Abstract
Background
Phase 2 trial endpoints that can be utilized in high-risk biochemical recurrence (BCR) after prostatectomy as a way of more rapidly identifying treatments for phase 3 trials are urgently needed. The efficacy of abiraterone acetate plus prednisone (AAP) in BCR is unknown.
Objective
To compare the rates of complete biochemical responses after testosterone recovery after 8 mo of AAP and degarelix, a gonadotropin-releasing hormone antagonist, alone or in combination.
Design, setting, and participants
Patients with BCR (prostate-specific antigen [PSA] ≥1.0 ng/ml, PSA doubling time ≤9 mo, no metastases on standard imaging, and testosterone ≥150 ng/dl) after prostatectomy (with or without prior radiotherapy) were included in this study.
Intervention
Patients were randomized to AAP (arm 1), AAP with degarelix (arm 2), or degarelix (arm 3) for 8 mo, and monitored for 18 mo.
Outcome measurements and statistical analysis
The primary endpoint was undetectable PSA with testosterone >150 ng/dl at 18 mo. Secondary endpoints were undetectable PSA at 8 mo and time to testosterone recovery.
Results and limitations
For the 122 patients enrolled, no difference was found between treatments for the primary endpoint (arm 1: 5.1% [95% confidence interval {CI}: 1–17%], arm 2: 17.1% [95% CI: 7–32%], arm 3: 11.9% [95% CI: 4–26%]; arm 1 vs 2, p = 0.93; arm 2 vs 3, p = 0.36). AAP therapy showed the shortest median time to testosterone recovery (36.0 wk [95% CI: 35.9–36.1]) relative to degarelix (52.9 wk [95% CI: 49.0–56.0], p < 0.001). Rates of undetectable PSA at 8 mo differed between AAP with degarelix and degarelix alone (p = 0.04), but not between AAP alone and degarelix alone (p = 0.12). Limitations of this study include a lack of long-term follow-up.
Conclusions
Rates of undetectable PSA levels with testosterone recovery were similar between arms, suggesting that increased androgen suppression with AAP and androgen deprivation therapy (ADT) is unlikely to eradicate recurrent disease compared with ADT alone.
Patient summary
We evaluated the use of abiraterone acetate plus prednisone (AAP) and androgen deprivation therapy (ADT), AAP alone, or ADT alone in men with biochemically recurrent, nonmetastatic prostate cancer. While more men who received the combination had an undetectable prostate-specific antigen (PSA) level at 8 mo on treatment, once men came off treatment and testosterone level rose, there was no difference in the rates of undetectable PSA levels. This suggests that the combination is not able to eradicate disease any better than ADT alone.
Keywords: Abiraterone, Androgen deprivation therapy, Androgen, Biochemical recurrence, Degarelix, Prostate cancer, Prostate-specific antigen
1. Introduction
The transformative and practice-changing trials in prostate cancer, first in metastatic castration-resistant prostate cancer and later in noncastrate or hormone-sensitive metastatic disease, used time-to-event endpoints that required large numbers of patients and years of follow-up [1], [2]. Now that multiple effective therapies are available—with even more in development—there are numerous potential combinations. Given the long natural history of biochemical recurrence (BCR) after prostatectomy, there is a need for response-based endpoints that occur early rather than time-to-event endpoints that occur late when evaluating new therapies in this population so that only the most promising ones are selected for more definitive study.
To address this, we employed a novel response endpoint—undetectable levels of prostate-specific antigen (PSA) with testosterone recovery—to assess the potential elimination of disease in patients with high-risk biochemically recurrent disease [3], [4]. Androgens are directly implicated in prostate cancer pathogenesis and progression; hence, an undetectable PSA level in the setting of recovered testosterone in a man with a history of BCR suggests that his disease is inactive.
BCR is defined as a rising PSA level with no visible disease on conventional imaging (computed tomography [CT], bone scan, or magnetic resonance imaging [MRI]), reflective of a low-volume setting where the disease is biologically less heterogeneous and oncogenic changes in the androgen receptor axis are infrequent, unlike what is found in castration-resistant disease [5], [6], [7], [8]. A wide range of prognoses is associated with this disease state. PSA doubling time is among the most consistent validated prognostic markers predicting for the development of overt metastases, and is frequently used to identify those at high risk of developing metastatic disease or symptoms, or dying of their cancer [8], [9], [10], [11], [12]. For men with a PSA doubling time of 3–9 mo, metastasis-free survival (MFS) is approximately 4 yr (95% confidence interval [CI]: 2–4), whereas for all men with BCR, the median MFS is 10 yr (95% CI: 8–14) [11].
Androgen deprivation therapy (ADT) is a standard first-line treatment for high-risk biochemically recurrent prostate cancer, but it is not curative. Cells that are resistant to ADT and survive in a low-androgen environment may ultimately regrow as castration-resistant tumors that, for most men, will be lethal. Direct profiling of residual disease in the prostate after neoadjuvant ADT demonstrates persistent PSA expression, reflecting incomplete inhibition of androgen receptor signaling, and tumor mutation profiles that may suggest early resistance [13], [14], [15], [16], [17], [18].
Abiraterone acetate is a prodrug of abiraterone, which is a selective CYP17 inhibitor of androgen biosynthesis that, when given in combination with prednisone and ADT, reduces blood and intratumoral testosterone by >1 log [14]. That the combination of abiraterone acetate plus prednisone (AAP) and ADT prolongs life is now well supported by phase 3 trials in both the castration-resistant and noncastrate/hormone-sensitive disease settings, and there are promising data in the neoadjuvant setting for high-risk localized disease, with improvements in complete pathologic response and 4-yr BCR-free survival [19], [20], [21], [22].
