Abstract
Objective
To examine the impact of men’s diet on outcomes of infertility treatment with assisted reproductive technology (ART) using an empirical score representing the relation of diet with semen quality.
Design
Prospective cohort study.
Setting
Fertility center at an academic medical center.
Patient(s)
We included 296 men (688 semen samples) to identify an empirical dietary pattern and 231 couples (406 ART cycles) to investigate the association of this diet pattern with ART outcomes.
Intervention(s)
Men’s diet was assessed at baseline using a validated questionnaire. An empirical dietary pattern reflecting the overall relation of diet with semen quality was identified using reduced rank regression.
Main Outcome Measure(s)
The primary outcome was live birth per treatment cycle. The secondary outcomes were fertilization, implantation, and clinical pregnancy.
Result(s)
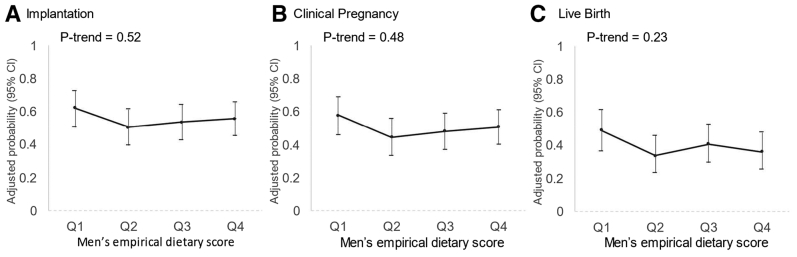
Men had a median baseline age and body mass index of 36.8 years and 26.9 kg/m2, respectively. Although the empirical diet pattern was significantly associated with all semen parameters, the empirical diet score was not related to any clinical outcome of infertility treatment after ART. The adjusted probabilities of relevant clinical outcomes in the lowest and highest quartiles of the empirical score were 0.62 (0.50–0.73) and 0.55 (0.45–0.66) for implantation, 0.57 (0.46–0.69) and 0.50 (0.40–0.61) for clinical pregnancy, and 0.49 (0.37–0.62) and 0.36 (0.25–0.48) for live birth. Analyses excluding couples with a diagnosis of male factor infertility and, separately, excluding intracytoplasmic sperm injection cycles yielded similar results.
Conclusion(s)
A dietary score representing the overall association of diet with semen quality parameters was not associated with ART outcomes.
Key Words: Male diet, semen quality, reduced rank regression, ART, live birth
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-21-00074
Infertility is an increasingly significant medical condition worldwide, affecting over 15% of couples of reproductive age (1). While much of the focus on understanding the causes of infertility is on women, male factors account for approximately half of the infertility burden (2). Standard semen analysis is not only an important biomarker of spermatogenesis and testicular function but also the cornerstone for the clinical diagnosis of male factor infertility (2, 3). However, it is known that semen analysis is not a perfect predictor of couples’ fertility, both in couples attempting conception on their own and in couples attempting conception with medical assistance (4, 5).
Men’s diet has been increasingly recognized as a potentially modifiable factor influencing semen quality. For example, intakes of n-3 fatty acids (6, 7), coenzyme Q10 (8), and carnitine (9), as well as foods such as fish, seafood, poultry, vegetables, fruits (10, 11, 12, 13), nuts, and whole cereals (14, 15, 16), have been positively related to semen quality. Similarly, adherence to healthier dietary patterns like the Mediterranean or prudent diet has been positively associated with semen parameters, whereas the opposite appears to be the case for adherence to unhealthy dietary patterns such as western pattern (10, 11, 12, 13). However, there are few data evaluating the impact of men’s diet on a couple’s fertility. This is a particularly significant knowledge gap because some data suggests that associations between diet and semen quality do not necessarily translate into associations with couple-based outcomes, such as fertility (17, 18, 19, 20, 21).
This study aimed to evaluate the extent to which men’s dietary factors associated with semen quality are also predictive of couples’ infertility treatment outcomes. To achieve this goal, we empirically derived a dietary score capturing the overall association of diet with semen quality and then examined this score in relation to the probability of achieving a live birth in the course of infertility treatment with assisted reproductive technology (ART).
Materials and methods
Study Population
Couples presenting to the Massachusetts General Hospital Fertility Center were invited to enroll in the Environment and Reproductive Health study, a prospective cohort study aimed at evaluating the impact of environmental and nutritional factors (22) on fertility and pregnancy outcomes. Women aged 18–45 years and men aged 18–55 years without a history of vasectomy, whose treating physician anticipated the use of their own gametes for infertility treatment, and who had not been administered any hormonal treatment at the enrollment of study were eligible. A total of 982 women, 553 men, and 513 couples enrolled between April 2004 and December 2019. Participants were encouraged, but not required, to take part in the study as a couple. All participants who joined signed written informed consent. Study staff administered a baseline questionnaire, including demographics, medical, reproductive, occupation histories, and lifestyle, and conducted anthropometric measurements. Physical activity was assessed with a previously validated questionnaire (23). Participants also provided blood and urine specimens during the first study visit (22). They also completed a Food Frequency Questionnaire (FFQ), introduced in April 2007, to assess habitual diet of participants. For the present study, we used data of two partially overlapping subgroups of patients in the Environment and Reproductive Health study. For analyses aimed at identifying empirical diet patterns related to semen, we included all men who enrolled in the study from April 2007 to June 2019, completed the FFQ, and produced at least one semen sample. We excluded men with azoospermia (n = 11 men) and men with missing values in any semen parameter or abstinence time (n = 22 men), leaving 296 men (688 semen samples) available for analysis (Supplemental Fig. 1, available online). For analyses aimed at evaluating the role of men’s diet on ART outcomes, we used data from all couples where the male partner completed the FFQ and the female partner underwent at least one ART cycle from April 2007 to April 2018 (Supplemental Fig. 1). The Institutional Review Boards of Massachusetts General Hospital and the Harvard T.H. Chan School of Public Health approved the study.
Semen Analysis
Men provided semen specimens on-site via masturbation. A 48-hour abstinence period before sample production was recommended, and actual abstinence time was recorded for each sample. Semen samples were maintained at 37 °C and allowed to liquefy. All assessments were performed within 30 minutes of collection following the 2010 World Health Organization (WHO) manual guidelines (3). Ejaculate volume was estimated by sample weight, assuming a semen density of 1 g/mL. Sperm concentration and motility were assessed by computer-assisted semen analysis (10HTM-IVOS; Hamilton Thorne Research, Beverly, MA) (24). Motile spermatozoa were defined according to the WHO four-category scheme: rapid progressive; slow progressive; nonprogressive; and immotile. Total sperm count (million/ejaculate) was calculated by multiplying ejaculated volume by sperm concentration. Sperm morphology (% normal) was assessed on two slides per specimen (with a minimum of 200 cells assessed per slide) via a microscope with an oil-immersion ×100 objective (Nikon, Tokyo, Japan). Strict Kruger scoring criteria were used to classify men as having normal or below normal morphology (25).
