Abstract
Purpose
To compare the dosimetric differences in stereotactic radiosurgery between use of passively scattered protons (PSRS) versus photons (XSRS) for pituitary adenomas.
Methods and Materials
Nine patients with pituitary adenomas were selected among patients receiving single-fraction proton stereotactic radiosurgery (PSRS) between 2016 and 2017. These cases were replanned with XSRS using volumetric-modulated arc therapy with 2.5 mm and 5 mm multileaf collimators (2.5XSRS and 5XSRS, respectively). PSRS was planned with a dedicated single scattering stereotactic proton unit delivered via 3 equally or unequally weighted isocentric fields. XSRS plans were created with optimization to spare organs at risk. Plans were generated using the original total treatment dose delivered in 1 fraction.
Results
Plans were evaluated for target volume dosimetry and estimated clinical toxicity. There was no significant difference in clinical target volume V100%, V95%, V90% or homogeneity index between treatment modalities. PSRS offered lower maximum dose (Dmax) to organs at risk and equivalent uniform dose (EUD) compared with 5XSRS and 2.5XSRS, respectively, for critical structures including optic nerve (right, Dmax 4.18, 5.32, 5.41; EUD 3.35, 4.08, 4.20) and hypothalamus (Dmax 1.71, 3.94, 3.77; EUD 0.94, 2.47, 2.39; P < .05 for PSRS vs 5XSRS and 2.5XSRS). The projected risk of secondary tumors in excess of baseline was lowest for PSRS plans (PSRS 5.28, 5XSRS 12.93, 2.5XSRS 12.66 cases per 10,000 patient-years; P = .008 for PSRS vs 5XSRS, PSRS vs 2.5XSRS, and P = .77 for 5XSRS vs 2.5XSRS).
Conclusions
We demonstrate that neither modality has empirically superior dosimetry and identify potential clinical advantages as well as limitations of each technique. PSRS, 5XSRS and 2.5XSRS demonstrate comparable target volume dosimetry for pituitary adenoma. PSRS compared with XSRS modalities offers modestly decreased maximum dose and EUD to critical proximal structures and decreases risk of radiation-induced secondary tumors by more than half.
Introduction
Pituitary adenomas are benign skull base tumors arising from the anterior pituitary gland. Common presentations include incidental radiographic identification, visual disturbances, headaches, and endocrinopathies. Adenomas may cause increased hormone secretion corresponding to the cell of origin or hypopituitarism through compression of neighboring cell types. The overall prevalence of pituitary adenomas is 16.7%,1 and the estimated prevalence of clinically relevant tumors is 68 to 98 cases per 100,000 population.2, 3, 4, 5 Whereas small incidental nonfunctioning adenomas can be observed, biochemically active and growing or symptomatic nonfunctioning adenomas require intervention. These lesions are primarily managed medically or surgically. Radiation therapy (RT) is used in the case of medically inoperable candidates, patient choice or when lesions are refractory to medical management, surgically inaccessible or recurrent.6
As tumor control with RT is upward of 90%7 and low risk of mortality is primarily secondary to comorbidities and endocrinopathy,8 minimization of late radiation toxicity including hypopituitarism, injury to adjacent structures, and risk of secondary malignancy is critical. While modern photon techniques deliver highly conformal dose to target, intensity modulated RT and volumetric-modulated arc therapy (VMAT) also deliver greater low-dose radiation to a large volume of nontarget tissue, raising concern for potentially increased risk of second cancers.9,10 This risk is especially concerning in treatment of benign neoplasms. Proton therapy offers several dosimetric benefits over photon therapy, including highly conformal dose distribution, preferential dose deposition within target due to the Bragg peak, sharper lateral penumbra, decreased scatter, and no exit dose. It remains unknown if these physical properties translate clinically to decreased dose to organs at risk (OAR) or lower risk of secondary tumors.
Conventional external beam radiation is typically delivered over 25 to 30 daily treatments. For appropriately selected cases, stereotactic radiosurgery (SRS), high-dose conformal RT using high-precision localization to treat a limited target volume, is convenient and has efficacy comparable to conventional fractionation.7,11 While clinical series report efficacy of both proton stereotactic radiosurgery (PSRS) and photon stereotactic radiosurgery (XSRS), advantages and limitations of each technique have yet to be elucidated as prior dosimetric comparisons of these modalities for skull base lesions evaluated older photon techniques and used conventional fractionation.12,13
The purpose of this study is to rigorously compare the dosimetric differences of PSRS and XSRS for pituitary adenomas.
Methods
Study population
A representative sample of patients with pituitary adenomas was selected among patients receiving single-fraction PSRS between 2016 and 2017. The study was approved by our institutional review board. At our institution, case selection for SRS of pituitary adenoma is contingent on treatment plans meeting constraints for maximum tolerated dose to OAR. Typically, these tumors are less than 3 cm in diameter and at least 3 to 5 mm removed from the optic chiasm. Maximum single fraction dose to the brainstem and optic chiasm and nerves used is 12 Gy and 8 Gy, respectively, adjusted using a standard relative biological effectiveness (RBE) of 1.1 for protons. Nine cases were selected and characteristics of selected cases included proximal to optic chiasm (n = 3), bone involvement (n = 1), cavernous sinus involvement (n = 2), left lateralization (n = 1), right lateralization (n = 1), and empty sella (n = 1).
Simulation
Patients were immobilized using a modified Gill-Thomas-Cosman head frame (Integra-Radionics), a noninvasive frame with custom dental and occipital molds connected with releasable Velcro straps.14 Stainless steel fiducial markers 1.1 mm in diameter were placed in the outer table of the skull as references to facilitate alignment of the target volume to the isocenter of the radiosurgical system immediately before same day simulation.15 Computed tomography (CT) simulation with intravenous contrast was performed and images were obtained at 1.25 mm axial intervals. Magnetic resonance imaging and CT fusions were generated to aid in target delineation.