We hypothesized that AAP in combination with degarelix, a gonadotropin-releasing hormone antagonist, could potentially eliminate disease in patients with high-risk, biochemically recurrent prostate cancer. To test this, we sought an endpoint that could be attained in the near term, recognizing the inherent difficulties associated with MFS and overall survival (OS) endpoints, which can be affected by subsequent therapy and require many years of follow-up. To circumvent these challenges, patients were randomized and treated for 8 mo with either AAP or degarelix, or the combination, and as a benchmark for success for this trial, we selected a binary endpoint of an undetectable serum PSA level with testosterone recovery at 18 mo from treatment initiation. Achieving this endpoint would not be definitive; however, it would support moving this combination forward into larger, definitive trials [3].
2. Patients and methods
This randomized phase 2 trial (ClinicalTrials.gov identifier: NCT01751451) was conducted at Memorial Sloan Kettering Cancer Center, Johns Hopkins University, Weill Cornell Medicine, Rutgers Cancer Institute of New Jersey, Karmanos Cancer Institute, University of North Carolina at Chapel Hill, Duke University, Oregon Health & Science University, New York University, NorthShore University HealthSystem, and Urology Cancer Center and GU Research Network. The protocol was approved by the respective institutional review boards; all patients signed a study-specific informed consent form.
2.1. Patients
All patients had undergone a radical prostatectomy (RP) for localized prostate cancer and had experienced a biochemical (ie, PSA) recurrence. All had rising serum PSA levels based on at least three time points taken at least 1 wk apart, with a minimum PSA level of 1 ng/ml. PSA doubling time at the time of trial entry was ≤9 mo. A testosterone level of at least 150 ng/dl was required. CT or MRI and bone scan were performed to exclude those with metastatic disease, although pelvic or retroperitoneal lymph nodes smaller than 2 cm in short axis were allowed. Prior salvage radiation therapy and ADT of <8 mo of duration in the neoadjuvant or salvage radiation therapy setting were allowed. Required laboratory values included hemoglobin >9 g/dl, platelets 100 000 mm3, creatinine ≤1.5 mg/dl, and alanine aminotransferase (ALT)/aspartate aminotransferase (AST)/total bilirubin ≤1.5 upper limit of normal. Uncontrolled hypertension, defined as systolic blood pressure ≥160 mmHg or diastolic pressure ≥95 mmHg, was exclusionary. Concomitant medications including CYP2D6 substrates and CYP3A4 inducers were not permitted. Prior ketoconazole, abiraterone acetate, and enzalutamide were not allowed.
2.2. Study design
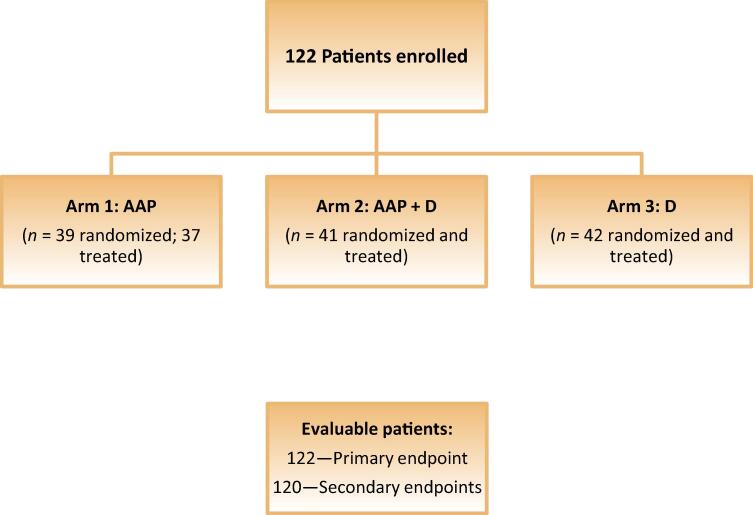
Patients were randomized in an open-label fashion 1:1:1 to receive AAP, AAP with degarelix, or degarelix alone (Fig. 1). Abiraterone acetate was administered orally as a fasting medication at 1000 mg daily, with prednisone 5 mg orally twice a day. A subcutaneous injection of degarelix 80 mg was given monthly after an initial loading dose of 240 mg. Treatment lasted for 8 mo, then patients entered follow-up and were seen monthly for toxicity assessments, serum PSA, and testosterone levels for up to 18 mo. If patients developed a confirmed rise in PSA (defined as a 25% rise above nadir) during the treatment period, they were taken off study for disease progression. During follow-up, patients were taken off study for disease progression if their PSA was detectable and confirmed detectable at a second time point. All laboratory tests were assessed locally. Five sites used an assay with a lower limit of detection of <0.06, and six sites’ assay used a lower limit of detection of 0.10.
Fig. 1.
CONSORT diagram. AAP = abiraterone acetate plus prednisone; D = degarelix.
The protocol provided guidance for dose holding and dose reductions for hepatotoxicity, hypertension, and pulmonary edema/anasarca. Serious adverse events were provided to the sponsor (Memorial Sloan Kettering Cancer Center, the lead site) and Janssen Scientific Affairs; local institutional review board notification occurred via standard site regulations.
The primary endpoint was undetectable PSA with testosterone >150 ng/dl, measured at 18 mo from the start of treatment. Secondary endpoints included the percentage of patients with undetectable PSA at 8 mo, adverse events, and testosterone recovery rates. A post hoc exploratory analysis was performed to assess PSA progression-free survival (PFS).
