterised by high avidity and endowed with neutralising activity.
Other studies have assessed the response to mRNA vaccines in patients with SARDs. As far as the antibody titres induced by vaccination are concerned, different and contrasting results have been found. In some studies, no differences between patients and the general population were observed.10 12
On the contrary, lower anti S1/S2 antibody titres were obtained in Israelian patients with SARDs, after the second dose of mRNA vaccine.9 Thus, the minor differences in antibody detection (anti S1/S2 vs anti-RBD) do not explain these discrepancies, that could be due to patients’ selection and background therapies.
In addition to the antibody titres, we have explored the quality of anti-RBD antibodies by assessing their neutralising ability and their avidity. Assays that detect antibodies inhibiting the binding of RBD to ACE2 are considered a surrogate of traditional virus neutralisation assays.17 However, it is worth mentioning that the SPIA based on the co-incubation of patient’s sera and ACE on RBD is able to detect inhibitory antibodies better than sequential inhibition assays, where sera are preincubated with RBD or viral particles before being added to ACE2. Neutralising antibodies are induced in most patients with SARDs, at levels lower than in controls, similarly to what has been previously reported.12 18 Ongoing therapies, and MMF in particular, are responsible for the low production of neutralising antibodies observed in a few patients. These subjects are most likely less protected and could be the first candidate for a third vaccination dose.
On the contrary, the analysis of antibody avidity disclosed the production of high avidity antibodies, comparable with controls. The relevance of this finding is strengthened by the observation that COVID-19-infected subjects develop incomplete avidity maturation.19 Under this respect, two-dose vaccination is more effective than natural infection in inducing high affinity antibody response also in patients with SARDs.
When the influence of therapies was tested, abatacept was associated with lower titre of anti-RBD antibodies, as previously reported.9 10 Although the number of treated patients was small and precluded more refined statistical analysis, MMF markedly affected the response to vaccine, as observed in other studies.9 10 On the contrary, steroid or cDMARD (including MTX) or anti-TNF treatment did not influence vaccine-induced immune response. Conflicting data are reported on steroids and MTX, probably due to dose differences in the cohorts of patients studied.9 12 14
On the whole, these data indicate that patients with SARDs treated with mild immune suppression are able to mount an efficient immune response after mRNA SARS-CoV-2 vaccines, with the production of neutralising antibodies characterised by high avidity.
This study suffers from a number of limitations. Even if COVID-19 was not observed in any patient, the absence of a blood sample prior to vaccination does not allow to exclude asymptomatic infection. Moreover, the impact of combined therapies over antibody responses cannot be evaluated accurately because of the limited size of this cohort of patients.
Further studies on larger cohorts are needed to evaluate not only humoral but also cellular immune responses and to establish the duration of protective immunity, to plan an efficient vaccination strategy for patients with SARDs.
Acknowledgments
The authors would like to thank all the patients who participated in the study, the nurses Sabrina Gori, Rosanna Lo Coco, Lucia Pedrocco, Carla Puccini, Pasqualina Semeraro, Manuela Terachi, Maria Tristano, Valentina Venturini, Catiuscia Zoina who took care of the patients and the project manager Rossella D'Urzo.
Footnotes
CT and FP contributed equally.
PM and MM contributed equally.
Contributors: CT and FP contributed equally to this paper. PM and MM contributed equally to this paper. CT, FP, RT, PM and MM conceived and planned experiments and wrote the manuscript. MLM carried out statistical analysis. TC performed the experiments. CC, FDC, MM, NI and EL provided and cared for study patients. PM and MM supervised the project. All authors critically revised the content and approved the final version.
Funding: The work was funded by the Italian Ministry of Health (grant COVID-2020-12371849).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by Comitato Etico Area Vasta Nord Ovest (approval no 17522). Participants gave informed consent to participate in the study before taking part.
References
- 1.Akiyama S, Hamdeh S, Micic D. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis 2020;13. 10.1136/annrheumdis-2020-218946 [DOI] [PubMed] [Google Scholar]
- 2.Strangfeld A, Schäfer M, Gianfrancesco MA. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis;72. 10.1136/annrheumdis-2020-219498 [DOI] [PubMed] [Google Scholar]
- 3.Furer V, Rondaan C, Agmon-Levin N, et al. Point of view on the vaccination against COVID-19 in patients with autoimmune inflammatory rheumatic diseases. RMD Open 2021;7:e001594. 10.1136/rmdopen-2021-001594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis JR, Johnson SR, Anthony DD, et al. American College of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol 2021;73:e60–75. 10.1002/art.41928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rondaan C, Furer V, Heijstek MW, et al. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open 2019;5:e001035. 10.1136/rmdopen-2019-001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ammitzbøll C, Bartels LE, Bøgh Andersen J, et al. Impaired antibody response to the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatol 2021;3:622–8. 10.1002/acr2.11299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prendecki M, Clarke C, Edwards H, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis 2021;80:1322–9. 10.1136/annrheumdis-2021-220626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun-Moscovici Y, Kaplan M, Braun M, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis 2021;80:1317–21. 10.1136/annrheumdis-2021-220503 [DOI] [PubMed] [Google Scholar]
- 9.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. 10.1136/annrheumdis-2021-220647 [DOI] [PubMed] [Google Scholar]
- 10.Ruddy JA, Connolly CM, Boyarsky BJ, et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1351–2. 10.1136/annrheumdis-2021-220656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyarsky BJ, Ruddy JA, Connolly CM. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021. 10.1136/annrheumdis-2021-220289. [Epub ahead of print: 23 Mar 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021;80:1306–11. 10.1136/annrheumdis-2021-220272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izmirly PM, Kim MY, Samanovic M, et al. Evaluation of immune response and disease status in SLE patients following SARS-CoV-2 vaccination. Arthritis Rheumatol 2021. 10.1002/art.41937. [Epub ahead of print: 04 Aug 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis 2021;80:1339–44. 10.1136/annrheumdis-2021-220597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pratesi F, Caruso T, Testa D, et al. BNT162b2 mRNA SARS-CoV-2 vaccine elicits high avidity and neutralizing antibodies in healthcare workers. Vaccines 2021;9:672. 10.3390/vaccines9060672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polanec J, Seppälä I, Rousseau S, et al. Evaluation of protein-denaturing immunoassays for avidity of immunoglobulin G to rubella virus. J Clin Lab Anal 1994;8:16–21. 10.1002/jcla.1860080105 [DOI] [PubMed] [Google Scholar]
- 17.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol 2020;38:1073–8. 10.1038/s41587-020-0631-z [DOI] [PubMed] [Google Scholar]
- 18.Simon D, Tascilar K, Fagni F, et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis 2021;80:1312–6. 10.1136/annrheumdis-2021-220461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Struck F, Schreiner P, Staschik E, et al. Vaccination versus infection with SARS-CoV-2: establishment of a high avidity IgG response versus incomplete avidity maturation. J Med Virol 2021;93:6765–77. 10.1002/jmv.27270 [DOI] [PMC free article] [PubMed] [Google Scholar]