Abstract
Purpose
The cohort of patients with locally advanced prostate cancer (PC) and positive surgical margin(s) at radical prostatectomy (RP) who would benefit from salvage or adjuvant treatment is unclear. This study examines the risk of prostate-specific antigen (PSA) relapse in a large population of men with PC after margin-positive RP.
Methods and Materials
Using a multi-institutional database, patients with clinically localized PC who underwent RP between 2002 and 2010 with recorded follow-up PSA were retrospectively selected. Patients were excluded for pathologic seminal vesicle or lymph node involvement, metastatic disease, pre-RP PSA ≥ 30, or adjuvant (nonsalvage) radiation therapy or hormone therapy. The primary endpoint was biochemical relapse free survival (bRFS), where PSA failure was defined as PSA > 0.10 ng/mL and rising, or at salvage intervention. The Kaplan-Meier method was employed for bRFS estimates; recursive partitioning analysis using cumulative or single maximal margin extent (ME) and Gleason grade (GG) at RP was applied to identify variables associated with bRFS.
Results
At median follow-up of 105 months, 210 patients with positive margins at RP were eligible for analysis, and 89 had experienced PSA relapse. Median age was 61 years (range, 43-76), and median pre-RP PSA 5.8 ng/mL (1.6-26.0). Recursive partitioning analysis yielded 5 discrete risk groups, with the lowest risk group (GG1, ≤ 2 mm ME) demonstrating a bRFS of 92% at 8 years compared with the highest risk group (GG3-5, ≥ 3 mm ME) of 11%.
Conclusions
This retrospective study suggests that it may be possible to risk-stratify patients undergoing margin-positive RP using commonly acquired clinical and pathologic variables. Patients with low-grade tumors and minimally involved margins have a very low recurrence risk and may be able to forego postprostatectomy radiation. Meanwhile, those with higher grade and greater involvement could benefit from adjuvant or early salvage radiation therapy.
Introduction
Surgical margin involvement at radical prostatectomy (RP) for prostate cancer (PC) is a well-established high-risk feature for recurrence.1,2 However, not all patients with involved surgical margins ultimately experience biochemical or clinical progression,3, 4, 5 particularly those with focal involvement6 or low-grade Gleason group (GG)6 without other high-risk features (eg, seminal vesicle or lymph node involvement).4,5 Randomized trials have suggested that adjuvant radiation therapy after RP may improve biochemical control and possibly survival for patients with pathologically advanced disease (inclusive of positive margins).7, 8, 9 However, radiation therapy has not been widely adopted, as many patients with high-risk features will never recur without any radiation therapy.10,11 Additionally, recent results of a trial and meta-analysis examining salvage and adjuvant radiation have not demonstrated significant differences in biochemical failure.12,13 As a result, the clinical benefit of improved biochemical control from postprostatectomy radiation is unclear. Despite this growing literature, some physicians are uncomfortable recommending observation for patients after margin-positive resection. Identifying which patients have a very low or high likelihood of recurrence after margin-positive RP could enable better selection of patients for observation or postprostatectomy radiation therapy. The present investigation seeks to identify factors associated with prostate-specific antigen (PSA) relapse within a population of patients with clinically localized PC who underwent prostatectomy alone (without adjuvant hormone or radiation therapy) and presented with involved margins, focusing on the effect of clinicopathologic features, including extent of margin (ME) involvement for risk substratification.
Methods and Materials
After institutional review board approval at each study institution, a research database was created with study-specific patient, treatment, and outcome data fields. Eligible cases were identified by review of medical records. After selection for prostate adenocarcinoma pathology with positive margins, patient records were reviewed to exclude patients at high risk for metastatic disease, including PSA ≥ 30 ng/mL at diagnosis or preoperative evidence of extraprostatic extension, seminal vesicle invasion, or pelvic lymph node involvement. Preoperative staging studies were performed at the discretion of the managing urologist, with bone scan and computed tomography scans generally performed for patients with Gleason score (GS) of 8 to 10 or PSA ≥ 20 ng/mL. All patients underwent retropubic prostatectomy (open or laparoscopic, with or without robot assistance) as primary curative-intent therapy. Patients with missing pathology, pT3b (seminal vesicle involvement), or pN1 (lymph node involvement) who received immediate adjuvant therapy (radiation or hormone) or who were lost to surveillance follow-up less than 1 year postprostatectomy (no PSA >1 2 months postoperatively) were excluded from the analysis. Additionally, those who completed surgery other than an RP, surgery elsewhere with missing records, or surgery outside of the study dates were not included.
