Abstract
Objective
To define the live birth rates in a large, population-based study of the most common reproductive-age cancers in women.
Design
Retrospective cohort study.
Setting
Population-based study.
Patients
Female cancer patients diagnosed with cancer at age 18 years old or older between 1952–2014 (n = 17,952) were compared to fertility of non-cancer controls (n = 89,436).
Interventions
Live births in cancer survivors were compared with those in healthy, age-matched controls. Cases and controls were matched in the ratio of 5:1 for birth year, birthplace (Utah, yes/no), and follow-up time in Utah.
Main Outcome Measure
Rate of at least one live birth, reported as an incidence rate ratio (IRR).
Results
Of all cancer survivors, 3,127 (17.4%) had at least 1 live birth after treatment in comparison to 19,405 healthy, age-matched controls (21.7%) with the same amount of time exposure for attempting pregnancy. Breast cancer was the most common cancer type (23.1% of patients in cohort). Compared with age-matched, healthy controls, IRR of live birth was 0.69 (95% confidence interval [CI], 0.67–0.70) for all cancer types, 0.25 (95% CI, 0.20–0.33) for leukemia, 0.40 (95% CI, 0.28–0.59) for gastrointestinal cancers, 0.44 (95% CI, 0.41–0.48) for breast cancer, 0.53 (95% CI, 0.47–0.59) for central nervous system cancers, and 0.57 (95% CI, 0.44–0.73) for soft tissue cancers. With all cancer types stratified by age at diagnosis, IRR for live births in cancer survivors aged >41 years at diagnosis was 0.48 (95% CI, 0.44–0.52); IRR was 0.64 (95% CI, 0.61–0.67) in the group aged 31–40 years and 0.71 (95% CI, 0.69–0.74) in the group aged 18–30 years after their cancer treatment.
Conclusions
Cancer and its treatment were associated with lower live birth rates when comparing women with cancer vs. age-matched, healthy controls.
Key Words: Fertility preservation, live births, cancer survivors, cancer, fertility
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-21-00067
With continued and substantial advancements in cancer treatment, there has been a rapid increase in the population of long-term survivors of adolescence and young adulthood cancer (1). Consequently, emphasis has increasingly been placed on the long-term effects of cancer therapies and the quality of life. The potential impact on fertility is one of the concerns that are of greatest importance to patients after their cancer treatment (2). Because of the adverse effects of cancer treatment on fertility, international guidelines have been established for recommendations for fertility preservation (3).
Although studies on the effect of cancer treatment on ovarian function are relatively numerous, the overall effect of cancer treatment on fertility at the population level remains understudied. A few studies have reported a detailed analysis of fertility and pregnancy outcomes after cancer treatment. The US Childhood Cancer Survivor Study, a self-reported questionnaire-based survey, has provided fertility outcomes in cancer survivors compared with their siblings (4, 5). In addition, the British Childhood Cancer Survivor Study has also reported fertility outcomes among childhood cancer survivors (6). However, these studies are confined to cancers diagnosed in the adolescent years. There are limited data available on the fertility outcome in survivors of cancer during the reproductive years (7). Studies on reproductive function in adult cancer survivors used amenorrhea or premature ovarian insufficiency as surrogate markers of their reproductive function. These measures would not be expected to fully examine the fertility potential after treatment (8, 9, 10). In a recent study from Scotland, investigators found fewer pregnancies among cancer survivors with standardized incidence ratios of 0.62 (95% confidence interval [CI], 0.60–0.63) (11). We wanted to replicate their findings in the United States in a large-scale, population-based study.
In Utah, the availability of linkable databases of cancer registries and birth certificate records offers the prospect to study whether women who are cancer survivors achieve live births after treatment less often than the age-matched, healthy controls who have the same amount of time for possible conception as women with cancer. We hypothesized that cancer survivors have a decrease in live births in comparison to the healthy population.
Materials and methods
We performed a retrospective cohort study within the Utah Population Database (UPDB). This study was approved by the University of Utah’s institutional review board and the Utah Resource for Genetic and Epidemiological Research.