Treatment planning and delivery
Passive scattering PSRS planning was performed with a forward 3-dimensional conformal approach using an in-house modified XiO planning system (Elekta Inc, Stockholm, Sweden) with a pencil beam algorithm. Beam incidence was selected by an experienced dosimetrist. XSRS plans used inverse planning techniques with VMAT multicriteria optimization in RayStation (RaySearch Laboratories, Stockholm, Sweden). Treatment planning system dose computation resolutions were set to 1 mm for both modalities.
PSRS was delivered using a lamination-based single scattering fixed beamline with a single-wavelength anomalous dispersion of 460 cm and 185 MeV maximum energy. Patient alignment was performed using a high precision robotic positioner with 6 df, STereotactic Alignment Radiosurgery (STAR). Comparison photon plans were generated using 2.5 mm (2.5XSRS) and 5 mm (5XSRS) multileaf collimators (MLC) delivered with 6 MV flattening filter-free Edge/TrueBeam linear accelerators (Varian, Palo Alto, CA).
The clinical target volume (CTV) was defined as grossly visible tumor, areas of proven or suspected microscopic positivity based on preoperative and postoperative scans available. No separate gross tumor volume was defined. OAR contours were verified by both a neuroanatomist and central nervous system specialized radiation oncologist. OAR included ocular globe, optic nerves, optic chiasm, temporal lobes, hypothalamus, whole brain, and brainstem. For both XSRS and PSRS, the planning target volume (PTV) was defined as the CTV with 0.5 mm isotropic expansion. For PSRS, per institutional protocol, a beam-specific end range margin was generated with a 3.5% CT density correction plus 1 mm for range uncertainty,16 as well as case-specific lateral margins for penumbra and set-up uncertainty. Proton radiation was delivered via 3 equally or unequally weighted isocentric fields. VMAT plans were constructed with up to 6 partial arcs to minimize dose to OAR and avoid direct irradiation or exit dose through the eyes. The prescribed dose was normalized to 90% of the PTV for both proton and photon plans. Plans for each case were generated using the original total treatment dose of 15 to 20 Gy (RBE) delivered in 1 fraction.
Endpoints
Plans were evaluated for target volume dosimetry and estimated clinical toxicity. Target coverage was quantified as percentage of the total CTV or PTV receiving a given percentage of the prescription dose using parameters of V90%, V95% and V100%. The maximal dose to the target, Dmax%, was defined as the highest percent of prescription dose to a 0.01 cm3 volume of CTV. Homogeneity index, a measure of pattern of dose distribution to the CTV, is quantified as maximum dose within the CTV divided by the prescription dose. We calculated the conformity index, the volume within the prescription isodose line divided by the volume of the PTV, following manual review of CT sections and dose-volume histograms (DVH) to ensure spatial overlay of the prescription isodose line and the PTV.17,18
Clinical toxicity risks included assessment of dose falloff, dose to OAR, and potential for radiation necrosis. Steep dose gradients ensure low dose outside the target and were quantified with the gradient index, defined as the volume within the 50% prescription isodose line divided by the volume within the prescription isodose line.19 Maximum dose to OAR (Dmax) is defined as the highest dose delivered to a 0.01 cm3 volume within the structure with a 0.2 Gy buffer. Dose to OAR was further quantified as the equivalent uniform dose (EUD). The EUD as first described by Niemierko is the dose that when uniformly distributed over a given volume causes the same radiobiologic effect as the delivered nonuniform dose.20 EUD is calculated as
where Di is the dose and vi is the partial volume of the i'th bin of the corresponding differential DVH, and a is a unitless model parameter specific to the organ at risk of interest.20 EUD volumes were calculated with the parameter a set to the following values: whole brain, 10; brainstem, 12; temporal lobes, 10; cochlea, 20; optic chiasm/nerves/ocular globes, 10; and hypothalamus, 5.21 The 12 Gy (RBE) and 16 Gy (RBE) isodose volumes (cm3) V12Gy and V16Gy were used as indicators of propensity to develop radiation necrosis.22, 23, 24, 25
The excess risk of radiation-associated secondary intracranial tumor was calculated based on organ equivalent dose (OED) using the method proposed by Schneider et al.26 The OED is the dose that when uniformly distributed over a given volume leads to the same radiation-induced tumor incidence as the delivered inhomogeneous dose. Organ equivalent dose is calculated based on the dose-volume histogram for whole brain as
where the sum is taken over N bins of a differential DVH, vi is the relative size of the i’th bin corresponding to dose Di, and α is an organ-specific cell sterilization parameter. We assume the secondary tumor incidence rate is proportional to the number of mutated cells relative to the number of stem cells before RT. Thus, the excess risk of tumors (‘I’) is an organ-specific tumor incidence rate for a low radiation dose (I0) multiplied by the OED
Model parameters were estimated by Schneider et al based on data published by the United Nations Scientific Committee on the Effects of Atomic Radiation. In the present study, we used I0 of 29.7 cancer cases per 10,000 patients per year per Sv and α = 0.08.26
Parameter values were statistically evaluated using the Wilcoxon matched-pairs signed-ranks test and paired t test to compare means, with P ≤ 0.05 considered statistically significant.