Pathologic specimen preparation technique involved differential inking of the peripheral margins, distinguishing right from left, and 10% buffered formalin fixation for 4 to 24 hours. The apex and base were excised, radially sectioned, and submitted entirely. The remaining tissue was serially sectioned in the transverse plane at 3- to 4-mm intervals. Alternate sections were routinely submitted with additional sections near close margins submitted at the discretion of the pathologist. Pathology reports were reviewed to identify cases with involvement of 1 or more surgical margin(s). A margin was considered positive if malignant cells were in contact with the inked margin in the absence of intervening benign tissue. Secondary pathologic review was employed in selected cases.
Postoperative evaluations included physical examination and PSA measurement every 3 to 6 months for the first 2 years postprostatectomy and every 6 to 12 months thereafter. In the setting of PSA or clinical relapse, restaging imaging and subsequent intervention(s) were performed at the discretion of the managing urologist and oncologist. The principal outcome measure of this retrospective study was PSA relapse-free survival (bRFS) after prostatectomy.
With the increased sensitivity of PSA detection, the definition of biochemical failure is controversial, as reflected by the various thresholds for PSA failure used in prospective trials.14, 15, 16 Because a lower PSA at the time of salvage intervention has been correlated with improved bRFS, a first rising PSA > 0.1 ng/mL (followed by confirmation with a second PSA) or initiation of salvage intervention for rising PSA was chosen as the definition of biochemical failure rather than 0.2 ng/mL.17,18 bRFS was measured from date of prostatectomy to date of failure. If no PSA rise or intervention occurred, then patients were censored at last follow-up or death if PSA had been drawn within 12 months or on date of most recent PSA if none had been documented within 12 months of last follow-up or death. Patients with detectable postoperative PSAs at ≤ 0.1 ng/mL were not considered disease failures in the absence of salvage intervention. Secondary objectives included analysis of factors associated with bRFS and identification of low- and/or high-risk subsets based upon this.
Statistical analysis
Cox proportional hazard regression was used to assess the effects of pathologic and postoperative variables on bRFS. Regression estimates are reported as hazard ratios and 95% confidence intervals (CIs). Plots of survival curves using the Kaplan-Meier method were constructed. Estimates and 95% pointwise CIs were reported for 5- and 8-year bRFS. To identify potential prognostic groups, a recursive binary partitioning by conditional inference analysis (RPA) was applied to identify patient cohorts representing varying levels of failure risk stratified by the GG and ME. ME was examined 2 ways: (1) the total length of all margins summed together (cumulative) and (2) the length of the largest involved margin (single maximum). The RPA was conducted with the specification Bonferroni adjusted and nodes with ≥ 20 patients. All statistical testing was 2-sided and assessed for significance at the 5% level using R (www.r-project.org) and SAS v9.4 (SAS Institute, Cary, NC).
Results
Between 2002 and 2010, 1041 patients underwent RP for PC at the study institutions, of whom 210 were identified for inclusion in the present study. Median age was 61 years (range, 43-76), and median highest preoperative PSA was 5.8 (1.6-26.0). Additional demographic and preprostatectomy tumor, staging, and workup characteristics are outlined in Table 1. Surgical and pathologic details are demonstrated in Table 2. No patient underwent postoperative radiation or hormone therapy in the absence of rising PSA.
Table 1.
Patient presurgical demographics and clinicopathologic characteristics
| Characteristic | n | % |
|---|---|---|
| Age (at diagnosis) | ||
| Median (range) | 61 (43-76) | |
| Preoperative PSA* | ||
| Median (range) | 5.8 ng/mL (1.6-26.0) | |
| Gleason score at biopsy | ||
| 6 | 126 | 60.0 |
| 7 | 72 | 34.3 |
| 8 | 8 | 3.8 |
| 9 | 4 | 1.9 |
| Interval biopsy to RP | ||
| Median (range) | 53 days (11-512) |
Abbreviations: PSA = prostate specific antigen; RP = radical prostatectomy.
One patient without pre-RP PSA.
Table 2.