Dietary Assessment and Dietary Score
Diet was assessed using a previously validated FFQ of 131 foods and beverages (26, 27). Participants were asked to report how often, on average, during the previous year they consumed each food item. Response options ranged from never or less than once per month to six or more times per day. The individual foods and beverage items were categorized into 42 predefined foods and beverages groups based on those proposed by Hu et al. (28).
Clinical Procedures
Women underwent one of three ovarian stimulation protocols for fresh in vitro fertilization (IVF) protocol: gonadotropin-releasing hormone (GnRH)-antagonist protocol; follicular phase GnRH-agonist/flare-up protocol; or luteal phase GnRH-agonist protocol. Embryologists classified oocytes as germinal vesicle, metaphase I, metaphase II, or degenerated. Metaphase II oocytes underwent conventional insemination (IVF) or intracytoplasmic sperm injection (ICSI) as clinically indicated. Embryologists evaluated fertilization status on day 1 after fertilization on the basis of the presence of two pronuclei. Fertilization rate was defined as the number of two pronuclei embryos divided by the number of metaphase II oocytes. Embryo transfer was performed either after stimulation and retrieval or after thawing of cryopreserved embryos (19, 22). Clinical outcomes were evaluated among women who underwent embryo transfer. An elevation of serum β-human chorionic gonadotropin level greater than 6 mIU/mL at approximately 2 weeks after embryo transfer was defined as successful implantation. The presence of an intrauterine gestational sac observed on ultrasonographic evaluation at approximately 6 gestational weeks was considered as a clinical pregnancy. Live birth was defined as the birth of a neonate at or after 24 weeks of gestation.
Statistical Analysis
To evaluate the overall impact of diet on all semen quality parameters simultaneously, we conducted a reduced rank regression (RRR) analysis (29). Reduced rank regression is a statistical procedure that is aimed at dimension reduction by simultaneously modeling the association of a set of predictors with a group of related outcome measures with the goal of obtaining a single (or a limited number of) summary response measure(s) (factors). In nutritional epidemiology, RRR has been used to identify how diet mediates health effects through specific biologic pathways by modeling the simultaneous association of multiple dietary factors on multiple biomarkers of the same underlying biologic process (e.g., inflammation) (30, 31). In this case, we used semen quality parameters as biologic intermediates between men’s diet and a couple’s fertility. To decrease variability in semen quality because of differences in abstinence time and incorporate all available semen analysis data from each man into a single value, we adjusted each semen parameter by abstinence time using the residual method with linear mixed regression models. Briefly, we fitted a linear mixed regression model for each semen parameter (total count, concentration, percent motile, percent progressively motile, and percent normal morphology) that included all semen analysis data available as observations (N = 688 samples, 296 men) and linear, quadratic, and cubic terms for abstinence time as predictors. The residuals for each man were then averaged to obtain a single time-integrated and abstinence time-independent measure of semen quality for each man. Then, we conducted an RRR analysis where the 42 predefined food and beverage groups were the predictive variables and the mean of the residual of each semen parameter (ejaculate volume, total sperm count, semen concentration, total motility, progressive motility, and percentage of sperm normal morphology) were the response variables. The first factor from this model was retained and interpreted as an empirical score capturing the overall relation of diet with semen quality.
Men were categorized into quartiles according to their empirical dietary score. Differences in the proportion or median of demographic, reproductive, and nutritional characteristics across quartiles of the empirical diet score were evaluated using the Kruskal-Wallis test for continuous variables and χ2 or Fisher exact test for categorical variables.
To corroborate that the solution from the RRR model captured the overall association of diet with semen quality, we fitted six separate linear regression models where the exposure of interest was the empirical dietary score and the outcome of interest was each semen parameter (ejaculate volume, total sperm count, total motility, progressive motility, and the percentage of sperm normal morphology). Total sperm count and sperm concentration were log-transformed to improve and more closely approximate a normal distribution. To allow direct comparisons of the magnitude of the relation of the diet score across all outcomes, we standardized the diet score and each of the outcomes by dividing each variable by its standard deviation. The results from these models can, therefore, be interpreted as the difference in each semen parameter, in original and standard deviation units, associated with a 1 standard deviation increase in the empirical diet score.
Then, to evaluate the association between the male diet score and ART outcomes, we fitted multivariable generalized linear mixed models with random intercepts to account for repeated ART cycles per couple while adjusting for potential confounders. A binomial distribution and logit link function were specified for fertilization rate and clinical outcomes (implantation, clinical pregnancy, and live birth). The primary outcome of this study was the probability of live birth per initiated treatment cycle. The secondary outcomes were fertilization rate, probabilities of implantation, and clinical pregnancy during the course of infertility treatment with ART. We used population marginal means to present results as probabilities and their corresponding 95% confidence intervals adjusted for all covariates in the model (32). We evaluated the linear trend across the quartiles of the dietary score by modeling the dietary score as a continuous variable. We chose the confounders using previous scientific knowledge and by assessment of the difference in patients’ baseline characteristics across the quartiles. The primary multivariable-adjusted model included terms for men’s and women’s age, men’s total calorie intake per day, total physical activity (minutes/week), couples’ primary infertility diagnosis, and treatment protocol and, in models for fertilization rate as the outcome, type of insemination (ICSI vs. conventional IVF). The second model included additional terms for men’s and women’s body mass index (BMI). For the second model, we had missing data on BMI for two men and three women. We decided to use complete data in the analysis resulting in the exclusion of five cycles. Using cross-product terms, we evaluated effect modification by insemination mode (ICSI vs. IVF).
Lastly, to evaluate the robustness of our findings, we performed a series of sensitivity analyses. We first performed analyses restricted to couples without a diagnosis of male factor infertility and, separately, excluding ICSI cycles. Then, we repeated the RRR analysis without adjusting semen parameters for abstinence time and using only semen samples produced within the WHO-recommended abstinence period of 2–7 days (3) and evaluated the relation of this new empirical diet score with all clinical outcomes. We conducted sensitivity analysis excluding the cycles of the couples for the 7 azoospermic men and sensitivity analysis excluding the cycles of the 29 couples who were not included in the RRR analysis for diet and semen quality (Supplemental Fig. 1). All analyses were performed using SAS University Edition with VirtualBox version 6.1.10.
