Abstract
Targeted mutagenesis of the glucocorticoid receptor has revealed an essential function for survival and the regulation of multiple physiological processes. To investigate the effects of an increased gene dosage of the receptor, we have generated transgenic mice carrying two additional copies of the glucocorticoid receptor gene by using a yeast artificial chromosome. Interestingly, overexpression of the glucocorticoid receptor alters the basal regulation of the hypothalamo-pituitary-adrenal axis, resulting in reduced expression of corticotropin-releasing hormone and adrenocorticotrope hormone and a fourfold reduction in the level of circulating glucocorticoids. In addition, primary thymocytes obtained from transgenic mice show an enhanced sensitivity to glucocorticoid-induced apoptosis. Finally, analysis of these mice under challenge conditions revealed that expression of the glucocorticoid receptor above wild-type levels leads to a weaker response to restraint stress and a strongly increased resistance to lipopolysaccharide-induced endotoxic shock. These results underscore the importance of tight regulation of glucocorticoid receptor expression for the control of physiological and pathological processes. Furthermore, they may explain differences in the susceptibility of humans to inflammatory diseases and stress, depending on individual prenatal and postnatal experiences known to influence the expression of the glucocorticoid receptor.
The glucocorticoid receptor (GR) is a ligand-dependent transcription factor which belongs to the nuclear hormone receptor superfamily. Due to its almost ubiquitous expression, GR plays an important role during development and in many physiological and pathological processes. These include regulation of energy homeostasis, adaptation to stress, and modulation of central nervous system functions (16). In addition, GR is a major modulator of the immune system due to its proficient antiinflammatory and immunosuppressive activities, thus serving a function which is frequently put to use in the treatment of inflammatory diseases, autoimmune disorders, and leukemia with glucocorticoids (2).
To study the role of GR in more detail, several mutant mouse strains have been generated by gene targeting (24, 32). The analysis of GR knockout mice has revealed a pivotal role for the receptor both in lung maturation and as the negative feedback control of the hypothalamo-pituitary-adrenal (HPA) axis (5, 14, 23). Furthermore, analysis of mice selectively lacking GR in the nervous system has revealed an important function of the GR in the brain for processes such as reacting to anxiety (31). A gene targeting approach was also taken to study the relative importance of different modes of action of the receptor. Specifically, mice that carried a point mutation in one of the dimerization domains of the GR, resulting in a DNA-binding-defective receptor, were generated (22). Surprisingly, analysis of these mice has shown that DNA-binding-dependent transactivation was dispensable not only for survival but also for the regulation of many physiological processes, such as thymocyte apoptosis.
In addition to a large number of loss-of-function experiments, there is a growing body of evidence suggesting that an increased gene dosage may also have profound effects on physiology and development. This was exemplified for the Pax-6 (28) and the Zipro-1 (36) genes. In the case of Pax-6, overexpression from a yeast artificial chromosome (YAC) led to abnormalities of the eyes, whereas additional copies of Zipro-1 expressed from a bacterial artificial chromosome caused a proliferation defect in cerebellum and skin. The latter observation was particularly unexpected given that Zipro-1 knockout mice lack an obvious phenotype (36). In the case of GR, evidence to date also suggests a gene dosage effect. Specifically, the magnitude of the transcriptional response elicited by GR in vitro was shown to be proportional to the number of receptor molecules per cell (34). Furthermore, heterozygous GR knockout mice show differences in the control of the HPA axis (5). Collectively, these data suggest that overexpression of GR by introduction of additional alleles into mice may lead to alterations in gene expression and physiological responses.
Expression of classical plasmid transgenes in mice is often variable and low and does not necessarily reflect the endogenous expression pattern of the gene. These limitations can be circumvented by using YACs (27). YACs span up to 1 Mb of genomic sequences and allow transfer of a transgene within an almost natural chromosomal context due to the large stretches of flanking sequences which protect the gene from position effects at the integration site (3, 17, 26). Therefore, this approach usually guarantees expression of the transgene in a copy number-dependent and position-independent manner (27). Furthermore, due to their large size, YACs are an ideal vector system for introduction of genes such as that for the GR, which spans at least 110 kb (29). Consequently, we have used a 290-kb YAC which covers the entire Gr locus to generate GR-overexpressing mice. Significantly, neuroendocrine regulation, the sensitivity of thymocytes to glucocorticoid-induced apoptosis, and the responses to stress and inflammation are severely altered in these transgenic mice. Thus, our results allow new insights into the mechanisms of GR in physiological and pathological processes.
MATERIALS AND METHODS
Isolation, characterization, and modification of Gr YAC.
A YAC library from C57BL/6 mouse DNA in the yeast strain AB1380 (Research Genetics, Huntsville, Ala.) was screened by PCR using two primers specific for Gr exon 2. This resulted in the isolation of three independent Gr YAC clones. One clone, designated YGR4, with an insert length of 620 kb, was transferred from the library's host strain to YPH925 by kar cross. The relative location of the Gr gene on the YAC could then be determined after electroporation with the fragmentation vector pHIS3-TelGR and subsequent analysis of His-autotroph colonies by pulsed-field gel electrophoresis (PFGE). The size of the homologously recombined YACs was compared to the size of YGR4 and from that comparison the relative position of the Gr locus was deduced. To shorten the YAC, a YPH925 clone carrying YGR4 was electroporated with the B1 element-containing fragmentation vector pCEN/HIS3-TelB1, and histidine-autotroph clones were analyzed by PFGE. Two clones contained a 290-kb YAC, and one of the two was chosen for microinjection.