Radical prostatectomy and surgical pathology characteristics
| Characteristic | n | % |
|---|---|---|
| Robot-assisted RP | ||
| Yes | 33 | 15.7 |
| No | 177 | 84.3 |
| Nerve-sparing RP | ||
| Yes | 156 | 74.3 |
| No | 54 | 25.7 |
| Prostate volume* | ||
| Median (range) | 44 cc (16-150) | |
| RP Gleason group | ||
| 1 | 80 | 38.1 |
| 2 | 82 | 39.0 |
| 3 | 30 | 14.3 |
| 4 | 10 | 4.8 |
| 5 | 8 | 3.8 |
| Pathologic tumor stage† | ||
| 2 | 140 | 67.0 |
| 3a | 69 | 33.0 |
| Extraprostatic extension‡ | ||
| No | 72 | 51.1 |
| Yes | 69 | 48.9 |
| Total foci§ | ||
| 1 | 140 | 69.3 |
| 2 + | 62 | 30.7 |
| Median maximal margin width (range)║ | 3.0 (0.1-23.0) | |
| Median cumulative margin width (range)║ | 4.0 (0.1-34.0) |
Abbreviation: RP = radical prostatectomy.
Three patients without prostate volume.
One patient without pathologic tumor stage.
Sixty-nine patients without extraprostatic extension information.
Eight patients with positive margins without total foci count.
Twelve patients with positive margins without maximal margin width and cumulative margin width.
For the overall population, at a median follow-up of 105 months (range, 13-182, with 90% followed ≥ 5 years and 58% followed ≥ 8 years), 89 patients (42.4%) had experienced PSA relapse, and 16 (7.6%) had died. The 5- and 8-year bRFS estimates were 63% (95% CI, 56%-69%) and 56% (48%-62%), respectively. The estimated 8-year overall survival was 97% (93%-99%). Univariate analysis identified higher GS at biopsy (4-6 vs ≥7 +), higher GG at RP (1-2 vs ≥3), extraprostatic extension, higher preprostatectomy PSA, larger cumulative extent of margin involvement, or larger single maximal extent of margin involvement associated with PSA failure (Table 3).
Table 3.
Univariate analysis of factors associated with disease control
| Characteristic | HR | 95% CI | P value |
|---|---|---|---|
| Univariate analysis | |||
| Age* | 1.00 | 0.97-1.03 | .72 |
| Preoperative PSA* | 1.09 | 1.06-1.14 | < .01 |
| Gleason score at biopsy | |||
| 4-6 | - | - | - |
| 7 | 1.93 | 1.24-3.02 | < .01 |
| 8-10 | 5.00 | 2.53-9.88 | < .01 |
| Interval from biopsy to RP* | 1.00 | 1.00-1.00 | .79 |
| Prostate volume* | 1.00 | 0.98-1.01 | .60 |
| Gleason score at RP | |||
| 5-6 | - | - | - |
| 7 | 2.41 | 1.45-4.02 | < .01 |
| 8-9 | 9.37 | 4.77-18.39 | < .01 |
| Gleason grade | |||
| 1-2 | - | - | - |
| 3 | 1.91 | 1.08-3.38 | .03 |
| 4-5 | 5.95 | 3.35-10.57 | < .01 |
| Robot-assisted RP, no vs yes | 0.83 | 0.47-1.48 | .53 |
| Nerve-sparing RP, no vs yes | 1.18 | 0.74-1.89 | .48 |
| Extraprostatic extension, yes vs no | 1.82 | 1.08-3.38 | .03 |
| Pathologic tumor stage | |||
| 2 | - | - | - |
| 3 | 1.46 | 0.95-2.24 | .08 |
| Foci of margin involvement | |||
| 1 | - | - | - |
| 2 + | 1.50 | 0.97-2.31 | .07 |
| Maximal margin width* | 1.09 | 1.04-1.14 | < .01 |
| Cumulative margin width* | 1.07 | 1.04-1.10 | < .01 |
Abbreviations: CI = confidence interval; HR = hazard ratio; PSA = prostate specific antigen; RP = radical prostatectomy.
bolding indicates statistically significant values.
*Continuous variables are denoted with an asterisk where HR equals 1 unit of change. All other variables are categorical.