Results
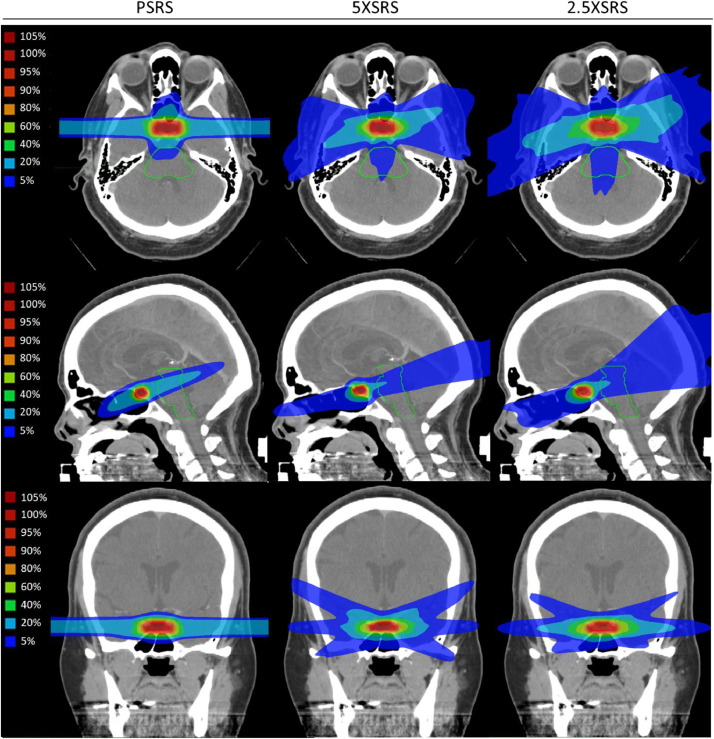
The 9 representative cases are diverse in patient and tumor characteristics, treatment volumes and dose (Table 1). All patients were initially managed with transsphenoidal surgery. Average target volume was 2.51 cm3 (range, 0.57-7.88) treated to an average dose of 17.5 Gy (RBE) (range, 15-20). Figure 1 shows the PSRS, 5XSRS and 2.5XSRS treatment plans for case 5, a patient with a functional adrenocorticotropic hormone-producing pituitary adenoma with an empty sella, 0.72 cm3 target, treated with 20Gy (RBE). The representative cross-sectional planning images show an example of the differential dose distribution between treatment modalities.
Table 1.
Patient and tumor characteristics, volume and treatment dose
| Case | Age | Sex | Anatomic characteristics | Functional | Hormone Produced | Target Volume (cm3) | Dose Gy (RBE) |
|---|---|---|---|---|---|---|---|
| 1 | 51 | F | Proximal to chiasm | Functional | Growth hormone | 0.57 | 20 |
| 2 | 68 | M | Proximal to chiasm | Nonfunctioning | 3.55 | 17 | |
| 3 | 57 | M | Bone involvement | Nonfunctioning | 7.88 | 17 | |
| 4 | 39 | M | Posterior sella | Nonfunctioning | 2.92 | 17 | |
| 5 | 45 | F | Empty sella | Functional | ACTH | 0.72 | 20 |
| 6 | 49 | F | Bilateral cavernous sinus involvement | Nonfunctioning | 1.32 | 16 | |
| 7 | 68 | F | Proximal to chiasm, Bilateral cavernous sinus involvement |
Nonfunctioning | 1.66 | 16 | |
| 8 | 71 | F | Left lateralization | Silent ACTH adenoma | 3.24 | 15 | |
| 9 | 57 | F | Right lateralization | Functional | ACTH | 0.73 | 20 |
Abbreviations: ACTH = adrenocorticotropic hormone; F = female; M = male; RBE = relative biological effectiveness.
All patients had prior transsphenoidal surgery.
Figure 1.
Cross sectional (axial, sagittal, coronal) stereotactic radiosurgery planning images for proton (PSRS) and volumetric-modulated arc therapy using 2.5 mm and 5 mm multileaf collimators (2.5XSRS and 5XSRS, respectively) for patient with functional pituitary adenoma, 0.72 cm3 target, treated with 20Gy (RBE), case 5 from Table 1. Images show an example of the differential dose distribution between treatment modalities.
Plans were evaluated for target volume dosimetry and estimated clinical toxicity. Target volume dosimetry metrics for the 3 treatment modalities are shown in Table 2. There was no statistically significant difference in CTV V100%, V95%, V90%, or homogeneity index between treatment modalities. Compared with PSRS, 5XSRS and 2.5XSRS offered equal (within 0.5%) or superior CTV and PTV V90% and V95% for all 9 cases.
Table 2.
Target volume dosimetry
| Modality | PSRS |
5XSRS |
2.5XSRS |
PSRS vs 5XSRS | PSRS vs 2.5XSRS | 5XSRS vs 2.5XSRS | |||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | SD | Mean | SD | Mean | SD | P value | P value | P value |
| CTV V100% | 91.0 | 12.9 | 93.3 | 9.1 | 95.3 | 6.9 | 0.11 | 0.07 | 0.053 |
| CTV V95% | 93.9 | 9.8 | 95.9 | 6.2 | 97.2 | 4.5 | 0.14 | 0.10 | 0.10 |
| CTV V90% | 96.3 | 5.9 | 97.3 | 4.3 | 98.3 | 3.0 | 0.15 | 0.08 | 0.14 |
| PTV V100% | 89.6 | 13.5 | 91.9 | 9.6 | 94.1 | 7.6 | 0.13 | 0.06 | 0.03* |
| PTV V95% | 93.2 | 10.2 | 95.2 | 6.7 | 96.8 | 5.0 | 0.12 | 0.09 | 0.08 |
| PTV V90% | 95.8 | 6.5 | 96.8 | 4.7 | 98.0 | 3.5 | 0.14 | 0.07 | 0.11 |
| Homogeneity index | 1.11 | 0.02 | 1.12 | 0.02 | 1.11 | 0.03 | 0.19 | 0.81 | 0.20 |
| Gradient index | 4.06 | 1.1 | 4.98 | 0.9 | 4.86 | 1.3 | 0.004* | 0.001* | 0.69 |
| Dmax% | 111.2 | 1.8 | 112.2 | 2.0 | 110.9 | 2.8 | 0.19 | 0.81 | 0.21 |
| Conformity index | 1.64 | 0.4 | 1.36 | 0.2 | 1.36 | 0.2 | 0.048* | 0.02* | 0.94 |
Abbreviations: 2.5XSRS, 5XSRS = volumetric-modulated arc therapy using 2.5 mm and 5 mm multileaf collimators, respectively; CTV = clinical target volume; Dmax% = dose maximum to 0.01 cm3 volume of CTV expressed as percentage of prescribed dose; PSRS = proton stereotactic radiosurgery; PTV = planning target volume; SD = standard deviation; VX% = volume (in percentage total volume) receiving X percentage of the prescribed dose.
