Abstract
Pathological choroidal angiogenesis, a salient feature of age-related macular degeneration, leads to vision impairment and blindness. Endothelial cell (EC) proliferation assays using human retinal microvascular endothelial cells (HRMECs) or isolated primary retinal ECs are widely used in vitro models to study retinal angiogenesis. However, isolating pure murine retinal endothelial cells is technically challenging and retinal ECs may have different proliferation responses than choroidal endothelial cells and different cell/cell interactions. A highly reproducible ex vivo choroidal sprouting assay as a model of choroidal microvascular proliferation was developed. This model includes the interaction between choroid vasculature (EC, macrophages, pericytes) and retinal pigment epithelium (RPE). Mouse RPE/choroid/scleral explants are isolated and incubated in growth-factor-reduced basal membrane extract (BME) (day 0). Medium is changed every other day and choroid sprouting is quantified at day 6. The images of individual choroid explant are taken with an inverted phase microscope and the sprouting area is quantified using a semi-automated macro plug-in to the ImageJ software developed in this lab. This reproducible ex vivo choroidal sprouting assay can be used to assess compounds for potential treatment and for microvascular disease research to assess pathways involved in choroidal micro vessel proliferation using wild type and genetically modified mouse tissue.
Introduction
Choroidal angiogenesis dysregulation is associated with neovascular age-related macular degeneration (AMD)1. The choroid is a microvascular bed present underneath the retinal pigment epithelium (RPE). It has been shown that reduced blood flow in the choroid is associated with progression of AMD2. The intricate relationship between vascular endothelium, RPE, macrophages, pericytes and other cells is responsible for the homeostasis of the tissue3, 4, 5. Therefore, a reproducible assay modeling choroidal microenvironment is critical for the study of neovascular AMD.
Ex vivo angiogenesis assays and in vitro endothelial cell cultures can complement studies of microvascular behavior in vivo, for testing new drugs and for studies of pathogenesis. Endothelial cells such as human retinal microvascular endothelial cells (HRMECs), Human Umbilical Vein Endothelial Cells (HUVEC), isolated primary animal brain or retinal ECs are often used in in vitro studies for ocular angiogenesis research6, 7, 8. HRMECs in particular have been widely used as a model of in vitro choroidal neovascularization (CNV)9 by assessing endothelial proliferation, migration, tubular formation, and vascular leakage to evaluate interventions6, 10. However, ECs in culture are limited as a model of CNV because of the lack of interactions with other cell types found in the choroid and because most EC used in these assays do not originate from choroid. Mouse choroidal ECs are difficult to isolate and maintain in culture.
The aortic ring assay is widely used as a model of macro vascular proliferation. Vascular sprouts from aortic explants include ECs, pericytes and macrophages11. The aortic ring assay models large vessel angiogenesis well12, 13, 14. However, it has limitations as a model of choroidal neovascularization as aortic rings are a macrovascular tissue lacking the characteristic choroidal microvascular environment, and sprouts from large vessels may differ from sprouts from capillary networks involved in microvascular pathology. Recently a group published an ex-vivo retinal assay15, 16. Although, it is suitable for retinal neovascular disease, it is not as appropriate for choroidal neovascularization as seen in AMD.
The choroidal sprouting assay using mouse RPE, choroid, and scleral explanted tissue was developed to better model CNV. The tissue can easily be isolated from mouse (or other species) eyes17. This assay allows reproducible evaluation of pro- and anti-angiogenic potential of pharmacologic compounds and evaluation of the role of specific pathways in choroidal neovascularization using tissue from genetically modified mice and controls18. This choroidal sprouting assay has been referenced in many subsequent publications9, 10, 18, 19, 20. Here, the method involved in the use of this assay are demonstrated.
Protocol
All animal experiments described were approved by the Institutional Animal Care and Use Committee at Boston Children’s Hospital (ARCH protocol number 19–04-3913R).