Generation of transgenic mice.
YAC DNA was purified for microinjection as previously described (10). Conditions for preparative PFGE were 1% agarose in 0.5× Tris-agarose-EDTA buffer, 14°C, 6 V/cm, 120°, and a time ramp of 10 to 60 s for 20 h (CHEF-DR III system; Bio-Rad).
RNA analysis.
Total RNA was isolated after guanidinium isothiocyanate extraction according to standard procedures. cDNA synthesis for reverse transcription (RT)-PCR analysis (20) and in situ hybridization (22) was performed as described previously.
Restriction fragment length polymorphism (RFLP) analysis.
An additional RsaI site in the 3′ region of the Gr gene due to the different origins of the YAC DNA (C57BL/6 mouse strain) and the oocytes used for microinjection (FVB/N mouse strain) was identified in the FVB/N allele. To distinguish between the endogenous Gr allele and the one derived from the YAC, either genomic DNA or a DNA fragment obtained by RT-PCR was digested with RsaI and analyzed by Southern blotting using a probe specific for the GR 3′ untranslated region.
Protein analysis.
For Western blot analysis, proteins were extracted in radioimmunoprecipitation assay buffer and 30 μg of the whole-cell extracts was resolved on a denaturing sodium dodecyl sulfate–7.5% polyacrylamide gel. Proteins were transferred to a polyvinylidene difluoride membrane and stained with a GR-specific antibody (M-20; Santa Cruz Biotechnology, Santa Cruz, Calif.), and immunoreactive bands were visualized by enhanced chemiluminescence.
Hormone and cytokine measurements.
Prior to the analyses, mice were kept in a quiet place under a constant dark-light cycle. Blood was quickly collected from the trunk after decapitation (at 9:00 a.m. for basal hormone measurements). The serum was isolated by centrifugation and stored in aliquots at −80°C. Concentrations of corticosterone and adenocorticotrope hormone (ACTH) were determined using commercially available radioimmunoassay kits (ICN, Meckenheim, Germany) according to the manufacturer's instructions. Titers of interleukin-6 (IL-6) were analyzed using an enzyme-linked immunosorbent assay kit (Endogen, Woburn, Mass.). Synthetic ACTH1-24 stimulation was performed by intraperitoneal injection of vehicle (phosphate-buffered saline [PBS]) or ACTH (10 μg/kg of body weight). Blood was collected 20 min after the injection by tail phlebotomy.
Immunohistochemistry.
Tissues were fixed in phosphorate-buffered 4% paraformaldehyde overnight at 4°C, dehydrated through an ascending ethanol series, and embedded in paraffin. Immunostaining was performed on 6-μm-wide sections using polyclonal antibodies against ACTH or corticotropin-releasing hormone (CRH). Antibody reactivity was visualized using a goat anti-rabbit immunoglobulin G conjugated with horseradish peroxidase and 3,3′-diaminobenzidine.
Quantification of in situ hybridization and immunohistochemistry.
The area positively immunostained or occupied by silver grains was taken as a measure for peptide and mRNA expression. Quantitative analysis of the signals was performed using an image processing system as described previously (14).
Thymocyte apoptosis.
Culturing of primary thymocytes as well as detection and evaluation of apoptosis was performed as previously described (18, 22). Briefly, aliquots of cells were cultivated at a concentration of 4 × 106 cells/ml in complete RPMI medium containing 10% heat-inactivated fetal calf serum plus the respective concentrations of dexamethasone (10−5 to 10−9 M) and ZK112,339 (10−6 M) (35) for 9 and 24 h each. Thymocytes were harvested and resuspended in 300 μl of a propidium iodide (PI) solution (50 μg/ml) containing 0.1% Triton X-100–0.1% sodium citrate. The samples were incubated overnight at 4°C and subsequently analyzed on a FACScalibur instrument (Becton Dickinson, San Jose, Calif.). Gated cells (excluding duplets) were evaluated by their FL-2 area versus side scatter (SSC) pattern. Based on low PI fluorescence and high SSC, cells considered sub-G1 and apoptotic were gated in region 2 and the percentage of apoptotic cells was calculated from the total number of gated cells.
RESULTS
Generation of YAC transgenic mice carrying two additional copies of the Gr gene.
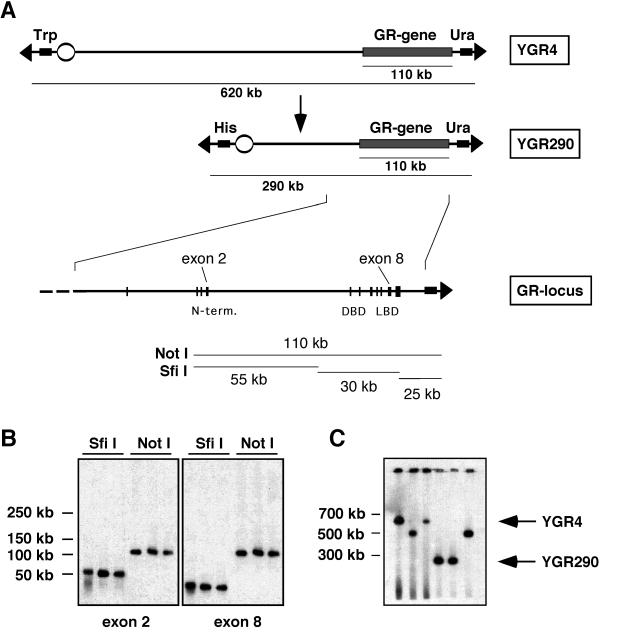
YAC YGR4 was isolated from a mouse C57BL/6 library using GR-specific primers. Briefly, YGR4 was 620 kb in length and contained the entire Gr locus of 110 kb, according to a genomic organization scheme based upon previously published results (Fig. 1A and B) (29). Using fragmentation analysis, the Gr gene was mapped close to the short arm of YGR4 (Fig. 1A). To facilitate microinjection, YGR4 was shortened. This was accomplished using homologous recombination of the YAC insert with a replacement vector targeting B1 elements frequently distributed over the mouse genome. Thereby we generated YACs 500 kb and 290 kb in length (Fig. 1A and C). YAC YGR290, which contained approximately 150 kb of sequence upstream of the Gr gene and 25 kb of sequence downstream of the Gr gene, was used to generate transgenic mice.
