Abstract
Background
The persistence of the SARS-CoV2 pandemic, partly due to the appearance of highly infectious variants, has made booster vaccinations necessary for vulnerable groups. Questions remain as to which cohorts require SARS-CoV2 boosters. However, there is a critical lack of data on the dynamics of vaccine responses in patients with chronic inflammatory diseases (CID) undergoing immunosuppressive/disease modifying anti-rheumatic (DMARD) treatment. Here, we present the first data regarding the decline of the vaccine-induced humoral immune responses in patients with CID.
Methods
23 patients with CID were monitored clinically and for anti-spike IgG and IgA levels, neutralization efficacy and antigen-specific CD4+ T cell responses over the first 6 months after SARS-CoV2 vaccination. 24 healthy individuals were included as controls.
Results
While anti-spike IgG-levels declined in CID patients and healthy controls, patients receiving anti-TNF treatment showed significantly greater declines at 6 months post second vaccination in IgG and especially neutralizing antibodies. IgA levels were generally lower in CID patients, particularly during anti-TNF therapy. No differences in SARS-CoV2 spike-specific CD4+ T-cell frequencies were detected.
Conclusion
Although the long-term efficacy of SARS-CoV2 vaccination in CID patients undergoing disease-modifying therapy is still not known, the pronounced declines in humoral responses towards SARS-CoV2 6 months after mRNA vaccination in the context of TNF blockade should be considered when formulating booster regimens. These patients should be considered for early booster vaccinations.
Keywords: vaccination, COVID-19, arthritis, tumor necrosis factor inhibitors
Key message.
Decline of the humoral immune response after SARS-CoV2 mRNA vaccine seems to be faster in patients undergoing tumour necrosis factor (TNF)-alpha blockade than in both patients receiving other disease-modifying antirheumatic drugs (DMARDs) and healthy controls in our however small cohort.
Indications on the dynamics of the immune response towards SARS-CoV2 mRNA vaccines in patients with DMARD therapy for chronic inflammatory diseases.
This rapid decline has to be confirmed in larger studies. Nevertheless, it should already be taken into consideration when considering timing of booster vaccines in patients on TNF blocking agents. This accelerated loss and/or defect in memory generation during anti-TNF therapy may also occur after other vaccinations.
In light of the current discussions regarding SARS-CoV2 booster vaccinations, information on the long-term persistence of protective immunity in different cohorts is now critical for booster allocation. There is a scarcity of data available on the duration of anti-SARS-CoV2 protection in healthy individuals and even less is known about the antivaccine immune kinetics in patients on immunosuppressive therapies. While patients treated with rituximab and mycophenolate have diminished antibody titres after vaccination and increased risks of severe COVID-19,1 we and others have also shown that the immune response to mRNA SARS-CoV2-vaccines in patients on tumour necrosis factor (TNF)-alpha blockade is delayed compared with healthy controls (HCo). Hence, anti-TNF-alpha patients had significantly lower anti-spike IgG and IgA titres as well as neutralising antibody levels at day 7 post second vaccination. While this difference became insignificant at day 14 for IgG and neutralising antibodies, anti-spike IgA titres remained significantly lower on day 14. In the follow-up study of our monocentric cohort,2 23 patients with chronic inflammatory diseases and 24 HCo were included. The patients had a mean age of 49 years and 66.6% were female. HCo had a mean age of 32.4 years and 62.5% were female. 13 of the patients were on TNF alpha blocking agents (online supplemental file 1), all others on a variety of conventional and biological disease-modifying antirheumatic drugs (DMARDs) with however no patient receiving B cell depleting therapy, methotrexate, mycophenolate or abatacept. Methods have been published previsouly.2 3
rmdopen-2021-002008supp001.pdf (89.7KB, pdf)
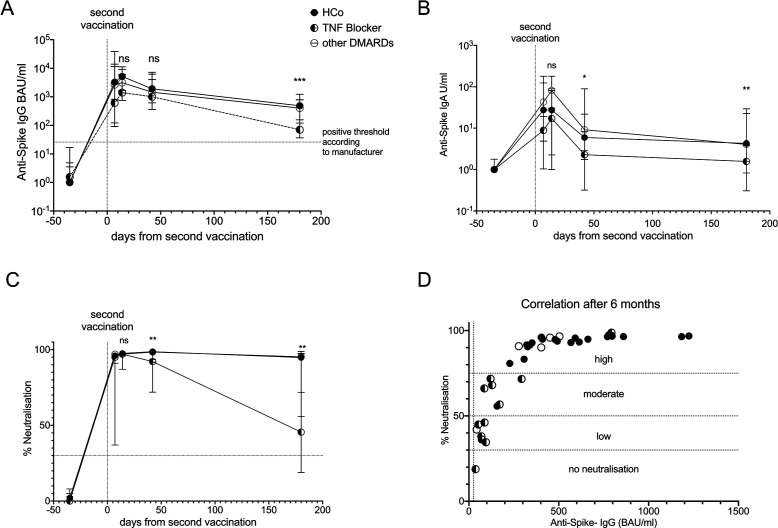
Follow-up of our monocentric cohort during the 6 months after the second vaccination revealed falls in anti-spike IgG and IgA titres in controls, anti-TNF-alpha patients and other DMARD patients. However, the 13 anti-TNF-alpha patients displayed more significant declines relative to the other groups, from a median peak anti-spike IgG titre of 1596 BAU/mL to 86.32 BAU/mL by 6 months in anti-TNF patients vs 5171 to 485 BAU/mL in controls (p<0.0001) and 3029 to 402 BAU/mL in other DMARD patients (p=0.0024) (figure 1A). Similarly, anti-spike IgA titres were consistently diminished in anti-TNF patients compared with the other groups, with anti-TNF patients falling from 17.1 to 1.6 U/mL by 6 months vs 51 to 5.0 U/mL in controls (p=0.0004) and 75.2 to 4.0 U/mL in other DMARD patients (p=0.002) (figure 1B).