1. Preparation
-
Add 5 mL of Penicillin/Streptomycin (10000 U/mL) and 5 mL and 10 mL of commercially available supplements to 500 mL of complete classic medium with serum. Aliquot 50 mL of the medium initially.
NOTE: Do not return any medium back to the stock to avoid contamination.
Put an aliquot of complete classic medium on ice.
Use 70% ethanol to clean the dissecting microscope, forceps, and scissors.
Prepare two cell culture dishes (10 cm), one on the dissection microscope, one on ice; put 10 mL of complete classic medium in each dish.
2. Experimental steps (Figure 1)
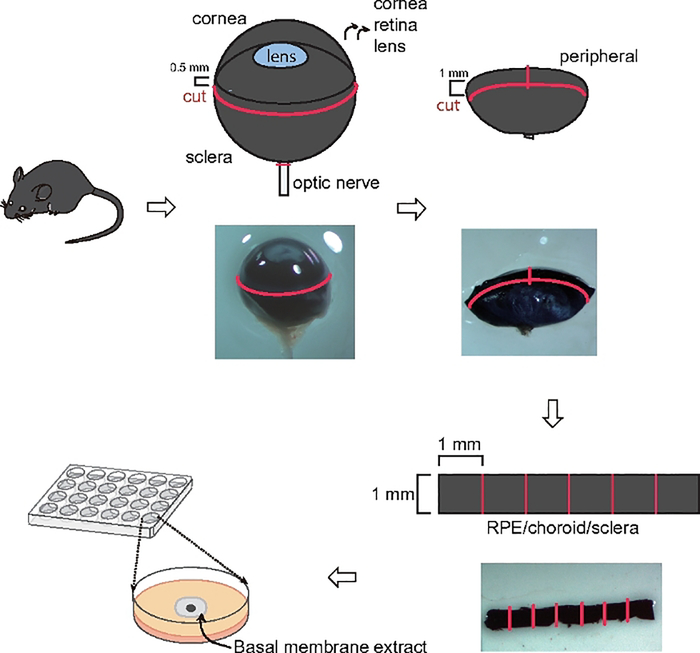
Figure 1: Schematic illustration showing choroid sprouting assay.
Eyes were first enucleated and cut circumferentially about 0.5 mm posterior to the limbus. The cornea, iris, lens and vitreous were removed. Then a 1 mm cut was made from the edge of the eye cup towards the optic nerve. A band was then cut circumferentially about 1.0 mm posterior to the cut edge and the band and the peripheral regions of the complex were separated. The band was cut into approximately 1 mm x 1 mm pieces and embedded in BME. Then using a microscope, the microvascular sprouts from the choroid were visualized.
Sacrifice C57BL/6J mice around postnatal (P) 20 using 75–100 mg/kg ketamine and 7.5–10 mg/kg xylazine injected intraperitoneally. Keep the eyes in complete classic medium on ice before dissection.
Remove the connective tissue (muscle and fatty tissue) and optic nerve on the eye.
Use a micro-scissor to circumferentially cut 0.5 mm posterior to the corneal limbus. Remove the cornea/iris complex, vitreous and the lens.
Make a 1 mm incision perpendicular to the cut edge towards the optic nerve and cut a circumferential band of 1 mm width. Separate the central and peripheral regions of the complex. Use forceps to peel off the retina from RPE/choroid/sclera complex.
Keep the peripheral choroid band in complete classic medium on ice; isolate the other eye and repeat the process to cut a second band.
-
Cut the circular band into 6 ~equal square pieces (~1 mm x 1 mm).
NOTE: Never touch any edge.
-
Thaw the basal membrane extract (BME) per manufacture’s instruction. Add 30 μL/well of BME into the center of each well of a 24 well tissue culture plate. Make sure the droplet of BME forms a convex dome at the bottom of the plate without touching the edges.
NOTE: Thaw the BME overnight in a refrigerator. BME should be on the ice any time after thawing.
