Abstract
Purpose
To examine the effectiveness and safety of single-isocenter multitarget stereotactic radiosurgery using a volume-adapted dosing strategy in patients with 4 to 10 brain metastases.
Methods and Materials
Adult patients with 4 to 10 brain metastases were eligible for this prospective trial. The primary endpoint was overall survival. Secondary endpoints were local recurrence, distant brain failure, neurologic death, and rate of adverse events. Exploratory objectives were neurocognition, quality of life, dosimetric data, salvage rate, and radionecrosis. Dose was prescribed in a single fraction per RTOG 90-05 or as 5 Gy × 5 fractions for lesions ≥3 cm diameter, lesions involving critical structures, or single-fraction brain V12Gy >20 mL.
Results
Forty patients were treated with median age of 61 years, Karnofsky performance status 90, and 6 brain metastases. Twenty-two patients survived longer than expected from the time of protocol SRS, with 1 living patient who has not reached that milestone. Median overall survival was 8.1 months with a 1-year overall survival of 35.7%. The 1-year local recurrence rate was 5% (10 of 204 of evaluable lesions) in 12.5% (4 of 32) of the patients. Distant brain failure was observed in 19 of 32 patients with a 1-year rate of 35.8%. Grade 1-2 headache was the most common complaint, with no grade 3-5 treatment-related adverse events. Radionecrosis was observed in only 5 lesions, with a 1-year rate of 1.5%. Rate of neurologic death was 20%. Neurocognition and quality of life did not significantly change 3 months after SRS compared with pretreatment.
Conclusions
These results suggest that volume-adapted dosing single-isocenter multitarget stereotactic radiosurgery is an effective and safe treatment for patients with 4 to 10 brain metastases.
Introduction
As systemic therapies for solid tumors improve and patients with metastatic cancer survive longer, the number of patients living with brain metastases has increased. Historically, whole-brain radiation therapy (WBRT) has been the primary treatment for multiple brain metastases. Although providing reasonable control of visible and occult lesions for many tumor types, WBRT is associated with cognitive decline1, 2, 3 and can only be delivered safely and efficaciously once at full dose. In contrast to WBRT, stereotactic radiosurgery (SRS) provides excellent local control of brain metastases while sparing normal brain, preserving cognitive function and quality of life (QOL). In addition, multiple courses of SRS can be delivered with minimal toxicity, which is particularly useful in the setting of new brain lesions after initial brain treatment.4
SRS is now widely considered the standard of care for patients with 1 to 3 brain metastases, and many institutions are exploring SRS to treat larger numbers of brain lesions. For example, a prospective observational trial investigating a multicentric, single-fraction SRS technique in patients with 2 to 10 brain metastases revealed no differences in survival, local recurrence, or toxicity in patients treated for 2 to 4 versus 5 to 10 brain metastases.5
The treatment of multiple brain metastases with SRS has several challenges. The traditional practice of delivering radiosurgery to each lesion with a unique isocenter plan can result in prolonged treatment times for patients with many brain lesions. Conversely, single-isocenter, multitarget (SIMT) techniques, in which all brain lesions are simultaneously treated using a common isocenter,6, 7, 8, 9, 10, 11, 12, 13 substantially reduce treatment time. Similarly, single-fraction SRS also is limited to treatment of modest individual and aggregate lesion volumes in noneloquent brain structures, as the prescribed dose must be reduced to prevent toxicity for large lesions or those located in structures such as the brainstem, which in turn may limit local control. Hypofractionated SRS (HF-SRS, SRS delivered in 2-5 fractions) potentially provides a broader therapeutic window than single-fraction SRS, permitting large lesions and aggregate lesion volumes, as well as lesions within critical structures, to be safely and effectively treated.14,15 Retrospective studies have shown that SIMT using 1 to 5 fractions is technically feasible and affords reasonable control of treated lesions with minimal toxicity,6,9,11,12 although additional systematic prospective studies of the indications, efficacy, and toxicities associated with this technique would be beneficial.
Combining an SIMT technique with dose adaptation enables safe treatment of a large number and volume of brain metastases in any brain location. In this study, “dose adapted” is defined as selection of either a 5-fraction SRS regimen for large treatment volumes or lesions near critical structures, or a single-fraction regimen with reduced dose prescribed to at least 1 lesion. We hypothesized that dose-adapted SIMT SRS in patients with 4 or more brain metastases is efficacious and can be delivered efficiently with minimal toxicity and no significant effect on quality of life and neurocognition and without adversely affecting survival predicted by the diagnostic-specific graded prognostic assessment (DS-GPA).16,17
Methods and Materials
Patient population
Patients with 4 to 10 untreated brain metastases were enrolled in this prospective, single-arm trial. Eligibility criteria included age ≥18 years Karnofsky performance status ≥70, and a life expectancy of >3 months. All patients were required to have brain magnetic resonance imaging (MRI). Patients with small cell carcinoma, germ-cell tumors, lymphoma, leukemia, and multiple myeloma or with leptomeningeal disease were excluded from this study. DS-GPA score, calculated using the original formulation of this system,16,17 needed to be 0.5 or greater. The largest metastatic lesion could be no more than 4 cm in maximum dimension, and all lesions were required to be >2 mm from the optic apparatus. Brain stem lesions were permitted. Previous cranial SRS/WBRT was allowed if >3 months before SIMT.
Study design
The institutional review board–approved study was registered at https://clinicaltrials.gov/ct2/show/NCT02886572. The primary endpoint was overall survival (OS). Secondary endpoints were local recurrence, distant brain recurrence, time to neurologic death, quality of life, prevalence of adverse events, and steroid use.
Radiation technique and treatment
For treatment planning, patients underwent computed tomography (CT) simulation using a frameless SRS thermoplastic mask (BrainLAB, Munich, Germany). A thin-cut (1-mm) CT scan of the brain was fused with a gadolinium-contrast-enhanced axial 3-dimensional spoiled-gradient MRI scan. Gross tumor volume included all contrast-enhancing lesions. The planning target volume (PTV) was created by adding a 1 mm margin to the gross tumor volume. Doses were prescribed to each lesion based on that lesion's size, location, and volume.
