Abstract
Background:
Atopic dermatitis (AD) is a common chronic-relapsing inflammatory skin disease hallmarked by epidermal barrier dysfunction, increased transepidermal water loss (TEWL) and decreased skin hydration. Recent findings on the T helper 2 (Th2)-driven pathogenesis of AD have led to the development of dupilumab, a monoclonal antibody directed against interleukin-4 and interleukin-13 that has been demonstrated to be effective in the treatment of moderate-to-severe AD. The effect of dupilumab on skin barrier dysfunction, however, has not yet been adequately investigated.
Objectives:
The primary endpoint of this study was to assess the status of the skin barrier in nonlesional skin of patients with severe AD treated with dupilumab, by evaluating the association between the relative variation of TEWL and the achievement of a 75% reduction of EASI (EASI75) over time.
Methods:
TEWL was measured below the antecubital fossae by means of the Vapometer® at baseline, at week 4 (T4), at week 16 (T16) and at week 32 after dupilumab starting. EASI and NRS-itch were measured at the same time points.
Results:
Seventy-eight patients with severe AD treated with dupilumab were enrolled. Median TEWL relative variation respect to baseline was significantly higher in patients who achieved EASI75 as compared with those who did not achieve EASI75 at T16 and at T32, but not at T4.
Conclusion:
During dupilumab treatment, TEWL on nonlesional skin tends to significantly improve 4 months after treatment initiation and could be a good tool for monitoring response to therapy.
Keywords: atopic dermatitis, dupilumab, skin barrier, transepidermal water loss, type 2 inflammation
Introduction
Atopic dermatitis (AD) is a common, chronic-relapsing type 2–driven inflammatory skin disease clinically characterized by intense pruritus and recurrent eczematous lesions with a heterogeneous clinical presentation. 1
Patients with moderate-to-severe AD often report a deterioration of their quality of life: severe pruritus leads to sleep disturbance, work productivity impairment, anxiety and depression. Although AD pathogenesis is complex and multifactorial, it is well established that two pivotal factors involved in its pathogenesis are (1) epidermal barrier disruption leading to an increase in transepidermal water loss (TEWL), a parameter related to the stratum corneum integrity,2–5 and (2) immune dysregulation mainly consisting of T helper 2 (Th2) and T helper 22 (Th22) pathway upregulation resulting in overproduction of type 2 inflammation-related cytokines, such as interleukin (IL)-4, IL-13, IL-5, IL-31 and IL-22.6,7 In particular, skin barrier abnormalities play a crucial role in the initiation and aggravation of AD and influence the immune dysfunction. 8 Genetic variants of filaggrin, a structural protein representing the major component of the keratohyalin granules involved in the keratinization, moisturization and antimicrobial properties of the human skin, are found in 15–40% of AD patients 9 and decreased levels of filaggrin and filaggrin-like proteins (hornerin and filaggrin family member 2) are found in lesional and nonlesional skin of AD patient who do not harbour the abovementioned genetic variants.10,11 Ceramides, cholesterol and free fatty acids are the main constituents of the extracellular lipid matrix of the stratum corneum and are essential to ensure a correct barrier function. It has been described that ceramide ratio and ceramide/cholesterol ratio are reduced in AD skin12,13 and hyperactivity of kallikrein along with increased levels of interferon α produce structural changes in free fatty acid and ceramide chains through an augmented degradation of very long chain fatty acid proteins (ELOV). 14 Elevated serine proteinase activity is also related to a reduction of the stratum corneum thickness and function due to the inactivation of acid sphingomyelinase and β-glucocerebrosidase, key enzymes in ceramide synthesis, and lamellar body secretion from keratinocytes, impairing keratinization process. 15 Tight junctions between keratinocytes of the granular layer act as a selective barrier, controlling cellular permeability; decreased levels of the transmembrane protein claudin-1, a major component of tight junctions, are strongly associated with AD leading to barrier function impairment and increased inflammation. 16 Although the AD standard treatment is based on emollients, topical and/or systemic corticosteroids, phototherapy and immunosuppressants [e.g. cyclosporine A (CsA)], recent findings on AD pathogenesis have led to the development of more targeted therapies. In particular, dupilumab is a monoclonal antibody that has been recently approved for the treatment of moderate-to-severe AD resistant to both topical agents and oral cyclosporine.17–19 The proven clinical benefit of dupilumab on AD is regarded as depending on the dual blockade of the IL-4/IL-13 pathway. These cytokines are critical in the induction and perpetuation of the type 2 inflammation, but a possible ancillary effect of dupilumab on skin barrier integrity still needs to be investigated. Recent studies have shown that in patients treated with dupilumab, after 16 weeks of treatment the gene expression profile of lesional skin had shifted towards that of nonlesional skin, with an important reduction in the gene expression of the related Th2 cytokines. This phenomenon was accompanied not only by a reduction in the expression of the genes involved in the Th17/Th22 pathways but also by an upregulation in the expression of barrier components (e.g. filaggrin, loricrin, claudin and fatty acids) and significant reduction in epidermal hyperplasia. 20
The primary objective of this prospective study was to assess the skin barrier status of nonlesional skin in patients with severe AD treated with dupilumab and investigate the association of TEWL improvement in nonlesional skin and achievement of 75% reduction of Eczema Area and Severity Index (EASI 75).