FIG. 1.
Isolation of a YAC carrying the Gr gene. (A) Structure of the unmodified YAC YGR4 and the shortened YAC YGR290. The position of the Gr gene on the YAC and its exon-intron structure (enlarged) is indicated. (B) Restriction analysis of YGR4 by digestion with the rare-cutting enzymes SfiI and NotI, PFGE, and Southern blotting with probes for GR exons 2 and 8. Alignment of fragments is shown in panel A. (C) PFGE and Southern blot analysis of YACs obtained after B1 fragmentation. The position of the original YAC YGR4 and its shortened derivative YGR290 are indicated by an arrow.
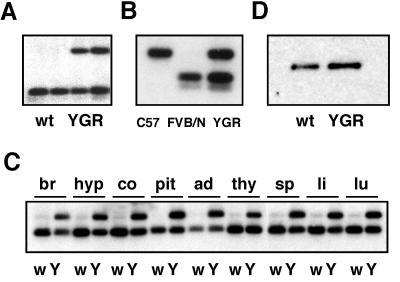
DNA from YGR290 was purified by PFGE and used for microinjection into FVB/N oocytes. Two founder mice were obtained, one of which, designated YGR, was analyzed in detail. An RFLP identified in the 3′ region of the Gr gene allowed the endogenous Gr allele to be distinguished from the YAC-derived one. Based on Southern blot analysis of tail DNA, the copy number of the YAC in YGR mice was determined to be two (Fig. 2A).
FIG. 2.
Expression analysis of YGR transgenic mice. (A) Determination of the YAC copy number in YGR mice by Southern blot analysis of tail DNA from WT (wt) mice and one transgenic mouse from each generation (F1 and F2). (B) Analysis of transgene expression in the hippocampus of a C57BL/6 mouse, an FVB/N mouse, and a YGR mouse by RT-PCR and RFLP. PCR products were cut with RsaI and analyzed by Southern blotting. (C) Analysis of transgene expression in various tissues by RT-PCR and RFLP. br, whole brain; hyp, hypothalamus; co, cortex; pit, pituitary; ad, adrenal; thy, thymus; sp, spleen; li, liver; lu, lung; w, wild type; Y, YGR transgenic mice. (D) Western blot analysis of GR protein expression in the hippocampus. wt, wild type.
RT-PCR followed by RFLP analysis was used to demonstrate expression of YAC-encoded GR mRNA in YGR mice. Based on this experiment we conclude that both YAC-derived and endogenous GR mRNA were expressed at equivalent levels in the hippocampus (Fig. 2B). Similar results were obtained in other organs and tissues known to contain GR, demonstrating faithful expression of GR from the YGR290 transgene (Fig. 2C).
Since autoregulation of GR has been reported to occur at the level of transcription (11, 12, 19), we also determined the total GR mRNA expression in several organs from YGR mice. Interestingly, the expression level of GR did not reach the twofold elevation predicted if the four alleles of GR were fully transcribed in transgenic mice. The highest level of overexpression in YGR mice was achieved in the brain and the pituitary, in which the GR mRNA was elevated by 60 and 43%, respectively, whereas in the spleen, thymus, and liver, GR was overexpressed by 20 to 24% (data not shown). This demonstrates that mRNA expression of GR is subject to autoregulatory downmodulation.
Finally, we tested whether the increase in GR mRNA expression was paralleled by higher receptor levels in transgenic mice. We found that YGR mice contained about 50% more GR protein in the hippocampus than the wild-type controls (Fig. 2D). This clearly shows that the higher copy number of the GR gene in YGR mice not only leads to increased transcription but also results in higher expression of GR protein.
Overexpression of GR causes multiple neuroendocrine changes and results in a blunted response to restraint stress.