All prescription doses were assigned per the protocol algorithm (Fig. E1). PTVs <2 cm in maximum dimension were prescribed 20 Gy, and those between 2.0 and <3.0 cm received 18 Gy.18 If 2 lesions were within 1 cm of each other, the prescribed dose to both lesions was based on the maximum diameter across these 2 lesions. PTVs >3 cm in maximum diameter received five 5-Gy fractions delivered on consecutive workdays. At the discretion of the treating physician, lesions in eloquent areas of the brain (eg, the motor strip or speech center) could be reduced from 20 Gy to 18 Gy for PTV <2 cm and 18 Gy to 16 Gy for PTV 2 to 2.9 cm. If the V12Gy of normal brain parenchyma (subtracting out the PTV) exceeded 20 mL on single-fraction treatment planning, treatment was replanned and administered with 5 Gy × 5 fractions to all lesions. In addition, if a patient had any lesions in the brain stem, all lesions were also treated with 5 Gy × 5. Patients were deemed to have had a “dose adjustment” if they underwent a 5-fraction regimen or a single-fraction treatment with at least 1 metastasis treated with reduced dose.
All patients were contoured using BrainLAB iPlan RT Planning software and planned in ECLIPSE (Varian Medical Systems, Palo Alto, CA) using volumetric modulated arc therapy with a single-isocenter technique detailed previously.19 All treatments were delivered on a Novalis TX or TrueBeam STx linear accelerator equipped with a micromultileaf collimator (2.5 mm leaf width, Varian Medical Systems) using orthogonal kV and cone beam CT imaging. Translational and rotational position deviations were detected and corrected using 2-dimensional/3-dimensional image matching and a 6 degrees of freedom couch.20
Follow-up
One month after SRS, patients returned for physical examination, QOL, and neurocognitive assessment. Subsequently, patients underwent brain MRI with physical examination, QOL/neurocognitive assessment every 3 months during the first year and every 6 months in the second year. After local or distant recurrence, patients no longer continued neurocognitive studies but were followed for survival, local control, and radiation necrosis. Radiation necrosis was identified either histologically when surgical intervention was appropriate or with the treating physician and principle investigator's clinical judgments of the appearance on MRI and rate of radiologic changes. Neurologic death was defined as death from neurologic causes related to tumor and categorized based on chart documentation. If neurologic death was not clearly noted but patients had documented MRI/CT progression of central nervous system disease within 3 months of death with no additional central nervous system treatment, this was also judged as neurologic death.
Neurocognition and quality of life
Neurocognitive and QOL testing was performed at baseline, 1 month after SIMT SRS, and at 3-month intervals from the time of SRS. Testing consisted of Mini-mental state examination (MMSE),21 Trail Making tests part A and B (TMT-A/B),22 Hopkins Verbal Learning Test,23 and the validated Functional Assessment of Cancer Therapy-Brain (FACT-Br).24 Testing was stopped either because of distant brain failure, patient refusal, or death.
Statistical design and analysis
The goal of this study was to determine that the use of SIMT SRS does not significantly reduce or compromise the outcome of patients with ≥4 brain metastases. The original DS-GPA score developed by Sperduto et al,16,17 dependent on primary histology and baseline characteristics, was used to make this assessment. Note that updated formulations of the DS-GPA provide more refined assessments of OS,25 but the molecular characteristics necessary to calculate these modified indices were usually not available when the patients were entered into the study. The expected survival calculated from the DS-GPA was used to help assess the efficacy of SIMT SRS. If the survival of patients treated with SIMT SRS is truly comparable to the survival of patients treated with currently available treatment regimens, we would expect that approximately half of the patients would live longer than expected. In designing this study, we planned to accrue 40 patients. If 17 or more lived longer than expected according to their GPA score, SIMT SRS would be considered noninferior to the treatment, given to the historical comparison cohort. Using this decision rule, there was 90% power to detect a noninferiority difference of −0.2 using a one-sided exact test with a target significance level of .10. The inferiority difference of −0.2 is the distance <0.5, the proportion that is expected to live longer than expected per the GPA score, that is considered statistically noninferior. A one-sided test was used because the study has a one-sided hypothesis that SIMT SRS does not significantly reduce or compromise the outcome of patients with ≥4 brain metastases.
Kaplan-Meier methods were used to estimate OS, with survival being defined as the time from the start of protocol SRS until death. Patients were censored at death or the time of last follow-up if still alive. When analyzing distant recurrence, the cumulative incidence function was used due to the presence of death before distant recurrence existing as a competing risk. This would have been the preferred analysis method of local recurrence as well, but with only 4 patients having local recurrence, the numbers did not allow for a meaningful cumulative incidence function to be calculated. Categorical variables were described with frequencies and percentages. Continuous variables were described using either means with standard deviations or medians with appropriate percentiles.
Results
Patient characteristics
From February 2017 to August 2019, a total of 40 patients were enrolled in this study. Patient characteristics are summarized in Table 1. Non-small cell lung cancer was the predominant histology (50%), followed by breast (27.5%), renal cell (7.5%), and melanoma (7.5%). Median age was 61 years (range, 30-80); 62.5% of participants were female. Median Karnofsky performance status was 90 (range, 70-100), median number of metastases 6, and total volume of brain metastases per patient ranged from 0.06 to 32.2 mL with a mean of 4.28 mL. In addition, 21 of 40 patients presented in their initial diagnosis of metastatic cancer. Eleven patients had previous brain SRS (9), whole brain (2), or both (1) before enrolling in this trial. Five of these patients had 2 or 3 previous courses of SRS.
Table 1.