P ≤ 0.05 considered significant.
Homogeneity index = maximum dose within CTV divided by prescription dose; gradient index = volume within the 50% prescription isodose line divided by the prescription isodose volume; conformity index = prescription isodose volume divided by the planning target volume.
Compared with PSRS, 5XSRS offered equal or superior CTV and PTV V100% for 8 and 7 of 9 cases, respectively, and 2.5XSRS offered equal or superior V100% for 9 and 8 of 9 cases, respectively. The gradient index was consistently lower with PSRS (4.06) than 5XSRS (4.98, P = .004) and 2.5XSRS (4.86, P = .001). The Dmax% was similar across treatment modalities (PSRS 111.2%, 5XSRS 112.2%, and 2.5XSRS 110.9%). The conformity index was significantly higher for PSRS (1.64) than 5XSRS (1.36, P = .048) and 2.5XSRS (1.36, P = .02).
Clinical toxicity was evaluated based on maximum dose and EUD for pertinent OAR and predicted risk of secondary tumors. The Dmax to OAR for each planning modality is shown in Table 3. Bilateral structures were analyzed according to right or left laterality. The Dmax to OAR was significantly lower with PSRS compared with 5XSRS and 2.5XSRS, respectively, for the ocular globes, optic nerves, hypothalamus, and cochlea. The Dmax did not significantly differ for the optic chiasm, temporal lobes, brainstem or whole brain. The mean whole brain V12Gy was higher for PSRS than 2.5XSRS (PSRS 4.69, 5XSRS 4.39, 2.5XSRS 4.14; P = .048 for PSRS vs 2.5 XSRS). There was no significant difference in the mean whole brain V16Gy (PSRS 2.23, 5XSRS 1.86, 2.5XSRS 1.82) or the mean temporal lobe V12Gy (PSRS 0.61, 5XSRS 0.59, 2.5XSRS 0.42).
Table 3.
Maximum dose in Gy (RBE) to organs at risk
| PSRS |
5XSRS |
2.5XSRS |
PSRS vs 5XSRS | PSRS vs 2.5XSRS | 5XSRS vs 2.5XSRS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Organ at risk | Mean | SD | Mean | SD | Mean | SD | P value | P value | P value |
| Right ocular globe | 0.00 | 0.0 | 0.15 | 0.1 | 0.14 | 0.1 | < 0.001* | < 0.001* | < 0.001* |
| Left ocular globe | 0.14 | 0.3 | 0.22 | 0.1 | 0.18 | 0.1 | 0.47 | 0.71 | 0.24 |
| Right optic nerve | 4.18 | 2.2 | 5.32 | 1.6 | 5.41 | 1.6 | 0.008* | 0.02* | 0.69 |
| Left optic nerve | 5.26 | 2.3 | 6.14 | 1.5 | 5.94 | 1.6 | 0.042* | 0.11 | 0.10 |
| Optic chiasm | 5.42 | 2.3 | 6.29 | 1.1 | 6.05 | 1.5 | 0.13 | 0.22 | 0.26 |
| Temporal lobes | 15.58 | 1.8 | 14.82 | 2.4 | 15.02 | 2.3 | 0.17 | 0.30 | 0.45 |
| Hypothalamus | 1.71 | 1.4 | 3.94 | 1.7 | 3.77 | 1.5 | 0.001* | <0.001* | 0.64 |
| Brainstem | 7.87 | 2.8 | 8.42 | 2.4 | 8.20 | 2.9 | 0.35 | 0.47 | 0.63 |
| Whole brain | 19.09 | 1.7 | 19.18 | 1.7 | 19.17 | 1.8 | 0.67 | 0.68 | 0.92 |
| Right cochlea | 0.00 | 0.0 | 1.68 | 1.0 | 1.66 | 1.2 | 0.001* | 0.003* | 0.88 |
| Left cochlea | 0.02 | 0.1 | 1.38 | 1.1 | 1.32 | 1.3 | 0.004* | 0.014* | 0.55 |
Abbreviations: 2.5XSRS, 5XSRS = volumetric-modulated arc therapy using 2.5 mm and 5 mm multileaf collimators, respectively; PSRS = proton stereotactic radiosurgery; SD = standard deviation.
P ≤ 0.05 considered significant.
Maximum dose to organs at risk = highest dose delivered to a 0.01 cm3 volume within the structure.
