Abstract
Purpose
Stereotactic body radiation therapy (SBRT) treatment planning for renal cell carcinoma requires accurate delineation of tumor from normal tissue due to the radiosensitivity of normal renal cortical tissue. Tc-99m dimercapto succinic acid (DMSA) renal imaging is a functional imaging technique that precisely differentiates normal renal cortical tissue from tumor. There are no prior publications reporting using this imaging modality for SBRT treatment planning.
Methods and Materials
A 59-year-old female with stage IV renal cell carcinoma progressed on systemic therapy and was dispositioned to primary cytoreduction with SBRT. She had baseline renal dysfunction and her tumor was 9 cm without clear delineation from normal tissue on conventional imaging. DMSA-single-photon emission computerized tomography (SPECT)/computed tomography (CT) was used for treatment planning.
Results
DMSA-SPECT/CT precisely delineated normal renal cortical tissue from tumor. Three months after treatment, labs were stable and DMSA-SPECT/CT was unchanged. The treated lesion had markedly decreased positron emission tomography avidity.
Conclusions
DMSA-SPECT or SPECT/CT can be incorporated into radiation therapy planning for renal lesions to improve target delineation and better preserve renal function.
Introduction
The standard of care for locoregional management of renal cell carcinoma (RCC) is partial or total nephrectomy.1 However, surgery is sometimes not feasible. For these tumors, alternative treatment approaches include cryotherapy, radiofrequency ablation, and radiation therapy.1 Cryotherapy and radiofrequency ablation are limited by both tumor size and location. In contrast, radiation therapy can be safely delivered for large tumors irrespective of location.2,3
Stereotactic body radiation therapy (SBRT) treatment planning for RCC involves fusion of diagnostic imaging to the noncontrast computed tomography (CT) obtained at the time of simulation.4,5 Diagnostic imaging modalities may include CT with or without contrast, magnetic resonance imaging (MRI) with or without contrast, and positron emission tomography (PET)-CT.5 Of note, PET-CT has reduced utility in these cases because normal renal tissue is PET-avid due to radiotracer excretion.6 As such, RCC can sometimes be difficult to differentiate from normal renal tissue under certain clinical scenarios.
We encountered a patient with baseline renal dysfunction who had received prior systemic therapy and had a residual large invasive primary RCC that could not be clearly delineated from normal renal tissue on conventional imaging. Due to the importance of sparing as much normal renal cortex as possible in this patient, we developed a treatment planning approach that incorporated dimercapto succinic acid (DMSA) imaging to differentiate the normal renal cortical tissue from the tumor. This is the first publication to report using DMSA-single-photon emission computerized tomography (SPECT)/CT for RCC radiation therapy treatment planning.
Case Report
The patient presented to the emergency room as a 59-year-old female with lightheadedness and increased shortness of breath. She had a past medical history of coronary artery disease, type 2 diabetes mellitus, hyperlipidemia, hypertension, and obstructive sleep apnea. At presentation, her oxygen saturation was 83%. CT scan showed an 8.7-cm renal lesion consistent with RCC, mediastinal lymphadenopathy up to 1.4 cm, and numerous pulmonary nodules up to 6 mm. MRI of the abdomen demonstrated invasion of the renal lesion into the renal vein and inferior vena cava. Endobronchial ultrasound guided biopsy of a right pulmonary lesion was consistent with metastatic RCC. She was staged as cT3b cN0 cM1, American Joint Committee on Cancer stage IV.
The patient began systemic therapy with pembrolizumab and axitinib. After 9 cycles of therapy, the renal lesion was 4.4 cm in size on CT scan and there were no visible pulmonary metastases. At that time, axitinib was stopped due to mouth sores. After an additional 3 cycles of immunotherapy, CT scan showed the renal mass had grown to 5.5 cm. Fluorodeoxyglucose PET-CT scan suggested a single site of metastasis at the left shoulder 1.7 cm in size with standard uptake value of 19.6. Immunotherapy was held due to increased liver function tests.