Figure 1.
Decline of humoral vaccine response over time in the first 6 months after second vaccination against SARS-CoV2. (A) Anti-spike IgG antibodies increase slightly delayed in patients and decline faster. The horizontal line indicates the manufacturer’s cut-off of 25.6 BAU/mL (B) IgA anti-spike antibodies are lower in patients as compared with controls at all time points. Neutralising antibodies show a steep decline especially in patients on TNF blocking therapies as compared with healthy controls (HCo) and the remaining patients of our cohort. (D) Correlating neutralisation and IgG-levels at 180 days post second vaccination reveals a significant correlation and a low to moderate neutralisation especially in patients on TNF blocking agents as defined by the manufacturer. Median and range are depicted for all groups over time. DMARD, disease-modifying antirheumatic drug; ns, not significant; TNF, tumour necrosis factor.
The most striking difference between anti-TNF-treated patients and the other groups was observed in neutralising antibody levels. While the neutralising antibody response remained stable in controls and other DMARDs patients 6 months after secondary vaccination, anti-TNF patients dropped from a median peak of 97% neutralisation to 45% by month 6 as compared with patients on other DMARDs (p=0.001) (figure 1C). Neutralisation activity below 30% is considered negative by the manufacturer. Initial data from breakthrough infections indicate that decreasing levels of neutralising antibodies correlate with declining protection against SARS-CoV2 (4 and manufacturer’s information). Of note, 12 out of 13 of our anti-TNF patients had less than 75% neutralisation along with IgG titres below 150 BAU/mL by 6 months which correlates to low IgG titres (figure 1D). Anti-spike IgG and Neutralisation were found to correlate significantly in patients and controls at 6 months (Pearson’s r=0.73, p<0.0001).
As T cell responses likely contribute to anti-SARS-CoV2 immunity, patients were also analysed for antigen-specific T cell numbers.3 The frequency of spike-specific CD4 +T cells remained unchanged between 6 weeks and 6 months after secondary vaccination, with comparable T-cell numbers in anti-TNF patients and HCo, even in patients with the lowest humoral responses (online supplemental file 2). At 6 months, only three of the patients with the most pronounced decline in IgG and neutralising antibodies were analysed.
rmdopen-2021-002008supp002.pdf (231.7KB, pdf)
In conclusion, patients with chronic inflammatory conditions undergoing TNF-alpha blockade, who are fortunately less likely to suffer from severe COVID-19,5 should nevertheless be considered candidates for early booster vaccinations due to their significant loss of neutralising antibodies in our small cohort. This is supposed to be linked to decreased protection against SARS-CoV2.4 The additionally low anti-spike IgA levels in these patients may represent a risk factor for SARS-CoV2 persistence at mucosal surfaces, increasing the chances of viral spread. Whether the dynamics of other vaccine immune responses are similarly impaired by anti-TNF-alpha agents should be the focus of future research. As the here analysed cohort is small, these results need to be confirmed in a larger setting.
Acknowledgments
We kindly thank medac (Rainer Ahrendt, Christine Knies) for providing cPASS Test kits. Without the organisatory help of Meike Zahnen, Ina Martens and Vanessa März, this study would not have been possible.
Footnotes
Twitter: @UlfGeisen, @rheuma_doktorin
SS, PB and BFH contributed equally.
Contributors: Study design: UMG, BFH, PB, SS, SG and FT. Sample collection: DKB, FT, MS, SG, AS, RZ, JHS, SN and ACL. Experiments and data analysis: UMG, DKB, LV, HMR, MC, PB and BFH. Tables and figure: BFH, SG and UMG. Data interpretation: BFH, PB and UMG. Writing of the manuscript: BFH, UMG, PH and PJM. Critical proof reading of the manuscript: all authors.
Funding: Funded by Bundesministerium für Bildung und Forschung (Grant GAIN_01GM1910D), DIO002/CoVispecT research grant from the Land Schleswig-Holstein and by the Deutsche Forschungs-Gemeinschaft Cluster of Excellence Precision Medicine in Chronic Inflammation and Transregio 130. BFH, PH and SS received funding from Pfizer and other companies.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
The study was reviewed and approved by the Kiel medical Faculty Ethics Board D 409/21. German Clinical Trial register DRKS00024214.
References
- 1.Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis 2021;80:1345–50. 10.1136/annrheumdis-2021-220781 [DOI] [PubMed] [Google Scholar]
- 2.Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021;80:1306–11. 10.1136/annrheumdis-2021-220272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacher P, Rosati E, Esser D, et al. Low-Avidity CD4+ T Cell Responses to SARS-CoV-2 in Unexposed Individuals and Humans with Severe COVID-19. Immunity 2020;53:1258–71. 10.1016/j.immuni.2020.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021;385:1474–84. 10.1056/NEJMoa2109072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician registry. Ann Rheum Dis 2021;80:1137–46. 10.1136/annrheumdis-2021-220418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002008supp001.pdf (89.7KB, pdf)
rmdopen-2021-002008supp002.pdf (231.7KB, pdf)