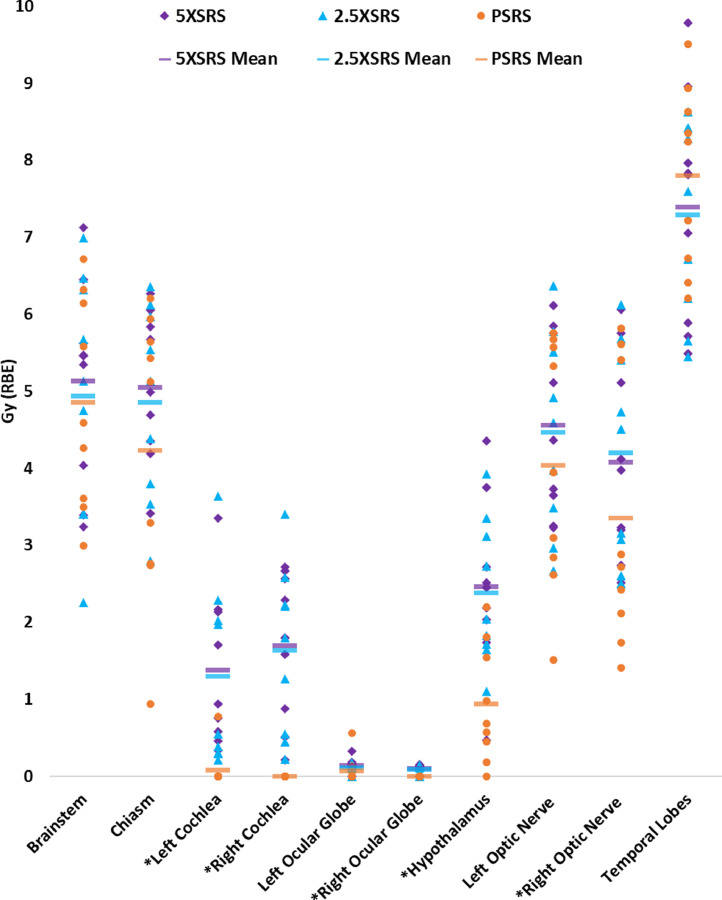
The EUD to OAR according to modality for each case is shown in Figure 2, and numerical values are provided in Supplementary Table 1. The following OAR received significantly lower dose in PSRS plans compared with paired 5XSRS or 2.5XSRS plans, respectively: cochlea (left 0.09, 1.38, 1.30; right 0.00, 1.69, 1.64), ocular globe (right 0.00, 0.10, 0.09), hypothalamus (0.94, 2.47, 2.39), and optic nerve (right 3.35, 4.08, 4.20), with P < .05 for PSRS vs 5XSRS and PSRS vs 2.5XSRS.
Figure 2.
Equivalent uniform dose (EUD) for organs at risk (OAR) for all 9 cases according to stereotactic radiosurgery treatment modality. OAR are listed on the horizontal axis and dose in Gy (RBE) is shown on the y-axis. EUD to a given OAR for each case is plotted for 5XSRS (purple diamond), 2.5XSRS (blue triangle) and PSRS (orange circle). The mean EUD averaged over the 9 cases for each OAR according to treatment modality is indicated with a horizontal bar as follows: 5XSRS (purple), 2.5XSRS (blue) and PSRS (orange).
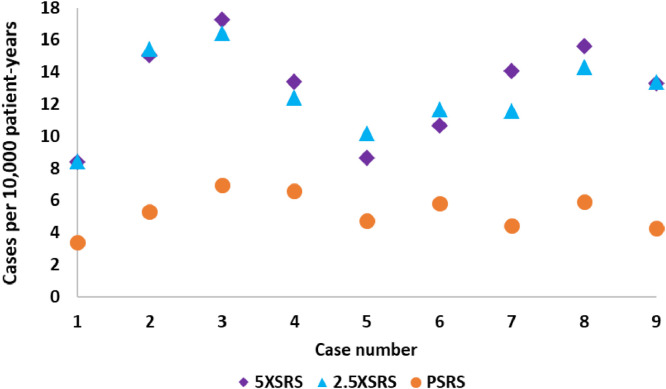
The projected excess risk of secondary tumor for each case according to treatment modality is shown in Figure 3 and numerical values are provided in Supplementary Table 2. The estimated incidence of radiation induced tumor by modality is as follows: PSRS 5.28, 5XSRS 12.93, 2.5XSRS 12.66 cases per 10,000 patient-years (PSRS vs 5XSRS, P = .008; PSRS vs 2.5XSRS, P = .008; 5XSRS vs 2.5XSRS, P = .77).
Figure 3.
Projected risk of secondary tumors (expressed as cases of secondary tumors per 10,000 patient-years) for all 9 cases according to stereotactic radiosurgery treatment modality.
Discussion
This study compared target volume dosimetry, injury to OAR, and risk of secondary tumor for stereotactic radiosurgery using modalities of protons, photons with 5 mm MLC VMAT, and photons with 2.5 mm MLC VMAT in treatment of pituitary adenoma. While the XSRS and PSRS treatment plans demonstrated no significant differences in target coverage, PSRS achieved better OAR sparing and reduced risk of secondary tumor. Target coverage, dose to OAR, and estimated risk of secondary tumor were similar between 5XSRS and 2.5XSRS. Our study findings are clinically significant as there is unlikely to be a clinical trial comparing PSRS and XSRS for pituitary adenoma, and, furthermore, discerning differences in secondary tumor and clinical toxicity rates would require both a large study population and decades of follow-up. In the absence of such clinical data, our study provides anticipated toxicity profile comparisons of commonly considered treatment modalities.