2.3. Statistical analysis
A total of 120 patients were planned for the three treatment groups. Each AAP group (monotherapy and in combination with degarelix) was compared with the degarelix-alone arm. With 40 patients per group, it was assumed that the probability of success in the degarelix-only group was <0.25 and the probability of success was at least 0.20 greater than degarelix alone in the AAP-based groups. Under these projections, there was a >80% chance of finding for either AAP-based treatment relative to degarelix alone using a Fisher’s exact test with a one-sided 0.20 significance level. The choice of a 0.20 significance level was intended to reduce the sample size required for this comparative trial. Owing to the high risk of a type I error, a significant outcome would not imply definitive evidence of superiority for either AAP-based treatment relative to degarelix alone; however, it would provide sufficient evidence that testing of AAP should proceed.
Fisher’s exact test was utilized to compare each AAP group (monotherapy or in combination with degarelix) relative to degarelix alone for the primary and secondary efficacy endpoints. In an exploratory analysis, the Kaplan-Meier estimate was used to compute PSA PFS estimates, and the cumulative incidence function was applied to estimate the probability of testosterone recovery over time. The logrank test and Gray’s test were used, respectively, to compare these endpoints across treatment arms. Fisher’s exact and Wilcoxon rank sum tests were conducted to assess for associations between select clinical factors (age, prior ADT, prior salvage radiation therapy, tumor stage at RP, and time from RP to study entry) and the primary endpoint.
3. Results
3.1. Patients
A total of 122 patients were randomized 1:1:1 to receive AAP, AAP with degarelix, or degarelix alone; 120 received at least one dose of study drug. Ten patients with undetectable PSA at 18 mo had not recovered testosterone, so their treatment was considered a failure. The intent-to-treat population (n = 122) was used to evaluate the primary endpoint, and the two patients who were not treated were not included in exploratory and secondary analyses.
The median age of the patients was 65 yr (range, 43–83 yr), and 43% (53/122) had Gleason ≥8 disease (Table 1). Of the patients, 57% (70/122) had pT3 or pT4 disease, and 61% (74/122) had received salvage radiotherapy. The median PSA level at trial entry was 4.4 ng/ml (range, 1.0–48.3 ng/ml).
Table 1.
Patient characteristics.
| Baseline characteristics | Arm 1: AAP (n = 39) | Arm 2: AAP + D (n = 41) | Arm 3: D (n = 42) | All cohorts(n = 122) |
|---|---|---|---|---|
| Age (yr) | 64 (43–83) | 65 (53–74) | 66 (46–78) | 65 (43–83) |
| Race Black or African American White Unknown |
4 (10) 35 (90) 0 (0) |
6 (15) 33 (80) 2 (5) |
3 (7) 39 (93) 0 (0) |
13 (11) 107 (88) 2 (1) |
| Laboratory values | ||||
| PSA (ng/ml) Testosterone ng/dl Albumin (g/dl) Alkaline phosphatase (units/l) LDH (units/l) Hemoglobin (g/dl) |
3.1 (1.2–35.4) 352 (162–739) 4.3 (3.3–4.8) 66 (29–134) 170 (96–554) 14.3 (12.3–18.9) |
5.8 (1.2–45.1) 318 (176–841) 4.3 (3.5–5.0) 66 (36–111) 170 (135–459) 14.3 (11.9–16.6) |
4.1 (1.0–48.3) 355.5 (151–904) 4.3 (3.4–4.9) 70 (42–139) 173 (121–539) 14.2 (12.4–17.2) |
4.4 (1.0–48.3) 331 (151–904) 4.3 (3.3–5.0) 67 (29–134) 170 (96–554) 14.3 (11.9–18.9) |
| ECOG performance status 1 0 |
4 (10) 35 (90) |
2 (5) 39 (95) |
3 (7) 39 (93) |
9 (7) 113 (93) |
| Prostatectomy Gleason score Unknown Total Gleason score 6 or 7 Total Gleason score ≥8 |
2 (5.1) 20 (51.3) 17 (43.6) |
– 22 (53.7) 19 (46.3) |
– 25 (59.5) 17 (40.5) |
2 (1.6) 67 (54.9) 53 (43.4) |
| TNM stage Unknown pT1-T2 N0 or NX pT3a N0 or NX pT3a N1 pT3b N0 or NX pT3b N1 pT4 NX |
2 (5) 8 (21) 13 (33) 2 (5) 10 (26) 3 (8) 1 (3) |
1 (2) 21 (51) 11 (27) 0 (0) 6 (15) 2 (5) 0 (0) |
2 (5) 18 (43) 9 (21) 1 (2) 10 (34) 2 (5) 0 (0) |
5 (4) 47 (39) 33 (27) 3 (2) 26 (21) 7 (6) 1 (1) |
| Prior therapies Salvage radiation therapy |
23 (59) |
24 (59) |
27 (64) |
74 (61) |
AAP = abiraterone acetate plus prednisone; D = degarelix; ECOG = Eastern Cooperative Oncology Group; LDH = lactate dehydrogenase; PSA = prostate-specific antigen; TNM = tumor node metastasis.
Data are given as n (%) or median (range).
3.2. Safety
Toxicities were graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [23]. The most common toxicities across all three arms were hot flashes (77%) and fatigue (51%; Table 2). Elevations in AST or ALT of any grade occurred in 15–16% of patients overall. In the AAP arms, 2–3% of patients experienced a grade 3 increase in AST and 5% experienced a grade 3 increase in ALT. Hypertension of any grade was identified in 19%, 20%, and 7% of patients in the AAP, AAP with degarelix, and degarelix arms, respectively. Ten patients experienced dose interruptions due to adverse events. Three patients had dose reductions of AAP. One patient discontinued because of transaminitis.
Table 2.