Results
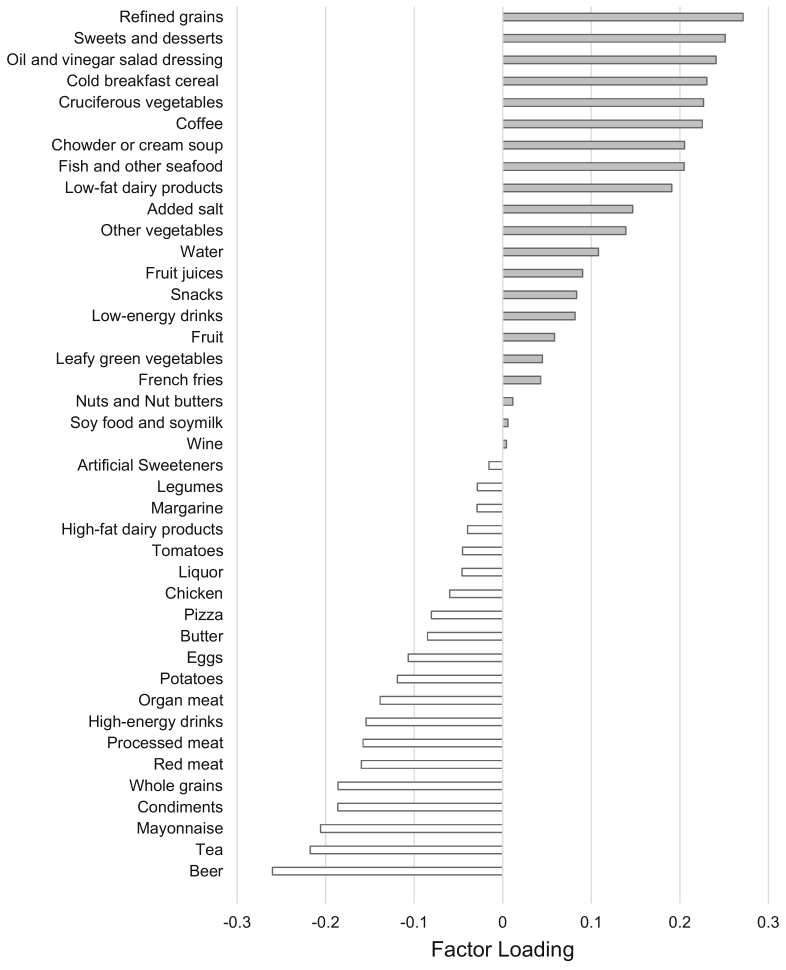
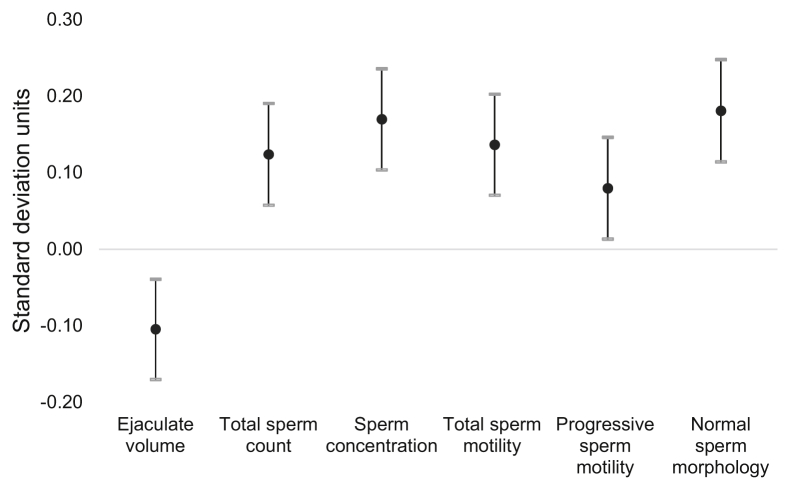
In total, we included 296 men (688 semen samples) in analyses aimed at identifying the empirical dietary pattern and 231 couples (406 ART cycles) in analyses aimed at evaluating the association of this diet pattern with ART outcomes. Supplemental Table 1 shows the distribution of semen quality parameters among study participants. Not surprisingly, men in the study had a high proportion of samples with values below the 2010 WHO reference values, particularly for sperm motility (total and progressive) (Supplemental Table 1). Figure 1 shows the results of the RRR model. Food groupings with positive factor loadings were positively associated with semen quality, whereas negative factor loadings had the opposite interpretation. As expected, the empirical diet pattern was significantly associated with all semen quality parameters. A one standard deviation increase in empirical dietary score was associated with lower ejaculate volume (−0.10 standard units [95% confidence interval, −0.17 to −0.04]) and higher total sperm count (0.12 standard units [0.06 to 0.19]), sperm concentration (0.17 standard units [0.10 to 0.24]), total sperm motility (0.14 standard units [0.07 to 0.20]), progressive sperm motility (0.08 standard units [0.01 to 0.15]), and normal sperm morphology (0.18 standard units [0.11 to 0.25]) (Fig. 2). The same pattern was observed when semen parameters were modeled in their measured units (Supplemental Table 2).
Figure 1.
Factor loadings for reduced rank regression with food groups as the predictive variables and semen parameters as the response variables. Gray bar: positive factor loading food groups. White bar: negative factor loading food groups.
Figure 2.
Association between empirical dietary pattern and individual semen quality parameters. All semen parameters and the empirical diet score have been standardized to allow direct comparison of the magnitude of association between the scores across semen parameters.
We then evaluated the relation of the empirical score with ART outcomes. The median (interquartile range) age and BMI of women were 35.0 years (32.0–38.0) and 23.1 kg/m2 (21.0–25.7), respectively. The corresponding values for men were 36.8 years (33.4–40.0) and 26.9 kg/m2 (24.1–29.1), respectively. The empirical diet score was positively associated with total energy intake and physical activity (Table 1). In addition, the frequency of male factor infertility as the primary infertility diagnosis decreased with increasing levels of the empirical score (P=.003 comparing male factor vs. not). The distribution of the initial stimulation protocol also differed according to quartiles of the empirical score (Table 1). No other demographic, nutritional, or reproductive characteristics were associated with the score (Table 1).
Table 1.