Subjects
To identify cancer survivors, we used data from UPDB. The UPDB is a comprehensive data resource of individuals in Utah that links not only cancer records but also birth, medical, and death information. The demographic and health information is obtained via birth, death, marriage, and divorce certificates and state driver’s license. Cancer data, including site, staging, and diagnosis dates, are obtained through linked information from the Utah Cancer Registry, a member of the National Cancer Institute Surveillance, Epidemiology, and End Results network of cancer registries since 1973. Women with a cancer diagnosis between the ages of 18 and 45 years between March 1952 and February 2014 in Utah were identified from the UPDB. Subsequent births in Utah after the first cancer diagnosis were ascertained using birth certificate records from March 1952 to December 2016. We excluded women who were not Utah residents at the time of the index diagnosis, defined as the first cancer diagnosis from March 1952 to February 2014. We also excluded women with unknown birthdates and diagnosis dates. The controls were women who were not diagnosed with any cancer and matched to the cases using a 5:1 unexposed-to-exposed ratio on birth year, whether born in or out of Utah and the follow-up time in Utah such that we could have equal exposure times for possible pregnancies for cases and controls, after the age of the cases’ cancer diagnoses.
Outcomes
The primary outcome of our study was the proportion of women with at least one live birth after cancer treatment or the corresponding dates in controls. We also examined the number of live births in three age groups (18–30 years, 31–40 years, and ≥41 years) and various cancer types. The secondary outcomes of this study were to measure the family size of cancer survivors compared with their healthy controls.
Data Analyses
Demographic characteristics were compared using Student's t tests for continuous variables and χ2 tests for categorical variables. The P value level of significance was set at ≤.05. The estimated effect of being diagnosed with cancer was investigated on the number of live births after cancer diagnosis using the conditional Poisson regression model, additionally adjusted for race and ethnicity and number of live births before the first cancer diagnosis. The incidence rate ratio (IRR) was used for Poisson regression models to analyze count data (i.e., the number of live births). We repeated the analysis in different age groups (18–30 years, 31–40 years, and ≥41 years) for various gynecologic and nongynecologic cancer types. We also performed a sensitivity analysis wherein we excluded patients diagnosed with carcinoma in situ, as women diagnosed with in situ cancers may be less likely to receive chemotherapy or radiation as a part of their cancer treatment.
Results
Patient Sample
We initially identified a total of 19,564 women, aged 18–45 years, who were newly diagnosed with cancer in Utah from March 1952 to February 2014. We excluded women who were diagnosed with cancer at <18 years of age (n = 1,604). We also excluded women for whom matched controls could not be identified (n = 8). Our final dataset included 17,952 women with cancer (Supplemental Fig. 1, available online).
Characteristics of reproductive-age cancer survivors and the comparison control cohort are shown in Supplemental Table 1 (available online). The mean ± SD age at the first cancer diagnosis was 35.1 ± 7.3 years in the group with cancer. Patients with cancer were slightly more likely to be identified as Caucasian (cancer cases, 95.5%; controls, 92.3%) and of Hispanic ethnicity (cancer cases, 13.0%; controls, 10.3%). Among the cases with cancer, 64.3% had a live birth before cancer diagnosis compared with 71% in the control group at the same age.
Among various nongynecologic cancer types, breast cancer was the most common cancer (23.1%). Other cancers that were prevalent among cancer survivors were central nervous system cancers (4.3%), Hodgkin (2.2%) and non-Hodgkin lymphoma (2.2%), leukemia (1.8%), soft tissue (<1%), and gastrointestinal cancers (<1%) (Supplemental Table 2, available online). Based on cancer staging, most cancer survivors had localized disease (43.5%). Approximately 25% of cancer survivors was diagnosed with carcinoma in situ (Supplemental Table 3, available online).
Live Births Among Cancer Survivors
Cancer survivors were less likely to have one or more live births after diagnosis than their controls (17.4% vs. 21.7%, respectively; Supplemental Fig. 2, available online). Incidence rate ratio after cancer diagnosis was estimated for various cancer types after adjusting for ethnicity and the number of live births before cancer diagnosis. For all cancer types, when compared with age-matched, healthy controls, IRR was 0.69 (95% CI, 0.67–0.70). Incidence rate ratio for each cancer type is as follows: leukemia, 0.25 (95% CI, 0.20–0.33); gastrointestinal cancers, 0.40 (95% CI, 0.28–0.59); breast cancer, 0.44 (95% CI, 0.41–0.48); central nervous system cancers, 0.53 (95% CI, 0.47–0.59); and soft tissue cancers, 0.57 (95% CI, 0.44–0.73) (Table 1).