Treatment-related adverse events.a
| Adverse event | ARM 1: AAP (n = 37) |
ARM 2: AAP + D (n = 41) |
ARM 3: D (n = 42) |
All cohorts (N = 120) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Any grade | |
| Hot flashes | 21 (57) | 1 (3) | – | 28 (68) | 5 (12) | – | 35 (83) | 2 (5) | – | 92 (77) |
| Fatigue | 16 (43) | 1 (3) | – | 16 (39) | 2 (5) | – | 24 (57) | 1 (2) | 1 (2) | 61 (51) |
| Injection site reaction | – | – | – | 9 (22) | 1 (2) | – | 9 (21) | – | – | 19 (16) |
| ALT increased | 4 (11) | 2 (5) | 2 (5) | 3 (7) | 2 (5) | 2 (5) | 4 (10) | – | – | 19 (16) |
| Hypertension | 2 (5) | 4 (11) | 1 (3) | 2 (5) | 4 (10) | 2 (5) | 1 (2) | 1 (2) | 1 (2) | 18 (15) |
| AST increased | 7 (19) | 1 (3) | 1 (3) | 4 (10) | 1 (2) | 1 (2) | 3 (7) | – | – | 18 (15) |
| Insomnia | 5 (14) | – | 1 (3) | 3 (7) | – | – | 6 (14) | 1 (2) | – | 16 (13) |
| Depression | 2 (5) | – | – | 2 (5) | 1 (2) | – | 3 (7) | 2 (5) | – | 10 (8) |
| Nausea | 4 (11) | – | – | 4 (10) | – | – | – | – | – | 8 (7) |
| Hyperglycemia | – | 1 (3) | – | 1 (2) | 1 (2) | 2 (5) | – | 1 (2) | 1 (2) | 7 (6) |
| Breast pain | 5 (14) | 2 (5) | – | – | – | – | – | – | – | 7 (6) |
| Urinary frequency | 3 (8) | 1 (3) | – | – | 1 (2) | – | 1 (2) | – | – | 6 (5) |
| Anemia | 1 (3) | – | 1 (3) | 1 (2) | – | – | 3 (7) | – | – | 6 (5) |
| Gynecomastia | 4 (11) | – | – | 1 (2) | – | – | 1 (2) | – | – | 6 (5) |
| Headache | 2 (5) | 1 (3) | – | 2 (5) | – | – | 1 (2) | – | – | 6 (5) |
AAP = abiraterone acetate plus prednisone; ALT = alanine aminotransferase; AST = aspartate transaminase; CTCAE = Common Terminology Criteria for Adverse Events; D = degarelix.
Data are presented as n (%).
Adverse events are reported only if they occurred in >5% of the overall patient population. CTCAE version 4.0 was used for this trial.
3.3. Efficacy
The primary endpoint, undetectable PSA with testosterone recovery at 18 mo, was achieved in 5.1% (95% CI: 1–17%) of patients in the AAP arm, 17.1% (95% CI: 7–32%) in the AAP with degarelix arm, and 11.9% (95% CI: 4–26%) in the degarelix-only arm (Table 3). No difference was found in either AAP arm relative to degarelix (arm 1 vs 3, p = 0.93; arm 2 vs 3, p = 0.36). Ten patients had an undetectable PSA level at 18 mo, but had not recovered testosterone, and two were never treated. Sensitivity analyses were performed, and whether these patients are included as meeting the endpoint, not meeting the endpoint, or excluded from the analysis did not change the overall primary endpoint results (Supplementary Table 1).
Table 3.
Efficacy as measured by PSA.
| Treatment | Secondary endpoint: undetectable PSA at 8 mo (n = 120) | Median time to testosterone recovery a (n = 120) | Primary endpoint: undetectable PSA at 18 mo with testosterone recovery (n = 122) |
|---|---|---|---|
| Arm 1: abiraterone acetate plus prednisone | 31 (83.8) | 36 wk | 2 (5.1) |
| Arm 2: abiraterone acetate plus prednisone with degarelix | 36 (87.8) | 56 wk | 7 (17.1) |
| Arm 3: degarelix | 28 (66.7) | 53 wk | 5 (11.9) |
PSA = prostate-specific antigen.
Data are presented as n (%).
Time to testosterone recovery was calculated from treatment start.
Secondary endpoints included the percentage of patients who achieved an undetectable PSA level at 8 mo, which was highest in the AAP with degarelix arm (87.8%) and lowest with degarelix alone (66.7%), and was achieved in 83.8% of patients treated with AAP alone (Table 3). The rate of undetectable PSA levels at 8 mo differed between AAP with degarelix and degarelix-alone arms (p = 0.04), but not between AAP-alone and degarelix-alone arms (p = 0.12).
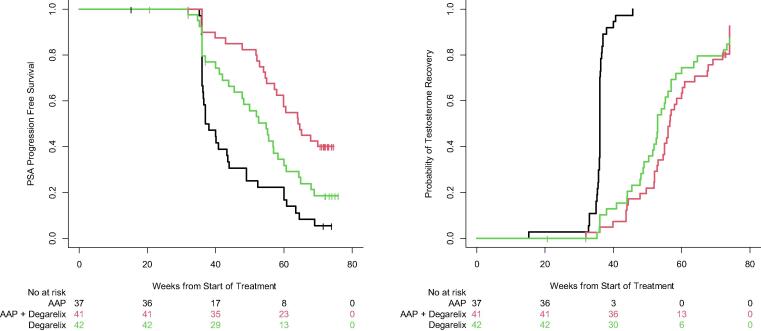
Among patients who recovered their testosterone within 18 mo, the time to PSA progression was longest in the AAP with degarelix arm (median 64.4 wk, 95% CI: 57.9–NA), and shortest in those receiving AAP alone (median 37.5 wk, 95% CI: 36.3–44.0); the degarelix-alone arm experienced PSA progression at a median of 54.9 wk (95% CI: 47.9–60.7; Fig. 2A). The median time from the start of treatment to testosterone recovery was 56 wk (95% CI: 54.6–60.3) in the combination arm, 52.9 wk (95% CI: 49.0–56.0) with degarelix, and 36 wk (95% CI: 35.9–36.1) in the AAP arm (Fig. 2B). The AAP-alone arm had a more rapid testosterone recovery than either degarelix arm (p < 0.001).