Our results demonstrate comparable target volume dosimetry between PSRS, 5XSRS, and 2.5XSRS, with similar CTV and PTV V90%, V95%, and V100%. Although our small sample size may limit the ability to detect statistically significant differences, in these paired plans VMAT photon SRS generally achieved equivalent or superior CTV and PTV coverage compared with proton SRS. Homogeneity index and Dmax% were similar between treatment modalities. The gradient index was lower for PSRS compared with XSRS, indicating a steeper dose gradient outside the target volume. Compared with XSRS, PSRS has a higher conformity index, indicating irradiation of a larger volume of tissue outside the PTV. This is unlikely to have clinical implications as at our institution, excess off-target dose is preferentially deposited in bone to minimize dose to more radiosensitive normal tissue structures. Correspondingly, although the conformity index is higher for PSRS, measures of whole brain Dmax, whole brain V16Gy, and temporal lobe V12Gy are similar across treatment modalities.
XSRS is delivered using linear accelerator (LINAC) or isotope-based systems. LINAC accelerate electrons that collide with a heavy metal to generate photons. In VMAT XSRS, the LINAC rotates around the patient while the radiation beam is continuously shaped by multileaf collimators to deliver conformal dose at variable dose rate. Gamma Knife uses approximately 200 Cobalt-60 sources housed within a helmet and delivers conformal dose to target selectively by allowing penetration of a combination of beams through the collimator helmet. We selected VMAT with 2 common MLC widths as a representative XSRS system in our study as LINAC SRS is a widely available treatment modality. Analysis of our VMAT plans showed clinically relevant parameters including risk of secondary tumor were similar between 2.5XSRS and 5XSRS, although 2.5XSRS offered the highest target coverage even in the setting of a relatively spherical and centrally located target. Other XSRS systems may achieve higher dose conformality than VMAT by increasing the number of beams or varying beam angles. However, our analysis supports that even with increased target conformality, these systems will have more extensive low-dose bath to OARs than PSRS and thus higher risk of secondary tumor. Our PSRS dosimetric assessment corresponds to a unique proton passive scattering system with optimized characteristics intended for small field delivery. Thus, our observations may not directly apply to all other passive scattering systems and are not directly transferable to pencil beam scanning with or without aperture collimation.
New pituitary hormone deficiencies are expectantly high following SRS for pituitary adenomas. In an institutional series of 165 patients with functional pituitary adenomas followed with rigorous surveillance, 92% of whom received PSRS with a median dose of 20Gy (RBE), actuarial 3-year and 5-year rates of development of new hypopituitarism were 45% and 62%, respectively, with median follow-up of 51 months.27 In 2 series of 418 and 76 patients treated with Gamma Knife SRS for pituitary adenoma, 24.4% and 23%, respectively, developed radiation-induced hypopituitarism often by 2 to 5 years post treatment.28,29 These rates are expected to continue to rise with time. In series with long-term follow-up, rates of hypopituitarism approach 80% at 10 to 15 years.30,31 A primary limitation of RT for pituitary adenoma is inclusion of the entire gland in the CTV. Accordingly, rates of secondary hypopituitarism are anticipated to be similar between photon and proton therapies.
Dose to critical OAR was lower with PSRS than XSRS plans with regard to maximum dose and EUD for several OARs. PSRS was particularly beneficial for sparing of optic structures, hypothalamus, and cochlea. Normal tissue complication probabilities were not calculated as the EUD values were relatively low for serious toxicity. The difference in OAR sparing between PSRS and XSRS is most likely to be clinically relevant in settings where the dose to optic structures is exceeding or approaching dose constraints. The risk of optic pathway injury is minimal at 8 Gy but estimated to be 1% at 12 Gy for SRS, and HyTEC recommends a maximum point dose of <10 Gy in 1 fraction.32, 33, 34 However, in a series of 512 patients treated with Gamma Knife SRS for nonfunctioning pituitary adenomas, 6.6% had worsening or new onset optic nerve dysfunction corresponding to maximum dose to optic apparatus of 6.6 ± 2.7 Gy.35 At our institution lesions appropriate for SRS are at least 3 to 5 mm removed from the optic chiasm to ensure safe collateral dose to the optic pathway. Yet in our series, 50% of cases had at least 1 optic structure with maximum dose between 7-8.5 Gy, demonstrating the importance of strategies to minimize dose to critical OAR even with stringent a priori selection criteria.
Radiation necrosis is a late toxicity related to radiation dose, volume, and location.25 Flickinger et al23 and Korytko et al24 showed V12Gy is significantly associated with development of symptomatic postradiosurgical imaging changes and symptomatic radiation necrosis, respectively, following SRS. In a brain metastasis series, Blonigen et al reported V8-V16Gy is predictive of symptomatic radiation necrosis.22 Furthermore, V12Gy of 1.6-4.7 cm3 corresponded to an 11.9% rate of overall radionecrosis, and V12Gy >7.9 cm3 was identified as a threshold volume for significant rise in the rate of radionecrosis. Similarly the QUANTEC analysis showed the risk of symptomatic radiation necrosis in SRS increases rapidly when V12Gy is >5 to 10 cm3.25 In our study, the mean V12Gy was less than 4.7 cm3 and there was no significant difference in V16Gy. Thus, the risk of radiation necrosis is likely similar between treatment modalities for appropriately selected lesions.
Our study shows the absolute risk of secondary tumors is low for each modality, yet PSRS does consistently offer lower excess risk of RT associated tumor. While limited long-term clinical data are available, several series show the risk of secondary tumors following RT for pituitary adenoma is relatively low. Minniti et al reported a cumulative risk of second brain tumors of 2.0% at 10 years and 2.4% at 20 years from date of radiation therapy among 426 patients treated from 1962 to 1994.36 In a Dutch series of postoperative radiation therapy versus surgery alone for pituitary adenoma, 236 of 462 patients received RT and 3 patients treated with RT developed an intracranial tumor compared with 1 patient treated with surgery alone.37 Furthermore, in a British retrospective study of 385 patients treated with RT for pituitary adenoma, the 20-year actuarial risk of intracranial tumor was 1.9%.38 Ascertaining risk of secondary tumors requires long-term follow-up - a systematic review showed average latency period to diagnosis of secondary tumor was 15.2 ± 8.7 years following RT for pituitary adenoma.39 In each of the above studies, patients were treated with conventional fractionation to a dose of approximately 45 to 50 Gy before development of 3-dimensional CT-based planning with larger treatment fields than would be prescribed using modern treatment techniques. Our data corroborate that the absolute risk of secondary tumors is low and should not preclude the use of photon or proton radiation therapy in treatment of pituitary adenoma. However, young patients have the highest lifetime risk of developing secondary tumors and thus should be the population to most strongly consider for proton therapy.