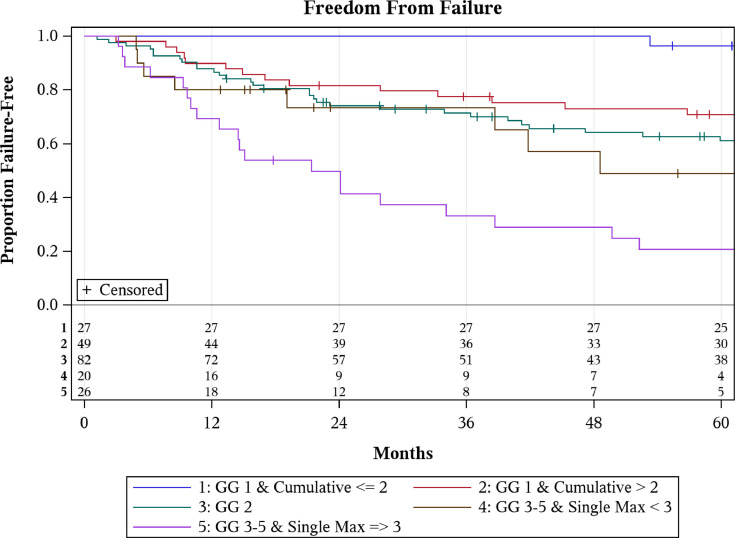
Of the 210 patients in this study, detailed pathology data including number of margin foci were available for 202 patients, with 62 demonstrating multiple foci of involvement. ME information was available for 198 patients with the median single maximal ME of 3 mm (0.1-23.0) and cumulative ME of 4 mm (0.1-34.0). Based upon cumulative and maximal single ME along with GG at RP, patients were stratified into 5 groups using RPA to characterize the differences in bRFS (Fig 1). RPA substratified GG1 patients based on cumulative ME (P = .02) and GG3 patients based on largest single ME (P = .01), whereas substratification of GG2 did not appreciate any differences in bRFS. Examining bRFS by RPA group, hazard ratio progressively increased with subsequent higher risk group (Table 4). Estimated 8-year bRFS for patients with GG1 at prostatectomy and cumulative ME of ≤ 2 mm was 92% (72%-98%) compared with 68% (52%-79%) for the same GG but cumulative ME ≥ 2 mm (Table 5). For those with GG3 or greater disease, estimated 8-year bRFS was 49% for patients with a largest single ME < 3 mm (22%-71%) compared with 11% for patients with a largest single ME ≥ 3 mm (2%-28%).
Fig. 1.
Kaplan-Meier curves comparing biochemical relapse free survival (bRFS) for stratified patient cohorts using recursive partitioning by conditional inference analysis (RPA). Cumulative = cumulative margins; GG = Gleason grade; Single Max = maximal extent of 1 involved margin.
Table 4.
Comparison of bRFS by risk group identified on RPA
| bRFS |
|||||
|---|---|---|---|---|---|
| Covariate | Level | n | Hazard ratio | 95% CI | |
| Group | GG 1 & cumulative* <= 2.0 | 27 | 0.06 | 0.02 | 0.19 |
| GG 1 & cumulative > 2.0 | 49 | 0.23 | 0.12 | 0.44 | |
| GG 2 | 82 | 0.35 | 0.20 | 0.59 | |
| GG 3-5 & single max† < 3.0 | 20 | 0.42 | 0.19 | 0.95 | |
| GG 3-5 & single max => 3.0 | 26 | Ref | - | - | |
Abbreviations: bRFS = biochemical relapse free survival; CI = confidence interval; GG = Gleason grade; RPA = recursive partitioning by conditional inference analysis.
Cumulative margins.
Maximal extent of 1 involved margin.
Table 5.
Five- and 8-year bRFS of positive-margin patients stratified by RPA
| Variable | Level | n | 5 Year | 8 Year |
|---|---|---|---|---|
| RPA group | GG 1 & cumulative* ≤ 2.0 | 27 | 96% (76%-99%) | 92% (72%-98%) |
| GG 1 & cumulative > 2.0 | 49 | 71% (56%-82%) | 68% (52%-79%) | |
| GG 2 | 82 | 61% (49%-71%) | 50% (38%-62%) | |
| GG 3-5 & single max† < 3.0 | 20 | 49% (22%-71%) | 49% (22%-71%)‡ | |
| GG 3-5 & single max ≥ 3.0 | 26 | 21% (8%-38%) | 11% (2%-28%) |
Abbreviations: bRFS = biochemical relapse free survival; GG = Gleason grade; RPA = recursive partitioning by conditional inference analysis.
Cumulative margins.
Maximal extent of 1 involved margin.
By 5 years, there are only 4 patients at risk still and none of them have had a failure by 8 years so it remains unchanged.
