Abstract
Purpose
We hypothesize treatment with nivolumab and stereotactic radiosurgery (SRS) will be feasible and well tolerated, and may improve intracranial tumor control rates compared with SRS alone.
Methods and Materials
The study was designed as a prospective, single-arm, nonrandomized, open-label, phase 1b trial of nivolumab and SRS among patients with metastatic breast cancer brain metastases. Key eligibility criteria included patients with breast cancer brain metastases of all subtypes, age ≥18, Eastern Cooperative Oncology Group Performance Status ≤2 with ≤10 brain metastases. Treatment was initiated with a dose of nivolumab (480 mg intravenously) that was repeated every 4 weeks. The initial dose of nivolumab was followed 1 week later by SRS. This study is closed to accrual and is registered with ClinicalTrials.gov, NCT03807765.
Results
Between February 2019 and July 2020, a total of 12 patients were treated to 17 lesions. No dose limiting toxicities were noted in our patient population. The most common neurologic adverse events included grade 1 to 2 headaches and dizziness occurring in 5 (42%) of patients. Median intracranial control was 6.2 months (95% confidence interval, 3-14 months) with 6- and 12-month control rates of 55% and 22%, respectively. A total of 4 patients had systemic progression during the study. Median time to systemic progression free survival has not been reached with 6- and-12 month rates of 63% and 51%, respectively.
Conclusions
Nivolumab and SRS is a safe and feasible treatment option in breast cancer brain metastases. Preliminary data reveals activity in certain breast cancer patients to study therapy.
Introduction
Patients with breast cancer brain metastases have a high unmet clinical need. Although a multitude of treatment options are available for the management of systemic disease, once metastases travel to the brain, patients lack treatment options aside from traditional local approaches. Standard-of-care treatments for these patients include local treatments, such as surgical resection, stereotactic radiosurgery (SRS), or whole brain radiation therapy (WBRT).1 In recent years, various systemic agents have been shown to have a role in the management of breast cancer brain metastases of various subtypes including tucatinib, lapatinib, and abemaciclib among others.2, 3, 4 Anti-programmed cell death protein-1 and programmed cell death ligand-1 (anti-PD-1/PD-L1) therapy has shown promise in the management of various subtypes of advanced metastatic breast cancer and in the neoadjuvant setting when combined with conventional chemotherapy.5, 6, 7
The use of pembrolizumab has demonstrated objective intracranial responses in one-third of patients with non-small cell lung cancer (NSCLC) brain metastases and a quarter of patients with melanoma brain metastases.8 In CheckMate 204, the combination of nivolumab and ipilimumab alone in patients with melanoma brain metastases produced an overall response rate of 56%.9 Given the encouraging data in NSCLC and melanoma brain metastases as well as data revealing systemic control in the management of breast cancer, particularly the triple-negative subtype,6,10 there is strong clinical rationale for investigating the utility of immune checkpoint inhibitors in the management of breast cancer brain metastases.
Combining radiation therapy with immune checkpoint inhibitors may hold promise.11 Preclinical data suggests the combination may upregulate PD-L1 expression and enhance immunogenicity.12 High dose-per-fraction radiation as is the case with SRS may also be an optimal regimen to stimulate the immune system based on preclinical evidence using fractionated high doses combined with antibodies against CTLA-4.13 In addition, there is evidence to suggest when sequenced appropriately radiation therapy may open the blood-brain barrier allowing for better penetration of systemic agents.14,15 The local control provided by SRS also has clear benefits.
Given the previously demonstrated role of immunotherapy in the management of brain metastases as well as the potential synergy and well-defined role of local therapy in the management of brain metastases, we conducted a phase 1b study to evaluate safety and preliminary activity of nivolumab and SRS in the management of breast cancer brain metastases.