-
Place the tissue in the middle of the BME.
NOTE: Do not flatten the choroid explant; generally, let the tissue expand within the BME. The orientation of the tissue (scleral side up or down) does not impact the experimental outcome.
Incubate the plate at 37 °C for 10 min to let the gel solidify.
Add 500 μL of the complete classic medium/well.
-
Change the classic medium every other day (500 μL). Choroid sprouting can be observed after 3 days with a microscope.
NOTE: For growth factor treatment, starve the tissue for 4 h. Dilute a trial compound in growth factor-reduced medium (1:200 boost instead of 1:50).
3. SWIFT-Choroid computerized quantification method 17 (Figure 2)
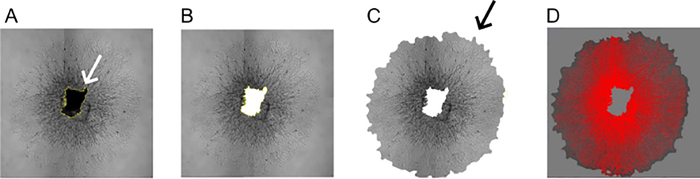
Figure 2: SWIFT-Choroid computerized quantification method.
(A,B) Magic wand function was used to outline the choroid tissue (white arrow) in the center of the sprouts and it was removed digitally (Shortcut key “F1”). (C, D) The background of the image was removed with the free selection tools (black arrow). Microvascular sprouts were then defined by using the threshold function against the background and periphery.
NOTE: A computerized method to measure the area covered by growing vessels was used. A macro plugin to ImageJ software is needed prior to quantification (see Supplemental Information for further detail).
Open the choroid sprouting image with ImageJ and check “Image | Type| 8-bit” with gray scale.
Go to “Image | Adjust | Brightness/Contrast (Ctrl/shift/C)” and optimize the contrast.
-
Use the magic wand function to outline and remove from the image the choroid tissue which are present in the center of the sprouts (using shortcut key “F1”) (Figure 2A,B).
NOTE: Set the tolerance rate of the magic wand to 20–30%.
Remove the background of the image with the free selection tools (Figure 2C). Go to “Image | Adjust | Threshold (Ctrl/shift/T)”. Use the threshold function to define the microvascular sprouts against the background and periphery (Figure 2D).
Click “F2” and a summary will appear. Save an image of the selected area by clicking “Save”. Save in the same folder as the original image for future reference.
-
After a group of samples is measured, copy the recorded for data analysis.
NOTE: It is also possible to measure the area (μm2) by “Analyze | Set Scale” using images with scale bars.
Representative Results
Comparison of choroid sprouting growth per day
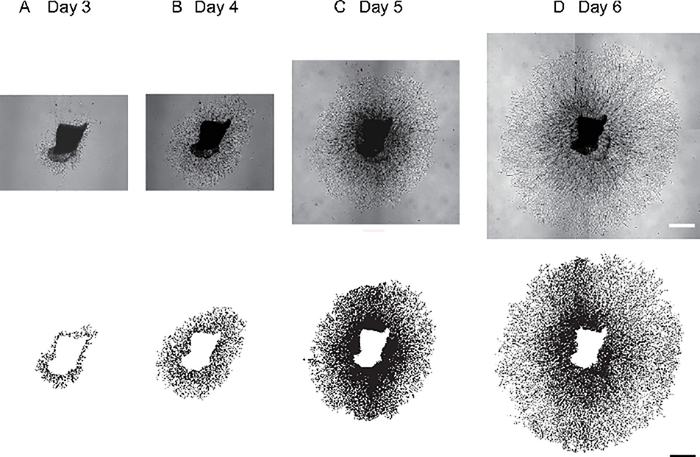
We dissected the choroid with sclera, embedded in BME and cultured them for 6 days (Figure 1). The choroid sprouting in C57BL/6J mice from day 3 to day 6 were examined with a microscope and quantified with SWIFT-Choroid a semi-automated quantification method in ImageJ. In a representative case, the choroidal sprouting area (the vessels extending from the explant, excluding the explant itself) was 0.38 mm2 at Day 3 (Figure 3A), 1.47 mm2 at Day 4 (Figure 3B), 5.62 mm2 at Day 5 (Figure 3C), and 10.09 mm2 at Day 6 (Figure 3D).