Discussion
We present 210 patients who underwent prostatectomy with positive margins. To our knowledge, this is the only study to implement RPA stratification to identify potential low- and high-risk cohorts with more than 200 margin-positive patients and median follow-up over 8 years. The bRFS at 8 years of those with GG1 disease with cumulative margin 2 mm or less was historically comparable (92%) to those with negative margins.6,19 Additionally, approximately 50% of patients with GG2 and positive margins experienced biochemical failure at 8 years. Although the findings from this study are unique in terms of population size and robust follow-up, they align with others observing that increasing ME, number of positive margins, and higher GS correlate with worse bRFS after prostatectomy.20, 21, 22, 23, 24
An initial report of short-term outcomes in this population observed differences in 5-year bRFS when a single or cumulative ME cutoff of 4 mm was used regardless of GS. However, our updated analysis presented in 2017 using the same cutoff was not able to achieve 95% CI separation, making it statistically insignificant.6 In a subsequent analysis with more positive- margin patients, RPA generated risk groups for PSA failure using GS (6-7 vs 8-9) and initial post-RP PSA (<0.1 and ≥0.1). However, margin involvement extent was not used in this analysis to further dissect subgroups within each GG.25 With longer follow-up and additional PSA relapses, we decided to perform an RPA, a more flexible modeling approach with the ability to uncover potential interactions and to generate significant differences in bRFS based on ME and pathologic GG.
By examining those patients with margin-positive PC after surgical resection, 1 aim of this study was to provide additional insight into which patients might be most amenable to observation after prostatectomy. Currently, national consensus guidelines recommend consideration of adjuvant radiation after prostatectomy for those with adverse pathologic features, including positive margins, seminal vesicle invasion, or extraprostatic extension.26,27 These recommendations are based on various prospective trials and retrospective studies demonstrating an improvement in bRFS after adjuvant radiation compared with observation.7, 8, 9,28, 29, 30 However, these studies also consistently find that 25% to 40% of patients who do not receive adjuvant radiation remain disease free and would not have benefited from adjuvant radiation. Leading professional organizations, including American Society for Radiation Oncology and American urologic association, recommend observation for most patients with an undetectable postop PSA regardless of pathologic risk factors, as adjuvant treatment can cause genitourinary toxicity even with modern radiation therapy delivery techniques and adjuvant radiation therapy has not consistently shown a survival benefit.31, 32, 33 Despite these recommendations, some radiation oncologists remain hesitant to recommend observation for patients after a margin-positive surgery.34,35 Our data build on prior observation and demonstrate that by using common pathologic variables and extent of margin involvement, it is possible to identify patients with a very low risk of recurrence who are unlikely to benefit from adjuvant radiation.
We observed an 8-year bRFS of 92% in patients with pathologic GG1 disease and positive cumulative ME of 2 mm or less. However, this dropped to 68% when cumulative ME was more than 2 mm in the same GS1 patients. Looking at a similar cohort of patients, Chapin and colleagues36 noted that patients with ME of 1 mm or less demonstrated improved bRFS at 5 years compared with those with ME greater than 1 mm regardless of GS. Other studies have also appreciated that increasing positive ME correlates with inferior bRFS.20, 21, 22, 23,37 One explanation for this observation is that the extent of focal postoperative tumor cell death at the margins from either surgical cauterization, ischemia, or local immune response that occurs is sufficient to sterilize low but not higher volumes of residual disease. Another possibility is that lower grade disease with less residual disease manifests as biochemical disease much later in higher risk patients. Regardless of the reason, these results suggest that patients with low GG disease, minimal involvement of margins at RP, and life expectancy less than 10 years are unlikely to develop biochemical failure and are less likely to benefit from adjuvant radiation therapy.
We investigated the bRFS of those with GG2 disease aiming to find a subgroup that would benefit the most from adjuvant radiation. At 8-years, the bRFS in this cohort was 50%, and RPA did not identify a lower risk subgroup with improved bRFS based on single or cumulative ME. It is worth noting our analysis only included 82 patients with positive margins and GG2 disease, which may have limited our ability to detect a true difference in this cohort. However, one previous study with the same number of patients (n = 82) found a significant effect of ME (> or <4 mm of involvement) in GG2 patients using Cox regression analysis.38 We observed that the single largest ME was an important predictor of bRFS in patients with GG3-5. Approximately half (49%) of patients with GG3-5 and <3 mm ME remained disease free with no further therapy, whereas almost 90% of patients with GG3-5 and >3 mm ME experienced a biochemical failure at 8 years. This observation suggests that the presence of focal GG3-5 at the margins is far from a guarantee of biochemical relapse and that many of these patients may be reasonably surveyed. Conversely, patients with larger ME and GG3-5 disease have a very high risk of progression, and consideration of adjuvant therapy may be more appropriate in this subset of patients.