Table 1.
Incidence rate ratios for the effect of different cancer diagnoses on subsequent live birth using conditional Poisson regression models, adjusted for ethnicity and the number of live births before cancer diagnosis.
| Cancer types | All |
||
|---|---|---|---|
| Incidence rate ratio | 95% CI | P value | |
| All cancers | 0.69 | 0.67–0.70 | <.001 |
| Nongynecologic cancer | |||
| Breast | 0.44 | 0.41–0.48 | <.001 |
| Hodgkin lymphoma | 0.68 | 0.60–0.76 | <.001 |
| Non-Hodgkin lymphoma | 0.75 | 0.65–0.87 | <.001 |
| Leukemia | 0.25 | 0.20–0.33 | <.001 |
| GI cancers | 0.40 | 0.28–0.59 | <.001 |
| Soft tissue | 0.57 | 0.44–0.73 | <.001 |
| Central nervous system cancers | 0.53 | 0.47–0.59 | <.001 |
| Gynecologic cancer | |||
| Vulva and vagina | 0.73 | 0.63–0.84 | <.001 |
| Cervix | 0.61 | 0.58–0.65 | <.001 |
| Uterine corpus | 0.18 | 0.13–0.24 | <.001 |
| Ovary | 0.50 | 0.42–0.60 | <.001 |
| Others | 0.88 | 0.85–0.91 | <.001 |
Note: CI = confidence interval; GI = gastrointestinal.
Incidence rate ratio among various cancer types was also estimated in different age groups (Table 2). For all cancers, IRR in cancer survivors aged ≥41 years was 0.48 (95% CI, 0.44–0.52). Incidence rate ratio in the group aged 31–40 years was 0.64 (95% CI, 0.61–0.67) and in the group aged 18–30 years was 0.71 (95% CI, 0.69–0.74) after their cancer treatment.
Table 2.
Incidence rate ratios for the effect of different cancer diagnoses on subsequent live birth in different age groups using conditional Poisson regression models, additionally adjusted for ethnicity and the number of live births before cancer diagnosis.
| Cancer types | 18–30 y |
31–40 y |
≥41 y |
||||||
|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% CI | P value | IRR | 95% CI | P value | IRR | 95% CI | P value | |
| All cancers | 0.71 | 0.69–0.74 | <.001 | 0.64 | 0.61–0.67 | <.001 | 0.48 | 0.44–0.52 | <.001 |
| Breast | 0.58 | 0.47–0.71 | <.001 | 0.37 | 0.32–0.42 | <.001 | 0.38 | 0.33–0.44 | <.001 |
| Hodgkin lymphoma | 0.64 | 0.55–0.74 | <.001 | 0.95 | 0.72–1.25 | .707 | - | - | <.001 |
| Non-Hodgkin lymphoma | 0.80 | 0.63–1.01 | .059 | 0.54 | 0.38–0.78 | <.001 | 1.71 | 1.20–2.43 | .003 |
| Leukemia | 0.26 | 0.18–0.37 | <.001 | 0.16 | 0.08–0.33 | <.001 | 1.18 | 0.75–1.85 | .475 |
| GI cancers | 0.45 | 0.20–1.01 | .058 | 0.28 | 0.12–0.66 | .004 | - | - | .941 |
| Soft tissue | 0.59 | 0.41–0.85 | .005 | 0.48 | 0.28–0.81 | .006 | - | - | - |
| Central nervous system cancers | 0.55 | 0.46–0.66 | <.001 | 0.46 | 0.36–0.59 | <.001 | - | - | .889 |
| Vulva and vagina | 0.71 | 0.57–0.88 | .002 | 0.73 | 0.52–1.03 | .072 | 0.78 | 0.47–1.29 | .339 |
| Cervix | 0.64 | 0.60–0.68 | <.001 | 0.56 | 0.50–0.63 | <.001 | 0.46 | 0.34–0.61 | <.001 |
| Uterine corpus | 0.32 | 0.16–0.67 | .002 | 0.06 | 0.02–0.14 | <.001 | - | - | .905 |
| Ovary | 0.51 | 0.41–0.64 | <.001 | 0.48 | 0.31–0.73 | <.001 | - | - | .916 |
| Others | 0.87 | 0.83–0.92 | <.001 | 0.91 | 0.85–0.98 | .012 | 0.58 | 0.51–0.67 | <.001 |
Note: CI = confidence interval; GI = gastrointestinal; IRR = incidence rate ratio.