Figure 3: Mouse peripheral choroid sprouting.
(A-D) Representative images of choroid sprouts with a C57BL/6J mouse and demonstrations of SWIFT-Choroid method quantifying the area of the sprouts. The choroidal sprouting area was 0.38 mm2 at Day 3 (A), 1.47 mm2 at Day 4 (B), 5.62 mm2 at Day 5 (C), and 10.09 mm2 at Day 6 (D). Scale bar; 500 μm.
Free fatty acid receptor (FFAR)4 suppression exacerbates choroidal neovascularization ex vivo.
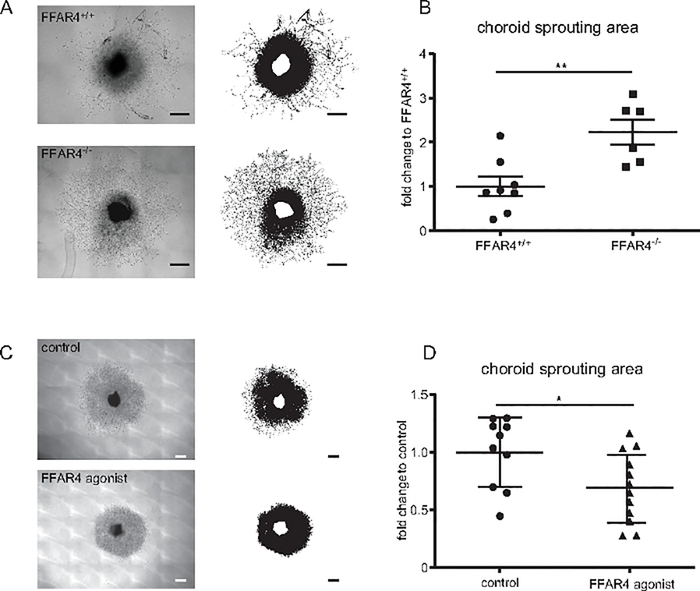
The effects of loss of FFAR4 (also known as G -protein coupled receptor 120) on choroidal vascular sprouting were evaluated using the choroid sprouting assay18. The choroid, RPE, and sclera complex were dissected from Ffar4 knock out (−/−) and Ffar4+/+ mice and cultured as described above. The sprouting area in Ffar4−/− increased choroidal vascular growth compared to Ffar4+/+ at day 6 (p = 0.004) (Figure 4A,B). The treatment of FFAR4 agonist (1 μM) reduced the choroidal sprouting area compared to untreated mice at day 6 (p = 0.03) (Figure 4C,D).
Figure 4: Free fatty acid receptor (FFAR) 4 suppression exacerbate choroidal neovascularization.
(A) Representative images of the sprouting assay with choroid from Free fatty acid receptor (Ffar)4+/+ compared with Ffar4 knock out (−/−) mice: upper image shows the choroid of Ffar4+/+ while lower image shows Ffar4−/− choroid. (B)Ffar4−/− showed increased choroid sprouting compared to Ffar4 +/+ mice (n = 6–8). (C) Representative images of choroid sprouting: upper image demonstrates vehicle treatment (control); lower image demonstrates FFAR4 agonist treatment. (D) FFAR4 agonist suppressed choroidal sprouting compared to control (n = 10–12). Scale bars = 500 μm. The data were analyzed by Student’s t-test and were expressed as mean ± SE. *p < 0.05; **p < 0.01. This figure has been modified from Tomita et al.18.