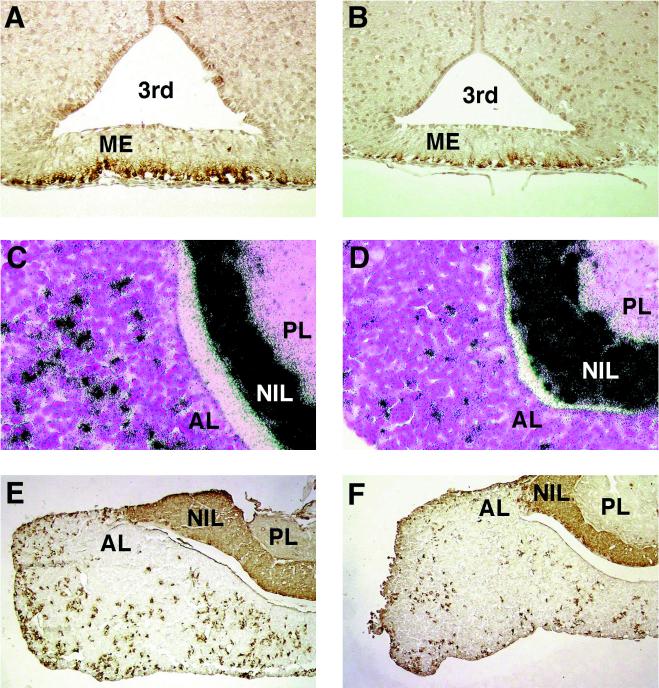
GR plays an important role in the negative feedback control of the HPA axis (8). This mechanism ensures proper regulation of serum glucocorticoid levels as well as a quick return to homeostasis after challenges such as stress. The two major targets for the repressive effect of GR in the regulation of the HPA axis are CRH in the hypothalamus and pro-opiomelanocortin (POMC) in the anterior pituitary. As a measure of CRH synthesis by the hypothalamus, the content of immunoreactive CRH in the median eminence was determined (14). YGR mice displayed a more-than-twofold reduction of CRH immunoreactivity (Fig. 3A and B and Table 1), suggesting that overexpression of GR in the brain leads to increased repression of CRH production. In addition, expression of POMC mRNA and its major peptide product, ACTH, in the anterior pituitary was reduced almost threefold in YGR mice (Fig. 3C to F and Table 1). This demonstrates that a higher GR level in the pituitary, possibly combined with reduced stimulation by CRH, leads to suppression of POMC and ACTH expression.
FIG. 3.
Expression analysis of genes involved in HPA axis regulation. (A) Immunohistochemistry of CRH in the median eminence of WT mice; (B) immunohistochemistry of CRH in the median eminence of YGR mice; (C) in situ hybridization of POMC in the anterior pituitary of WT mice; (D) in situ hybridization of POMC in the anterior pituitary of YGR mice; (E) immunohistochemistry of ACTH in the anterior pituitary of WT mice; (F) immunohistochemistry of ACTH in the anterior pituitary of YGR mice. 3rd, third ventricle; ME, median eminence; AL, anterior lobe of the pituitary; NIL, intermediate lobe of the pituitary; PL, posterior lobe of the pituitary.
TABLE 1.
Quantitative alterations of some of the components of the HPA axis
| Componenta | Result forb:
|
Pc | n | |
|---|---|---|---|---|
| WT mice | YGR mice | |||
| CRH immunoreactivity in the ME | 10.7 ± 1.8 | 4.9 ± 0.8 | <0.001 | 5 |
| POMC mRNA in the AL | 34.5 ± 9.6 | 12.6 ± 3.3 | <0.01 | 5 |
| ACTH immunoreactivity in the AL | 24.0 ± 7.3 | 10.4 ± 3.7 | <0.01 | 7 |
| ACTH levels in the serum | 43 ± 33 | 110 ± 46 | <0.04 | 6 |
| CORT levels in the serum | 41 ± 18 | 9 ± 9 | <0.005 | 7 |
ME, median eminence; AL, anterior lobe of the pituitary; CORT, corticosterone.
Values are given in arbitrary units, except that ACTH levels are given in picograms per milliliter and CORT levels are given in nanograms per milliliter.
Determined by the Student t test.
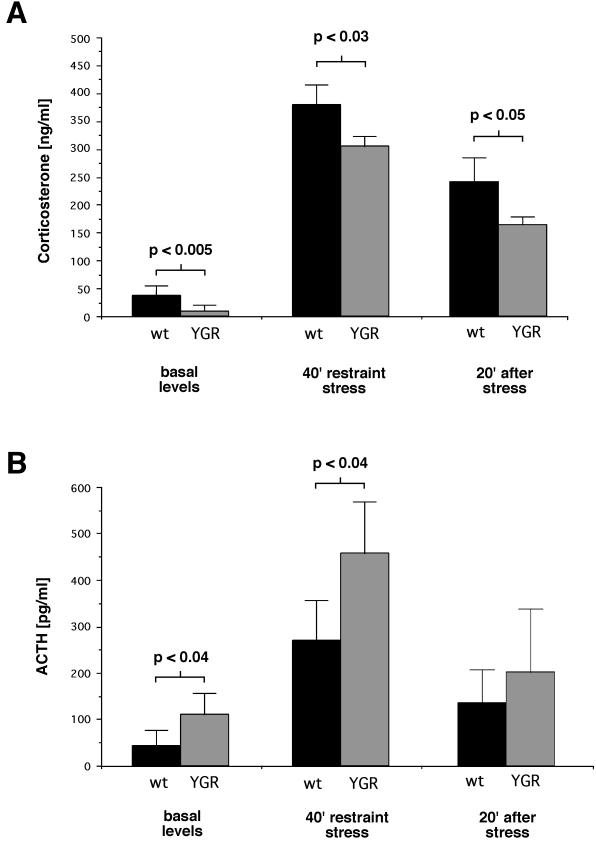
Next, we analyzed whether the observed changes in gene expression would translate into altered corticosterone and ACTH levels in serum. Corticosterone levels in YGR mice were more than fourfold lower than those in wild-type (WT) controls, whereas ACTH levels were elevated almost threefold (Table 1). Thus, basal secretion of the two hormones is altered in opposite directions. In addition, mRNA expression of the mineralocorticoid receptor was also reduced by 33% (P < 0.05, n = 5), indicating reciprocal regulation of the two GRs in the brain (6).