Family Size After Cancer Treatment
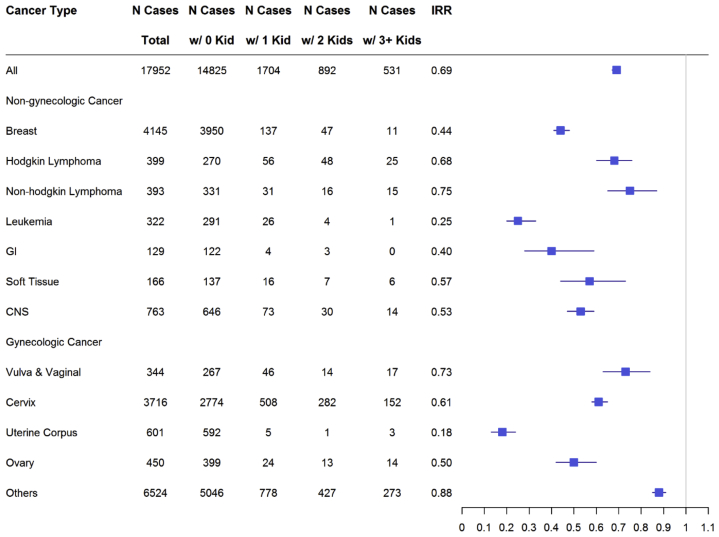
We estimated the effect of cancer diagnosis on parity after cancer diagnosis using conditional Poisson regression models, additionally adjusted for ethnicity and the number of live births before cancer diagnosis. Among nongynecologic cancers, the group with non-Hodgkin lymphoma had the highest IRR in parity after a cancer diagnosis. Incidence rate ratio for postcancer parity for non-Hodgkin lymphoma was 0.75. Among other nongynecologic cancers, IRR for Hodgkin lymphoma was 0.68, soft tissue cancers was 0.57, central nervous system cancers was 0.53, breast cancer was 0.44, gastrointestinal cancers was 0.40, and leukemia was 0.25 (Fig. 1).
Figure 1.
Estimated effect of being diagnosed with cancer on parity after the first cancer diagnosis using conditional Poisson regression models, additionally adjusted for whether Caucasian and Hispanic and the number of live births before the first cancer diagnosis. CNS = central nervous system; GI = gastrointestinal; IRR = incidence rate ratio; w/ = with.
Among gynecologic cancers, IRR for vulvar and vaginal cancers was 0.73, cervical cancers was 0.61, ovarian cancers was 0.50, and uterine cancer was 0.18 (Fig. 1).
Sensitivity Analysis Excluding Carcinoma In Situ
Based on conditional Poisson regression analysis additionally adjusted for ethnicity and the number of live births before cancer diagnosis and excluding the patients diagnosed with carcinoma in situ, there was still a significant reduction in live births after cancer diagnosis with IRR of 0.69 (95% CI, 0.67–0.70; P<.001) among all cancer types (Table 3). The exclusion of patients with carcinoma in situ did not change any of the disease-specific IRRs.
Table 3.
Incidence rate ratios for the effect of a cancer diagnosis on subsequent parity using conditional Poisson regression models, additionally adjusted for race and/or ethnicity and the number of live births before cancer diagnosis. Carcinoma in situ and their controls were excluded from this analysis.