Discussion
The choroidal sprouting assay aids research in neovascular AMD9, 10, 18, 19, 20. Choroid explants can be isolated from mice as well as rats and humans17, 21. The choroid explant includes ECs, macrophages, and pericytes17. In this assay the interaction between choroidal ECs and adjacent cells such as RPE cells, help elucidate the mechanisms involved in choroidal vascular growth17. Furthermore, this reproducible and semi-automated assessment method decreases interobserver variability17.
The first published study of ex vivo choroidal tissue used isolated choroid to test pharmacological interventions with the potential to treat DR and AMD22, 23, 24, 25.
The assay counted the number and length of sprouting vessels, which can be subject to interobserver variability. In contrast, the quantification method described here has been standardized17. This assay of microvascular choroid angiogenesis includes interactive partner cells and extracellular matrix. ECs in culture may lose many of their physiological properties, such as the ability to form vascular tubes26 which may be due to the loss of interaction with other cells such as RPE. Therefore, EC in vitro cultures may not reflect all aspects of choroidal neovascularization. The aortic ring assay includes large vessel endothelial cells and interactive cells to evaluate sprouting from large vessels. But large vessel sprouts may not accurately reflect choroidal microvascular disease27. The choroidal sprouting assay is a closer representation of choroidal microvascular responses.
There are important caveats. First, the peripheral choroid complex explant sprouts are more consistent and grow much faster than explants from the central sections17. Second, the RPE from choroid was not removed because choroid sprouts with RPE grow much faster than without RPE17. To understand the impact of a drug on choroid alone, the assay without RPE may be used. Finally, when outlining the image of choroid tissue and sprouting area by using the magic wand function, it is sometimes difficult to trace the area of choroid sprouting because of high background noise. There can be variation among images. Therefore, for consistency, it is important to digitally create sufficient contrast between choroid and background as seen in Figure 2.
In summary, ex vivo choroid sprouting assay has been characterized and semi-automated. This method provides an experimental tool for AMD research. This assay can be used to screen compounds as potential treatments or to assess pathways involved in proliferating choroidal micro vessels using wild type and genetically modified mouse tissue.
Supplementary Material
Acknowledgments
The work was supported by Grants from the Manpei Suzuki Diabetic Foundation (YT), Boston Children's Hospital OFD/BTREC/CTREC Faculty Career Development Grant, Boston Children's Hospital Ophthalmology Foundation, BCH Pilot Award, BCH Manton Center Fellowship, and Little Giraffe Foundation (ZF), The German Research Foundation (DFG; to BC [CA1940/1-1]), NIH R24EY024868, EY017017, R01EY01717-13S1, EY030904-01, BCH IDDRC (1U54HD090255), Massachusetts Lions Eye Foundation (LEHS).
Footnotes
Disclosures
The authors have no financial disclosures. The computerized method is available free of charge to academic institutions through the authors.
Supplemental File 1: How to create plugins and shortcuts for the choroid sprouting assay program. Please click here to download this file.