Baseline demographic, nutritional, and reproductive characteristics of study participants, overall and by quartiles of the empirical dietary score.a
| Empirical dietary score |
Total |
Q1 |
Q2 |
Q3 |
Q4 |
P valueb |
|---|---|---|---|---|---|---|
| −0.33 to 0.43 |
0.44 to 0.78 |
0.79 to 1.07 |
1.08 to 2.98 |
|||
| n | 231 | 57 | 58 | 58 | 58 | |
| Demographics, men | ||||||
| Age (y) | 36.8 (33.4–40.0) | 37.2 (34.4–40.0) | 37.4 (33.5–40.5) | 35.9 (31.9–39.2) | 37.2 (34.1–40.4) | .45 |
| BMI (kg/m2) | 26.9 (24.1–29.1) | 27.4 (24.8–30.0) | 26.7 (23.8–28.7) | 26.7 (24.5–29.3) | 26.7 (23.7–28.6) | .36 |
| Race (white) | 206 (89.2) | 49 (86.0) | 51 (87.9) | 53 (91.4) | 53 (91.4) | .75 |
| Smoking status (never smoker) | 153 (66.2) | 41 (71.9) | 38 (65.5) | 35 (60.3) | 39 (67.2) | .63 |
| Education (college or higher) | 183 (84.7) | 43 (84.3) | 44 (84.6) | 45 (79.0) | 51 (91.1) | .36 |
| Total physical activity (min/week) | 347 (150–629) | 270 (90–612) | 210 (84–510) | 472 (252–750) | 372 (221–600) | .005 |
| Calories (kcal/day) | 1,934 (1,586–2,384) | 1,906 (1,547–2,189) | 1,794 (1,341–2,221) | 1,910 (1,571–2,384) | 2,233 (1,886–2,724) | .0003 |
| Reproductive history | ||||||
| History of varicocele | 19 (8.2) | 5 (8.8) | 6 (10.3) | 5 (8.6) | 3 (5.2) | .81 |
| Previous infertility examination | 188 (83.6) | 46 (82.1) | 49 (86.0) | 49 (86.0) | 44 (80.0) | .79 |
| Previous infertility treatment | 107 (51.7) | 21 (40.4) | 29 (54.7) | 29 (55.8) | 28 (56.0) | .31 |
| History of past pregnancy | 86 (37.4) | 19 (33.3) | 23 (39.7) | 17 (29.8) | 27 (46.6) | .23 |
| Primary infertility diagnosis | ||||||
| Male factor | 85 (36.8) | 28 (49.1) | 28 (48.3) | 15 (25.9) | 14 (24.1) | .10 |
| Female factor | 84 (36.4) | 20 (35.1) | 19 (32.8) | 22 (37.9) | 23 (40.0) | |
| Unexplained | 62 (26.8) | 9 (15.8) | 11 (19.0) | 21 (36.2) | 21 (36.2) | |
| Initial stimulation protocol | ||||||
| Antagonist | 35 (15.2) | 6 (10.5) | 13 (22.4) | 8 (13.8) | 8 (13.8) | .05 |
| Flare | 22 (9.5) | 11 (19.3) | 3 (5.2) | 4 (6.9) | 4 (6.9) | |
| Luteal phase agonist | 152 (65.8) | 38 (66.7) | 38 (65.5) | 40 (69.0) | 36 (62.1) | |
| Cryo/donor | 22 (9.5) | 2 (3.5) | 4 (6.9) | 6 (10.3) | 10 (17.2) | |
| Demographics, female partner | ||||||
| Age(y) | 35.0 (32.0–38.0) | 35.0 (33.0–38.0) | 36.0 (33.0–38.0) | 34.5 (32.0–37.0) | 35.5 (32.0–39.0) | .45 |
| BMI (kg/m2) | 23.1 (21.0–25.7) | 23.5 (21.6–26.2) | 23.2 (21.6–25.5) | 22.2 (20.1–24.2) | 22.8 (21.1–25.4) | .15 |
| Race (white) | 194 (84.4) | 47 (82.5) | 47 (81.0) | 53 (93.0) | 47 (81.0) | .19 |
| Smoking status (never smoker) | 166 (72.2) | 39 (68.4) | 38 (65.5) | 44 (77.2) | 45 (77.6) | .37 |
| Dietary score for women | 0.82 (0.57–1.10) | 0.76 (0.54–1.01) | 0.80 (0.56–1.14) | 0.79 (0.51–1.14) | 0.90 (0.66–1.13) | .46 |
Note: BMI = body mass index; cryo = cryopreservation embryo; Q = quartile.
Data are presented as median (interquartile range) for continuous variables or n (%) for categorical variables.
From the Kruskal-Wallis test for continuous variables and Fisher exact test for categorical variables except for primary infertility diagnosis and in vitro fertilization treatment protocol where the χ2 test was used.
We found no association between the empirical dietary score and fertilization rate overall nor when examined separately in IVF and ICSI cycles (Supplemental Fig. 2). Similarly, the empirical score was unrelated to the probability of implantation, clinical pregnancy, and live birth per initiated treatment cycle (Fig. 3). The adjusted probabilities of implantation, clinical pregnancy, and live birth for couples with the primary model in the lowest and highest quartiles of the empirical score were 0.62 (0.50–0.73) and 0.55 (0.45–0.66), 0.57 (0.46–0.69) and 0.50 (0.40–0.61), and 0.49 (0.37–0.62) and 0.36 (0.25–0.48), respectively. The results were nearly identical after additional adjustment for men’s and women’s BMI (data not shown). In addition, we found no evidence of effect modification by type of insemination.
Figure 3.
Men’s empirical dietary score in relation to clinical outcomes of infertility treatment with assisted reproductive technology (N = 231 couples, 406 cycles). Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, primary infertility diagnosis, and stimulation protocol. Q = quartile.
We found no evidence of an association between the empirical diet score and any clinical ART outcome in analyses excluding couples with a primary diagnosis of male factor infertility (Supplemental Figs. 3 and 4) and in analyses excluding ICSI cycles (Supplemental Fig. 5). Similarly, when we revised the RRR model to not account for abstinence time (Supplemental Fig. 6), the revised empirical diet score was also unrelated to all ART outcomes evaluated (Supplemental Figs. 7 and 8). Lastly, the sensitivity analysis excluding couples whose male partners had azoospermia and were not included in the RRR analysis for the dietary score demonstrated almost identical results (Supplemental Figs. 9–12).
Discussion
We investigated the association between men’s diet, using an empirical dietary score capturing the overall impact of diet on semen quality, and ART outcomes among subfertile couples undergoing infertility treatment at an academic fertility center. We found that, despite being associated with all standard semen parameters, this empirical dietary score was not related to any ART outcome. Our findings suggest that the extent to which diet impacts semen quality among men in subfertile couples does not influence ART outcomes, including fertilization, implantation, clinical pregnancy, and live birth. More broadly, these data suggest that in the setting of infertility treatment with ART, any impact that men’s diet—and possibly other environmental factors—may have on couple-based outcomes are unlikely to be a result of their effect on semen quality parameters.