Whether the highest risk patients in this study should receive adjuvant or salvage radiation remains to be determined. Initial landmark trials investigating patients with adverse pathologic features including positive margins noted lower rates of PSA failure with adjuvant radiation compared with observation.7, 8, 9 Approximately 30% of patients in 2 of these trials presented with a detectable PSA at the time of initiating radiation, which raised the question of whether improved biochemical control was attributable to adjuvant radiation therapy in high-risk patients or early salvage radiation in the sizable portion of patients with a detectable postop PSA. Preliminary results from 2 prospective studies examining adjuvant and salvage radiation have been released in abstract form. The RADICALS trial enrolled those with undetectable PSA with at least 1 risk factor (positive margins or preoperative PSA ≥ 10 ng/nL, GS 7-10, pT3/4).14 At median follow-up of 5 years, no differences in bRFS or overall survival were noted and patients in the adjuvant arm demonstrated higher rates of urinary incontinence and urethral stricture. The ARTISTIC meta-analysis combined the RADICALS trial and 2 similar trials, GETUG-AFU 17 and RAVES, and was also unable to find a difference in PSA relapse.15 Both studies support the use observation with early salvage rather than adjuvant radiation.
There multiple limitations of our study including its retrospective nature. Less than 10% of patients studied presented with GG4 or GG5 disease, which can introduce small sample bias when performing subgroup analysis. Additionally, the GG at the margin was not recorded, which could affect bRFS rates if different than the primary tumor specimen. The results of this study highlight the shortcoming of using GG and margin status alone rather than higher precision methods. The cancer of the prostate risk assessment postsurgical score, which incorporates 6 tumor characteristics to determine a score from zero to 12, has demonstrated high accuracy rates toward predicting biochemical recurrence.39 Other approaches including computational modeling with Deep Neural Networks and genomic assays such as Decipher have also shown promise.16,40 Finally, liquid biopsies assessing minimal residual disease by detecting either circulating tumor DNA or cells are also being investigated.41,42 However, their roles in specific subsets of patients with localized prostate cancer after prostatectomy is currently unclear, and we provide evidence for a discerning RPA using regularly available clinical and pathologic variables.
Conclusions
In patients with PC with pT2-3aN0 disease who underwent prostatectomy, employing an RPA of pathologic data, with specific focus on a margin-involved subset of patients, ME, in combination with GG at prostatectomy, demonstrated an opportunity for long-term bRFS risk substratification. We observed over 90% of patients with GG1 disease and a minimal cumulative ME did not develop PSA relapse at 8 years. Additionally, only 50% of GG2 or GG3-5 disease with small ME experienced biochemical failure. This may assist urologists and radiation oncologists in clinical decision-making, specific to adjuvant or salvage therapy interventions.
Footnotes
Sources of support: Provided by the University of Iowa Department of Radiation Oncology.
Disclosures: none.
Data Sharing Statement: Research data are not available at this time.