Methods and Materials
Study design and participants
The study was designed as a prospective, single-arm, nonrandomized, open-label, phase 1b trial of nivolumab and SRS among patients with metastatic breast cancer brain metastases. The study was performed at Moffitt Cancer Center. Key eligibility criteria included patients with breast cancer brain metastases of all subtypes, age ≥18, Eastern Cooperative Oncology Group ≤2 with ≤10 brain metastases, maximum diameter of the largest intact brain metastases ≤4 cm. Patients on a stable dose of steroids (dexamethasone ≤8 mg/daily) could enroll. Patients needed to be eligible to receive stereotactic radiation to the intact brain metastases or postoperative cavity. Patients were required to have adequate organ function including absolute neutrophil count ≥1000/mm3, hemoglobin ≥8 g/dL, platelets ≥50,000/mm3, serum total bilirubin <1.5 times upper limits of normal (ULN) except patients with Gilbert syndrome, who were excluded if total bilirubin was 3 times ULN, aspartate aminotransferase and alanine aminotransferase <2.5 times ULN or <5 times ULN if liver metastases are present, amylase and lipase ≤1.5 ULN unless there are signs of pancreatitis. Any number of previous systemic therapies was allowed. A 2-week wash-out period for investigational systemic treatments was required before starting study treatment.
Exclusion criteria included receipt of prior WBRT, presence of leptomeningeal disease (LMD), prior treatment with anti-PD-1, anti-PD-L1, anti-PD-L2, anti-CD137, or anti-CTLA-4 therapy as well as interstitial lung disease that was symptomatic or may interfere with the detection or management of suspected drug-related pulmonary toxicity. The study was approved by our central institutional review board.
Procedures
Nivolumab (480 mg intravenously) was given every 4 weeks, starting 1 week before SRS. SRS was given at sites of brain metastases or postoperative cavities. Patients were allowed to continue prior endocrine and HER2-targeted therapies per treating physicians’ discretion if brain metastases progression was noted on these agents for continued systemic disease control.
Treatment continued until disease progression, toxicity that precluded continuing study drug, withdrawal from study, or death. Patients were allowed to continue study therapy despite radiologic progression if they were deriving clinical benefit according to investigator assessment or if progressive lesions (brain or systemic) could be controlled with local therapy or receipt of an additional systemic agent for systemic progression after the first intracranial response assessment at 2 months.
A brain magnetic resonance imaging (MRI) and computed tomography (CT) thorax, abdomen, and pelvis was obtained every 8 weeks for the first year followed by every 12 weeks thereafter to assess intracranial and systemic responses, respectively. Brain metastases response was assessed via Response Assessment in Neuro-Oncology (RANO) and extracranial disease was assessed via immune-related Response Evaluation Criteria in Solid Tumors criteria.16,17 Common Terminology Criteria for Adverse Events version 5.0 was used to grade adverse events. Laboratory monitoring including a complete blood count and serum chemistries were performed before receipt of every dose of nivolumab; thyroid function studies were performed every 8 weeks.
Dose-limiting toxicities (DLTs) were defined as neurologic toxicities attributable to SRS by the treating radiation oncologist, neuro-oncologist, or breast oncologist in the first 8 weeks of treatment including symptomatic radionecrosis, defined by surgical pathology or multidisciplinary evaluation, grade ≥3 headaches, grade ≥3 memory impairment, and new onset grade ≥3 seizures. DLTs and AEs continued to be assessed throughout the treatment and follow-up period. Patients were assessed at the time of SRS, before each nivolumab infusion at 4-week intervals, and postimaging at 8-week intervals.
Stereotactic radiation technique
Patient immobilization was achieved by using a head mask fixation system (Brainlab, Feldkirchen, Germany). Brain metastases were evaluated using MRI- (Siemens Sonata, Siemens Medical Systems, Erlangen, Germany) with 1-mm slices for treatment planning purposes. The MRI image was coregistered and fused with CT simulation imaging (General Electric Medical System, Milwaukee, WI). Treatments were delivered using multiple dynamic conformal arcs or intensity modulated radiation therapy. Doses were prescribed to assure coverage of at least 95% of the planning target volume (PTV) with the dose prescribed.