Numerous studies have reported that men’s diet has an impact on semen quality or other biomarkers of testicular function. In general, healthier dietary patterns, such as the Mediterranean diet pattern, which is characterized by higher intake of olive oil, fruits, vegetables, fruit, seafood, poultry, and whole grains, have been associated with favorable semen quality in observational studies among healthy and subfertile men (33, 34, 35) and more recently in a randomized controlled trial (RCT) among healthy men (36). Another dietary factor with strong evidence of benefit is intake of long-chain n-3 fatty acids. For example, in a large study of military recruits in Denmark, the use of fish oil supplements was associated with higher semen volume, total sperm count, and testis size and lower follicle-stimulating hormone and luteinizing hormone levels (37). These findings are similar to those of RCTs of fish oil supplementation among subfertile men (38) and RCTs of supplementation with nuts, which are also rich in n-3 fatty acids, among young healthy men (14, 15).
Nevertheless, the literature linking men’s diet to couple-based outcomes, such as fertility, is scant and inconsistent. On one hand, there are some studies that have documented consistent associations of diet with semen quality and fertility. For example, studies in independent populations have documented inverse associations of men’s intake of sugary beverages with both semen quality (39, 40) and fertility (41). Similarly, studies in independent populations have documented positive associations of men’s fish intake with semen quality (42) and fertility (18). Of note, in these cases, the associations of men’s diet with a couple’s fertility were documented in studies of pregnancy planners without a history of infertility. On the other hand, there are multiple other studies where the consistency of associations of diet with semen quality and fertility is not present, particularly in studies among couples undergoing infertility treatment. For example, we have previously reported that men’s intakes of processed meats, dairy, soy, and carotenoids are associated with semen quality (21, 42, 43, 44) but not with the outcomes of infertility treatment with ART (17, 19, 20, 45). Conversely, we have found associations between men’s intakes of alcohol, caffeine, and vitamin C and ART outcomes in the absence of an association with semen quality in the same population (45, 46). Clearly, it is significant that additional studies evaluate the extent to which predictors of semen quality overlap with predictors of couple-based outcomes like fertility, both in couples attempting conception naturally and in those with medical assistance.
It is significant to consider the study findings in light of their strengths and limitations. First and most saliently, the study was conducted among subfertile couples undergoing infertility treatment. As a result, the frequency of men with semen quality below the WHO reference limits was higher than expected in a population of pregnancy planners without a history of infertility. However, sensitivity analyses excluding couples with a primary infertility diagnosis of male factor showed nearly identical results suggesting that the overrepresentation of men with poor semen quality alone does not explain the findings. Perhaps of greater relevance is the possibility that infertility treatment with ART itself may completely negate any effects that environmental factors, including diet, could have on a couple’s fertility by influencing semen quality. This interpretation is consistent with the scant evidence to date on the relation between diet and a couple’s fertility described earlier. In other words, given the stringent sperm selection procedures built into ART, especially ICSI, any effect that diet or other environmental factors may have on a couple’s chances of conceiving is unlikely to reflect environmental impacts on semen quality. In fact, the results of the sensitivity analysis excluding ICSI cycles suggest that even conventional IVF poses enough selective pressure on sperm to the point of nullifying the effect that environmental and behavioral factors may have on fertility through their effect on semen quality. Therefore, it is unclear whether findings from this study can be generalized to couples trying to conceive without ART. Moreover, our findings further highlight that bulk semen parameters are far from perfect biomarkers of men’s reproductive potential (4, 5), and it is, thus, important to examine whether other characteristics of sperm such as deoxyribonucleic acid integrity (47, 48, 49), ribonucleic acid elements (50), proteomics (51, 52), or others yet to be identified are better able to capture how men’s environment and behavior influence a couple’s fertility in the general population and in the setting of infertility treatment. Second, we need to consider misclassification and measurement error in diet, which is a concern even when using extensively validated questionnaires. Nevertheless, this problem would apply uniformly to all outcomes. Hence, because the empirical diet score was predictive of semen quality in the same group of men, it is unlikely that measurement error alone is responsible for the lack of association with clinical ART outcomes.
There are also significant strengths of this study. The use of a completely agnostic and data-driven approach to characterize the impact on men’s diet on semen quality as our exposure variable has several advantages. First, it eliminates the impact of any prior beliefs on how diet might impact a couple’s fertility. Second, given that decision-making on male factor infertility, including lifestyle recommendations patients may receive, is driven by the current knowledge on predictors of semen quality parameters, this approach approximates the type of advice men in couples facing difficulties conceiving may receive from their physicians. The availability of preconception information on the male partner, which, unfortunately, remains a rarity in studies of fertility, is a significant strength of the study. Additional strengths include the prospective study design with a live birth rate as a primary outcome and complete follow-up of study participants for all study outcomes.
Conclusion
We found that an empirical dietary score capturing the overall impact of diet on semen quality was not related to infertility treatment outcomes with ART. Given that ART incorporates robust sperm selection procedures, it is possible that ART itself may negate the effects of environmental factors on a couple’s ability to conceive with these treatments that are mediated through semen quality. As a result, it is unclear to what extent these findings can be generalized to couples attempting conception without medical assistance. In addition, these results further highlight the limitations of semen quality parameters as a predictor of a couple’s fertility. Additional studies are necessary to understand how men’s diet, environment, and behaviors impact on a couple’s fertility, naturally and with medical assistance, and the extent to which men’s reproductive potential can be captured through biomarkers other than bulk semen parameters.
Acknowledgments
The authors acknowledge all members of the Environment and Reproductive Health study team and extend a special thanks to all the study participants.
Footnotes
M.M. has nothing to disclose. A.S.H. has nothing to disclose. L.M.A. has nothing to disclose. J.A.A. has nothing to disclose. J.B.F. has nothing to disclose. M.K. has nothing to disclose. I.S. has nothing to disclose. J.E.C. reports grants from the National Institutes of Health.
Supported by grants R01ES009718, R01ES022955, P30ES000002, and P30DK46200 from the US National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary data
Flowchart showing participant flow and exclusions for analyses of diet with semen quality and outcomes of infertility treatment with assisted reproductive technology.
Supplemental Figure 2. Men’s empirical dietary score in relation to fertilization rate, overall (A) and in in vitro fertilization (B) or intracytoplasmic sperm injection (ICSI) (C) cycles. Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, ICSI (yes/no), primary infertility diagnosis, and stimulation protocol. Stratified models (B and C) do not include a term for ICSI. Q = quartile.