Her case was presented at tumor board with consensus recommendation for cytoreduction of the primary while on systemic therapy holiday, followed by resumption of systemic therapy once liver function tests returned to baseline. She was determined to be a poor surgical candidate due to her cardiovascular and pulmonary comorbidities. Therefore, SBRT was proposed. Because her PET-CT could not clearly differentiate the renal lesion from the normal functioning kidney and her creatinine was 1.2 mg/dL at this time (but as high as 2.2 mg/dL 4 months prior while on systemic therapy), DMSA-SPECT/CT was ordered to better delineate the malignancy so that dose to the normal right renal cortical tissue could be minimized. Although planar DMSA imaging is routine, SPECT/CT imaging was performed due to the need for tomographic images and ease of image fusion. Imaging was performed 2 hours after the intravenous injection of 5 mCi (185 MBq) of Tc-99m DMSA. Images were obtained on a Siemens Symbia T6 gamma camera with SPECT/CT capability.
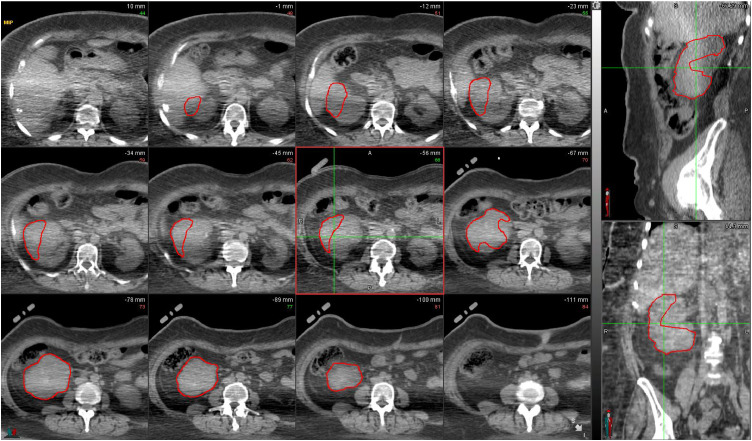
Our center does not use breathhold, so the patient underwent a 4-dimensional (4D)–CT simulation with an abdominal compression belt. The lesion had grown to 9 cm in largest diameter at the time of simulation. The PET-CT and DMSA-SPECT/CT were fused to the 4D-CT average sequence using MIM Maestro Version 6.6.14 (MIM Sofware Inc, Cleveland, OH). To reduce the effect of distortions caused by the compression belt, region of interest coregistration was used. Other anatomic references such as bony anatomy further aided in the coregistration. An internal target volume (ITV) was contoured based on the 4D-CT (Fig 1), with PET-CT overlaid to distinguish viable tumor (Fig 2A) and DMSA-SPECT/CT overlaid to distinguish normal renal cortical tissue (Fig 3A). The contoured target was reviewed with nuclear medicine colleagues to ensure accuracy. There was a portion of the mass that was neither PET-avid nor demonstrating normal activity on the DMSA images. Review of prior imaging suggested this region had responded to systemic therapy and now represented nonviable tumor. However, this region was still included in the target out of precaution because there could be microscopic viable cells. A 5-mm planning target volume (PTV) expansion was placed on the ITV. Because the CT simulation used an abdominal compression belt while the PET-CT and DMSA-SPECT/CT did not, the PTV margin helped manage uncertainties related to coregistering images generated with and without abdominal compression. The Eclipse treatment planning system was used (Varian Medical Systems, Palo Alto, CA).
Figure 1.
Maximum intensity projection (MIP) images from 4-dimensional computed tomography (4D-CT) scan. The 12 panes on the left are axial cross-sections, while the upper right pane is a sagittal cross-section and the lower right pane is a coronal cross-section. Internal target volume (ITV) of right renal tumor contoured in red.