Patients received single session SRS to intact brain metastases and postoperative cavities. For intact brain metastases, this was 15 Gy to lesions between 31 to 40 mm, 18 Gy to 21 to 30 mm, and 24 Gy to lesions measuring ≤20 mm.18 If predefined stopping boundaries were met, the radiation dose would be modified to dose level 1 and would proceed with 25 Gy in 5 fractions.19 A 1-mm expansion was made from the gross tumor volume to the PTV. For postoperative lesions, the clinical target volume was defined as the edge of the resection cavity including areas of contrast enhancement and the overlying dura. A clinical target volume to PTV expansion of 2 mm was used for postoperative cavities. Doses prescribed to resection cavities were based on the size of the surgical cavity volume <4.2 mL 20 Gy, 4.2 to 7.9 mL 18 Gy, 8 to 14.3 mL 17 Gy, 14.4 to 19.9 mL 15 Gy, 20 to 29.9 mL 14 Gy, ≥30 mL up to 5 cm 12 Gy.20 Modifications to the above dosing criteria for intact and postoperative brain metastases were allowed by the treating radiation oncologist if lesions were located within an eloquent area of the brain. Single fraction dosing constraints included the optic pathway 0.2 cm3 <8 Gy, Dmax 10 Gy; cochlea Dmax 9 Gy; brain stem 0.5 cm3 <10 Gy, Dmax 15 Gy; and spinal cord and medulla 0.35 cm3 < 10 Gy, Dmax 14 Gy.
Statistical analysis
The study followed a standard 3 + 3 design with a predefined dose expansion to 12 patients. Local brain metastasis failure was defined by RANO Brain Metastases criteria in which there was a ≥20% increase that remained consistent or demonstrated continued progression on subsequent imaging, while local brain metastasis control (LC) included all treated lesions not meeting these criteria.16 Distant brain metastasis failure was defined as new brain metastases or leptomeningeal enhancement outside the previously irradiated field. Distant intracranial control (DIC) was defined as freedom from development of new brain metastases outside of the irradiated field and freedom from development of LMD. Intracranial control was defined as freedom from local and distant failure. Systemic progression free survival (PFS) was defined by extracranial progression by immune-related Response Evaluation Criteria in Solid Tumors Criteria17 or death. DIC, LC, and systemic PFS were calculated from the start date of trial therapy and assessed to the end of trial therapy. Overall survival (OS) was calculated from the start of trial therapy to last available survival follow-up or death. The rate of LC and DIC of brain lesions at 6 and 12 months was calculated along with median and 95% confidence intervals (CI) using the Kaplan-Meier curve method. Events were summarized descriptively using frequencies and percentages. Demographics and baseline laboratory results were summarized using descriptive statistics for all participants. This report was conducted once all twelve patients had enrolled and the first intracranial response assessment at 2 months was completed.