Supplemental Figure 3. Men’s empirical dietary score in relation to fertilization rate, overall (A) and in in vitro fertilization (B) and intracytoplasmic sperm injection (ICSI) (C) cycles, excluding couples with a primary diagnosis of male factor infertility. Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, ICSI (yes/no), primary infertility diagnosis, and stimulation protocol. Stratified models (B and C) do not include a term for ICSI. Q = quartile.
Supplemental Figure 4. Men’s empirical dietary score in relation to clinical outcomes of infertility treatment with assisted reproductive technology, excluding couples with a primary diagnosis of male factor infertility (N = 146 couples, 272 cycles). Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, primary infertility diagnosis, and stimulation protocol. Q = quartile.
Supplemental Figure 5. Men’s empirical dietary score in relation to clinical outcomes of infertility treatment with assisted reproductive technology, excluding intracytoplasmic sperm injection cycles (N = 92 couples, 124 cycles).
Supplemental Figure 6. Factor loadings for reduced rank regression with food groups as the predictive variables and semen parameters as the response variables, without adjustment for abstinence time. Gray bar: positive factor loading food groups. White bar: negative factor loading food groups.
Supplemental Figure 7. Men’s empirical dietary score (without adjustment for abstinence time) in relation to fertilization rate, overall (A) and in in vitro fertilization (B) or intracytoplasmic sperm injection (ICSI) (C) cycles. Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, ICSI (yes/no), primary infertility diagnosis, and stimulation protocol. Stratified models (B and C) do not include a term for ICSI. Q = quartile.
Supplemental Figure 8. Men’s empirical dietary score (without adjustment for abstinence time) in relation to clinical outcomes of infertility treatment with assisted reproductive technology (N = 231 couples, 406 cycles). Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, primary infertility diagnosis, and stimulation protocol. Q = quartile.
Supplemental Figure 9. Men’s empirical dietary in relation to fertilization rate, overall (A) and in in vitro fertilization (B) or intracytoplasmic sperm injection (ICSI) (C) cycles, excluding couples whose male factor had azoospermia. Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, ICSI (yes/no), primary infertility diagnosis, and stimulation protocol. Stratified models (B and C) do not include a term for ICSI. Q = quartile.
Supplemental Figure 10. Men’s empirical dietary score in relation to clinical outcomes of infertility treatment with assisted reproductive technology, excluding couples whose male factor had azoospermia (N = 224 couples, 393 cycles). Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, primary infertility diagnosis, and stimulation protocol. Q = quartile.
Supplemental Figure 11. Men’s empirical dietary in relation to fertilization rate, overall (A) and in in vitro fertilization (B) or intracytoplasmic sperm injection (ICSI) (C) cycles, excluding couples whose male partners were not included in the reduced rank regression analysis for the dietary score. Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, ICSI (yes/no), primary infertility diagnosis, and stimulation protocol. Stratified models (B and C) do not include a term for ICSI. Q = quartile.
Supplemental Figure 12. Men’s empirical dietary score in relation to clinical outcomes of infertility treatment with assisted reproductive technology, excluding couples whose male partners were not included in the reduced rank regression analysis for the dietary score (N = 202 couples, 361 cycles). Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, primary infertility diagnosis, and stimulation protocol. Q = quartile.
References
- 1.Thoma M.E., McLain A.C., Louis J.F., King R.B., Trumble A.C., Sundaram R., et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–1331.e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Practice Committee of the American Society for Reproductive Medicine Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103:e18–e25. doi: 10.1016/j.fertnstert.2014.12.103. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization, editor. WHO laboratory manual for the examination and processing of human semen. 5th ed. World Health Organization; Geneva: 2010. p. 271. [Google Scholar]
- 4.Jedrzejczak P., Taszarek-Hauke G., Hauke J., Pawelczyk L., Duleba A.J. Prediction of spontaneous conception based on semen parameters. Int J Androl. 2008;31:499–507. doi: 10.1111/j.1365-2605.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- 5.Buck Louis G.M., Sundaram R., Schisterman E.F., Sweeney A., Lynch C.D., Kim S., et al. Semen quality and time to pregnancy: the Longitudinal Investigation of Fertility and the Environment Study. Fertil Steril. 2014;101:453–462. doi: 10.1016/j.fertnstert.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attaman J.A., Toth T.L., Furtado J., Campos H., Hauser R., Chavarro J.E. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27:1466–1474. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen T.K., Heitmann B.L., Jensen M.B., Halldorsson T.I., Andersson A.M., Skakkebæk N.E., et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr. 2013;97:411–418. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- 8.Lafuente R., González-Comadrán M., Solà I., López G., Brassesco M., Carreras R., et al. Coenzyme Q10 and male infertility: a meta-analysis. J Assist Reprod Genet. 2013;30:1147–1156. doi: 10.1007/s10815-013-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigman M., Glass S., Campagnone J., Pryor J.L. Carnitine for the treatment of idiopathic asthenospermia: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2006;85:1409–1414. doi: 10.1016/j.fertnstert.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 10.Salas-Huetos A., Bulló M., Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371–389. doi: 10.1093/humupd/dmx006. [DOI] [PubMed] [Google Scholar]
- 11.Ricci E., Al-Beitawi S., Cipriani S., Alteri A., Chiaffarino F., Candiani M., et al. Dietary habits and semen parameters: a systematic narrative review. Andrology. 2018;6:104–116. doi: 10.1111/andr.12452. [DOI] [PubMed] [Google Scholar]
- 12.Salas-Huetos A., James E.R., Aston K.I., Jenkins T.G., Carrell D.T. Diet and sperm quality: nutrients, foods and dietary patterns. Reprod Biol. 2019;19:219–224. doi: 10.1016/j.repbio.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Benatta M., Kettache R., Buchholz N., Trinchieri A. The impact of nutrition and lifestyle on male fertility. Arch Ital Urol Androl. 2020;92 doi: 10.4081/aiua.2020.2.121. [DOI] [PubMed] [Google Scholar]
- 14.Robbins W.A., Xun L., FitzGerald L.Z., Esguerra S., Henning S.M., Carpenter C.L. Walnuts improve semen quality in men consuming a Western-style diet: randomized control dietary intervention trial. Biol Reprod. 2012;87:101. doi: 10.1095/biolreprod.112.101634. [DOI] [PubMed] [Google Scholar]