Patient characteristics
| All patients |
||
|---|---|---|
| N | % | |
| Total | 40 | 100.00 |
| Sex | ||
| Female | 25 | 62.50 |
| Male | 15 | 37.50 |
| Primary disease site | ||
| Lung | 20 | 50.00 |
| Breast | 11 | 27.50 |
| Renal | 3 | 7.50 |
| Melanoma | 3 | 7.50 |
| Other | 3 | 7.50 |
| Were brain metastasis present at initial cancer diagnosis? | ||
| No | 28 | 70.00 |
| Yes | 12 | 30.00 |
| Immunotherapy at baseline? | ||
| No | 32 | 80.00 |
| Yes | 8 | 20.00 |
| Race | ||
| White | 33 | 82.50 |
| Black or African-American | 7 | 17.50 |
| Ethnicity | ||
| Hispanic or Latino | 1 | 2.50 |
| Not Hispanic or Latino | 38 | 95.00 |
| Unknown/not reported | 1 | 2.50 |
| Karnofsky Performance Score | ||
| 100 | 3 | 7.50 |
| 90 | 21 | 52.50 |
| 80 | 11 | 27.50 |
| 70 | 5 | 12.50 |
| No. brain metastasis | ||
| 4 | 8 | 20.00 |
| 5 | 9 | 22.50 |
| 6 | 5 | 12.50 |
| 7 | 7 | 17.50 |
| 8 | 4 | 10.00 |
| 9 | 6 | 15.00 |
| 10 | 1 | 2.50 |
| Metastatic involvement | ||
| Brain only | 11 | 27.50 |
| Extracranial | 29 | 72.50 |
| Prior brain surgery for metastasis | ||
| No | 36 | 90.00 |
| Yes | 4 | 10.00 |
| SRS technique | ||
| VMAT | 39 | 97.50 |
| Static conformal | 1 | 2.50 |
| Received prior SRS/WBRT | ||
| No | 29 | 77.50 |
| Yes | 11 | 22.50 |
Abbreviations: SRS = stereotactic radiosurgery; VMAT = volumetric modulated arc therapy; WBRT = whole-brain radiation therapy.
Twenty-four patients were treated in 1 fraction (total of 125 lesions), and 16 patients were treated in 5 fractions (total of 79 lesions). Of the patients who had single-fraction treatment, 4 received reduced dose because of proximity to critical structures such as optic nerve and brain stem; thus, a total of 20 patients had dose adjustment.
Thirty-two patients had at least 1 follow-up MRI, and only those patients were included in the analyses for all calculations and data points. The other 8 patients died before their first MRI. All 40 patients were included for survival analysis.
Dosimetric data
Twenty-four patients were treated in 1 fraction and 16 in 5 fractions. Median maximum dose was 22.7 Gy (19.8-25.2 Gy) for single-fraction cases and 29.0 Gy (26.7-32.4 Gy) for 5-fraction cases. Mean prescribed dose was 19.9 Gy (18-20 Gy) for single fraction and 25 Gy for 5 fractions. For those treated in 1 or 5 fractions, median V12Gy (brain – PTV) was 11.7 mL (3.0-21.20 mL) and V24Gy was 3.5 mL (0.93-10.97 mL), respectively. Of the patients who underwent single-fraction SRS, 4 had reduced dose because of proximity to critical structures such as optic nerve and brain stem. Median longest distance between metastases was 12.0 cm (6.7-15.1 cm) with a median distance from the isocenter to the most distant metastasis of 7.0 cm (4.2-9.6 cm), mean overall conformity index 1.44 (1.1-2.0), and median number of arcs 4 (3-6). Largest PTV lesion diameter for each patient had a mean of 1.62 cm (0.50-3.80 cm). Mean integral whole brain dose was 3.27 Gy (1.02-14.15 Gy). Median total hippocampal dose was 2.4 Gy with right and left hippocampal doses of 2.20 Gy and 2.76 Gy, respectively. The median maximal hippocampal doses were 4.27 Gy and 4.93 Gy for the right and left hippocampi, respectively.
Delivery time
Median total time on the treatment table was 31.5 min/d (13-70 minutes), including a median beam-on time of 8 minutes (3-13 minutes).
Survival and local and distant control
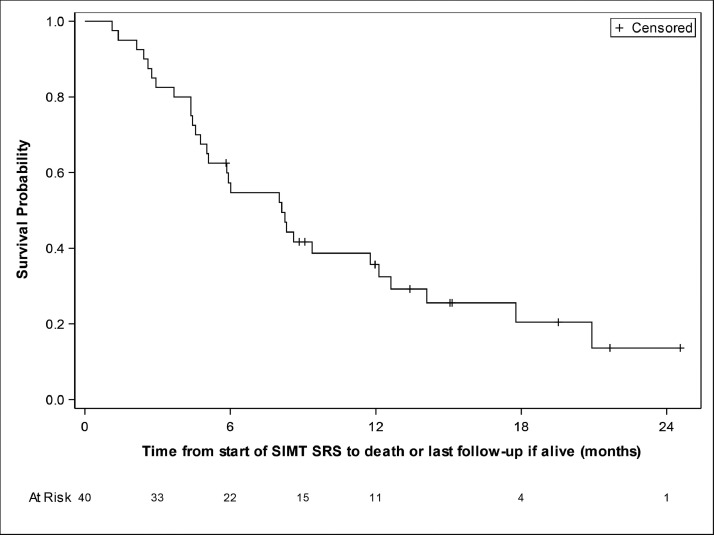
From the time of protocol SRS, median follow-up time was 15.2 months. Median OS was 8.1 months with a 6-month OS of 57.3% and a 1-year OS 35.7% (Fig. 1). OS by histology is presented in Table 2. For comparison, based on the DS-GPA, the median OS for our cohort was calculated to be 5.5 months with a 1-year survival rate of 20% (see Tables E1-E3 for stratification by histology). From the time of protocol SRS, 22 patients survived longer than predicted by DS-GPA, surpassing the minimum of 17 patients needed for non-inferiority (Table E2). In addition, 65% of lung patients and 18% of breast patients lived longer than predicted. From the time of any initial brain metastases–directed radiation therapy (including those treated for brain metastases before the SRS delivered in this SIMT SRS study), 29 patients survived longer than predicted by DS-GPA, with 80% of lung and 55% breast patients living longer than predicted (Table E3). From any brain radiation treatment including those before this study, median OS was 11.8 months with a 1-year OS of 49.5% (33.2%-63.9%).