Results
Patient and treatment characteristics
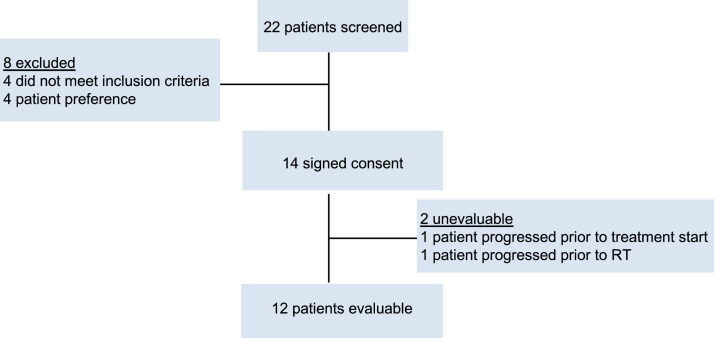
Between February 2019 and July 2020, a total of 14 patients consented after the screening of 22 patients according to the trial profile in Figure 1. One patient progressed before receiving nivolumab and SRS in the liver such that they no longer met eligibility in liver function tests. An additional patient was found to have LMD on repeat brain MRI for SRS treatment planning after receiving the first dose of nivolumab and was no longer protocol eligible. Data from the study was assessed in November 2020. Patient and treatment characteristics are detailed in Table 1. A total of 12 patients were enrolled and treated to 17 lesions. The median age of patients was 58 (range, 26-67). The majority of patients were triple negative (n = 6; 50%) followed by HR+/HER2– (n = 4; 33%), and HR–/HER2+ (n = 2; 17%). The majority of patients had a singular lesion treated (n = 8; 67%). A total of 6 patients (50%) underwent prior CNS directed local therapy. Two patients who were HR–/HER2+ continued trastuzumab with study therapy. Three patients who were HR+/HER2– continued hormone therapy while one patient continued hormone therapy with abemaciclib for continued systemic control.
Fig. 1.
Trial profile.
Table 1.
Patient and treatment characteristics
| Variable | N | % |
|---|---|---|
| No. of patients | 12 | |
| No. of lesions irradiated | 17 | |
| Age, median (range) | 58 (26-67) | |
| ECOG performance status | ||
| 0 | 8 | 67 |
| 1 | 4 | 33 |
| Receptors | ||
| HR+/HER2– | 4 | 33 |
| HR–/HER2+ | 2 | 17 |
| TN | 6 | 50 |
| No. of previous systemic therapy regimens | ||
| 1 | 8 | 67 |
| 2 | 2 | 17 |
| ≥4 | 2 | 17 |
| Previous CNS therapy | ||
| None | 6 | 50 |
| Surgical resection + stereotactic Radiation therapy | 1 | 8 |
| Stereotactic radiation therapy | 2 | 17 |
| Surgery | 3 | 25 |
| No. of lesions irradiated | ||
| 1 | 8 | 67 |
| 2 | 3 | 25 |
| 3 | 1 | 8 |
Abbreviations: CNS = central nervous system; HR = hormone receptor; TN = triple negative.
Of the 17 lesions treated on the study, the majority were treated with single fraction radiosurgery (n = 15; 88%) to a median dose of 21 Gy (range, 16-24 Gy) while 2 lesions were treated to a dose of 30 Gy in 5 fractions given size, location adjacent to eloquent areas, and receipt of previous radiation therapy. Doses and volumes of treated lesions are detailed in Table E1. Four postoperative cavities were treated. A total of 5 patients were on short courses of steroids while receiving radiation treatment and completed tapers approximately 2 weeks after radiation completion.
Toxicity
Overall, nivolumab and stereotactic radiation was well tolerated with no DLTs noted in our patient population (Table 2). One patient experienced grade 4 encephalopathy and grade 3 seizures, which were unrelated to study treatment and due to disease progression. Another patient experienced grade 3 syncope thought to be unrelated to study treatment. One patient experienced grade 3 cerebral edema leading to word finding difficulties, lethargy, and decreased oral intake thought to be possibly related to study treatment during week 9 of treatment which resolved 2 days later with oral steroids. No other patient required an increase in steroids after start of study therapy. Five (42%) patients experienced grade 1 to 2 headaches and dizziness thought to be related to study therapy, disease, or both. No grade 3 or 4 nonneurologic side effects thought to be at least partially attributable to study therapy were noted. The most common nonneurologic adverse effects at least possibly attributable to study therapy included grade 1 to 2 nausea (n = 10; 83%), hypothyroidism (n = 6; 50%), fatigue (n = 5; 42%), and anemia (n = 5; 42%). No cases of radionecrosis have been noted. There have been no treatment-related deaths.
Table 2.