To further study the divergent regulation of basal corticosterone and ACTH secretion, an ACTH stimulation test was performed. Injections of exogenous ACTH (10 μg/kg) into WT mice led to a level of circulating corticosterone that was increased fourfold (88 ± 27 ng/ml) compared to the level in vehicle-injected controls (23 ± 4 ng/ml). In YGR mice a fivefold increase in serum corticosterone levels was observed after ACTH injection (9 ± 2 ng/ml for vehicle-injected controls versus 46 ± 16 ng/ml for ACTH-injected mice). Thus, following appropriate stimulation, a similar increase in glucocorticoid secretion is achieved in WT and transgenic mice. This clearly indicates that YGR mice are able to mount a normal glucocorticoid response. Finally, histological analysis of the adrenal gland did not reveal any abnormalities, such as a hypotrophy of the cortex (data not shown).
Since a major function of GR in the control of the HPA axis is its downregulation after challenges such as stress (15, 31), we analyzed the response of YGR mice to acute restraint stress. Immobilization for 40 min led to a strong elevation of serum corticosterone levels in mice of both genotypes (Fig. 4A); 20 min after the relief of stress the corticosterone levels were significantly decreased. Despite the qualitatively similar response in YGR mice, the elevation of the corticosterone levels during stress was significantly smaller and the serum corticosterone concentrations also declined faster, demonstrating a weaker stress response in YGR mice. In addition, immobilization stress caused a strong increase in ACTH secretion, which returned to moderate levels after 20 min (Fig. 4B). Interestingly, at all time points measured, ACTH levels were higher in YGR mice than in WT mice. Again, this confirms the divergence of ACTH and corticosterone secretion in YGR mice.
FIG. 4.
Consequences of restraint stress on hormone secretion in WT (wt) and YGR mice. (A) Serum corticosterone levels. Basal levels, levels after 40 min of restraint stress, and levels 20 min after the removal of the stressor are shown. (B) Serum ACTH levels at the same time points as described for panel A. Statistical significance was determined by the Student t test (n ≥ 5).
Increased sensitivity of primary thymocytes to glucocorticoid-induced apoptosis in YGR mice.
A well-studied function of GR is the induction of apoptosis in response to glucocorticoid exposure (1, 4, 21). This GR action is thought to be involved in modulating positive and negative selection during T-cell maturation in the thymus, thereby influencing the responsiveness of the immune system to autoantigens (13, 30). Importantly, induction of thymocyte apoptosis was demonstrated to require DNA-binding-dependent gene activation by GR (22).
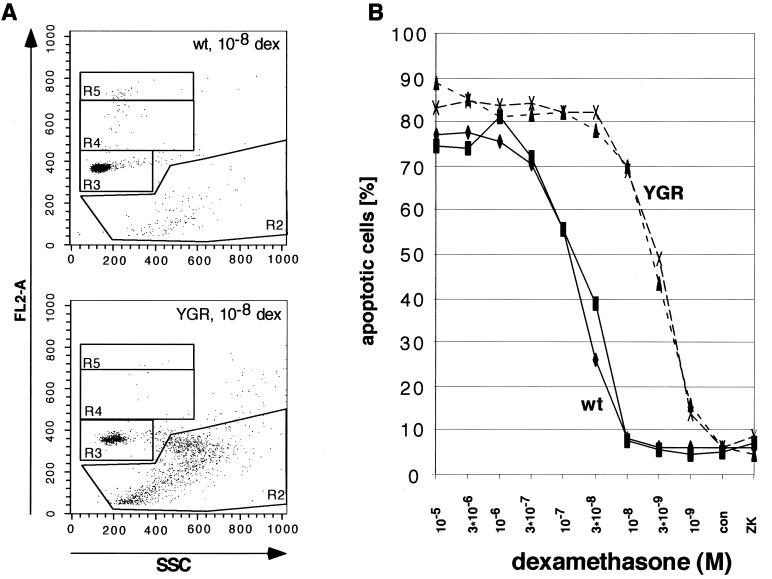
To study the impact of increased GR expression on apoptosis, we cultivated primary thymocytes for 9 h in the presence of the GR agonist dexamethasone, with concentrations ranging from 10−9 to 10−5 M (Fig. 5). Interestingly, WT cells started to undergo apoptosis at 3 · 10−8 M, whereas thymocytes from YGR mice did so at 3 · 10−9 M. Furthermore, maximal apoptosis in WT cells was reached at around 3 · 10−7 M, compared to 3 · 10−8 M in YGR cells. Finally, the maximal level of apoptosis achieved in YGR cells was higher than the one in controls (Fig. 5). Qualitatively similar results were obtained after 24 h of incubation (data not shown). Taken together, the data indicate that overexpression of GR in thymocytes results in a shift of the dose-response curve to the right and an increase of the maximal level of apoptosis. Consequently, the expression level of GR critically determines the magnitude of activation-dependent events such as thymocyte apoptosis.
FIG. 5.
Glucocorticoid-induced apoptosis of primary thymocytes of WT (wt) and YGR mice. (A) Flow cytometric analysis of thymocytes cultivated for 9 h in the presence of 10−8 M dexamethasone (dex) and stained with PI. Analysis by gating the cells in region 2 on the basis of their DNA content (FL2-A) and the granulation (SSC) pattern is exemplified. (B) Dose-response curves of dexamethasone-treated thymocytes from four individual mice, two WT and two YGR, are depicted. Cells were cultivated for 9 h in the absence (con) or presence of various concentrations of the GR agonist dexamethasone or after treatment with the GR antagonist ZK112,339 (10−6 M). The degree of apoptosis was determined as described in Materials and Methods and plotted against the concentration of dexamethasone.
Reduced inflammatory responses in YGR mice.