| Cancer types | All |
18–30 y |
31–40 y |
≥41 y |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% CI | P value | IRR | 95% CI | P value | IRR | 95% CI | P value | IRR | 95% CI | P value | |
| All cancers | 0.69 | 0.67–0.70 | <.001 | 0.72 | 0.69–0.75 | <.001 | 0.61 | 0.57–0.64 | <.001 | 0.45 | 0.40–0.49 | <.001 |
| Nongynecologic cancer | ||||||||||||
| Breast | 0.42 | 0.38–0.45 | <.001 | 0.57 | 0.45–0.71 | <.001 | 0.34 | 0.29–0.39 | <.001 | 0.24 | 0.20–0.30 | <.001 |
| Hodgkin lymphoma | 0.68 | 0.60–0.76 | <.001 | 0.64 | 0.55–0.74 | <.001 | 0.95 | 0.72–1.25 | .707 | - | - | <.001 |
| Non-Hodgkin lymphoma | 0.75 | 0.65–0.87 | <.001 | 0.80 | 0.63–1.01 | .059 | 0.54 | 0.38–0.78 | <.001 | 1.71 | 1.20–2.43 | .003 |
| Leukemia | 0.25 | 0.20–0.33 | <.001 | 0.26 | 0.18–0.37 | <.001 | 0.16 | 0.08–0.33 | <.001 | 1.18 | 0.75–1.85 | .475 |
| GI cancers | 0.41 | 0.28–0.60 | <.001 | 0.45 | 0.20–1.01 | .058 | 0.29 | 0.12–0.68 | .005 | - | - | .941 |
| Soft tissue | 0.57 | 0.44–0.73 | <.001 | 0.59 | 0.41–0.85 | .005 | 0.48 | 0.28–0.81 | .006 | - | - | - |
| Central nervous system cancers | 0.53 | 0.47–0.59 | <.001 | 0.55 | 0.46–0.66 | <.001 | 0.46 | 0.36–0.59 | <.001 | - | - | .889 |
| Gynecologic cancer | ||||||||||||
| Vulva and vagina | 0.72 | 0.54–0.96 | .027 | 0.63 | 0.36–1.07 | .091 | 2.08 | 1.02–4.27 | .049 | - | - | - |
| Cervix | 0.30 | 0.25–0.35 | <.001 | 0.36 | 0.27–0.49 | <.001 | 0.22 | 0.17–0.30 | <.001 | 0.26 | 0.14–0.47 | <.001 |
| Uterine corpus | 0.18 | 0.13–0.24 | <.001 | 0.31 | 0.15–0.65 | .002 | 0.06 | 0.02–0.14 | <.001 | - | - | .908 |
| Ovary | 0.50 | 0.42–0.59 | <.001 | 0.51 | 0.41–0.64 | <.001 | 0.46 | 0.30–0.72 | <.001 | - | - | .917 |
| Others | 0.86 | 0.83–0.90 | <.001 | 0.85 | 0.81–0.90 | <.001 | 0.89 | 0.83–0.96 | .004 | 0.66 | 0.57–0.76 | <.001 |
Note: CI = confidence interval; GI = gastrointestinal; IRR = incidence rate ratio.
Discussion
This study provides robust, population-based evidence for the effect of cancer and its treatment on subsequent live birth in women aged 18–45 years. We found a reduction in live births in cancer survivors for all cancer types compared with age-matched, healthy controls from the general population. This reduction in live birth was the highest in the group with leukemia among nongynecologic cancers and in the group with uterine cancer among gynecologic cancers. When compared within various age groups, the reduction in live births was the highest in cancer survivors aged ≥41 years. This may be due to the already-diminished ovarian reserve in this age group, along with the aggravating effect of cancer radiotherapy and chemotherapy. Indeed, older women are more likely to be amenorrheic after chemotherapy than younger women (12).
The reduction in subsequent pregnancy after cancer treatment showed marked differences among cancer types, with leukemia being the most common cancer among nongynecologic cancers maximally affecting live births among cancer survivors. This observation is likely due to the relatively high risk of loss of fertility linked with total body irradiation or high-dose alkylating agent chemotherapy as conditioning management before bone marrow transplantation for the treatment of acute leukemia (13, 14). We were not able to stratify for bone marrow transplantation vs. no bone marrow transplantation in our data for leukemia. These findings are in line with those of another study conducted in Scotland on the impact of the chance of pregnancy among cancer survivors after their cancer treatment (11). Among gynecologic cancers, uterine cancer maximally affected the live births after the treatment, which is likely due to the removal of the uterus as a common historical approach to uterine cancer management and loss of the ability to become pregnant. Hysterectomy along with salpingo-oophorectomy is the main treatment of uterine cancer, with fertility-sparing techniques (e.g., dilation and curettage, progestin therapy) recently becoming more common in reproductive-age women (15).
The reduced chance of pregnancy in reproductive-age female cancer survivors demonstrated here reinforces the importance of identifying those women at significant risk of reduced fertility and offering them timely access to fertility preservation options (16). These include oocyte or embryo cryopreservation or ovarian tissue cryopreservation (17). Appropriate reproductive counseling, diagnosis and treatment of premature ovarian insufficiency, and access to timely assisted reproductive technologies should be a priority for young and reproductive-age female cancer survivors who are estimated to be at a high risk of a decreased chance of pregnancy after cancer treatment (3, 18).