- 15.Salas-Huetos A., Moraleda R., Giardina S., Anton E., Blanco J., Salas-Salvadó J., et al. Effect of nut consumption on semen quality and functionality in healthy men consuming a Western-style diet: a randomized controlled trial. Am J Clin Nutr. 2018;108:953–962. doi: 10.1093/ajcn/nqy181. [DOI] [PubMed] [Google Scholar]
- 16.Vujkovic M., de Vries J.H., Dohle G.R., Bonsel G.J., Lindemans J., Macklon N.S., et al. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod. 2009;24:1304–1312. doi: 10.1093/humrep/dep024. [DOI] [PubMed] [Google Scholar]
- 17.Xia W., Chiu Y.H., Williams P.L., Gaskins A.J., Toth T.L., Tanrikut C., et al. Men’s meat intake and treatment outcomes among couples undergoing assisted reproduction. Fertil Steril. 2015;104:972–979. doi: 10.1016/j.fertnstert.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaskins A.J., Sundaram R., Buck Louis G.M., Chavarro J.E. Seafood intake, sexual activity, and time to pregnancy. J Clin Endocrinol Metab. 2018;103:2680–2688. doi: 10.1210/jc.2018-00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia W., Chiu Y.H., Afeiche M.C., Williams P.L., Ford J.B., Tanrikut C., et al. Impact of men’s dairy intake on assisted reproductive technology outcomes among couples attending a fertility clinic. Andrology. 2016;4:277–283. doi: 10.1111/andr.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mínguez-Alarcón L., Afeiche M.C., Chiu Y.H., Vanegas J.C., Williams P.L., Tanrikut C., et al. Male soy food intake was not associated with in vitro fertilization outcomes among couples attending a fertility center. Andrology. 2015;3:702–708. doi: 10.1111/andr.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassan F.L., Chiu Y.H., Vanegas J.C., Gaskins A.J., Williams P.L., Ford J.B., et al. Intake of protein-rich foods in relation to outcomes of infertility treatment with assisted reproductive technologies. Am J Clin Nutr. 2018;108:1104–1112. doi: 10.1093/ajcn/nqy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messerlian C., Williams P.L., Ford J.B., Chavarro J.E., Mínguez-Alarcón L., Dadd R., et al. The Environment and Reproductive Health (EARTH) study: a prospective preconception cohort. Hum Reprod Open. 2018;2018 doi: 10.1093/hropen/hoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf A.M., Hunter D.J., Colditz G.A., Manson J.E., Stampfer M.J., Corsano K.A., et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 24.Mínguez-Alarcón L., Williams P.L., Chiu Y.H., Gaskins A.J., Nassan F.L., Dadd R., et al. Secular trends in semen parameters among men attending a fertility center between 2000 and 2017: Identifying potential predictors. Environ Int. 2018;121:1297–1303. doi: 10.1016/j.envint.2018.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruger T.F., Acosta A.A., Simmons K.F., Swanson R.J., Matta J.F., Oehninger S. Reprint of: predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 2019;112:e61–e66. doi: 10.1016/j.fertnstert.2019.08.074. [DOI] [PubMed] [Google Scholar]
- 26.Yuan C., Spiegelman D., Rimm E.B., Rosner B.A., Stampfer M.J., Barnett J.B., et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185:570–584. doi: 10.1093/aje/kww104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan C., Spiegelman D., Rimm E.B., Rosner B.A., Stampfer M.J., Barnett J.B., et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187:1051–1063. doi: 10.1093/aje/kwx328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu F.B., Rimm E.B., Stampfer M.J., Ascherio A., Spiegelman D., Willett W.C. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–921. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann K., Schulze M.B., Schienkiewitz A., Nöthlings U., Boeing H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol. 2004;159:935–944. doi: 10.1093/aje/kwh134. [DOI] [PubMed] [Google Scholar]
- 30.Tabung F.K., Liu L., Wang W., Fung T.T., Wu K., Smith-Warner S.A., et al. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol. 2018;4:366–373. doi: 10.1001/jamaoncol.2017.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabung F.K., Smith-Warner S.A., Chavarro J.E., Wu K., Fuchs C.S., Hu F.B., et al. Development and validation of an empirical dietary inflammatory index. J Nutr. 2016;146:1560–1570. doi: 10.3945/jn.115.228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Searle S.R., Speed F.M., Milliken G.A. Population marginal means in the linear model: an alternative to least squares means. Am Statistician. 1980;34:216–221. [Google Scholar]
- 33.Salas-Huetos A., Babio N., Carrell D.T., Bulló M., Salas-Salvadó J. Adherence to the Mediterranean diet is positively associated with sperm motility: a cross-sectional analysis. Sci Rep. 2019;9:3389. doi: 10.1038/s41598-019-39826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cutillas-Tolín A., Mínguez-Alarcón L., Mendiola J., López-Espín J.J., Jørgensen N., Navarrete-Muñoz E.M., et al. Mediterranean and western dietary patterns are related to markers of testicular function among healthy men. Hum Reprod. 2015;30:2945–2955. doi: 10.1093/humrep/dev236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karayiannis D., Kontogianni M.D., Mendorou C., Mastrominas M., Yiannakouris N. Adherence to the Mediterranean diet and IVF success rate among non-obese women attempting fertility. Hum Reprod. 2018;33:494–502. doi: 10.1093/humrep/dey003. [DOI] [PubMed] [Google Scholar]
- 36.Montano L., Ceretti E., Donato F., Bergamo P., Zani C., Viola G.C.V., et al. Effects of a lifestyle change intervention on semen quality in healthy young men living in highly polluted areas in Italy: the FASt randomized controlled trial. Eur Urol Focus. 2021 doi: 10.1016/j.euf.2021.01.017. S2405-4569(21)00041-00049. [DOI] [PubMed] [Google Scholar]
- 37.Jensen T.K., Priskorn L., Holmboe S.A., Nassan F.L., Andersson A.M., Dalgård C., et al. Associations of fish oil supplement use with testicular function in young men. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2019.19462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Safarinejad M.R. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia. 2011;43:38–47. doi: 10.1111/j.1439-0272.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 39.Chiu Y.H., Afeiche M.C., Gaskins A.J., Williams P.L., Mendiola J., Jørgensen N., et al. Sugar-sweetened beverage intake in relation to semen quality and reproductive hormone levels in young men. Hum Reprod. 2014;29:1575–1584. doi: 10.1093/humrep/deu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nassan FL, Prikson L, Salas-Huetos A, Halldorsson TI, Jensen TK, Jørgensen N, et al. Association between intake of soft drinks and testicular function in young men. Hum Reprod. In press. [DOI] [PMC free article] [PubMed]