Fig. 2.
(A) Kaplan-Meier curves for PSA progression-free survival across treatment arms. The probability of developing a detectable serum PSA level once treatment was discontinued varied by study arm. On average, the abiraterone acetate arm was the soonest to develop a detectable PSA level, followed by degarelix alone, and then the combination arm. (B) Kaplan-Meier curves for the probability of testosterone recovery across treatment arms. On average, testosterone recovery occurred first in the abiraterone acetate plus prednisone arm, and then recovered at similar rates between the degarelix-alone arm and the combination arm. AAP = abiraterone acetate plus prednisone; PSA = prostate-specific antigen.
Patients who met the primary endpoint, as compared with those who did not, were more likely to be older (median age 69.5 vs 64.0 yr; p = 0.004) and have had prior salvage radiation (79% vs 47%; p = 0.04), with a longer time between RP and starting on trial (median time 270 vs 175 d; p = 0.06). There was no association between meeting the primary endpoint and prior ADT or tumor stage at the time of RP.
Data are available for bona fide researchers who request it from the authors.
4. Discussion
The more complete androgen suppression resulting from the combination of AAP and degarelix enabled 17.1% (95% CI: 7–32%) of patients with BCR to achieve an undetectable PSA level after testosterone recovery. However, this effect did not differ between treatment arms, and 11.9% (95% CI: 4–26%) of patients who received ADT alone achieved a similar outcome. In an exploratory analysis, PSA PFS was longest with combination therapy, which may in part be attributable to the longer time to testosterone recovery. The group with the shortest PSA PFS was the AAP arm that experienced testosterone recovery approximately 4–5 mo earlier than patients in the other arms. Additionally, although the PSA PFS curves separate, the difference in time to PSA PFS between the degarelix arm (median 54.9 wk, 95% CI: 47.9–60.7) and the AAP and degarelix combination arm (median 64.4 wk, 95% CI: 57.9–NA) was 10 wk, which is arguably not clinically meaningful.
The primary endpoint of the trial—undetectable PSA with testosterone recovery at 18 mo—was utilized to address the recognized need for earlier readouts of efficacy, shifting from late time-to-event-progression endpoints to earlier response endpoints based on the principles outlined by the Prostate Cancer Working Group 3 [24]. This endpoint will require more extensive study and is not a surrogate for survival; however, given the long natural history of this disease and the biologic rationale as a tumor driven by androgens, we felt it appropriate for use in this phase 2 setting [8]. A contemporary study in the phase 2 setting of salvage radiotherapy utilized a fixed course of ADT with enzalutamide and employed a 2-yr PSA PFS endpoint with expectations of testosterone recovery at that time point [25]; although the treatment and population differ from our trial, this study also utilized detectable PSA as a near-term endpoint after testosterone recovery [25]. For comparison, the pivotal phase 3 trial using a survival endpoint in high-risk biochemically recurrent disease initiated by the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) randomized 1386 men to intermittent versus continuous ADT and took nearly 14 yr to conduct [26]. Between 2003 and 2020, ten drugs were approved by the Food and Drug Administration for the treatment of castration-resistant prostate cancer; many of these are now being used or evaluated for use in earlier disease states. With more agents and combinations available for testing, the demand for efficient phase 2 trial design has increased. New life-prolonging therapies that can be administered upon progression on a clinical trial can also blunt OS outcomes.
In the contemporary setting of androgen receptor inhibitors, the EMBARK trial (NCT02319837) is enrolling 1860 patients with BCR across 200 international sites and evaluating the continuous use of ADT alone, enzalutamide alone, and enzalutamide in combination with ADT, and uses a primary endpoint of MFS. Another trial in BCR, AFT-19 (NCT03009981), will randomize 504 patients to ADT alone, ADT with apalutamide and AAP, and ADT with apalutamide for 12 mo. This trial will use a primary endpoint of PSA PFS, with MFS as a secondary endpoint. Smaller phase 2 trials such as the one reported here (122 patients) are not intended to be definitive trials; rather these utilize an economical design and response endpoint to help inform decisions for the development of these larger-scale, costly studies with long maturity times.
Despite the modest sample size, this study took longer to complete than anticipated, with 5 yr elapsing between the trial opening and data cutoff. A primary reason was prolonged accrual time due to difficulty in identifying high-risk patients. Opening additional study sites might have helped mitigate. A limitation of this trial is a lack of long-term follow-up, so the durability of PSA response and time to subsequent treatment, as well as whether this endpoint predicts for MFS or OS, are unknown. Patients who met the primary endpoint were older, and more likely to have had prior salvage radiation therapy, than those who did not meet the primary endpoint, but the sample size is too small to draw firm conclusions.
It should be noted that our understanding and characterization of biochemically recurrent disease, previously defined by conventional bone scan and CT/MRI, are changing with advances in positron emission tomography (PET) imaging such as Ga-68-labeled Glu-NH-CO-NH-Lys-(Ahx)-[Ga-68(HBED-CC)] prostate-specific membrane antigen (PSMA) PET/CT and 18F-fluciclovine PET/CT [27], [28], [29], [30], [31]. While it is recognized that patients with BCR likely harbor micrometastases, this becomes more obvious with greater imaging sensitivity and provides opportunities for trial designs. Contemporary phase 2 trials may enroll PSMA-positive/conventional scan–negative (negative for metastatic disease) patients alongside PSMA-negative/conventional scan–negative patients provided that the endpoint, such as the one utilized in this trial, captures both populations [6].