Methods
Patients
We performed a prospective study on 78 patients with the primary endpoint of assessing the skin barrier status in patients with severe AD treated with dupilumab. Inclusion criteria were (1) age ⩾18 years, (2) severe disease defined by a baseline Eczema Area and Severity Index (EASI) ⩾24 and (3) inadequate response/intolerance to CsA or medical inadvisability of CsA treatment. Patients with any documented psychiatric comorbidity were excluded from the study. All patients were given self-administered subcutaneous dupilumab 300 mg every other week following a loading dose of 600 mg. Traditional immunosuppressive agents (e.g. CsA, azathioprine and methotrexate) were discontinued at least 4 weeks before dupilumab initiation in all patients, while systemic corticosteroids were maintained in a minority of patients, with progressive tapering and subsequent withdrawal within 2 weeks. Concomitant topical corticosteroids or calcineurin inhibitors were allowed except for the antecubital fossa of the right arm, where TEWL was measured.
The full protocol was approved by the institutional review board of the ethics committee of the principal investigator’s centre (Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; protocol no. 696_2021). All the participants enrolled in the study gave their written informed consent. The study was conducted between October 2020 and April 2021.
TEWL measurement and itch/disease severity assessment
TEWL (range = 0–300 g/m2/h) was measured using Vapometer® (Delfin Technologies Ltd., Finland) by placing the instrument on a nonlesional area immediately below the antecubital fossa of the right arm, according to the standardized procedure described by Hon et al. 21
Vapometer is a closed unventilated chamber system, validated by Nuutinen et al. 22 The reproducibility of Vapometer TEWL measures was assessed by Nuutinen et al. 22 and De Paepe et al. 23 A coefficient of variation equal to 8% was found in the initial work by Nuutinen et al., relative to the very body area that we studied (volar forearm). TEWL measures are highly dependent on room microenvironment. Measurement conditions were standardized as follows: 40–50% relative humidity and 20°C room temperature. All measures were performed by a trained, dedicated dermatologist (SF). According to current literature, a ‘normal’ TEWL range does exist. Akdeniz et al. 24 , however, reviewed the evidence on TEWL in healthy adults and found that the pooled TEWL estimate for proximal volar right forearm (corresponding to the area that we selected in this study) was 5.3 (4.3–6.3) g/m2/h.
The final TEWL value was calculated averaging out the values obtained through three subsequent measurements. After this procedure, we evaluated the extension and severity of the disease by means of EASI. Itch was quantified through a Numeric Rating Scale (NRS) ranging from 0 to 10. TEWL, NRS-itch and EASI were collected at baseline (T0), week 4 (T4), week 16 (T16) and week 32 (T32) after dupilumab initiation.
Statistical analysis
Categorical variables were reported as frequencies and percentages while continuous variables were reported as medians and interquartile range (IQR). Relative variation of TEWL from baseline (T0) to T4, T16 and T32 was calculated. The nonparametric Wilcoxon two-sample test was used to assess if relative variation of TEWL, from t0 to the three time points considered, was different in patients who achieved 75% reduction of EASI (EASI75) from t0 to each time point, compared with those who did not achieve EASI75 at the same time point.