Another interesting finding of this study was that women with cancer were less likely than their matched controls to have had a live birth before their cancer diagnosis. Potential factors contributing to this may be that nulliparity increases the risk of certain cancers, including breast and ovarian cancers, that cancer may have impacted fertility before diagnosis, or an association between infertility and cancer risk (19, 20, 21, 22).
Although major strengths of this study are its size and population-based data and the inclusion of women up to the age of 45 years, weaknesses include the lack of detailed treatment information regarding live births based on radiotherapy, chemotherapy, and surgery. Specific cancer treatment information is not collected in the database used in this study; however, it is important to perform a more detailed analysis of the effects of various cancer treatment regimens on fertility. In addition, another limitation of datasets is that we could not evaluate the fertility of cancer survivors in light of their personal choices. Some women may not have chosen to become pregnant after their cancer treatment due to the psychologic, social, or medical effects of the cancer therapies (23, 24). We were also unable to determine whether the reduction in live births occurred at the level of conception, miscarriage, or stillbirth. Information on the live births of cancer survivors who were diagnosed and treated for cancers in Utah but moved out of the state after their treatment was also not available. In addition, cancer treatments have changed dramatically since the beginning of the inclusion date of this study. Due to incomplete data regarding fertility treatments, we could not measure the fertility trends based on the time period of treatment.
Conclusion
This analysis shows the association between cancer and its treatment and a decreased chance of subsequent live birth in women of the reproductive age group. A reduction in live birth was seen across all reproductive age groups at diagnosis and was most pronounced among those aged ≥40 years at diagnosis. We also demonstrated disease-specific reductions in live birth, with the most impacted cancer type being leukemia. These data highlight the need for fertility preservation counseling and interventions to safeguard fertility in the reproductive-age women with cancer at the time of cancer diagnosis.
Footnotes
D.G. has nothing to disclose. H.D.M. has nothing to disclose. E.J. has nothing to disclose. S.L.B. has nothing to disclose. K.R.S. has nothing to disclose. J.H. has nothing to disclose. J.M.L. has nothing to disclose.
Supplementary data
Flowchart of selected cases.
Supplemental Figure 2. Percentage of women who had at least one live birth after index diagnosis, defined as the first cancer diagnosis dates from March 1952 to February 2014 for cancer survivors and index diagnosis dates for matching cases for controls.
Supplemental Table 1. Demographic characteristics of women with cancer their matched controls.
Supplemental Table 2. Cancer types of women with cancer by age at diagnosis.
Supplemental Table 3. Staging of all cancers among cancer survivors.
References
- 1.Skinner R., Wallace W.H.B., Levitt G.A. UK Children's Cancer Study Group Late Effects Group. Long-term follow-up of people who have survived cancer during childhood. Lancet Oncol. 2006;7:489–498. doi: 10.1016/S1470-2045(06)70724-0. [DOI] [PubMed] [Google Scholar]
- 2.Peate M., Meiser B., Hickey M., Friedlander M. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat. 2009;116:215–223. doi: 10.1007/s10549-009-0401-6. [DOI] [PubMed] [Google Scholar]
- 3.Oktay K., Harvey B.E., Partridge A.H., Quinn G.P., Reinecke J., Taylor H.S., et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36:1994–2001. doi: 10.1200/JCO.2018.78.1914. [DOI] [PubMed] [Google Scholar]
- 4.Green D.M., Kawashima T., Stovall M., Leisenring W., Sklar C.A., Mertens A.C., et al. Fertility of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller B.A., Chow E.J., Kamineni A., Daling J.R., Fraser A., Wiggins C.L., et al. Pregnancy outcomes in female childhood and adolescent cancer survivors: a linked cancer-birth registry analysis. Arch Pediatr Adolesc Med. 2009;163:879–886. doi: 10.1001/archpediatrics.