References
- 1.Blute ML, Bergstralh EJ, Iocca A, Scherer B, Zincke H. Use of Gleason score, prostate specific antigen, seminal vesicle and margin status to predict biochemical failure after radical prostatectomy. J Urol. 2001;165:119–125. doi: 10.1097/00005392-200101000-00030. [DOI] [PubMed] [Google Scholar]
- 2.Swindle P, Eastham JA, Ohori M, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005;174:903–907. doi: 10.1097/01.ju.0000169475.00949.78. [DOI] [PubMed] [Google Scholar]
- 3.Kang JH, Ha Y-S, Kim S, et al. Concern for overtreatment using the AUA/ASTRO guideline on adjuvant radiotherapy after radical prostatectomy. BMC Urol. 2014;14:30. doi: 10.1186/1471-2490-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ploussard G, Agamy MA, Alenda O, et al. Impact of positive surgical margins on prostate-specific antigen failure after radical prostatectomy in adjuvant treatment-naive patients. BJU Int. 2011;107:1748–1754. doi: 10.1111/j.1464-410X.2010.09728.x. [DOI] [PubMed] [Google Scholar]
- 5.Blute ML, Bostwick DG, Bergstralh EJ, et al. Anatomic site-specific positive margins in organ-confined prostate cancer and its impact on outcome after radical prostatectomy. Urology. 1997;50:733–739. doi: 10.1016/S0090-4295(97)00450-0. [DOI] [PubMed] [Google Scholar]
- 6.Watkins JM, Laszewski M, Watkins PL, Dufan TA, Adducci C. Margin involvement at prostatectomy for clinically localized prostate cancer: does a low-risk group exist. Pract Radiat Oncol. 2015;5:e31–6. doi: 10.1016/j.prro.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: Long-term followup of a randomized clinical trial. J Urol. 2009;181:956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–2027. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 9.Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66:243–250. doi: 10.1016/j.eururo.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Ghia AJ, Shrieve DC, Tward JD. Adjuvant radiotherapy use and patterns of care analysis for margin-positive prostate adenocarcinoma with extracapsular extension: Postprostatectomy adjuvant radiotherapy: A SEER analysis. Urology. 2010;76:1169–1174. doi: 10.1016/j.urology.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman KE, Nguyen PL, Chen M-H, et al. Recommendations for post-prostatectomy radiation therapy in the United States before and after the presentation of randomized trials. J Urol. 2011;185:116–120. doi: 10.1016/j.juro.2010.08.086. [DOI] [PubMed] [Google Scholar]
- 12.Vale CL, Fisher D, Kneebone A, et al. Adjuvant or salvage radiotherapy for the treatment of localised prostate cancer? A prospectively planned aggregate data meta-analysis. Ann Oncol. 2019;30:v883. doi: 10.1016/S0140-6736(20)31952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker C, Clarke NW, Cook A, et al. Timing of radiotherapy (RT) after radical prostatectomy (RP): First results from the RADICALS RT randomised controlled trial (RCT) [ NCT00541047] Ann Oncol. 2019;30:v883–v884. [Google Scholar]
- 14.Parker CC, Clarke NW, Cook AD, et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): A randomised, controlled phase 3 trial. Lancet. 2020;396:1413–1421. doi: 10.1016/S0140-6736(20)31553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vale CL, Fisher D, Kneebone A, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet. 2020;396:1422–1431. doi: 10.1016/S0140-6736(20)31952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sargos P, Leduc N, Giraud N, et al. Deep Neural Networks outperform the CAPRA score in predicting biochemical recurrence after prostatectomy. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.607923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tendulkar RD, Agrawal S, Gao T, et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016;34:3648–3654. doi: 10.1200/JCO.2016.67.9647. [DOI] [PubMed] [Google Scholar]
- 18.King CR. The timing of salvage radiotherapy after radical prostatectomy: A systematic review. Int J Radiat Oncol Biol Phys. 2012;84:104–111. doi: 10.1016/j.ijrobp.2011.10.069. [DOI] [PubMed] [Google Scholar]
- 19.Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–4305. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochiai A, Sotelo T, Troncoso P, Bhadkamkar V, Babaian RJ. Natural history of biochemical progression after radical prostatectomy based on length of a positive margin. Urology. 2008;71:308–312. doi: 10.1016/j.urology.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 21.Servoll E, Vlatkovic L, Saeter T, et al. The length of a positive surgical margin is of prognostic significance in patients with clinically localized prostate cancer treated with radical prostatectomy. Urol Int. 2014;93:289–295. doi: 10.1159/000362342. [DOI] [PubMed] [Google Scholar]
- 22.Shikanov S, Song J, Royce C, et al. Length of positive surgical margin after radical prostatectomy as a predictor of biochemical recurrence. J Urol. 2009;182:139–144. doi: 10.1016/j.juro.2009.02.139. [DOI] [PubMed] [Google Scholar]
- 23.van Oort IM, Maxim Bruins H, Kiemeney LALM, et al. The length of positive surgical margins correlates with biochemical recurrence after radical prostatectomy. Histopathology. 2010;56:464–471. doi: 10.1111/j.1365-2559.2010.03497.x. [DOI] [PubMed] [Google Scholar]
- 24.Stephenson AJ, Wood DP, Kattan MW, et al. Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol. 2009;182:1357–1363. doi: 10.1016/j.juro.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 25.Seyedin SN, Mott SL, Snow AN, Russo JK, Watkins J. The impact of prostate cancer (PC) margin extent (ME) at radical prostatectomy (RP) on biochemical relapse-free survival (bRFS). J Clin Oncol. 2018;36(6_suppl):83-83.