Adverse events in all treated patients
| Grade 1-2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|
| Neurologic adverse events, regardless of attribution | ||||
| Stroke | 1 (8%) | |||
| Muscle weakness right-sided | 1 (8%) | |||
| Memory impairment | 1 (8%) | |||
| Headache | 5 (42%) | |||
| Dizziness | 5 (42%) | |||
| Seizure | 1 (8%) | 1 (8%) | ||
| Peripheral sensory neuropathy | 2 (17%) | |||
| Nystagmus | 1 (8%) | |||
| Dysgeusia | 1 (8%) | |||
| Syncope | 1 (8%) | |||
| Cerebral edema | 1 (8%) | |||
| Encephalopathy | 1 (8%) | 1 (8%) | ||
| Nonneurologic adverse events, treatment related | ||||
| Hypotension | 1 (8%) | |||
| Decreased lymphocyte count | 9 (75%) | 1 (8%) | ||
| Weight loss | 1 (8%) | |||
| Thromboembolic event | 1 (8%) | |||
| Pneumonitis | 1 (8%) | |||
| Adrenal insufficiency | 2 (17%) | |||
| Alanine aminotransferase increased | 2 (17%) | |||
| Anemia | 5 (42%) | |||
| Aspartate aminotransferase increased | 2 (17%) | |||
| Blood lactate dehydrogenase increased | 2 (17%) | |||
| Diarrhea | 3 (25%) | |||
| Dyspnea | 2 (17%) | |||
| Fatigue | 5 (42%) | |||
| Hyperglycemia | 2 (17%) | |||
| Hypothyroidism | 6 (50%) | |||
| Elevated chloride | 2 (17%) | |||
| Nausea | 10 (83%) | |||
| Neutrophil count decreased | 3 (25%) | |||
| Platelet count decreased | 2 (17%) | |||
Adverse events are included if they are grade 3 to 5 severity or occurred in at least 10% of patients and were considered at least possibly related to study therapy. Neurologic events are included regardless of attribution to study therapy.
Intracranial and systemic response
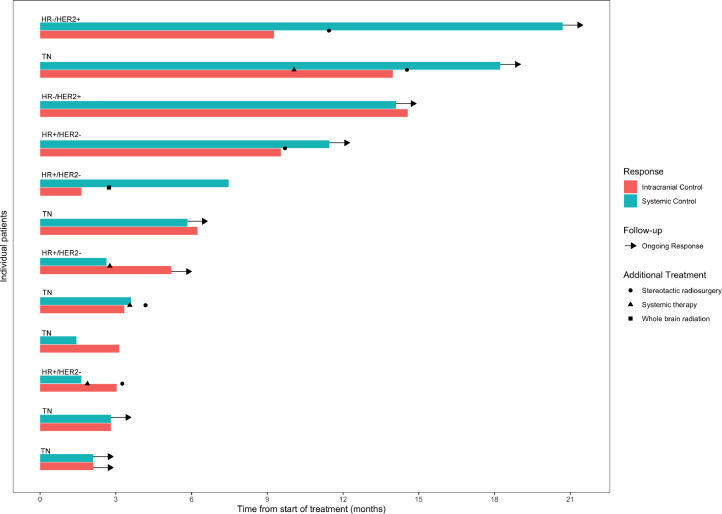
Median follow-up from start of protocol therapy at time of analysis is 9.6 months (range, 2.8-18.7 months). Two (12%) of 17 treated lesions have been noted to undergo local failure both of which were confirmed by surgical resection. Median LC has not been reached (95% CI, 6.2; not reached). Six- and 12-month rates of LC were 100% and 89%, respectively. Median DIC on study therapy is 9.3 months (95% CI, 3-14 months) with 6- and 12-month control rates of 55% and 28%, respectively. A total of 8 patients had distant intracranial failure, of which 5 patients were amenable to additional SRS and 2 received WBRT one for dural based failure and one for LMD. Median intracranial control was 6.2 months (95% CI, 3.0-14 months) with 6- and 12-month control rates of 55% and 22%, respectively (Fig. 1). Best RANO intracranial responses on study therapy were categorized as CR in 6 patients (50%), PR in 5 patients (42%), and PD in 1 patient (8%); intracranial response (92%).