P values less than .05, two-sided, were considered statistically significant. The statistical software SAS (release 9.4, SAS Institute, Inc., Cary, NC, USA) was used to perform all the statistical analyses.
Results
Seventy-eight patients (29 males and 49 females) were enrolled. Clinical features of patients included in the study are summarized in Table 1. Disease onset in adult age (>18 years) was observed in 17 (21.79%) patients. Median age at dupilumab initiation was 35 (IQR = 24.75–46.5) years. At baseline, median EASI was 28 (IQR = 24–30.25), median NRS-itch was 8 (IQR =8–10) and median TEWL was 14 (IQR = 8–20) g/m2/h (Figure 1). At baseline, only seven patients had a TEWL value within the normal range for the assessed area (<6.3 g/m2/h).
Table 1.
Clinical features of patients with atopic dermatitis included in the study.
| Median (IQR) | ||
|---|---|---|
| Disease onset in adult age a | 17 (21.79) | |
| Age at dupilumab initiation (years) | 35 (24.75–46.5) | |
| Males a | 18 (23.07) | |
| Median clinical parameters at baseline | EASI | 28 (24–30.25) |
| NRS-itch | 8 (8–10) | |
| TEWL | 14 (8–20) | |
| Median clinical parameters at T4 | EASI | 7 (3–11) |
| NRS-itch | 4 (3–5.25) | |
| TEWL | 13.8 (9–19) | |
| Median clinical parameters at T16 | EASI | 5 (2–7) |
| NRS-itch | 3 (2–5) | |
| TEWL | 10 (7–14.25) | |
| Median clinical parameters at T32 | EASI | 4 (1–6) |
| NRS-itch | 3 (1–5) | |
| TEWL | 9 (7–12) |
EASI, Eczema Area and Severity Index; IQR, interquartile range; NRS, Numeric Rating Scale; TEWL, transepidermal water loss.
Data are reported as n (%).
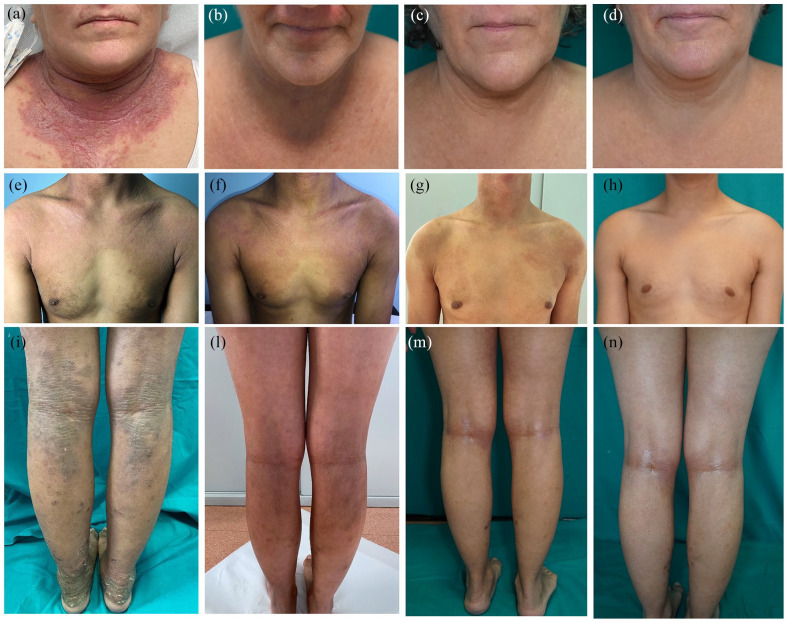
Figure 1.
Clinical images showing improvement of atopic dermatitis in dupilumab-treated patients included in the study. (a–d) Face and neck: baseline, T4, T16 and T32. (e–h) Chest: baseline, T4, T16 and T32. (i–n) Popliteal fossa: baseline, T4, T16 and T32.