5. Conclusions
Although the primary endpoint did not differ between treatment arms, the knowledge that 12% of men achieved an undetectable PSA level after testosterone recovery with standard ADT sets a benchmark for future trials. Further study of this endpoint as it relates to OS and MFS will be necessary. Our understanding of intratumoral androgens, genomic alterations, and alternative pathways, coupled with advances in drug development, has the potential to transform the management of BCR. Efficient phase 2 trial designs with meaningful response measures will enable drug development to keep abreast of the science.
Author contributions: Karen A. Autio had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autio, Morris, Danila, Scher.
Acquisition of data: Autio, Antonarakis, Mayer, Shevrin, Stein, Vaishampayan, Morris, Slovin, Heath, Tagawa, Rathkopf, Milowsky, Harrison, Beer, Armstrong, George, Paller, Apollo, Danila, Graff, Nordquist, Dayan Cohn.
Analysis and interpretation of data: Autio, Antonarakis, Vaishampayan, Morris, Slovin, Tagawa, Rathkopf, Milowsky, Balar, Armstrong, Paller, Danila, Graff, Tse, Schreiber, Scher.
Drafting of the manuscript: Autio, Antonarakis, Slovin, Tagawa, Balar, Armstrong, Danila, Graff, Scher.
Critical revision of the manuscript for important intellectual content: Autio, Antonarakis, Mayer, Stein, Vaishampayan, Morris, Heath, Tagawa, Rathkopf, Milowsky, Harrison, Beer, Balar, Armstrong, George, Paller, Apollo, Graff, Nordquist, Schreiber, Scher.
Statistical analysis: Heller.
Obtaining funding: Scher.
Administrative, technical, or material support: Autio, Antonarakis, Beer, Morris, Tagawa, Armstrong, Dayan Cohn, Tse, Schreiber.
Supervision: Autio, Beer, Armstrong, Paller, Scher.
Other: None.
Financial disclosures: Karen A. Autio certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Antonarakis: consultant and honorarium recipient—Amgen, Astellas, AstraZeneca, Clovis, Dendreon, Eli Lilly and co., ESSA, Janssen, Medivation, and Sanofi; consultant without honorarium—Bayer; research funding to institution—AstraZeneca, BMS, Celgene, Clovis, Dendreon, Genetech, Janssen, Merck, Novartis, Sanofi, and Tokai; patent holder/licenser: Qiagen. Apollo: no disclosures. Armstrong: research funding to institution—Janssen, Pfizer/Astellas, BMS, Merck, AstraZeneca, Genentech/Roche, Forma, Celgene, Amgen, Beigene, Constellation, Bayer, and Dendreon; consulting—Janssen, Pfizer, Astellas, BMS, Merck, AstraZeneca, Bayer, Dendreon, and Forma. Autio: research funding to institution—CytomX, AstraZeneca, Pfizer, Amgen, GlaxoSmithKline, Parker Institute for Cancer Immunotherapy, and Trishula. Balar: consultant/advisory role—Incyte, BMS, Janssen, Merck, Pfizer, Astra Zeneca, Nektar, Seagen, and Immunogenics/Gilead; research funding to institution—Genetech, Merck, AstraZeneca, Nektar, Seagen, and Immunogenics/Gilead; equity—EpiVax Oncology. Beer: stock and other ownership interests—Arvinas and Salarius Pharmaceuticals; consulting or advisory role—Astellas, Arvinas, AstraZeneca, Bayer, BMS, Clovis, Constellation Pharmaceuticals, GRAIL, Janssen, Novartis, Pfizer, Sanofi, Tolero, and Myovant Sciences; research funding to institution—Alliance Foundation Trials, Astellas, Boehringer Ingelheim, Corcept Therapeutics, Endocyte, Freenome, GRAIL, Harpoon Therapeutics, Janssen, Medivation, Sotio, Theraclone Sciences, Zenith Epigenetics, and Bayer. Danila: research funding to institution—US Department of Defense, American Society of Clinical Oncology, Prostate Cancer Foundation, Stand Up 2 Cancer, Janssen, Astellas, Medivation, Agensys, Genentech, and CreaTV; consulting—Angle LLT, Axiom LLT, Janssen Research & Development, Astellas, Medivation, Pfizer, Genzyme, and Agensys. Dayan: no disclosures. George: consultant—Astellas, AstraZeneca, Bayer, BMS, Constellation Pharmaceuticals, Exelixis, Flatiron, Janssen, Merck Sharp and Dohme, Michael J Hennessey Associates, Myovant Sciences Inc., Physician Educational Research LLC, Pfixer, Propella TX, RevHealth, and Sanofi; research funding to institution—Astellas, AstraZeneca, BMS, Calithera, Exelixis, Janssen, Novartis, Pfizer, and Sanofi; advisory board—Astellas, AstraZeneca, Capio Biosciences, Modra Pharmaceuticals BV, UroGPO, and UroToday; honorarium—Bayer, EMD Serono, Exelixis, Ipsen, Michael J Hennessey Associates, and Sanofi; speaker—Bayer, Exelixis, Pfizer, and Sanofi; travel—Bayer, Exelixis, UroToday, and Sanofi. Graff: research funding to institution—Merck, Sanofi, Astellas/Pfizer, and Janssen. Harrison: research funding to institution—Acerta, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Exelixis, Clovis Oncology. Merck, Pfizer, and Seattle Genetics; consulting—AstraZeneca, Bayer, Bristol Myers Squibb, Exelixis, FujiFilm, Genentech, Immunogenics, Janssen, Pfizer, and