A total of 4 patients had systemic disease progression during the study. Median time to systemic PFS has not been reached (95% CI, 1.6; not reached) with 6- and 12-month rates of 63% and 51%, respectively (Fig. E2). At time of systemic progression, one triple negative patient started taxol, one HR+/HER2– patient stated abemaciclib, and another HR+/HER2– patient started eribulin while continuing nivolumab for continued intracranial control. One patient had passed away at the time of this analysis. Median OS has not been reached (95% CI, 6.8; not reached) with a 12-month OS rate 89%. Individual patient responses are detailed in Figure 2.
Fig. 2.
Bars represent individual intracranial and systemic control assessed by Response Assessment in Neuro-Oncology Brain Metastases Criteria and immune-related Response Evaluation Criteria in Solid Tumors Criteria, respectively. Also detailed are additional treatments received by patients and ongoing responses as well as receptor subtypes. Abbreviations: HR = hormone receptor; TN = triple negative.
Discussion
In this analysis of a prospective phase 1b study of stereotactic radiation and nivolumab in the management of breast cancer brain metastases, we note several findings. First, study therapy was well tolerated with no cases of radionecrosis noted to date. Second, several patients were noted to have continued intracranial responses at the time of this analysis with the majority amenable to additional local therapy at the time of intracranial failure. Several patients remain on study therapy with responses continuing to be assessed.
Trials have demonstrated significant response in the treatment of brain metastasis with immune checkpoint inhibition in the setting of melanoma and NSCLC brain metastases.8,9 CheckMate 204 revealed an intracranial efficacy of 57% with receipt of nivolumab and ipilimumab in the management of melanoma brain metastases.9 Goldberg et al reported an approximate 30% response rate with pembrolizumab in NSCLC brain metastases with PD-L1 expression of ≥1%.8 Stereotactic radiation is standard of care in the management of localized brain metastases.21 In addition, there has been significant interest in combining radiation therapy with immune checkpoint inhibition due to an immune priming effect noted in preclinical studies from radiation therapy upregulating PD-L112,13 and enhanced tumor reduction with data suggesting clinical improvement including in the setting of brain metastases.22, 23, 24, 25, 26 Given this data, as well as the evolving role of immunotherapy in the management of breast cancer,6,7,10 the present study was undertaken in patients with breast cancer brain metastases.
Radionecrosis can be one of the most problematic side effects after receipt of stereotactic radiation. There have been a number of mechanisms proposed for radiation necrosis including vascular injury and hypoxia, injury to oligodendrocytes, and chronic inflammation in response to these injuries.27 Evaluating pathology in the Radiation Therapy Oncology Group 9005 study revealed radiation necrosis to occur at rates of 8% and 11% at 12 and 24 months, respectively, after single fraction radiosurgery.18 T-cell activation with checkpoint inhibitors might be expected to increase the risk of radiation injury.28 The administration of immune checkpoint inhibitors with stereotactic radiation in brain metastases of melanoma, NSCLC, and renal cell carcinoma origin has been reported to potentially increase the risk of radionecrosis.29 These risks might be higher with ipilimumab as the study from Martin et al revealed an increased risk of symptomatic radiation necrosis in melanoma brain metastases treated with ipilimumab but not a statistically significant increase with anti-PD-1 inhibition.29 A small phase 1 study of whole brain radiation therapy or SRS with ipilimumab as well as retrospective reports of immune checkpoint inhibitors with stereotactic radiation have reported adequate safety profiles without an increased risk of radionecrosis.22,23,30,31
In our study, we have noted no cases of radionecrosis to date. This is the first prospective evidence that stereotactic radiation combined with anti-PD-1 therapy does not increase the risk of radionecrosis. Radiation necrosis occasionally requires surgical intervention for symptom control or histologic confirmation to distinguish necrosis from tumor regrowth.32 In our study, both lesions with MRI features consistent with tumor regrowth underwent surgical resection with pathology confirming tumor progression and not radionecrosis. This further confirms our safety findings. At this time, there are ongoing prospective trials examining atezolizumab and stereotactic radiation in triple negative breast brain metastases (NCT03483012), pembrolizumab and SRS for brain metastases in multiple histologies (NCT02886585), and pembrolizumab with SRS (NCT03449238) in breast brain metastases. As we await the results of these studies to confirm our findings in breast cancer brain metastases management, our data does show safety and feasibility of this concurrent treatment approach.