Figure 1.
Overall survival from start of protocol stereotactic radiosurgery (SRS) treatment. Kaplan-Meier methods were used to estimate overall survival, with survival being defined as the time from the start of protocol SRS until death. Follow-up time was 2 years.
Table 2.
Overall survival from protocol SRS by histology
| Primary disease site | Total | No. dead | Median survival (95% CI), mo | 6-mo survival (95% CI) | 9-mo survival (95% CI) | 12-mo survival (95% CI) |
|---|---|---|---|---|---|---|
| Overall | 40 | 30 | 8.1 (5-12.1) | 57.3% (40.6%-70.9%) | 41.7% (26.3%-56.4%) | 35.7% (21%-50.7%) |
| Lung | 20 | 15 | 8.4 (3.7-17.8) | 60% (35.7%-77.6%) | 45% (23.1%-64.7%) | 39.4% (18.6%-59.7%) |
| Breast | 11 | 9 | 8.3 (2.9-12.6) | 54.5% (22.9%-78%) | 45.5% (16.7%-70.7%) | 36.4% (11.2%-62.7%) |
| Renal | 3 | 2 | 5.9 (5,.) | 33.3% (0.9%-77.4%) | 33.3% (0.9%-77.4%) | 33.3% (0.9%-77.4%) |
| Melanoma | 3 | 3 | 5.1 (2.1-8.1) | 33.3% (0.9%-77.4%) | - | - |
| Other | 3 | 1 | - | 100% (100%-100%) | 50% (0.6%-91%) | - |
Abbreviations: CI = confidence interval; SRS = stereotactic radiosurgery.
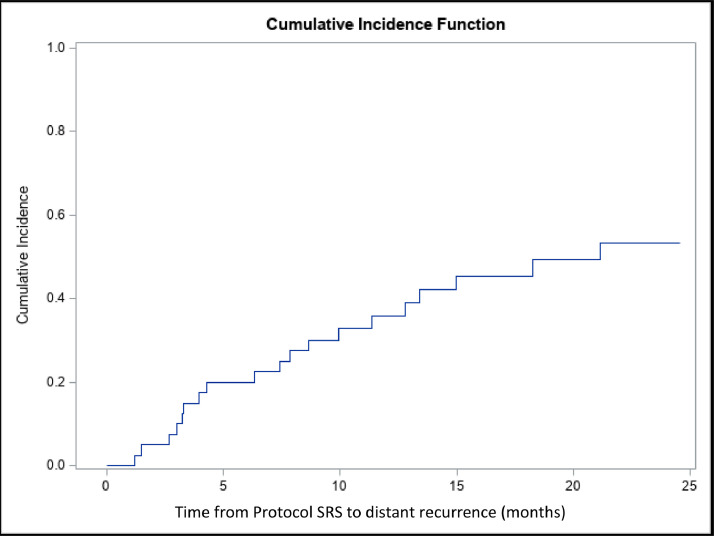
To date, local recurrence has been observed in 4 patients, involving 10 of 204 treated lesions for a 1-year local failure rate of 5% (2.6%, 8.7%) in 4 of 32 patients. One patient with NSCLC exhibited recurrence in 5 lesions. Eight of the 10 recurrent lesions had been treated as prescribed by RTOG 90-05, with no dose adjustment. The other 2 lesions had been treated with 5 fractions. Thus, for lesions where there was no dose adjustment and treatment with a single fraction, local failure occurred in 8 of 119, for 93% local control. For those lesions treated in a 5-fraction regimen, 2 of 79 lesions failed, for 98% local control. Of the 40 patients, 19 experienced distant brain failure and 16 died without documentation of distant failure. The 6- and 12-month cumulative incidence for distant recurrence was 20% (95% CI, 9.3%-33.7%), and 35.8% (95% CI, 20.9%-50.9%), respectively (Fig. 2). Five patients recurred with >10 metastases, although only 1 had diffuse leptomeningeal disease. The remaining 14 patients had 1 to 8 metastases at recurrence with a median of 4 lesions.
Figure 2.
Time from protocol single-isocenter, multitarget to distant recurrence within the brain. Magnetic resonance imaging was obtained every 3 months. Cumulative incidence function was used due to the presence of death before distant recurrence existing as a competing risk.
Of the 15 patients who received salvage therapy after the protocol SRS, 11 received SRS and 4 whole-brain radiation therapy, with a median OS of 5.8 months and a 9-month survival of 37% (95% CI, 13.8%-60.8%) after salvage therapy.
OS did not significantly differ between those who had 4 to 5 metastases versus 6 to 10 metastases. Similarly, there were no differences in OS between patients who received 1 versus 5 fractions. Among the 31 patients who died, 6 experienced a neurologic death (20%) with 1 unknown cause of death. Nine patients were alive at the time this article was submitted.
Toxicity
Adverse events that were possibly, probably, or definitely related to SIMT were reported in 73% of patients, all grade 1 or 2. The most common were headache (50%), fatigue (45%), nausea (25%), and alopecia (20%). No grade 3-5 treatment-related adverse events attributable to SIMT were observed (Table 3.) Additionally, when comparing patients who had no dose adjustment versus those that had dose adjustment, there were no significant differences in the relevant side effect profiles.
Table 3.