Seattle Genetics; promotional speaking—Exelixis and Genentech. Heath: honoraria—Bayer, Sanofi, and Seattle Genetics; consulting/advisory role—Astellas; advisory board—Astra Zeneca, BMS, and Sanofi; paid travel—Astellas, Caris Life Sciences, Sanofi, and Seattle Genetics; research funding to institution—Astellas Pharma, AstraZeneca, Boehringer Ingelheim, BMS, Caris Life Sciences, Celgene, Celldex, Corcept, Curemeta, Dendreon, eFFECTOR Therapeutics, Esanik, Fortis Therapeutics, Genetech/Roche, GSK, Ignyta, Inovio Pharmaceuticals, Medivation, Merck Sharp and Dohme, Merck, Millenium, Oncolys BioPharm, Plexxicon, Seattle Genetics, Synta, Tokai Pharmaceuticals, and Zenith Epigenetics. Heller: no disclosures. Mayer: honoraria—EMD Serono, and Exelixis; consulting—AstraZeneca; research funding to institution—Sotio, Merck, and Pfizer/EMD Serono. Milowsky: research funding to institution—Merck, Roche/Genetech, BMS, Astellas, Clovis, Inovio, Mirata, Constellation Pharmaceuticals, Syndax, Incyte, Amgen, Regeneron, Arvinas, Seagen, Pfizer, Johnson, and Johnson/Janssen. Morris: uncompensated consultant: Advanced; accelerator applications—Johnson and Johnson, and Novartis; consultant (<5K): NCCN, Curium, and Oric; research funding to institution—Bayer, Sanofi, Endoycte, Progenics, Corcept, Roche/Genentech, and Janssen. Nordquist and Paller: no disclosures. Rathkopf: consulting or advisory role (all uncompensated)—Genentech/Roche, Janssen Oncology, AstraZeneca, and Myovant; research funding to institution—AstraZeneca, Celgene, Ferring, Genentech/Roche, Janssen Oncology, Medivation, Millennium, Novartis, Taiho Pharmaceutical, Takeda, TRACON Pharma, and Phosplatin. Scher: compensated Board of Directors’ member—Asterias Biotherapeutics; compensated consultant/advisor—Ambry Genetics Corp, Konica Minolta Inc., Bayer, Pfizer Inc., Sun Pharmaceuticals, and WCG Oncology; uncompensated consultant/advisory—Amgen, ESSA Pharma Inc. Janssen Research & Development, LLC, Janssen Biotech, Inc., and Sanofi Aventis; research funding (to institution)—Epic Sciences, Illumina, Inc., Janssen, Menarini Silicon Biosystems, Prostate Cancer Foundation, and ThermoFisher; intellectual property rights—BioNTech, Elucida Oncology, MaBVAX, and Y-mAbs Therapeutics, Inc.; nonfinancial support—Amgen, Asterias Biotherapeutics, Bayer, ESSA Pharma Inc., Menarini Silicon Biosystems, Phosplatin, Pfizer Inc., Prostate Cancer Foundation, and WCG Oncology. Schreiber: no disclosures. Shevrin: speakers—Sanofi and Bayer. Slovin: honoraria for advisory boards/educational programs—Novartis, Janssen, Pfizer, Merck, Sanofi-Aventis, Clovis, and Physician Education Resource; research funding to institution—Sanofi-Aventis, Novartis, Poseida Pharma, Immunomedex, and Prostate Cancer Foundation. Stein: consulting/advisory role—Merck Sharp and Dohme, Exelixis, Xencor, Janssen, and Oxford Oncology; research funding to institution—Oncoceutics, Merck Sharp and Dohme, Janssen, Medivation/Astellas, Advaxis, Suzhou Kintor Pharmaceuticals, Harpoon, BMS, Genococea Biosciences, Lilly, Nektar, Seattle Genetics, Xencor, Tmunity, and Exelixis. Tagawa: honoraria—Sanofi, Medivation/Astellas, Dendreon, Janssen, Genentech, Bayer, Endocyte, Eisai, Immunomedics, Karyopharm, Abbvie, Tolmar, Seattle Genetics, Amgen, Clovis, QED, Pfizer, AAA/Novartis, Clarity, Genomic Health, POINT Biopharma, Blue Earth Diagnostics, AIkido Pharma, and Gilead Sciences; research funding to institution—Sanofi, Medivation, Astellas, Janssen, Amgen, Progenics, Dendreon, Lilly, Genentech, Newlink, BMS, Inovio, AstraZeneca, Immunomedics, Aveo, Rexahn, Atlab, Boehringer Ingelheim, Millennium, Bayer, Merck, Abbvie, Karyopharm, Endocyte, Clovis, Seattle Genetics, AAA/Novartis, POINT Biopharma, and Gilead Sciences. Tse: no disclosures. Vaishampayan: research funding to institution—BMS, Exelixis, and Merck; consulting and honoraria—AAA, Alkermes, Bayer, BMS, EMD Serono, Pfizer, Sanofi, Exelixis, and Merck.
Funding/Support and role of the sponsor: This research was funded in part through an NIH/NCI Cancer Center Support Grant to Memorial Sloan Kettering (P30 CA008748) and Janssen Pharmaceuticals, Inc. Memorial Sloan Kettering authors were supported in part by grants from the NIH/NCI (Cancer Center Support Grant P30 CA008748, the SPORE in Prostate Cancer P50 CA092629) and Congressionally Directed Medical Research Programs (W81XWH-18-2-0060) as well as the Sidney Kimmel Center for Prostate and Urologic Cancers, and David H. Koch through the Prostate Cancer Foundation. Emmanuel S. Antonarakis was partially funded by Department of Defense (Congressionally Directed Medical Research Program) grants W81XWH-13-PCRP-CCA and W81XWH-16-PCRP-CCRSA, and by National Institutes of Health (Cancer Center support grant P30 CA006973).