- 26.Valicenti RK, Thompson I, Albertsen P, et al. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys. 2013;86:822–828. doi: 10.1016/j.ijrobp.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 27.National Comprehensive Cancer Network, Prostate Cancer (Version 2.2020). Available at: www.nccn.org. Accessed September 16, 2021.
- 28.Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–2930. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 29.Trabulsi EJ, Valicenti RK, Hanlon AL, et al. A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3-4N0 prostate cancer. Urology. 2008;72:1298–1302. doi: 10.1016/j.urology.2008.05.057. [discussion 1302-1304] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leibovich BC, Engen DE, Patterson DE, et al. Benefit of adjuvant radiation therapy for localized prostate cancer with a positive surgical margin. J Urol. 2000;163:1178–1182. [PubMed] [Google Scholar]
- 31.Gandaglia G, Briganti A, Clarke N, et al. Adjuvant and salvage radiotherapy after radical prostatectomy in prostate cancer patients. Eur Urol. 2017;72:689–709. doi: 10.1016/j.eururo.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Jensen L, Yuh B, Wong JYC, et al. Outcomes and toxicity of 313 prostate cancer patients receiving helical tomotherapy after radical prostatectomy. Adv Radiat Oncol. 2017;2:597–607. doi: 10.1016/j.adro.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ost P, Fonteyne V, Villeirs G, et al. Adjuvant high-dose intensity-modulated radiotherapy after radical prostatectomy for prostate cancer: clinical results in 104 patients. Eur Urol. 2009;56:669–675. doi: 10.1016/j.eururo.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 34.Kalbasi A, Swisher-McClure S, Mitra N, et al. Low rates of adjuvant radiation in patients with nonmetastatic prostate cancer with high-risk pathologic features. Cancer. 2014;120:3089–3096. doi: 10.1002/cncr.28856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniels CP, Millar JL, Spelman T, et al. Predictors and rate of adjuvant radiation therapy following radical prostatectomy: A report from the Prostate Cancer Registry. J Med Imaging Radiat Oncol. 2016;60:247–254. doi: 10.1111/1754-9485.12407. [DOI] [PubMed] [Google Scholar]
- 36.Chapin BF, Nguyen JN, Achim MF, et al. Positive margin length and highest Gleason grade of tumor at the margin predict for biochemical recurrence after radical prostatectomy in patients with organ-confined prostate cancer. Prostate Cancer Prostatic Dis. 2018;21:221–227. doi: 10.1038/s41391-017-0019-4. [DOI] [PubMed] [Google Scholar]
- 37.Emerson RE, Koch MO, Jones TD, Daggy JK, Juliar BE, Cheng L. The influence of extent of surgical margin positivity on prostate specific antigen recurrence. J Clin Pathol. 2005;58:1028–1032. doi: 10.1136/jcp.2005.025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sooriakumaran P, Ploumidis A, Nybert T, et al. The impact of length and location of positive margins in predicting biochemical recurrence after robot-assisted radical prostatectomy with a minimum follow-up of 5 years. BJU Int. 2015;115:106–113. doi: 10.1111/bju.12483. [DOI] [PubMed] [Google Scholar]
- 39.Punnen S, Freedland SJ, Presti JC, et al. Multi-institutional validation of the CAPRA-S score to predict disease recurrence and mortality after radical prostatectomy. Eur Urol. 2014;65:1171–1177. doi: 10.1016/j.eururo.2013.03.058. [DOI] [PubMed] [Google Scholar]
- 40.Marascio J, Spratt DE, Zhang J, et al. Prospective study to define the clinical utility and benefit of Decipher testing in men following prostatectomy. Prostate Cancer Prostatic Dis. 2020;23:295–302. doi: 10.1038/s41391-019-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pantel K, Alix-Panabieres C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16:409–424. doi: 10.1038/s41571-019-0187-3. [DOI] [PubMed] [Google Scholar]
- 42.Azad TD, Chaudhuri AA, Fang P, et al. Circulating tumor DNA analysis for detection of minimal residual disease after chemoradiotherapy for localized esophageal cancer. Gastroenterology. 2019;158:494–505. doi: 10.1053/j.gastro.2019.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]