EASI75 was achieved by 42 (53.85%), 57 (73.08%) and 63 (80.77%) patients at T4, T16 and T32, respectively. Median EASI reduction respect to baseline was of 21 (IQR = 18–25), 23 (IQR = 20–27) and 24 (IQR = 22–27) points at T4, T16 and T32, respectively. Median TEWL reduction respect to baseline was of 0 (IQR: −3.5 to −2), −3.7 (IQR: −8 to 0) and −5 (IQR: −11 to −0.6) points at T4, T16 and T32, respectively. Of the 71 patients with baseline TEWL values above the mentioned range (>6.3 g/m2/h), 6 (8.5%), 9 (12.7%) and 11 (15.5%) patients achieved a normal TEWL at T4, T16 and T32, respectively.
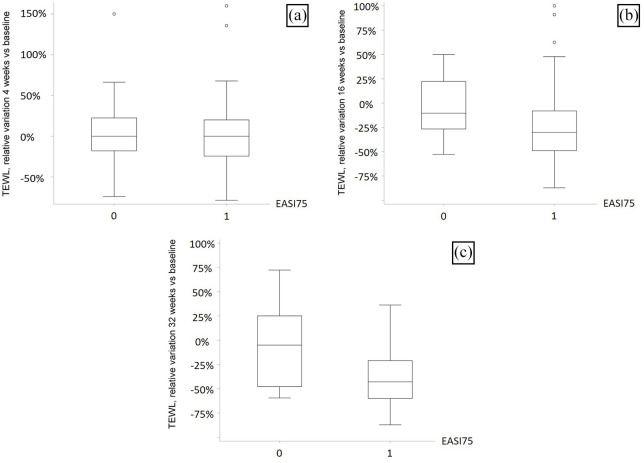
A median 0% reduction of TEWL was documented in both the 53.85% patients achieving EASI75 at T4 and those who did not achieve EASI75 at the same timepoint.
A median 30% reduction of TEWL was documented in the 73.08% patients achieving EASI75 at T16, while only a 11% median reduction was noted in those who did not achieve EASI75 at the same timepoint (p = .0209).
A median 43% reduction of TEWL was documented in the 80.77% patients achieving EASI75 at T32, while only a 5% median reduction was noted in those that did not achieve EASI75 at the same timepoint (p = .0088) (Figure 2).
Figure 2.
Box plots for TEWL relative variation in EASI75 achievers versus non-EASI75 achievers, at (a) 4, (b) 16 and (c) 32 weeks.
Discussion
Successful management of AD relies on restoring barrier function and decreasing local and systemic inflammation. In the daily practice, topical application of emollients is the cornerstone barrier repair therapy. 25 Ceramide-dominant emollients, which can help restore lipid barrier abnormalities, have been shown to improve TEWL values and upregulate antimicrobial peptides in the skin of atopic patients with similar efficacy compared with the topical calcineurin inhibitor tacrolimus. 26 Moreover, the reduction in TEWL has been demonstrated to parallel improvement of the severity scoring SCORAD (Scoring Atopic Dermatitis) together with the restoration of stratum corneum in a children population. 27 Other moisturizers containing α-hydroxyacid, pseudo-ceramides, urea and hyaluronic acid have been demonstrated to be effective in ameliorating skin xerosis, itch and barrier permeability representing a safe and efficacy adjuvant therapeutic option to restore the barrier and sparring the use of topical corticosteroids, calcineurin inhibitors or both. 25 Currently, there are no data available investigating the role of systemic therapy on barrier function.
If, on the one hand, it is well acknowledged that skin barrier abnormalities, mainly resulting from decreased levels of filaggrin and ceramides, are major contributors in the pathogenesis of AD, on the other data on the status of skin barrier in patients with AD treated with dupilumab are limited. A study similar to ours has already been performed in a cohort of 30 AD patients treated with dupilumab, showing that there was an inverse proportional correlation between TEWL and disease severity after 8 weeks of dupilumab treatment. 28 Moreover, Rohner et al. 29 demonstrated in 10 AD patients that dupilumab led to a significant increased expression of epithelial barrier proteins [filaggrin, lymphoepithelial-Kazal-type-related inhibitor (LEKTI), human β-defensin-3 and cathelicidin LL-37]. Similar conclusions were drawn by Guttman-Yassky et al., 20 who found increased expression of the epidermal differentiation, barrier and lipid metabolism genes filaggrin, loricrin, claudins and ELOVL3. These results support that dupilumab therapy has the potential, over time, to change the history of this disease.