Acknowledgments
Acknowledgments
We thank Margaret McPartland, BA, an independently supported editor at Memorial Sloan Kettering.
Portions of this work were previously presented at the American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, June 1–5, 2018.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2021.09.015.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Fizazi K., Scher H.I., Molina A., et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 2.Ryan C.J., Smith M.R., de Bono J.S., et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teo M.Y., O'Shaughnessy M.J., McBride S.M., Vargas H.A., Scher H.I. Drug development for noncastrate prostate cancer in a changed therapeutic landscape. Nat Rev Clin Oncol. 2018;15:168–182. doi: 10.1038/nrclinonc.2017.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris M.J., Hilden P., Gleave M.E., et al. Efficacy analysis of a phase III study of androgen deprivation therapy (ADT) +/- docetaxel (D) for men with biochemical relapse (BCR) after prostatectomy. J Clin Oncol. 2015;33:5011. [Google Scholar]
- 5.Abeshouse A., Ahn J., Akbani R., et al. The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson D., Van Allen E., Wu Y.-M., et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abida W., Armenia J., Gopalan A., et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. 2017;2017 doi: 10.1200/PO.17.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher H.I., Eisenberger M., D'Amico A.V., et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22:537–556. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 9.Freedland S.J., Humphreys E.B., Mangold L.A., et al. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25:1765–1771. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 10.Slovin S.F., Wilton A.S., Heller G., Scher H.I. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res. 2005;11:8669–8673. doi: 10.1158/1078-0432.CCR-05-1668. [DOI] [PubMed] [Google Scholar]
- 11.Antonarakis E.S., Feng Z., Trock B.J., et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109:32–39. doi: 10.1111/j.1464-410X.2011.10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brockman J.A., Alanee S., Vickers A.J., et al. Nomogram predicting prostate cancer-specific mortality for men with biochemical recurrence after radical prostatectomy. Eur Urol. 2015;67:1160–1167. doi: 10.1016/j.eururo.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan C.J., Smith A., Lal P., et al. Persistent prostate-specific antigen expression after neoadjuvant androgen depletion: an early predictor of relapse or incomplete androgen suppression. Urology. 2006;68:834–839. doi: 10.1016/j.urology.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Mostaghel E.A., Cho E., Zhang A., et al. Association of tissue abiraterone levels and SLCO genotype with intraprostatic steroids and pathologic response in men with high-risk localized prostate cancer. Clin Cancer Res. 2017;23:4592–4601. doi: 10.1158/1078-0432.CCR-16-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzbeierlein J., Lal P., LaTulippe E., et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montgomery R.B., Mostaghel E.A., Vessella R., et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Titus M.A., Schell M.J., Lih F.B., Tomer K.B., Mohler J.L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 18.Stanbrough M., Bubley G.J., Ross K., et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 19.Fizazi K., Chi K.N. Abiraterone in metastatic prostate cancer. N Engl J Med. 2017;377:1697–1698. doi: 10.1056/NEJMc1711029. [DOI] [PubMed] [Google Scholar]
- 20.McKay R.R., Montgomery B., Xie W., et al. Post prostatectomy outcomes of patients with high-risk prostate cancer treated with neoadjuvant androgen blockade. Prostate Cancer Prostatic Dis. 2018;21:364–372. doi: 10.1038/s41391-017-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taplin M.-E., Montgomery B., Logothetis C.J., et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol. 2014;32:3705–3715. doi: 10.1200/JCO.2013.53.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fizazi K., Tran N., Fein L., Matsubara N., Rodriguez-Antolin A., Alekseev B.Y., et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 23.NIH. National Cancer Institute. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
- 24.Scher H.I., Morris M.J., Stadler W.M. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bitting RL, Healy P, George DJ, et al. Phase II trial of enzalutamide and androgen deprivation therapy with salvage radiation in men with high-risk prostate-specific antigen recurrent prostate cancer: the STREAM trial. Eur Urol Oncol. In press. 10.1016/j.euo.2020.01.005. [DOI] [PubMed]
- 26.Crook J.M., O'Callaghan C.J., Duncan G., Dearnaley D.P., Higano C.S., Horwitz E.M., et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bashir U., Tree A., Mayer E., et al. Impact of Ga-68-PSMA PET/CT on management in prostate cancer patients with very early biochemical recurrence after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2019;46:901–907. doi: 10.1007/s00259-018-4249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu S.Y., Boreta L., Shinohara K., et al. Impact of staging (68)Ga-PSMA-11 PET scans on radiation treatment plans in patients with prostate cancer. Urology. 2019;125:154–162. doi: 10.1016/j.urology.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 29.Andriole G.L., Kostakoglu L., Chau A., et al. The impact of positron emission tomography with 18F-fluciclovine on the treatment of biochemical recurrence of prostate cancer: results from the LOCATE trial. J Urol. 2019;201:322–331. doi: 10.1016/j.juro.2018.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fendler W.P., Calais J., Eiber M., et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856. doi: 10.1001/jamaoncol.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceci F., Castellucci P., Graziani T., et al. (68)Ga-PSMA-11 PET/CT in recurrent prostate cancer: efficacy in different clinical stages of PSA failure after radical therapy. Eur J Nucl Med Mol Imaging. 2019;46:31–39. doi: 10.1007/s00259-018-4189-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.