References
- 1.Zarbin MA Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 122 (4), 598–614 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Pemp B, Schmetterer L Ocular blood flow in diabetes and age-related macular degeneration. Canadian Journal of Ophthalmology. 43 (3), 295–301 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Murakami Y, Ishikawa K, Nakao S, Sonoda KH Innate immune response in retinal homeostasis and inflammatory disorders. Progress in Retinal and Eye Research. 74, 100778 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Fu Z et al. Dyslipidemia in retinal metabolic disorders. EMBO Molecular Medicine. 11 (10), e10473 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daruich A et al. Mechanisms of macular edema: Beyond the surface. Progress in Retinal and Eye Research. 63, 20–68 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Tomita Y et al. Long-Acting FGF21 Inhibits Retinal Vascular Leakage in In Vivo and In Vitro Models. International Journal of Molecular Sciences. 21 (4), e21041188 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maisto R et al. ARPE-19-derived VEGF-containing exosomes promote neovascularization in HUVEC: the role of the melanocortin receptor 5. Cell Cycle. 18 (4), 413–424 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzoni J et al. The Wnt Inhibitor Apcdd1 Coordinates Vascular Remodeling and Barrier Maturation of Retinal Blood Vessels. Neuron. 96 (5), 1055–1069 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Z et al. Adiponectin Mediates Dietary Omega-3 Long-Chain Polyunsaturated Fatty Acid Protection Against Choroidal Neovascularization in Mice. Investigative Ophthalmology and Visual Sciences. 58 (10), 3862–3870 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Y et al. Cytochrome P450 Oxidase 2C Inhibition Adds to omega-3 Long-Chain Polyunsaturated Fatty Acids Protection Against Retinal and Choroidal Neovascularization. Arteriosclerosis, Thrombosis and Vascular Biology. 36 (9), 1919–1927 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicosia RF, Zorzi P, Ligresti G, Morishita A, Aplin AC Paracrine regulation of angiogenesis by different cell types in the aorta ring model. International Journal of Developmental Biology. 55 (4–5), 447–453 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Bellacen K, Lewis EC Aortic ring assay. Journal of Visulaized Experiments. (33), e1564 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masson VV et al. Mouse Aortic Ring Assay: A New Approach of the Molecular Genetics of Angiogenesis. Biological Procedures Online. 4, 24–31 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katakia YT et al. Ex vivo model for studying endothelial tip cells: Revisiting the classical aortic-ring assay. Microvascular Research. 128, 103939 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Rezzola S et al. In vitro and ex vivo retina angiogenesis assays. Angiogenesis. 17 (3), 429–442 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Rezzola S et al. A novel ex vivo murine retina angiogenesis (EMRA) assay. Experimental Eye Research. 112, 51–56 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Shao Z et al. Choroid sprouting assay: an ex vivo model of microvascular angiogenesis. PLoS One. 8 (7), e69552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomita Y et al. Free fatty acid receptor 4 activation protects against choroidal neovascularization in mice. Angiogenesis. 23, 385–394 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J et al. Endothelial TWIST1 promotes pathological ocular angiogenesis. Investigative Ophthalmology and Vision Science. 55 (12), 8267–8277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CH et al. Endothelial microRNA-150 is an intrinsic suppressor of pathologic ocular neovascularization. Proceedings of the National Academy of Science U. S. A. 112 (39), 12163–12168 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Q et al. LncEGFL7OS regulates human angiogenesis by interacting with MAX at the EGFL7/miR-126 locus. Elife. 8, e40470, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi S, Fukuta M, Kontani H, Yanagita S, Kimura I A quantitative assay for angiogenesis of cultured choroidal tissues in streptozotocin-diabetic Wistar and spontaneously diabetic GK rats. Japanese Journal of Pharmacology. 78 (4), 471–478 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi S et al. Inhibitory effects of tetrandrine and related synthetic compounds on angiogenesis in streptozotocin-diabetic rodents. Biological and Pharmaceutical Bulletin. 22 (4), 360–365 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi S, Shinohara H, Tsuneki H, Nagai R, Horiuchi S N(epsilon)-(carboxymethyl)lysine proliferated CD34(+) cells from rat choroidal explant in culture. Biological and Pharmaceutical Bulletin. 27 (9), 1382–1387 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi S et al. Overproduction of N(epsilon)-(carboxymethyl)lysine-induced neovascularization in cultured choroidal explant of streptozotocin-diabetic rat. Biological and Pharmaceutical Bulletin. 27 (10), 1565–1571 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Bergers G, Song S The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology. 7 (4), 452–464 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Browning AC, Stewart EA, Amoaku WM Reply to: Phenotypic plasticity of human umbilical vein endothelial cells. British Journal of Ophthalmology. 96 (9), 1275–1276 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.