Our results confirmed the abovementioned findings and showed that dupilumab-induced EASI reduction is associated with improvement of skin barrier integrity measured through TEWL after the first 4 months from treatment initiation. It may be hypothesized that the reduction of skin inflammation related to dupilumab rapidly determines a reduction in EASI, while restoration of the barrier secondary to the anti-inflammatory action of dupilumab takes a longer period of time. More experimental evidence, however, is needed to unravel the mechanisms underlying skin barrier changes during dupilumab therapy.
The main limitation of this study is the small sample size, which was mainly due to its single-centre design. Selection bias due to the fact that the study included only patients with severe AD might be considered another limitation. Furthermore, even if we did our best to minimize any source of variation, emotional sweating, ambient relative humidity and skin surface temperature could have represented difficult-to-control environmental factors possibly influencing TEWL measurements.
Conclusion
In conclusion, our study demonstrates that TEWL, and thus skin barrier function in nonlesional skin, tends to significantly improve 4 months after treatment initiation paralleling disease severity amelioration measured by EASI.
Footnotes
Author contributions: SF, GG and AVM participated in generating original ideas and in study design. FG, ST, LA, GC and MR participated in conception and design or analysis and interpretation of data, or both. FG, ST and LA participated in revising the manuscript critically for important intellectual content. GC participated in data acquirement and statistics performing. All the authors approved the final version of the manuscript.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Conflict of interest: The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: SF has been principal investigator in clinical trials by AbbVie, Sanofi-Genzyme and Lilly and has served on advisory board and received honoraria for lectures from Novartis, Menarini and Almirall. The other authors reported no conflicts of interests.
ORCID iD: Angelo Valerio Marzano  https://orcid.org/0000-0002-8160-4169
https://orcid.org/0000-0002-8160-4169
Contributor Information
Silvia Ferrucci, Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy.
Maurizio Romagnuolo, Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy.
Carlo Alberto Maronese, Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy.
Francesca Germiniasi, Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy.
Simona Tavecchio, Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy.
Luisa Angileri, Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy.
Giovanni Casazza, Dipartimento di Scienze Biomediche e Cliniche ‘L. Sacco’, Università degli Studi di Milano, Milan, Italy.
Angelo Valerio Marzano, Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Via Pace 9, 20122 Milan, Italy. Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy.
Giovanni Genovese, Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy.
References
- 1. Jung T, Stingl G. Atopic dermatitis: therapeutic concepts evolving from new pathophysiologic insights. J Allergy Clin Immunol 2008; 122: 1074–1081. [DOI] [PubMed] [Google Scholar]
- 2. Holm EA, Wulf HC, Thomassen L, et al. Instrumental assessment of atopic eczema: validation of transepidermal water loss, stratum corneum hydration, erythema, scaling, and edema. J Am Acad Dermatol 2006; 55: 772–780. [DOI] [PubMed] [Google Scholar]
- 3. Seidenari S, Giusti G. Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm Venereol 1995; 75: 429–433. [DOI] [PubMed] [Google Scholar]
- 4. Tagami H, Yoshikuni K. Interrelationship between water barrier and reservoir functions of pathologic stratum corneum. Arch Dermatol 1985; 121: 642–645. [PubMed] [Google Scholar]
- 5. Kim DW, Park JY, Na GY, et al. Correlation of clinical features and skin barrier function in adolescent and adult patients with atopic dermatitis. Int J Dermatol 2006; 45: 698–701. [DOI] [PubMed] [Google Scholar]
- 6. Leung DYM, Berdyshev E, Goleva E. Cutaneous barrier dysfunction in allergic diseases. J Allergy Clin Immunol 2020; 145: 1485–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Langan SM, Irvine AD, Weidinger S. Atopic dermatitis [Erratum in: Lancet 2020; 396: 758]. Lancet 2020; 396: 345–360. [DOI] [PubMed] [Google Scholar]