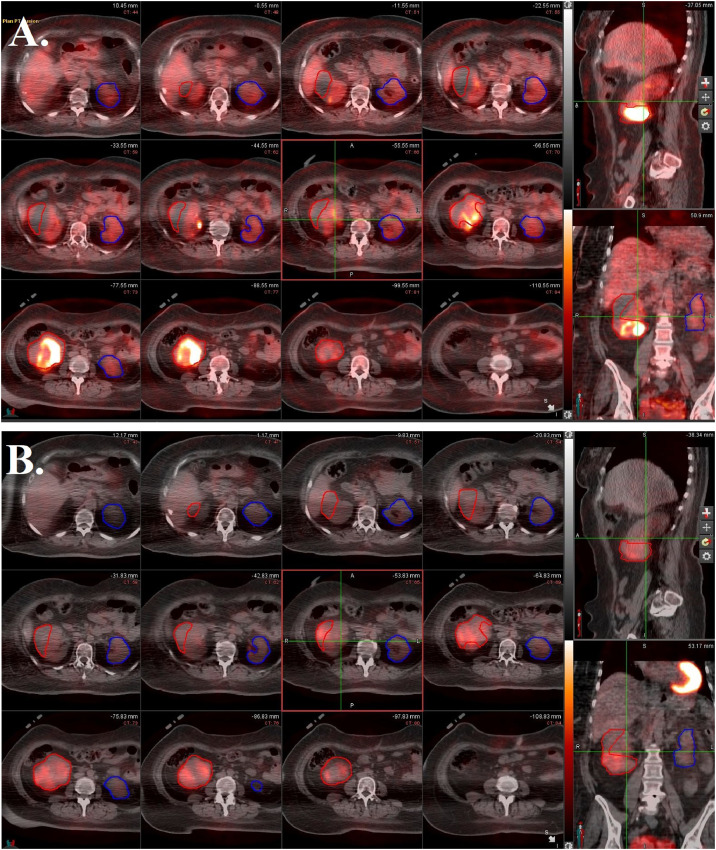
Figure 2.
Positron emission tomography (PET) images fused with computed tomography (CT) average images from 4-dimensional (4D)-CT scan. The 12 panes on the left are axial cross-sections, while the upper right pane is a sagittal cross-section and the lower right pane is a coronal cross-section. (A) Before stereotactic body radiation therapy (SBRT). Internal target volume (ITV) of right renal tumor (red) has high avidity, but normal right renal tissue has mild avidity, preventing clear delineation. Left kidney (blue) has mild diffuse avidity. (B) Three months after SBRT. The avidity of treated tumor (red) has markedly decreased.
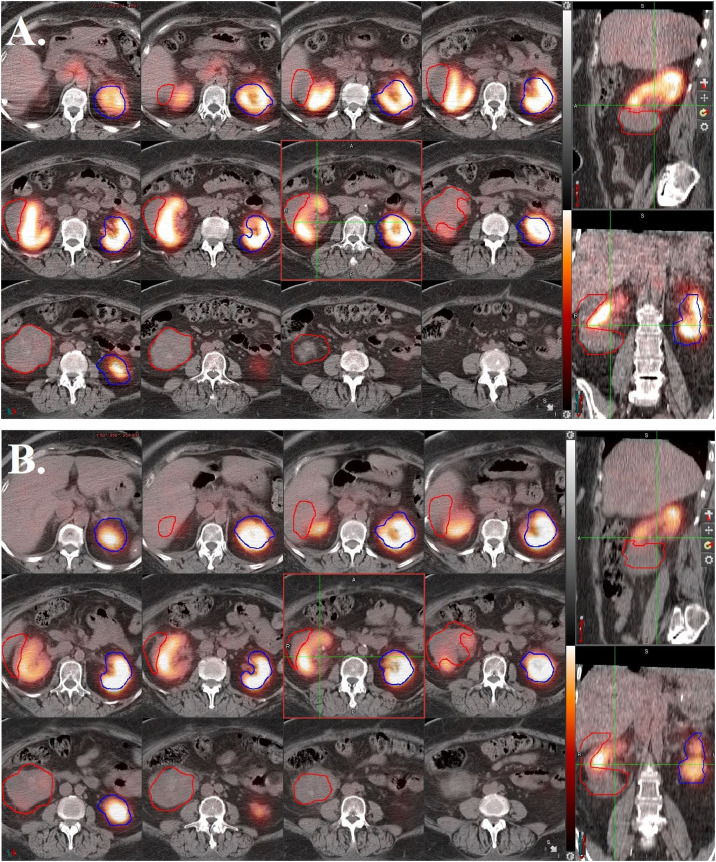
Figure 3.