Glucocorticoids are powerful immunosuppressive and antiinflammatory drugs which achieve their effects by repression of cytokine synthesis and downregulation of other immune functions via GR (2). A suitable model to study this GR function in vivo is injection of lipopolysaccharides (LPS) into mice. Whereas low doses of LPS are used to study regulation of immune mediators such as cytokines (33), injection of high doses is considered a good paradigm for the pathogenesis of endotoxic shock (9).
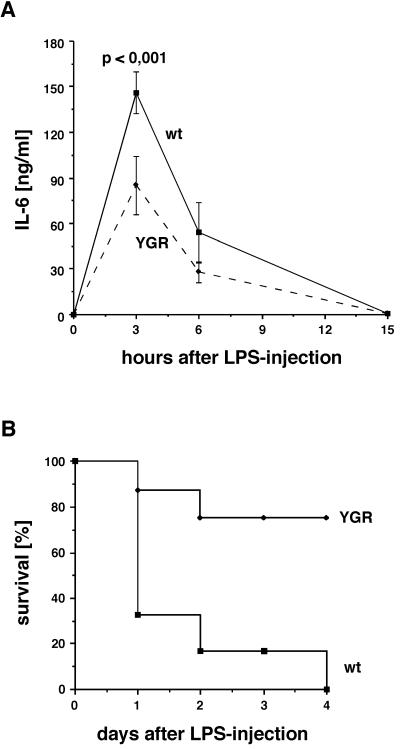
Injection of WT mice with a comparatively low concentration of LPS (4 mg/kg) caused a strong increase of serum IL-6 levels, which peaked at around 3 h after injection and returned to basal levels after 15 h (Fig. 6A). In YGR mice the same kinetics were observed, although with reduced intensity. At both 3 and 6 h after injection, serum IL-6 levels in YGR mice were 40 to 50% lower than those observed in WT mice. Importantly, this difference cannot be attributed to differences in glucocorticoid secretion, since corticosterone was strongly induced to a level of 700 to 800 ng/ml in both genotypes and similarly returned to moderate levels after 15 h (data not shown). This suggests that overexpression of GR in cells mediating systemic inflammation, notably macrophages and neutrophils, leads to an increased sensitivity of these cells to repression by glucocorticoids.
FIG. 6.
LPS-induced inflammation and endotoxic shock in WT (wt) and YGR mice. (A) LPS injection (4 mg/kg intraperitoneally) and analysis of IL-6 levels in the serum at the given time points. The difference at 3 h is highly significant. (B) Survival after injection of a high dose of LPS (40 mg/kg intraperitoneally). The percentage of surviving mice was determined at 24-h intervals (for WT mice, n = 6; for YGR mice, n = 8).
To study the possible impact of the altered cytokine response on pathology, we analyzed the resistance of YGR mice to the lethal effects of endotoxic shock (9). To this end, mice were injected with a high dose of LPS (40 mg/kg) and the percentage of survivors was determined (Fig. 6B). Within the first 24 h 67% of the WT mice but only 13% of the YGR mice died. After 4 days all WT animals were dead, whereas 75% of the YGR mice were still alive, without any obvious signs of illness. In contrast, mock injections of vehicle did not cause death in any case. We conclude that overexpression of GR leads to a strongly increased resistance to endotoxic shock, underscoring the importance of GR signaling for protection against an overshooting inflammatory response.
DISCUSSION
The presence of GR is essential for survival at birth, and its function is important for proper regulation of many physiological processes. This is known from human pathology (16), in vitro and in vivo experiments, and the analysis of genetically manipulated mice (5, 22, 31). However, in contrast to the large body of data describing the physiological consequences of loss of GR function, to our knowledge, analyses of the physiological impact of ubiquitous GR overexpression have not yet been undertaken. We previously established that position-independent and copy number-dependent expression of a transgene can be achieved by the use of YACs (27). Consequently, to address the question of GR overexpression, we have introduced two additional Gr alleles on YACs into the germ line of mice. As predicted, the YAC-derived GR is faithfully expressed in YGR mice in all expected tissues at levels equivalent to the endogenous GR. However, despite the presence of four functional Gr alleles, the absolute expression level in YGR mice varied in different organs, suggesting tissue-specific autoregulatory mechanisms (11, 12, 19).
Both prenatal and postnatal experiences are known to influence the regulation of the HPA axis and the response to stress (15, 25). Prenatal immune challenges were shown to result in increased corticosterone secretion under basal as well as stress conditions, which was correlated with a reduction of GR expression in the hippocampus (25). Conversely, postnatal handling of mice results in reduced glucocorticoid secretion during immobilization stress, an effect which is associated with an increase in GR expression in the hippocampus (15). Taken together, these data suggest a relationship between HPA axis activity, stress responsiveness, and central GR expression. This model is now strongly supported by our findings in GR-overexpressing mice. YGR mice display a strongly reduced expression of the main components of the HPA axis, a fourfold lower secretion of corticosterone, and a significantly weaker response to restraint stress. Thus, an altered expression of GR represents a potential mechanism by which prenatal and postnatal experiences might influence the regulation of the HPA axis and responsiveness to stress in adults. Given the importance of a disturbed control of the HPA axis for the development of psychiatric disorders, our data also point towards a possible explanation for the influence of brain development on the pathogenesis of diseases such as major depression.