Several HER2 tyrosine kinase inhibitors have shown efficacy in the management of HER2+ brain metastases.2,3,33 In the recently reported HER2CLIMB study, the intracranial response rate was 47.3% in the tucatinib arm versus 20% in the control arm, among 75 patients with active brain metastases.2 However, systemic treatment options remain limited for patients with triple negative brain metastases and HR+/HER2– brain metastases. The majority of patients (50%) in this trial were triple negative followed by HR+/HER2– (33%). Median DIC on study therapy is 9.3 months with the majority of patients amenable to additional local therapy at the time of intracranial failure. Given the lack of systemic intracranial treatment options and the defined role of immune checkpoint inhibitors for systemic management in triple negative patients,7 the current regimen may be particularly beneficial for these patients.
Although the study has strengths with regards to its prospective nature and protocol defined therapy, there are several limitations. These include its small sample size of various breast subtypes conducted at a single center, heterogeneity in patients, receipt of prior intracranial radiation therapy, and its follow-up of 9.6 months.
Conclusions
In summary, although limited follow-up in our small cohort, the data reveals the combination of stereotactic radiation with nivolumab to be safe and well tolerated in breast cancer brain metastases. No cases of radionecrosis have been noted to date. Further evaluation in phase 2 studies is warranted.
Footnotes
Sources of support: This study was funded by Bristol-Myers Squibb (CA209-8NK) and Moffitt Cancer Center.
Disclosures: The authors report the following disclosures outside of the current work: Dr Yu has received speaker's honoraria from BrainLab and is on the advisory boards of Novocure and Abbvie. Dr Soliman serves as a consultant for Astrazeneca, Celgene, Novartis, PUMA, and Eisai. Dr Czerniecki has intellectual property on a HER2 dendritic cell vaccine. Dr Forsyth has received research funding from Pfizer and Celgene and is on the advisory boards of Novocure, BTG, Inovio, AbbVie, Ziopharm, Tocagen, and Pfizer. Dr Han declares that she has received a speaker's honorarium from Lilly Pharmaceuticals, research funding to the institution from Arvinas, Abbvie, GSK, Marker therapeutics, Novartis, Bristol-Myers Squibb, Pfizer, SeattleGenetics, Prescient, Horizon, Zymeworks and Karyopharm. Dr Ahmed has received research funding from Eli Lilly and Genentech. Dr Caudell has received research funding, consulting fees, and honoraria from Varian Medical Systems. Dr Kim has received research funding from Bristol-Myers Squibb and Astrazeneca. Dr Costa has received consulting honoraria from Bristol-Meyers Squibb.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2021.100798.