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reulen R.C., Zeegers M.P., Wallace W.H.B., Frobisher C., Taylor A.J., Lancashire E.R., et al. Pregnancy outcomes among adult survivors of childhood cancer in the British Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2009;18:2239–2247. doi: 10.1158/1055-9965.EPI-09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haggar F.A., Pereira G., Preen D., Holman C.D., Einarsdottir K. Adverse obstetric and perinatal outcomes following treatment of adolescent and young adult cancer: a population-based cohort study. Plos One. 2014;9 doi: 10.1371/journal.pone.0113292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letourneau J.M., Ebbel E.E., Katz P.P., Oktay K.H., McCulloch C.E., Ai W.Z., et al. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2012;118:1933–1939. doi: 10.1002/cncr.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton S.E., Najita J.S., Ginsburg E.S., Leisenring W.M., Stovall M., Weathers R.E., et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013;14:873–881. doi: 10.1016/S1470-2045(13)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganz P.A., Rowland J.H., Desmond K., Meyerowitz B.E., Wyatt G.E. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 11.Anderson R.A., Brewster D.H., Wood R., Nowell S., Fischbacher C., Kelsey T.W., et al. The impact of cancer on subsequent chance of pregnancy: a population-based analysis. Hum Reprod. 2018;33:1281–1290. doi: 10.1093/humrep/dey216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruddy K.J., Schaid D.J., Partridge A.H., Larson N.B., Batzler A., Häberle L., et al. Genetic predictors of chemotherapy-related amenorrhea in women with breast cancer. Fertil Steril. 2019;112:731–739.e1. doi: 10.1016/j.fertnstert.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin-Epstein R., Oliai C., Schiller G. Allogeneic hematopoietic stem cell transplantation for older patients with acute myeloid leukemia. Curr Treat Options Oncol. 2018;19:63. doi: 10.1007/s11864-018-0577-2. [DOI] [PubMed] [Google Scholar]
- 14.Salooja N., Szydlo R.M., Socie G., Rio B., Chatterjee R., Ljungman P., et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358:271–276. doi: 10.1016/s0140-6736(01)05482-4. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society Treating endometrial cancer. https://www.cancer.org/content/dam/CRC/PDF/Public/8612.00.pdf Available at:
- 16.Anderson R.A., Mitchell R.T., Kelsey T.W., Spears N., Telfer E.E., Wallace W.H.B. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015;3:556–567. doi: 10.1016/S2213-8587(15)00039-X. [DOI] [PubMed] [Google Scholar]
- 17.Loren A.W., Mangu P.B., Beck L.N., Brennan L., Magdalinski A.J., Partridge A.H., et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace W.H.B., Critchley H.O.D., Anderson R.A. Optimizing reproductive outcome in children and young people with cancer. J Clin Oncol. 2012;30:3–5. doi: 10.1200/JCO.2011.38.3877. [DOI] [PubMed] [Google Scholar]
- 19.Kelsey J.L., Gammon M.D., John E.M. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 20.Koushik A., Grundy A., Abrahamowicz M., Arseneau J., Gilbert L., Gotlieb W.H., et al. Hormonal and reproductive factors and the risk of ovarian cancer. Cancer Causes Control. 2017;28:393–403. doi: 10.1007/s10552-016-0848-9. [DOI] [PubMed] [Google Scholar]
- 21.Opdahl S., Alsaker M.D.K., Janszky I., Romundstad P.R., Vatten L.J. Joint effects of nulliparity and other breast cancer risk factors. Br J Cancer. 2011;105:731–736. doi: 10.1038/bjc.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundberg F.E., Iliadou A.N., Rodriguez-Wallberg K., Gemzell-Danielsson K., Johansson A.L.V. The risk of breast and gynecological cancer in women with a diagnosis of infertility: a nationwide population-based study. Eur J Epidemiol. 2019;34:499–507. doi: 10.1007/s10654-018-0474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard-Anderson J., Ganz P.A., Bower J.E., Stanton A.L. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson J., Jervaeus A., Lampic C., Eriksson L.E., Widmark C., Armuand G.M., et al. ‘Will I be able to have a baby?’ Results from online focus group discussions with childhood cancer survivors in Sweden. Hum Reprod. 2014;29:2704–2711. doi: 10.1093/humrep/deu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart of selected cases.
Supplemental Figure 2. Percentage of women who had at least one live birth after index diagnosis, defined as the first cancer diagnosis dates from March 1952 to February 2014 for cancer survivors and index diagnosis dates for matching cases for controls.
Supplemental Table 1. Demographic characteristics of women with cancer their matched controls.
Supplemental Table 2. Cancer types of women with cancer by age at diagnosis.
Supplemental Table 3. Staging of all cancers among cancer survivors.