Toxicities possibly, probably, or definitely related to SIMT SRS treatment
| Grade of adverse event |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1: Mild |
2: Moderate |
3: Severe |
4: Life Threatening |
5: Lethal |
Total | ||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | N | |
| Nonhematologic adverse events | |||||||||||
| Eye disorders | |||||||||||
| Eye disorders: other, specify (decrease visual acuity) | 2 | (5%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Gastrointestinal disorders | |||||||||||
| Nausea | 9 | (23%) | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Vomiting | 0 | (0%) | 2 | (5%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| General disorders and administration site conditions | |||||||||||
| Fatigue | 16 | (40%) | 2 | (5%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Gait disturbance | 3 | (8%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| General disorders and administration site conditions: other, specify (unsteadiness without vertigo) | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Irritability | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Localized edema | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Musculoskeletal and connective tissue disorders | |||||||||||
| Generalized muscle weakness | 0 | (0%) | 2 | (5%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Nervous system disorders | |||||||||||
| Dizziness | 2 | (5%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Dysgeusia | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Dysphasia | 0 | (0%) | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Headache | 16 | (40%) | 4 | (10%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Memory impairment | 3 | (8%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Nervous system disorders: other, specify (minor nuisance discomfort in her head) | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Nervous system disorders: other, specify (odd sensation in head behind eyes) | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Nervous system disorders: other, specify (right mandibular numbness) | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Nervous system disorders: other, specify (syncope with seizure like activity and loss of consciousness) | 0 | (0%) | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Paresthesia | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Peripheral sensory neuropathy | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Tremor | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Psychiatric disorders | |||||||||||
| Confusion | 0 | (0%) | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Personality change | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Psychiatric disorders: other, specify (decrease concentration) | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Psychiatric disorders: other, specify (mental fogginess) | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Restlessness | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Skin and subcutaneous tissue disorders | |||||||||||
| Alopecia | 7 | (18%) | 1 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Scalp pain | 3 | (8%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| Nonhematologic adverse events | |||||||||||
| Summary | |||||||||||
| Maximum nonhematologic AE | 18 | (45%) | 11 | (28%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
| All adverse events | |||||||||||
| Summary | |||||||||||
| Maximum overall AE | 18 | (45%) | 11 | (28%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 40 |
Abbreviations: AE = adverse events; CI = confidence interval; SIMT = single-isocenter, multi-target; SRS = stereotactic radiosurgery.
Among 204 lesions treated, radionecrosis was observed in 5 lesions in 4 patients, and the 1-year local recurrence rate was 1.5% (0.4%, 4%). Four lesions had been treated with standard (unaltered) single-fraction dosing and one with 5 fractions. PTV diameters ranged from 0.9 to 1.78 cm with a median of 1.3 cm. Mean maximum doses did not exceed 113.9% for the metastases treated with single fraction. Total V12Gy values were 11.4 mL, 18.7 mL, and 14 mL for these single-fraction SRS cases. Mean maximum dose for the 5-fraction treatment was 124% with a total V24Gy of 4 mL.
Steroid use
Twelve patients were receiving steroids immediately before SIMT. All patients were able to discontinue steroids after SIMT at a mean time of 1.55 months. Immediately after SRS treatment, 3 patients not previously on steroids began receiving steroids. One patient received 0.5 mg daily for 5 days; another 4 mg 4 times a day followed by a slow taper for 2 months; and the last 1 mg QD to 4 mg twice daily for 3 weeks. The median duration of steroid therapy for these patients was 1.3 months.
All 4 patients with radiation necrosis were placed on steroids when they developed new neurologic symptoms at a median time of 12.3 months after SRS; none had received steroids at brain metastasis diagnosis or immediately after SRS. Two patients (total of 3 lesions) with radiation necrosis required surgical intervention. One patient received surgery for one lesion and laser interstitial thermal therapy for another. Another patient received surgery alone, after which steroids were discontinued. Pathology showed necrosis alone in each of those 3 procedures. Both patients are still alive at the time of the article; the first survived 15.3 months after surgery and 9.1 months after lesion and laser interstitial thermal therapy, and the other lived 7.8 months after surgery.
Neurocognition
As shown in Tables E4 to E7 and Figures E2 to E5, scores on the MMSE, Trail Making, and Hopkins Verbal Learning tests did not change significantly from baseline with a mean follow-up time of 4.2 months. A 3 months post-SRS, the following scores were observed: MMSE, 29 pre-SRS and 30 post-SRS, with a mean change of 0.1; Trail Making A, 37.04 pre-SRS and 33.88 post-SRS, with a mean change of −1.17; Trail Making B, 75 pre-SRS to 75.50 post-SRS, with a mean change of −4.41; and HVLT, 23.5 pre-SRS and 25 post-SRS, with a mean change of 0.76. FACT-BR revealed no statistically significant changes in QOL after treatment, with scores of 142.50 (pre-SRS) and 136 (post-SRS), exhibiting a mean change of −2.25.
Discussion
As advances in systemic therapies allow patients with advanced cancer to live longer, brain metastases appear to be a growing problem with rising prevalence.26,27 There is increasing need for local control of brain disease that preserves long-term QOL and neurocognition. SRS has been demonstrated to better preserve neurocognition than WBRT and is the preferred treatment for patients with limited numbers of brain metastases. Larger numbers of brain lesions (>4) can present several logistical and technical hurdles when treating multiple isocenters with traditional SRS techniques. Likewise, increased toxicity can be associated with single-fraction SRS of large lesions or those in or near critical structures. The volume-adapted dosing strategy described and used in this prospective study addresses many of these concerns.
This dose-adapted SIMT strategy effectively controlled the local growth of brain metastases. Local control rates were comparable with our previous prospective study examining SRS margin, which showed 93% LC at 1 year.28 In the present study, even with a 12 cm median maximum distant between targets (maximum 15 cm), excellent local control was observed, highlighting the accuracy of SIMT delivery, as Kraft et al have also shown.29 In addition, local control rates per lesion with hypofractionated SRS were similar to that for single-fraction treatment (98% vs 93%, P = NS), in line with results from previous studies.30, 31, 32 Thus, hypofractionated SRS techniques appear appropriate for larger lesions and those near critical structures without compromise of local tumor control.