- 8. Yang G, Seok JK, Kang HC, et al. Skin barrier abnormalities and immune dysfunction in atopic dermatitis. Int J Mol Sci 2020; 21: 2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006; 38: 441–446. [DOI] [PubMed] [Google Scholar]
- 10. Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol 2014; 134: 792–799. [DOI] [PubMed] [Google Scholar]
- 11. Pellerin L, Henry J, Hsu CY, et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol 2013; 131: 1094–1102. [DOI] [PubMed] [Google Scholar]
- 12. Di Nardo A, Wertz P, Giannetti A, et al. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol 1998; 78: 27–30. [DOI] [PubMed] [Google Scholar]
- 13. Jungersted JM, Scheer H, Mempel M, et al. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy 2010; 65: 911–918. [DOI] [PubMed] [Google Scholar]
- 14. Elias PM, Wakefield JS. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol 2014; 134: 781–791.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolf R, Wolf D. Abnormal epidermal barrier in the pathogenesis of atopic dermatitis. Clin Dermatol 2012; 30: 329–334. [DOI] [PubMed] [Google Scholar]
- 16. Bergmann S, von Buenau B, Vidal-Y-Sy S, et al. Claudin-1 decrease impacts epidermal barrier function in atopic dermatitis lesions dose-dependently. Sci Rep 2020; 10: 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seegraber M, Srour J, Walter A, et al. Dupilumab for treatment of atopic dermatitis. Expert Rev Clin Pharmacol 2018; 2: 467–474. [DOI] [PubMed] [Google Scholar]
- 18. De Bruin-Weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroids in adult patients with AD who are not adequately controlled with or are intolerant to ciclosporin A, or when this treatment is medically inadvisable: a placebo-controlled, randomized phase 3 clinical trial (LIBERTY AD CAFE). Br J Dermatol 2018; 178: 1083–1101. [DOI] [PubMed] [Google Scholar]
- 19. Ferrucci S, Casazza G, Angileri L, et al. Clinical response and quality of life in patients with severe atopic dermatitis treated with dupilumab: a single-center real-life experience. J Clin Med 2020; 9: 791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guttman-Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol 2019; 143: 155–172. [DOI] [PubMed] [Google Scholar]
- 21. Hon KL, Wong KY, Leung TF, et al. Comparison of skin hydration evaluation sites and correlations among skin hydration, transepidermal water loss, SCORAD Index, Nottingham Eczema Severity Score, and quality of life in patients with atopic dermatitis. Am J Clin Dermatol 2008; 9: 45–50. [DOI] [PubMed] [Google Scholar]
- 22. Nuutinen J, Alanen E, Autio P, et al. A closed unventilated chamber for the measurement of transepidermal water loss. Skin Res Technol 2003; 9: 85–89. [DOI] [PubMed] [Google Scholar]
- 23. De Paepe K, Houben E, Adam R, et al. Validation of the VapoMeter, a closed unventilated chamber system to assess transepidermal water loss vs. The open chamber Tewameter. Skin Res Technol 2005; 11: 61–69. [DOI] [PubMed] [Google Scholar]
- 24. Akdeniz M, Gabriel S, Lichterfeld-Kottner A, et al. Transepidermal water loss in healthy adults: a systematic review and meta-analysis update. Br J Dermatol 2018; 179: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 25. Hon KL, Leung AK, Barankin B. Barrier repair therapy in atopic dermatitis: an overview. Am J Clin Dermatol 2013; 14: 389–399. [DOI] [PubMed] [Google Scholar]
- 26. Park KY, Kim DH, Jeong MS, et al. Changes of antimicrobial peptides and transepidermal water loss after topical application of tacrolimus and ceramide-dominant emollient in patients with atopic dermatitis. J Korean Med Sci 2010; 25: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol 2002; 47: 198–208. [DOI] [PubMed] [Google Scholar]
- 28. Cristaudo A, Pigliacelli F, Sperati F, et al. Instrumental evaluation of skin barrier function and clinical outcomes during dupilumab treatment for atopic dermatitis: an observational study. Skin Res Technol 2021; 27: 810–813. [DOI] [PubMed] [Google Scholar]
- 29. Rohner MH, Thormann K, Cazzaniga S, et al. Dupilumab reduces inflammation and restores the skin barrier in patients with atopic dermatitis. Allergy 2021; 76: 1268–1270. [DOI] [PubMed] [Google Scholar]