- 41.Hatch E.E., Wesselink A.K., Hahn K.A., Michiel J.J., Mikkelsen E.M., Sorensen H.T., et al. Intake of sugar-sweetened beverages and fecundability in a North American preconception cohort. Epidemiology. 2018;29:369–378. doi: 10.1097/EDE.0000000000000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afeiche M.C., Gaskins A.J., Williams P.L., Toth T.L., Wright D.L., Tanrikut C., et al. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr. 2014;144:1091–1098. doi: 10.3945/jn.113.190173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Afeiche M.C., Bridges N.D., Williams P.L., Gaskins A.J., Tanrikut C., Petrozza J.C., et al. Dairy intake and semen quality among men attending a fertility clinic. Fertil Steril. 2014;101:1280–1287.e2. doi: 10.1016/j.fertnstert.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zareba P., Colaci D.S., Afeiche M., Gaskins A.J., Jørgensen N., Mendiola J., et al. Semen quality in relation to antioxidant intake in a healthy male population. Fertil Steril. 2013;100:1572–1579. doi: 10.1016/j.fertnstert.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M.C., Chiu Y.H., Gaskins A.J., Mínguez-Alarcón L., Nassan F.L., Williams P.L., et al. Men’s intake of vitamin C and β-carotene is positively related to fertilization rate but not to live birth rate in couples undergoing infertility treatment. J Nutr. 2019;149:1977–1984. doi: 10.1093/jn/nxz149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karmon A.E., Toth T.L., Chiu Y.H., Gaskins A.J., Tanrikut C., Wright D.L., et al. Male caffeine and alcohol intake in relation to semen parameters and in vitro fertilization outcomes among fertility patients. Andrology. 2017;5:354–361. doi: 10.1111/andr.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cissen M., Wely M.V., Scholten I., Mansell S., Bruin J.P., Mol B.W., et al. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al Omrani B., Al Eisa N., Javed M., Al Ghedan M., Al Matrafi H., Al Sufyan H. Associations of sperm DNA fragmentation with lifestyle factors and semen parameters of Saudi men and its impact on ICSI outcome. Reprod Biol Endocrinol. 2018;16:49. doi: 10.1186/s12958-018-0369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L., Fang J., Jiang W., Wang J., Li D. Effects of the sperm DNA fragmentation index on the clinical and neonatal outcomes of intracytoplasmic sperm injection cycles. J Ovarian Res. 2020;13:52. doi: 10.1186/s13048-020-00658-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salas-Huetos A., Blanco J., Vidal F., Godo A., Grossmann M., Pons M.C., et al. Spermatozoa from patients with seminal alterations exhibit a differential micro-ribonucleic acid profile. Fertil Steril. 2015;104:591–601. doi: 10.1016/j.fertnstert.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 51.Batruch I., Smith C.R., Mullen B.J., Grober E., Lo K.C., Diamandis E.P., et al. Analysis of seminal plasma from patients with non-obstructive azoospermia and identification of candidate biomarkers of male infertility. J Proteome Res. 2012;11:1503–1511. doi: 10.1021/pr200812p. [DOI] [PubMed] [Google Scholar]
- 52.Pilch B., Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart showing participant flow and exclusions for analyses of diet with semen quality and outcomes of infertility treatment with assisted reproductive technology.
Supplemental Figure 2. Men’s empirical dietary score in relation to fertilization rate, overall (A) and in in vitro fertilization (B) or intracytoplasmic sperm injection (ICSI) (C) cycles. Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, ICSI (yes/no), primary infertility diagnosis, and stimulation protocol. Stratified models (B and C) do not include a term for ICSI. Q = quartile.
Supplemental Figure 3. Men’s empirical dietary score in relation to fertilization rate, overall (A) and in in vitro fertilization (B) and intracytoplasmic sperm injection (ICSI) (C) cycles, excluding couples with a primary diagnosis of male factor infertility. Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, ICSI (yes/no), primary infertility diagnosis, and stimulation protocol. Stratified models (B and C) do not include a term for ICSI. Q = quartile.
Supplemental Figure 4. Men’s empirical dietary score in relation to clinical outcomes of infertility treatment with assisted reproductive technology, excluding couples with a primary diagnosis of male factor infertility (N = 146 couples, 272 cycles). Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, primary infertility diagnosis, and stimulation protocol. Q = quartile.
Supplemental Figure 5. Men’s empirical dietary score in relation to clinical outcomes of infertility treatment with assisted reproductive technology, excluding intracytoplasmic sperm injection cycles (N = 92 couples, 124 cycles).
Supplemental Figure 6. Factor loadings for reduced rank regression with food groups as the predictive variables and semen parameters as the response variables, without adjustment for abstinence time. Gray bar: positive factor loading food groups. White bar: negative factor loading food groups.
Supplemental Figure 7. Men’s empirical dietary score (without adjustment for abstinence time) in relation to fertilization rate, overall (A) and in in vitro fertilization (B) or intracytoplasmic sperm injection (ICSI) (C) cycles. Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, ICSI (yes/no), primary infertility diagnosis, and stimulation protocol. Stratified models (B and C) do not include a term for ICSI. Q = quartile.
Supplemental Figure 8. Men’s empirical dietary score (without adjustment for abstinence time) in relation to clinical outcomes of infertility treatment with assisted reproductive technology (N = 231 couples, 406 cycles). Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, primary infertility diagnosis, and stimulation protocol. Q = quartile.
Supplemental Figure 9. Men’s empirical dietary in relation to fertilization rate, overall (A) and in in vitro fertilization (B) or intracytoplasmic sperm injection (ICSI) (C) cycles, excluding couples whose male factor had azoospermia. Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, ICSI (yes/no), primary infertility diagnosis, and stimulation protocol. Stratified models (B and C) do not include a term for ICSI. Q = quartile.
Supplemental Figure 10. Men’s empirical dietary score in relation to clinical outcomes of infertility treatment with assisted reproductive technology, excluding couples whose male factor had azoospermia (N = 224 couples, 393 cycles). Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, primary infertility diagnosis, and stimulation protocol. Q = quartile.
Supplemental Figure 11. Men’s empirical dietary in relation to fertilization rate, overall (A) and in in vitro fertilization (B) or intracytoplasmic sperm injection (ICSI) (C) cycles, excluding couples whose male partners were not included in the reduced rank regression analysis for the dietary score. Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, ICSI (yes/no), primary infertility diagnosis, and stimulation protocol. Stratified models (B and C) do not include a term for ICSI. Q = quartile.
Supplemental Figure 12. Men’s empirical dietary score in relation to clinical outcomes of infertility treatment with assisted reproductive technology, excluding couples whose male partners were not included in the reduced rank regression analysis for the dietary score (N = 202 couples, 361 cycles). Adjusted for men’s age, women’s age, men’s total calorie intake, total exercise, primary infertility diagnosis, and stimulation protocol. Q = quartile.