Our findings are consistent with prior dosimetric comparisons of PSRS and XSRS in treatment of intracranial lesions. For conventional fractionation in treatment of pituitary adenoma, Winkfield et al showed PSRS plans achieved the best therapeutic ratio and lowest risk of secondary tumors compared with static field intensity modulated RT.13 Similarly, for benign intracranial meningioma treated with conventional fractionation, Arvold et al showed proton therapy compared with photon therapy decreased risk of secondary tumors and dose to neurocognitive and critical structures.12 Cao et al compared multiple modalities of SRS including protons (double scattering proton therapy and intensity modulated proton therapy) and photons (Gamma Knife, CyberKnife, and coplanar- and noncoplanar-arc VMAT) for hypofractionated treatment (2-5 fractions) of intracranial tumors >3 cm. They found PSRS offered the highest gradient index and lowest integral dose to normal brain and thus is likely to offer dosimetric advantages for tumors that are irregularly shaped or adjacent to critical structures.40 To our knowledge, the present study is the first dosimetric comparison of PSRS with modern LINAC-based XSRS techniques for treatment of pituitary adenoma.
Conclusions
In clinical practice, with careful patient selection, single-fraction SRS is a convenient and safe approach to treatment. Several clinical studies show long-term effectiveness of SRS for nonfunctioning and secretory pituitary adenomas treated with either proton or photon therapy; however, clinical comparisons between the modalities have been lacking. We demonstrate that neither PSRS nor XSRS is empirically superior dosimetrically and identify potential clinical advantages and limitations of each technique. This comparison is useful when selecting between treatment modalities and importantly for providers considering whether to refer to a proton center with the associated treatment burden for the patient.
Footnotes
Presented at The Radiosurgery Society Annual Conference, March 21-23, 2019; San Diego, California.
Sources of support: This work had no specific funding.
Disclosures: Dr Shih has served as a writer for UpToDate and on the Board of Directors of The Radiosurgery Society. All other authors have no disclosures to declare.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2021.100806.
Appendix. Supplementary materials
References
- 1.Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: A systematic review. Cancer. 2004;101:613–619. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 2.Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: A cross-sectional study in the province of Liège, Belgium. J Clin Endocrinol Metab. 2006;91:4769–4775. doi: 10.1210/jc.2006-1668. [DOI] [PubMed] [Google Scholar]
- 3.Day PF, Loto MG, Glerean M, Picasso MFR, Lovazzano S, Giunta DH. Incidence and prevalence of clinically relevant pituitary adenomas: Retrospective cohort study in a health management organization in Buenos Aires, Argentina. Arch Endocrinol Metab. 2016;60:554–561. doi: 10.1590/2359-3997000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raappana A, Koivukangas J, Ebeling T, Pirilä T. Incidence of pituitary adenomas in northern Finland in 1992-2007. J Clin Endocrinol Metab. 2010;95:4268–4275. doi: 10.1210/jc.2010-0537. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez A, Karavitaki N, Wass JAH. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK) Clin Endocrinol (Oxf) 2010;72:377–382. doi: 10.1111/j.1365-2265.2009.03667.x. [DOI] [PubMed] [Google Scholar]
- 6.Molitch ME. Diagnosis and treatment of pituitary adenomas: A review. JAMA. 2017;317:516–524. doi: 10.1001/jama.2016.19699. [DOI] [PubMed] [Google Scholar]
- 7.Loeffler JS, Shih HA. Radiation therapy in the management of pituitary adenomas. J Clin Endocrinol Metab. 2011;96:1992–2003. doi: 10.1210/jc.2011-0251. [DOI] [PubMed] [Google Scholar]
- 8.Sherlock M, Ayuk J, Tomlinson JW, et al. Mortality in patients with pituitary disease. Endocr Rev. 2010;31:301–342. doi: 10.1210/er.2009-0033. [DOI] [PubMed] [Google Scholar]
- 9.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Hall EJ, Wuu C-S. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83–88. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 11.Minniti G, Clarke E, Scaringi C, Enrici RM. Stereotactic radiotherapy and radiosurgery for non-functioning and secreting pituitary adenomas. Rep Pract Oncol Radiother. 2016;21:370–378. doi: 10.1016/j.rpor.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arvold ND, Niemierko A, Broussard GP, et al. Projected second tumor risk and dose to neurocognitive structures after proton versus photon radiotherapy for benign meningioma. Int J Radiat Oncol. 2012;83:e495–e500. doi: 10.1016/j.ijrobp.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 13.Winkfield KM, Niemierko A, Bussiere MR, et al. Modeling intracranial second tumor risk and estimates of clinical toxicity with various radiation therapy techniques for patients with pituitary adenoma. Technol Cancer Res Treat. 2011;10:243–251. doi: 10.7785/tcrt.2012.500199. [DOI] [PubMed] [Google Scholar]
- 14.Winey B, Daartz J, Dankers F, Bussière M. Immobilization precision of a modified GTC frame. J Appl Clin Med Phys. 2012;13:12–19. doi: 10.1120/jacmp.v13i3.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gall KP, Verhey LJ, Wagner M. Computer-assisted positioning of radiotherapy patients using implanted radiopaque fiducials. Med Phys. 1993;20:1153–1159. doi: 10.1118/1.596969. [DOI] [PubMed] [Google Scholar]