Dimercapto succinic acid (DMSA)- single-photon emission computerized tomography (SPECT)/computed tomography (CT) images with projection of right renal tumor internal target volume (ITV) (red) and left kidney (blue). The 12 panes on the left are axial cross-sections, while the upper right pane is a sagittal cross-section and the lower right pane is a coronal cross-section. Normal renal cortical tissue demonstrates tracer uptake, while ITV demonstrates absolutely no uptake. (A) Before stereotactic body radiation therapy (SBRT). (B) Three months after SBRT with no significant changes noted.
The patient was treated on a Varian Halcyon LINAC using 6 MV photons and volumetric-modulated arc therapy technique with 4 full arcs. The PTV was prescribed 40 Gy in 5 fractions, delivered every other day over the course of 10 days. Over 97% of the target volume received at least 100% of the dose and 50% of the target volume received 45 Gy. The left kidney, which was 226 cm3, received a max dose of 10 Gy and a mean of 4 Gy. The duodenum had a max dose of 30 Gy, and the large bowel had a max dose of 36.5 Gy. The patient experienced no acute toxicity and resumed immunotherapy 1 month later. At 3 months follow-up, labs were stable, the treated lesion had markedly decreased PET-avidity (Fig 2B), and normal renal function on DMSA-SPECT/CT was unchanged (Fig 3B).
Discussion
The kidneys are the dose-limiting organ for abdominal radiation therapy.7 Emami et al8 estimated a 5% risk of clinical nephritis when 23 Gy is delivered to the entire renal tissue via conventional fractionation. This risk jumps to 50% when 28 Gy is administered. Because the functional unit of the kidney is the nephron, which operates in a parallel fashion, in contemporary literature the volume of kidneys spared from irradiation is considered a better dosimetric measure of normal tissue tolerance.9 Current guidelines stipulate that for 1 and 5 fraction SBRT, at least 200 cc of renal cortical tissue must receive ≤8.4 Gy and ≤17.5 Gy, respectively.9 More recently, functional imaging after SBRT has confirmed radiation nephritis to be dose dependent, with renal function preserved in areas that receive <13 Gy in 1 fraction.10,11
There are no well-defined constraints that take into account baseline renal function or prior renal morbidity. Our patient had an elevated creatinine of 1.2 mg/dL at the time of radiation therapy, but this value had been as high as 2.2 mg/dL 4 months prior while on systemic therapy. Thus, it was necessary to consider novel techniques to spare as much normal renal cortical tissue as possible.
Functional imaging such as PET-CT is commonplace in radiation treatment planning. Because most tumors are PET-avid compared with the normal surrounding tissue, delineating the tumor is generally straightforward. However, this is not the case for RCC because the kidneys are responsible for excreting the radionuclide used in PET-CT.
Renal DMSA imaging is a functional imaging modality using the radionuclide technetium-99m DMSA. It was first described in 1974 as a novel method for renal cortical imaging.12 The molecule binds to intact proximal convoluted tubules of nephrons. Although most often used to distinguish renal scarring from normal tissue, in our case, it was used to distinguish tumor from normal renal tissue. Imaging is routinely performed with planar images only, but SPECT/CT imaging was chosen for ease of image fusion and from the need for tomographic images. Although other radiotracer studies such as PET-CT are less accurate for border delineation, DMSA is an excellent modality for border delineation when combined with SPECT/CT and displayed with normal image intensity. Because DMSA imaging was not done during the time of the CT simulation scan, there exists uncertainties regarding registration with the CT simulation scan.
For this patient, PET-CT suggested a region of viable tumor and a region of nonviable tumor. Although this nonviable tumor could represent a response to prior immunotherapy and/or targeted therapy, the meaning of 18F-fluorodeoxyglucose suppression with immunotherapy has not been investigated for RCC and is not fully understood for other malignancies.13 For this reason, our ITV included the region that may represent nonviable tumor.
DMSA imaging, on the other hand, was useful for differentiating normal renal cortical tissue from tumor. Therefore, having both functional imaging tests enables clear elucidation of viable tumor. Owing to the simplicity of the study and the added value in treatment planning, we advocate for widespread use of DMSA-SPECT or SPECT/CT imaging in the target delineation of RCC. PET-CT should not be discarded because it retains value in potentially distinguishing viable from nonviable tumor.