Although there is growing acceptance of SRS for multiple metastases, the risk of distant brain failure from occult metastatic brain disease not visualized on MRI has raised concerns about the appropriate therapeutic space for SRS alone. However, note that the number of brain metastases appears to be one of many factors in predicting distant brain failure. Other factors must be considered, including histology, control of systemic disease, existing systemic therapy options, and predicted survival.33,34 For example, Barrett et al35 analyzed outcomes of SRS in patients with 5 or more brain metastases and demonstrated that neither tumor volume nor tumor number were predictive of distant failure in multivariate analysis. Risk of distant brain failure in 6 months in our study was 58%, which is comparable with historical controls, where risk of distant brain failure in patients after SRS alone was similar and ranged from 35% to 64%3,28,35 In addition, Nam et al demonstrated no differences in distant brain failure between those who had 1 to 3 versus 4 to 10 brain metastases.36 More than half of our patients did not experience a distant recurrence (21 of 40), and the cumulative incidence at 1 year for distant recurrence was 35.8%. Of the 19 patients who failed after protocol SRS, 15 patients received salvage therapy (11 with SRS and 4 with WBRT), exhibiting a median OS of 5.8 months after salvage therapy with a 9-month survival of 37% (95% CI, 13.8%-60.8%). Salvage SRS appeared well tolerated and effective, recognizing that regular post-SRS imaging and follow-up are necessary. Consequently, SRS provided reasonable overall disease control in the majority of patients, most of whom would have otherwise received WBRT.
In SRS trials treating multiple brain metastases, median survival after SRS has ranged from 6.2 to 8.6 months (Table 4). In our study, survival was comparable at 8.1 months. When patients were stratified by number of brain metastases, OS did not significantly differ between those who had 4 to 5 metastases versus 6 to 10 metastases. Also, there were there no differences in OS between the group of patients who received single-fraction SRS and those who received hypofractionated treatment, despite the fact that HF-SRS was associated much higher treatment volumes, a characteristic that should adversely affect local control and brain-specific survival.37,38
Table 4.
Summary of studies of radiosurgery in the simultaneous treatment of ≥4 brain metastases
| Study published, y | No. of patients | Nature of study | Lesions/patient median (range) | Max lesion diameter (cm)/ aggregate lesion vol (mL) | Karnofsky performance status median (range) | Median overall survival (mo) |
|---|---|---|---|---|---|---|
| Yamamoto et al5,49 2014, 2017 | 1194 | Prospective | 3 (1-10) | 3/<15 | (<70->70) | 13.9 |
| Nichol et al11 2016 | 60 | Retrospective | (1-10) | 3/<15 | 90 | 10.1 |
| Limon et al37 2017 | 59 | Retrospective | 5 (4-23) | No maximum | ≥70 (93.2%) | 5.8 |
| Palmer et al12 2019 | 173 | Retrospective | 4 (1-30) | No maximum | 80 (70-90) | 13.2 |
| Minniti et al47 2020 | 40 | Retrospective | 13 (10-21) | 3/< 15 | (>60) | 14.1 |
| Alongi et al6 2021 | 172 | Retrospective | 4 (2-22) | 3/50 | - | 12 |
| Present study | 40 | Prospective | 6 (4-10) | 4/no maximum | 90 (70 - 100) | 8.1 |
OS in this study was also similar to published reports when adjusted for known risk factors for SRS patients. Using the DS-GPA as a benchmark, our trial design required that 17 patients surpass the DS-GPA expected survival to demonstrate noninferiority of the SIMT approach. From the time of protocol SIMT, 22 patients exceeded their expected survival time with 1 patient still alive with the potential to exceed their expected survival, meeting this noninferiority mark. When comparing histologies of primary tumors, 65% of lung and 18% of patients with breast cancer lived longer than expected. The benchmark used in this study is imperfect, as Sperduto et al calculated survival time based on time of initial brain metastases.16, 17 Eleven of the patients enrolled in this study had prior SRS/WBRT. If we calculated survival from first presentation of brain metastases, median survival was 11.8 months with a 1-year survival of 49.5% (95% CI, 33.2%-63.9%), and 29 patients living longer than predicted by the DS-GPA. Based on our results, we conclude that SIMT does not significantly compromise the survival of patients with 4 to 10 brain metastases. Moreover, this SRS strategy also appears to preserve neurocognition and QOL in at least the subset of patients who elected to return for detailed testing after SRS.
SRS potentially offers benefits compared with WBRT with regard to integrated treatment logistics and preservation of QOL and neurocognition. In this study, 77.4% of patients died of systemic failure or nonneurocognitive death. SRS can positively affect this by enabling more rapid initiation or a shorter break from systemic therapy. This could allow better extracranial tumor control, especially as systemic therapies continue to improve. In addition, as many patients have worsening performance statuses on systemic therapy, this study is consistent with the observation that patients undergoing SRS experience smaller declines in QOL compared with those receiving WBRT.3
Toxicity was extremely low in this study. In the literature, radiation necrosis rates have ranged from 2% to 24%.39, 40, 41, 42 The rate of radiation necrosis per lesion was 2.5%, which is similar to a retrospective experience involving SIMT to multiple metastases delivered in both single and hypofractionated radiation schemes.16 In this study, rates were 3.4% among lesions treated with a single fraction (4 of 119 lesions) and 1.3% among lesions treated with hypofractionation (1 of 79 lesions). Using this hypofractionated regimen, no serious adverse effects and excellent local control were observed, even though the aggregate PTV was up to 32 mL. Finally, no treatment-related grade 3 or 4 toxicities were observed in any patient. Rates of grade 1 and 2 toxicities also did not appear to differ between hypofractionated and single-fraction schemes. In this trial, hypofractionation was an effective and safe method to treat a large number of brain metastases.