Appendix. Supplementary materials
References
- 1.Witzel I, Oliveira-Ferrer L, Pantel K, et al. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. 2016;18:8. doi: 10.1186/s13058-015-0665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated her2-positive breast cancer with brain metastases in the her2climb trial. J Clin Oncol. 2020;38:2610–2619. doi: 10.1200/JCO.20.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from her2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 4.Tolaney SM, Sahebjam S, Le Rhun E, et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin Cancer Res. 2020;26:5310–5319. doi: 10.1158/1078-0432.CCR-20-1764. [DOI] [PubMed] [Google Scholar]
- 5.Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: An analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6:676–684. doi: 10.1001/jamaoncol.2019.6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase ib keynote-012 study. J Clin Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg SB, Schalper KA, Gettinger SN, et al. Pembrolizumab for management of patients with nsclc and brain metastases: Long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21:655–663. doi: 10.1016/S1470-2045(20)30111-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J Clin Oncol. 2017;35(Suppl):15. [Google Scholar]
- 11.Ahmed KA, Stallworth DG, Kim Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol. 2016;27:434–441. doi: 10.1093/annonc/mdv622. [DOI] [PubMed] [Google Scholar]
- 12.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-l1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y, Tsien CI, Shen Z, et al. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol. 2005;23:4127–4136. doi: 10.1200/JCO.2005.07.144. [DOI] [PubMed] [Google Scholar]
- 15.Teng F, Tsien CI, Lawrence TS, et al. Blood-tumor barrier opening changes in brain metastases from pre to one-month post radiation therapy. Radiother Oncol. 2017;125:89–93. doi: 10.1016/j.radonc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: Proposal from the rano group. Lancet Oncol. 2015;16:e270–e278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 17.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 18.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed KA, Sarangkasiri S, Chinnaiyan P, et al. Outcomes following hypofractionated stereotactic radiotherapy in the management of brain metastases. Am J Clin Oncol. 2016;39:379–383. doi: 10.1097/COC.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 20.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills MN, Figura NB, Arrington JA, et al. Management of brain metastases in breast cancer: A review of current practices and emerging treatments. Breast Cancer Res Treat. 2020;180:279–300. doi: 10.1007/s10549-020-05552-2. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed KA, Abuodeh YA, Echevarria MI, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol. 2016;27:2288–2294. doi: 10.1093/annonc/mdw417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed KA, Kim S, Arrington J, et al. Outcomes targeting the PD-1/PD-l1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J Neurooncol. 2017;133:331–338. doi: 10.1007/s11060-017-2437-5. [DOI] [PubMed] [Google Scholar]
- 24.Qian JM, Yu JB, Kluger HM, et al. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer. 2016;122:3051–3058. doi: 10.1002/cncr.30138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the keynote-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theelen W, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Rhun E, Dhermain F, Vogin G, et al. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev Neurotherapeutics. 2016;16:903–914. doi: 10.1080/14737175.2016.1184572. [DOI] [PubMed] [Google Scholar]
- 28.Rauch PJ, Park HS, Knisely JP, et al. Delayed radiation-induced vasculitic leukoencephalopathy. Int J Radiat Oncol Biol Phys. 2012;83:369–375. doi: 10.1016/j.ijrobp.2011.06.1982. [DOI] [PubMed] [Google Scholar]
- 29.Martin AM, Cagney DN, Catalano PJ, et al. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol. 2018;4:1123–1124. doi: 10.1001/jamaoncol.2017.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams NL, Wuthrick EJ, Kim H, et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys. 2017;99:22–30. doi: 10.1016/j.ijrobp.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Hubbeling HG, Schapira EF, Horick NK, et al. Safety of combined pd-1 pathway inhibition and intracranial radiation therapy in non-small cell lung cancer. J Thoracic Oncol. 2018;13:550–558. doi: 10.1016/j.jtho.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Alomari A, Rauch PJ, Orsaria M, et al. Radiologic and histologic consequences of radiosurgery for brain tumors. J Neurooncol. 2014;117:33–42. doi: 10.1007/s11060-014-1359-8. [DOI] [PubMed] [Google Scholar]
- 33.Freedman RA, Gelman RS, Anders CK, et al. Tbcrc 022: A phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37:1081–1089. doi: 10.1200/JCO.18.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.