Of note, there are alternative imaging modalities currently being explored for RCC SBRT. The MRI-Guided Radiation Therapy for the Treatment of Early-Stage Kidney Cancer (MRI-MARK Trial [NCT04580836]) at MD Anderson Cancer Center is investigating MRI-guided radiation therapy using an MRI linear accelerator to treat early-stage kidney cancer.14 Although MRI-guided treatment certainly provides value in minimizing set-up error and facilitating adaptive radiation therapy, DMSA-SPECT retains value in treatment planning because it is uniquely capable of differentiating normal renal cortical tissue from tumor.
Conclusions
DMSA renal imaging is a functional imaging technique that precisely differentiates normal renal cortical tissue from tumor. Because PET-CT is inept in this regard, DMSA-SPECT or SPECT/CT can be incorporated into radiation therapy planning for renal lesions to improve target delineation and better preserve renal function.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Restricted access (legal/ethical): Because this is a case report with a single patient, access to data cannot be provided outside the treating institution as it would violate patient privacy laws in the United States.
References
- 1.Motzer RJ, Jonasch E, Agarwal N, et al. NCCN guidelines kidney cancer version 1.2021. Available at: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed November 27, 2020. [DOI] [PMC free article] [PubMed]
- 2.Correa RJM, Louie AV, Staehler M, et al. Stereotactic radiotherapy as a treatment option for renal tumors in the solitary kidney: A multicenter analysis from the IROCK. J Urol. 2019;201:1097–1104. doi: 10.1097/JU.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 3.Siva S, Correa RJM, Warner A, et al. Stereotactic ablative radiotherapy for ≥T1b Primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK) Int J Radiat Oncol Biol Phys. 2020;108:941–949. doi: 10.1016/j.ijrobp.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Teh BS, Bloch C, Galli-Guevara M, et al. The treatment of primary and metastatic renal cell carcinoma (RCC) with image-guided stereotactic body radiation therapy (SBRT) Biomed Imaging Interv J. 2007;3:e6. doi: 10.2349/biij.3.1.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siva S, Ellis RJ, Ponsky L, et al. Consensus statement from the International Radiosurgery Oncology Consortium for Kidney for primary renal cell carcinoma. Future Oncol. 2016;12:637–645. doi: 10.2217/fon.16.2. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y. The place of FDG PET/CT in renal cell carcinoma: Value and limitations. Front Oncol. 2016;6:201. doi: 10.3389/fonc.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson LA, Kavanagh BD, Paulino AC, et al. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S108–S115. doi: 10.1016/j.ijrobp.2009.02.089. [DOI] [PubMed] [Google Scholar]
- 8.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 9.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101 [published correction appears in Med Phys. 2012 Jan;39(1):563. Dosage error in article text] Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 10.Jackson P, Foroudi F, Pham D, et al. Short communication: timeline of radiation-induced kidney function loss after stereotactic ablative body radiotherapy of renal cell carcinoma as evaluated by serial (99m)Tc-DMSA SPECT/CT. Radiat Oncol. 2014;9:253. doi: 10.1186/s13014-014-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siva S, Jackson P, Kron T, et al. Impact of stereotactic radiotherapy on kidney function in primary renal cell carcinoma: Establishing a dose-response relationship. Radiother Oncol. 2016;118:540–546. doi: 10.1016/j.radonc.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Enlander D, Weber PM, dos Remedios LV. Renal cortical imaging in 35 patients: Superior quality with 99mTc-DMSA. J Nucl Med. 1974;15:743–749. [PubMed] [Google Scholar]
- 13.Tan AC, Emmett L, Lo S, et al. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann Oncol. 2018;29:2115–2120. doi: 10.1093/annonc/mdy330. [DOI] [PubMed] [Google Scholar]
- 14.ClinicalTrials.gov. National Library of Medicine (US). (2000, February 29-). MRI-guided radiation therapy for the treatment of early-stage kidney cancer, the MRI-MARK Trial. Identifier NCT04580836. Available at: https://www.clinicaltrials.gov/ct2/show/NCT04580836. Accessed February 26, 2021.