Regarding neurocognitive function, in a prospective study of low-grade gliomas treated with conventional fractionation, 7.3 Gy to at least 40% of hippocampal volume was associated with long-term delayed recall.43 In addition, a higher integral brain dose has been associated with leukoencephalopathy and neurocognitive dysfunction.44 In our study, integral brain and hippocampal doses were low, and there were no clear changes in neurocognition from baseline, as measured by the HVLT, MMSE, and Trail Making Tests. In addition, QOL was well preserved after SRS in patients who underwent testing. The results in this study are consistent with other studies which have found no significant changes in neurocognition or QOL a few months after SRS.28,45, 46, 47, 48, 49
Limitations of the study
The study's number of patients and population heterogeneity prevented multivariate analysis of survival. There also were not enough events to calculate a statistically relevant time to neurologic death, local failure, or radiation necrosis. In addition, an analysis of metastases volume effect on survival was not feasible given the number of patients on study. Finally, compliance with neurocognitive and QOL testing dropped off quickly after 6 months. Many patients had died (18 before the 6-month mark and another 7 patients in the following 3 months) or did not undergo the planned neurocognitive testing. However, in the 0- to 6-month timeframe, there were no obvious changes from baseline. Lastly, 11 patients had prior radiation, primarily SRS. Patients were chosen sequentially for consideration as they came into our clinic, whether or not they had previous radiation. Enrolling patients who have had previous brain radiation could be a confounding factor.
Conclusions
This study demonstrates that a dose-adapted SIMT SRS strategy including hypofractionation can be used to effectively and efficiently treat 4 to 10 brain metastases for a broad range of lesion sizes and locations. In this setting, it appears to be an attractive treatment option compared with WBRT, as outlined. Nonetheless, validation of these findings in a larger trial is merited. In particular, randomized trials evaluating SRS versus WBRT for patients with multiple metastases are currently being conducted: NCT03550391 (5-15 metastases; hippocampal-avoiding WBRT), NCT03075072 (5-20 metastases), and NCT01592968 (4-15 metastases). We strongly encourage enrollment in these trials. It would also be worthwhile to determine the potential benefits of hypofractionated versus single-fraction SRS in the large aggregate volume, multiple brain metastasis space, similar to the Alliance trial in resected brain metastases (NCT04114981) in which single-fraction versus hypofractionated SRS is being tested.
Footnotes
Sources of support: Work was completed in Durham, NC, and was funded by a grant from Varian Medical Systems, Inc.
Disclosures: Grace Kim and John P. Kirkpatrick report funding from Varian Medical Systems, which was directly related to this study. John P. Kirkpatrick and Justus Adamson report ownership of Clearsight RT LLC, which is unrelated to this study. Carey Anders has research funding from PUMA, Lilly, Merck, Seattle Genetics, Nektar, Tesaro, G1-Therapeutics, ZION, Novartis, and Pfizer; has a compensated consultant role for Genentech (1/2019 to present), Eisai (1/2019 to present), IPSEN (2/2019 to present), Seattle Genetics (11/15/2019 to 11/15/2020); Astra Zeneca (3/2020 to 6/2020), Novartis (5/2020 to 5/2022), Immunomedics (10/1/2020 to 9/22/2021), Elucida (9/2020); and receives royalties from UpToDate, Jones and Bartlett, which are unrelated to this study.
Jordan A. Torok, Jr, is currently at the Department of Radiation Oncology, University of Pittsburgh Medical Center.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2021.100760.
Appendix. Supplementary materials
References
- 1.Aoyama H, Shirato H, Onimaru R, et al. Hypofractionated stereotactic radiotherapy alone without whole-brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol Biol Phys. 2003;56:793–800. doi: 10.1016/s0360-3016(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 2.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minniti G, Scaringi C, Paolini S, et al. Repeated stereotactic radiosurgery for patients with progressive brain metastases. J Neurooncol. 2016;126:91–97. doi: 10.1007/s11060-015-1937-4. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 6.Alongi F, Nicosia L, Figlia V, et al. Long-term disease outcome and volume-based decision strategy in a large cohort of multiple brain metastases treated with a mono-isocentric linac-based stereotactic radiosurgery technique. Clin Transl Oncol. 2021;23:1561–1570. doi: 10.1007/s12094-020-02550-0. [DOI] [PubMed] [Google Scholar]
- 7.Cui Y, Gao H, Zhang J, Kirkpatrick JP, Yin FF. Retrospective quality metrics review of stereotactic radiosurgery plans treating multiple targets using single-isocenter volumetric modulated arc therapy. J Appl Clin Med Phys. 2020;21:93–99. doi: 10.1002/acm2.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwai Y, Ozawa S, Ageishi T, Pellegrini R, Yoda K. Feasibility of single-isocenter, multi-arc non-coplanar volumetric modulated arc therapy for multiple brain tumors using a linear accelerator with a 160-leaf multileaf collimator: A phantom study. J Radiat Res. 2014;55:1015–1020. doi: 10.1093/jrr/rru042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau SK, Zakeri K, Zhao X, et al. Single-isocenter frameless volumetric modulated arc radiosurgery for multiple intracranial metastases. Neurosurgery. 2015;77:233–240. doi: 10.1227/NEU.0000000000000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Andrews DW, Evans JJ, et al. Plan quality and treatment efficiency for radiosurgery to multiple brain metastases: Non-coplanar RapidArc vs Gamma Knife. Front Oncol. 2016;6:26. doi: 10.3389/fonc.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichol A, Ma R, Hsu F, et al. Volumetric radiosurgery for 1 to 10 brain metastases: A multicenter, single-arm, phase 2 study. Int J Radiat Oncol Biol Phys. 2016;94:312–321. doi: 10.1016/j.ijrobp.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Palmer JD, Sebastian NT, Chu J, et al. Single-isocenter multitarget stereotactic radiosurgery is safe and effective in the treatment of multiple brain metastases. Adv Radiat Oncol. 2020;5:70–76. doi: 10.1016/j.adro.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas EM, Popple RA, Wu X, et al. Comparison of plan quality and delivery time between volumetric arc therapy (RapidArc) and Gamma Knife radiosurgery for multiple cranial metastases. Neurosurgery. 