Stimulation of the HPA axis usually results in parallel changes in glucocorticoid and ACTH secretion. Consequently, immobilization stress and ACTH injection led to a strong increase in both serum corticosterone and ACTH levels, irrespective of the presence of the transgene. However, under basal conditions both hormones were altered in opposite directions in YGR mice. This divergence of the regulation of the two hormones might be explained by developmental influences causing a new equilibrium of basal hormone secretion due to altered GR expression. This interpretation is supported by the finding that the components of the HPA axis are fully responsive to glucocorticoids already by day 16.5 of fetal development (23). Furthermore, stimulation of the adrenal gland by extrapituitary factors, including neuronal input, e.g., via the splanchnic nerve, might also participate in this phenomenon. Interestingly, a similar divergence has been observed in nervous system-specific GR knockout mice (31) and in patients suffering from major depression. Thus, our finding appears to be of general significance.
Thymocyte apoptosis can be induced by glucocorticoid treatment via GR-dependent gene activation. This function is thought to play an important role in determining the T-cell repertoire by modulating positive and negative selection of thymocytes during the maturation process in the thymus (1, 21). Notably, mice expressing reduced amounts of GR in the thymus show decreased apoptosis of thymocytes following glucocorticoid treatment and a leftward shift of the dose-response curve (13). Furthermore, mice deficient in DNA-binding-dependent gene activation by GR are completely resistant to thymocyte apoptosis (22). Taken together, these findings are in line with the observation that higher GR expression in YGR mice causes an increased sensitivity of thymocytes to glucocorticoid-induced apoptosis. Since the shift of the dose-response curve to the right and the overall elevated level of apoptosis after 9 h are just the opposite of what was observed in mice with reduced GR expression (13), the magnitude of activation-dependent processes appears to be linked to the expression level of GR.
The strength of the inflammatory response is at least in part determined by the HPA axis and the cellular sensitivity to glucocorticoids. From animal studies as well as clinical observations it is known that increased glucocorticoid signaling confers strong resistance to the development of TH1-mediated diseases (7). Fisher rats which have a hyperactive stress system are less prone to develop experimental allergic encephalomyelitis, and women in the third trimester of pregnancy, who have increased cortisol levels, frequently experience remission of rheumatoid arthritis and multiple sclerosis (7). Taken together, this suggests that enhanced GR signaling protects the organism from an overshooting immune response. The finding that an increased gene dosage of GR enhances the resistance of mice to inflammation provides strong genetic support for this hypothesis. Significantly, YGR mice are less prone to the deleterious effects of endotoxin, which, in its most extreme form, leads to endotoxic shock. Whereas in WT mice, LPS injection results in a strong increase in IL-6 secretion and at high concentrations causes death, YGR mice secrete significantly less IL-6 during the inflammatory response and are highly resistant to endotoxic shock. Thus, the ability of glucocorticoids to repress expression and secretion of cytokines and other factors involved in inflammation and the pathogenesis of endotoxic shock depends on the level of GR and therefore on the strength of glucocorticoid signaling.
Collectively, our results argue that higher expression of GR is likely to be of significant adaptive value due to the increased resistance to stress and inflammation. Given that prenatal and postnatal experiences seem to influence the expression level of GR and that this in turn alters physiological responses in the adult, individual differences in humans with regard to their susceptibility to infections, inflammatory diseases, and stress may, at least in part, be accounted for by differences in GR levels.
ACKNOWLEDGMENTS
We thank Brenda Stride for careful reading of the manuscript and Heike Glaser and Nadine Sold for expert technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft through SFB 405; by the Fonds der Chemischen Industrie; by the European Community, through the grants PL 96 0179 and Marie Curie QLK2-CD-1999-51404; by the BMBF through the HGP grant 01 KW 9606/7; by the Hermann von Helmholtz-Gemeinschaft Deutscher Forschungszentren (HGF); by the Alexander von Humboldt-Stiftung; by the Volkswagen-Stiftung; and by Boehringer Ingelheim.
H.M.R. and T.U. contributed equally to this work.
REFERENCES
- 1.Ashwell J D, Lu F W, Vacchio M S. Glucocorticoids in T cell development and function. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 2.Barnes P J. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 3.Burke D T, Carle G F, Olson M V. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science. 1987;236:806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J J. Glucocorticoid-induced apoptosis in the thymus. Semin Immunol. 1992;4:363–369. [PubMed] [Google Scholar]
- 5.Cole T J, Blendy J A, Monaghan A P, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 6.De Kloet E R, Vreugdenhil E, Oitzl M S, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 7.Elenkov I J, Chrousos G P. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 8.Fink G. Mechanism of negative and positive feedback of steroids in the hypothalamic-pituitary system. In: Bittar E E, Bittar N, editors. Principles of medical biology. 10A. London, England: JAI Press; 1997. pp. 30–100. [Google Scholar]