- 16.Paganetti H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol. 2012;57:R99–R117. doi: 10.1088/0031-9155/57/11/R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw E, Kline R, Gillin M, et al. Radiation therapy oncology group: Radiosurgery quality assurance guidelines. Int J Radiat Oncol. 1993;27:1231–1239. doi: 10.1016/0360-3016(93)90548-a. [DOI] [PubMed] [Google Scholar]
- 18.Feuvret L, Noël G, Mazeron J-J, Bey P. Conformity index: A review. Int J Radiat Oncol. 2006;64:333–342. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Paddick I, Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J Neurosurg. 2006;105(suppl):194–201. doi: 10.3171/sup.2006.105.7.194. [DOI] [PubMed] [Google Scholar]
- 20.Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys. 1997;24:103–110. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 21.Gay HA, Niemierko A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys Med. 2007;23:115–125. doi: 10.1016/j.ejmp.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Flickinger JC, Kondziolka D, Pollock BE, Maitz AH, Lunsford LD. Complications from arteriovenous malformation radiosurgery: Multivariate analysis and risk modeling. Int J Radiat Oncol Biol Phys. 1997;38:485–490. doi: 10.1016/s0360-3016(97)89481-3. [DOI] [PubMed] [Google Scholar]
- 24.Korytko T, Radivoyevitch T, Colussi V, et al. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64:419–424. doi: 10.1016/j.ijrobp.2005.07.980. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol. 2010;76:S20–S27. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider U, Zwahlen D, Ross D, Kaser-Hotz B. Estimation of radiation-induced cancer from three-dimensional dose distributions: Concept of organ equivalent dose. Int J Radiat Oncol. 2005;61:1510–1515. doi: 10.1016/j.ijrobp.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 27.Wattson DA, Tanguturi SK, Spiegel DY, et al. Outcomes of proton therapy for patients with functional pituitary adenomas. Int J Radiat Oncol Biol Phys. 2014;90:532–539. doi: 10.1016/j.ijrobp.2014.06.068. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan JP, Pouratian N, Steiner L, Laws ER, Vance ML. Gamma knife surgery for pituitary adenomas: Factors related to radiological and endocrine outcomes. J Neurosurg. 2011;114:303–309. doi: 10.3171/2010.5.JNS091635. [DOI] [PubMed] [Google Scholar]
- 29.Castinetti F, Nagai M, Morange I, et al. Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J Clin Endocrinol Metab. 2009;94:3400–3407. doi: 10.1210/jc.2008-2772. [DOI] [PubMed] [Google Scholar]
- 30.van den Bergh ACM, van den Berg G, Schoorl MA, et al. Immediate postoperative radiotherapy in residual nonfunctioning pituitary adenoma: Beneficial effect on local control without additional negative impact on pituitary function and life expectancy. Int J Radiat Oncol Biol Phys. 2007;67:863–869. doi: 10.1016/j.ijrobp.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 31.Minniti G, Osti M, Jaffrain-Rea ML, Esposito V, Cantore G, Maurizi Enrici R. Long-term follow-up results of postoperative radiation therapy for Cushing's disease. J Neurooncol. 2007;84:79–84. doi: 10.1007/s11060-007-9344-0. [DOI] [PubMed] [Google Scholar]
- 32.Tishler RB, Loeffler JS, Lunsford LD, et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215–221. doi: 10.1016/0360-3016(93)90230-s. [DOI] [PubMed] [Google Scholar]
- 33.Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76(suppl 3):S28–S35. doi: 10.1016/j.ijrobp.2009.07.1753. [DOI] [PubMed] [Google Scholar]
- 34.Milano MT, Grimm J, Soltys SG, et al. Single- and multi-fraction stereotactic radiosurgery dose tolerances of the optic pathways. Int J Radiat Oncol. 2021;110:87–99. doi: 10.1016/j.ijrobp.2018.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheehan JP, Starke RM, Mathieu D, et al. Gamma knife radiosurgery for the management of nonfunctioning pituitary adenomas: A multicenter study. J Neurosurg. 2013;119:446–456. doi: 10.3171/2013.3.JNS12766. [DOI] [PubMed] [Google Scholar]
- 36.Minniti G, Traish D, Ashley S, Gonsalves A, Brada M. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: Update after an additional 10 years. J Clin Endocrinol Metab. 2005;90:800–804. doi: 10.1210/jc.2004-1152. [DOI] [PubMed] [Google Scholar]
- 37.Sattler MGA, van Beek AP, Wolffenbuttel BHR, et al. The incidence of second tumours and mortality in pituitary adenoma patients treated with postoperative radiotherapy versus surgery alone. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2012;104:125–130. doi: 10.1016/j.radonc.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Erridge SC, Conkey DS, Stockton D, et al. Radiotherapy for pituitary adenomas: Long-term efficacy and toxicity. Radiother Oncol. 2009;93:597–601. doi: 10.1016/j.radonc.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Yamanaka R, Abe E, Sato T, Hayano A, Takashima Y. Secondary intracranial tumors following radiotherapy for pituitary adenomas: A systematic review. Cancers (Basel) 2017;9:103. doi: 10.3390/cancers9080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao H, Xiao Z, Zhang Y, et al. Dosimetric comparisons of different hypofractionated stereotactic radiotherapy techniques in treating intracranial tumors >3 cm in longest diameter. J Neurosurg. 2019;132:1024–1032. doi: 10.3171/2018.12.JNS181578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.