2014;75:409–418. doi: 10.1227/NEU.0000000000000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkpatrick JP, Soltys SG, Lo SS, Beal K, Shrieve DC, Brown PD. The radiosurgery fractionation quandary: Single fraction or hypofractionation? Neuro Oncol. 2017;19(Suppl 2):ii38–ii49. doi: 10.1093/neuonc/now301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minniti G, Scaringi C, Paolini S, et al. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: A comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95:1142–1148. doi: 10.1016/j.ijrobp.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 19.Stanhope C, Chang Z, Wang Z, et al. Physics considerations for single-isocenter, volumetric modulated arc radiosurgery for treatment of multiple intracranial targets. Pract Radiat Oncol. 2016;6:207–213. doi: 10.1016/j.prro.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Chang Z, Wang Z, Ma J, Wu Q, McMahon R. Six degree-of-freedom image guidance for frameless intracranial stereotactic radiosurgery with kilo-voltage cone-beam CT. J Nucl Med Radiat Ther. 2010;1:2. [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Lezak MD, Howieson DB, Loring DW, Fischer JS. Oxford University Press; New York, NY: 2004. Neuropsychological Assessment. [Google Scholar]
- 23.Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 24.Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75:1151–1161. doi: 10.1002/1097-0142(19950301)75:5<1151::aid-cncr2820750515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Sperduto PW, Mesko S, Li J, et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. 2020;38:3773–3784. doi: 10.1200/JCO.20.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fecci PE, Champion CD, Hoj J, et al. The evolving modern management of brain metastasis. Clin Cancer Res. 2019;25:6570–6580. doi: 10.1158/1078-0432.CCR-18-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valiente M, Ahluwalia MS, Boire A, et al. The evolving landscape of brain metastasis. Trends Cancer. 2018;4:176–196. doi: 10.1016/j.trecan.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkpatrick JP, Wang Z, Sampson JH, et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: Results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91:100–108. doi: 10.1016/j.ijrobp.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Kraft J, van Timmeren JE, Mayinger M, et al. Distance to isocenter is not associated with an increased risk for local failure in LINAC-based single-isocenter stereotactic radiosurgery (SRS) or radiotherapy (SRT) for multiple brain metastases. Radiother Oncol. 2021;159:168–175. doi: 10.1016/j.radonc.2021.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Fokas E, Henzel M, Surber G, Kleinert G, Hamm K, Engenhart-Cabillic R. Stereotactic radiosurgery and fractionated stereotactic radiotherapy: Comparison of efficacy and toxicity in 260 patients with brain metastases. J Neurooncol. 2012;109:91–98. doi: 10.1007/s11060-012-0868-6. [DOI] [PubMed] [Google Scholar]
- 31.Kim YJ, Cho KH, Kim JY, et al. Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys. 2011;81:483–489. doi: 10.1016/j.ijrobp.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 32.Remick JS, Kowalski E, Khairnar R, et al. A multi-center analysis of single-fraction versus hypofractionated stereotactic radiosurgery for the treatment of brain metastasis. Radiat Oncol. 2020;15:128. doi: 10.1186/s13014-020-01522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayala-Peacock DN, Attia A, Braunstein SE, et al. Prediction of new brain metastases after radiosurgery: Validation and analysis of performance of a multi-institutional nomogram. J Neurooncol. 2017;135:403–411. doi: 10.1007/s11060-017-2588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 35.Barrett OC, McDonald AM, Thompson JW, et al. Distant brain recurrence in patients with five or more newly diagnosed brain metastases treated with focal stereotactic radiotherapy alone. J Radiosurg SBRT. 2017;4:255–263. [PMC free article] [PubMed] [Google Scholar]
- 36.Nam T-K, Lee J-I, Jung Y-J, et al. Gamma Knife surgery for brain metastases in patients harboring four or more lesions: Survival and prognostic factors. J Neurosurg. 2005;102(Special Supplement):147–150. doi: 10.3171/jns.2005.102.s_supplement.0147. [DOI] [PubMed] [Google Scholar]
- 37.Limon D, McSherry F, Herndon J, et al. Single fraction stereotactic radiosurgery for multiple brain metastases. Adv Radiat Oncol. 2017;2:555–563. doi: 10.1016/j.adro.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moraes FY, Winter J, Atenafu EG, et al. Outcomes following stereotactic radiosurgery for small to medium-sized brain metastases are exceptionally dependent upon tumor size and prescribed dose. Neuro Oncol. 2019;21:242–251. doi: 10.1093/neuonc/noy159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chin LS, Ma L, DiBiase S. Radiation necrosis following gamma knife surgery: A case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 2001;94:899–904. doi: 10.3171/jns.2001.94.6.0899. [DOI] [PubMed] [Google Scholar]
- 40.Kohutek ZA, Yamada Y, Chan TA, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125:149–156. doi: 10.1007/s11060-015-1881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varlotto JM, Flickinger JC, Niranjan A, Bhatnagar AK, Kondziolka D, Lunsford LD. Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2003;57:452–464. doi: 10.1016/s0360-3016(03)00568-6. [DOI] [PubMed] [Google Scholar]
- 43.Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2012;83:e487–e493. doi: 10.1016/j.ijrobp.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trifiletti DM, Lee C-C, Schlesinger D, Larner JM, Xu Z, Sheehan JP. Leukoencephalopathy after stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2015;93:870–878. doi: 10.1016/j.ijrobp.2015.07.2280. [DOI] [PubMed] [Google Scholar]
- 45.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 46.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 47.Minniti G, Capone L, Nardiello B, et al. Neurological outcome and memory performance in patients with 10 or more brain metastases treated with frameless linear accelerator (LINAC)-based stereotactic radiosurgery. J Neurooncol. 2020;148:47–55. doi: 10.1007/s11060-020-03442-7. [DOI] [PubMed] [Google Scholar]
- 48.Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: Quality-of-life results. J Clin Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto M, Serizawa T, Higuchi Y, et al. A multi-institutional prospective observational study of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901 Study Update): Irradiation-related complications and long-term maintenance of mini-mental state examination scores. Int J Radiat Oncol Biol Phys. 2017;99:31–40. doi: 10.1016/j.ijrobp.2017.04.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.