- 9.Gutierrez-Ramos J, Bluethmann H. Molecules and mechanisms operating in septic shock: lessons from knockout mice. Immunol Today. 1997;18:329–334. doi: 10.1016/s0167-5699(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 10.Hiemisch H, Schütz G, Kaestner K H. Transcriptional regulation in endoderm development: characterization of an enhancer controlling Hnf3g expression by transgenesis and targeted mutagenesis. EMBO J. 1997;16:3995–4006. doi: 10.1093/emboj/16.13.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollenberg S M, Weinberger C, Ong E S, Cerelli G, Oro A, Lebo R, Thompson E B, Rosenfeld M G, Evans R M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalinyak J E, Dorin R I, Hoffman A R, Perlman A J. Tissue-specific regulation of glucocorticoid receptor mRNA by dexamethasone. J Biol Chem. 1987;262:10441–10444. [PubMed] [Google Scholar]
- 13.King L B, Vacchio M S, Dixon K, Hunziker R, Margulies D H, Ashwell J D. A targeted glucocorticoid receptor antisense transgene increases thymocyte apoptosis and alters thymocyte development. Immunity. 1995;3:647–656. doi: 10.1016/1074-7613(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 14.Kretz O, Reichardt H M, Schütz G, Bock R. Corticotropin-releasing hormone expression is the major target for glucocorticoid feedback-control at the hypothalamic level. Brain Res. 1999;818:488–491. doi: 10.1016/s0006-8993(98)01277-3. [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky P M, Meaney M J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 16.Miller W L, Blake Tyrrel J. The adrenal cortex. In: Felig P, Baxter J D, Frohman L A, editors. Endocrinology and metabolism. New York, N.Y: McGraw-Hill, Inc.; 1995. pp. 555–711. [Google Scholar]
- 17.Montoliu L, Umland T, Schütz G. A locus control region at −12 kb of the tyrosinase gene. EMBO J. 1996;15:6026–6034. [PMC free article] [PubMed] [Google Scholar]
- 18.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 19.Okret S, Poellinger L, Dong Y, Gustafsson J A. Down-regulation of glucocorticoid receptor mRNA by glucocorticoid hormones and recognition by the receptor of a specific binding sequence within a receptor cDNA clone. Proc Natl Acad Sci USA. 1986;83:5899–5903. doi: 10.1073/pnas.83.16.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otto C, Reichardt H M, Schütz G. Absence of glucocorticoid receptor-beta in mice. J Biol Chem. 1997;272:26665–26668. doi: 10.1074/jbc.272.42.26665. [DOI] [PubMed] [Google Scholar]
- 21.Penninger J M, Kroemer G. Molecular and cellular mechanisms of T lymphocyte apoptosis. Adv Immunol. 1998;68:51–144. doi: 10.1016/s0065-2776(08)60558-1. [DOI] [PubMed] [Google Scholar]
- 22.Reichardt H M, Kaestner K H, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schütz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 23.Reichardt H M, Schütz G. Feedback control of glucocorticoid production is established during fetal development. Mol Med. 1996;2:735–744. [PMC free article] [PubMed] [Google Scholar]
- 24.Reichardt H M, Schütz G. Glucocorticoid signalling—multiple variations of a common theme. Mol Cell Endocrinol. 1998;146:1–6. doi: 10.1016/s0303-7207(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 25.Reul J M, Stec I, Wiegers G J, Labeur M S, Linthorst A C, Arzt E, Holsboer F. Prenatal immune challenge alters the hypothalamic-pituitary-adrenocortical axis in adult rats. J Clin Investig. 1994;93:2600–2607. doi: 10.1172/JCI117272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schedl A, Larin Z, Montoliu L, Thies E, Kelsey G, Lehrach H, Schütz G. A method for the generation of YAC transgenic mice by pronuclear microinjection. Nucleic Acids Res. 1993;21:4783–4787. doi: 10.1093/nar/21.20.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schedl A, Montoliu L, Kelsey G, Schütz G. A yeast artificial chromosome covering the tyrosinase gene confers copy number-dependent expression in transgenic mice. Nature. 1993;362:258–261. doi: 10.1038/362258a0. [DOI] [PubMed] [Google Scholar]
- 28.Schedl A, Ross A, Lee M, Engelkamp D, Rashbass P, van Heyningen V, Hastie N D. Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell. 1996;86:71–82. doi: 10.1016/s0092-8674(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 29.Strähle U, Schmidt A, Kelsey G, Stewart A F, Cole T J, Schmid W, Schütz G. At least three promoters direct expression of the mouse glucocorticoid receptor gene. Proc Natl Acad Sci USA. 1992;89:6731–6735. doi: 10.1073/pnas.89.15.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolosa E, King L B, Ashwell J D. Thymocyte glucocorticoid resistance alters positive selection and inhibits autoimmunity and lymphoproliferative disease in MRL-lpr/lpr mice. Immunity. 1998;8:67–76. doi: 10.1016/s1074-7613(00)80459-8. [DOI] [PubMed] [Google Scholar]
- 31.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban P C, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 32.Tronche F, Kellendonk C, Reichardt H M, Schütz G. Genetic dissection of glucocorticoid receptor function in mice. Curr Opin Genet Dev. 1998;8:532–538. doi: 10.1016/s0959-437x(98)80007-5. [DOI] [PubMed] [Google Scholar]
- 33.Ulich T R, Guo K Z, Remick D, del Castillo J, Yin S M. Endotoxin-induced cytokine gene expression in vivo. III. IL-6 mRNA and serum protein expression and the in vivo hematologic effects of IL-6. J Immunol. 1991;146:2316–2323. [PubMed] [Google Scholar]
- 34.Vanderbilt J N, Miesfeld R, Maler B A, Yamamoto K R. Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Mol Endocrinol. 1987;1:68–74. doi: 10.1210/mend-1-1-68. [DOI] [PubMed] [Google Scholar]
- 35.Wessely O, Deiner E, Beug H, von Lindern M. The glucocorticoid receptor is a key regulator of the decision between self-renewal and differentiation in erythroid progenitors. EMBO J. 1997;16:267–280. doi: 10.1093/emboj/16.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X W, Wynder C, Doughty M L, Heintz N. BAC-mediated gene-dosage analysis reveals a role for Zipro1 (Ru49/Zfp38) in progenitor cell proliferation in cerebellum and skin. Nat Genet. 1999;22:327–335. doi: 10.1038/11896. [DOI] [PubMed] [Google Scholar]